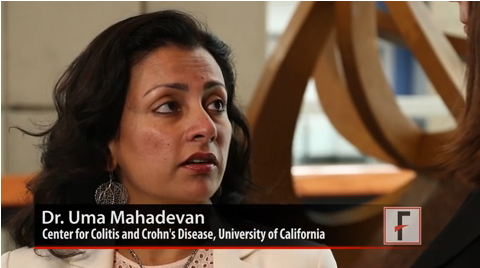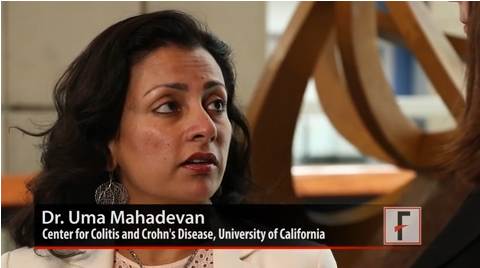User login
Digestive Disease Week (DDW 2014)
VIDEO: Oral drugs replacing interferon for hepatitis C
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
EXPERT ANALYSIS FROM DDW 2014
VIDEO: Oral drugs tackle hepatitis C genotypes 2, 3
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The oral drug duo of sofosbuvir and ribavirin produced good cure rates in a study of 419 patients with hepatitis C genotype 2 or 3.
Dr. Bruce R. Bacon, who was not involved in the study, highlighted the regimen’s efficacy even in patients with cirrhosis or who had failed previous therapy. Hear his perspective on treating genotypes 2 and 3 in this interview at the annual Digestive Disease Week.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University.
He reported financial associations with Gilead Sciences, which funded the study, and with AbbVie and Janssen Pharmaceuticals.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
VIDEO – IBD meds found safe during pregnancy
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At DDW 2014
IBD Meds Found Safe During Pregnancy
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Medications to treat inflammatory bowel disease were well tolerated in pregnancy and weren’t linked to worse maternal-fetal outcomes, according to a study of more than 1,000 pregnant women.
Despite many physicians’ concerns about the safety of azathioprine and biologic agents during pregnancy, maternal exposure to those IBD medications was not associated with an increase in adverse outcomes such as preterm birth or congenital anomalies, explained Dr. Uma Mahadevan of the University of California, San Francisco Center for Colitis and Crohn’s Disease.
"If you continue [the medications] and control your disease and have a healthy pregnancy, that’s the key to having a healthy baby," Dr. Mahadevan observed.
In a video interview at the annual Digestive Disease Week, Dr. Mahadevan discussed the study’s findings, whether combination therapy has an impact on the risk of preterm birth, and what effect IBD medication exposure may have had on children’s achievement of developmental milestones up to age 4 years.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At DDW 2014
VIDEO: New directions in bariatric surgery quality measures
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Bariatric surgery has made great strides in safety and efficacy – so many, in fact, that the field is ready to focus on a new range of quality goals, explained Dr. John Morton.
In an interview at the annual Digestive Disease Week, Dr. Morton, chief of bariatric surgery at Stanford (Calif.) University, discussed how new quality targets, such as 30-day readmissions, could improve bariatric surgery outcomes and boost patient satisfaction.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At DDW 2014
Interferon-free Regimen Improves Response in HCV
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
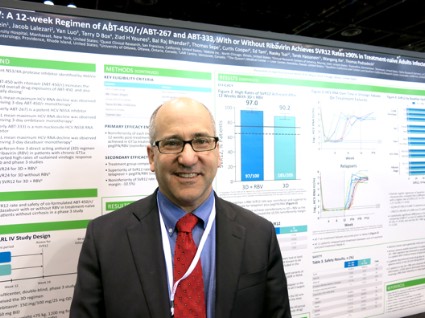
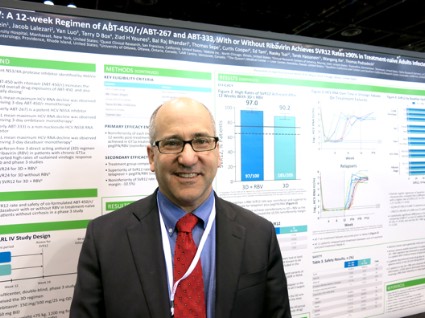
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Interferon-free regimen improves response in HCV
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
With evidence from these trials and other recent studies suggesting that interferon-free regimens will revolutionize treatment of hepatitis C infection, "the future is here," Dr. T. Jake Liang and Dr. Marc G. Ghany wrote in an editorial accompanying the study (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMe1403619]).
"The side effects associated with interferon-based therapy have prevented many patients from undergoing treatment and are a major reason for treatment failure. Perhaps the more important achievement of these interferon-free regimens is the lower rate and severity of side effects associated with treatment," they wrote.
Much remains to be learned, they added. The new interferon-free regimens have been tested mainly in white, middle-aged men without cirrhosis, and not as much in more difficult to treat patients.
Dr. T. Jake Liang and Dr. Marc G. Ghany, both of the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Md., reported having no financial disclosures.
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
CHICAGO – Treating patients with hepatitis C virus genotype 1a or 1b with 12 weeks of an experimental interferon-free, triple-drug oral regimen produced good sustained virologic response rates with or without the use of ribavirin, but the addition of ribavirin appeared helpful to those with genotype 1a infection, according to results of two separate phase III studies.
Both studies recruited previously untreated patients with no cirrhosis to undergo a regimen of ABT-450 with ritonavir and ombitasvir (ABT-450/r-ombitasvir) plus dasabuvir and either ribavirin or a matching placebo. The PEARL-III study randomized 419 patients with hepatitis C virus genotype 1b, and the PEARL-IV study randomized 305 patients with genotype 1a infection. Patients received a daily dose of 150 mg ABT-450, 100 mg ritonavir, and 25 mg ombitasvir, twice-daily doses of 250 mg dasabuvir, and either placebo or ribavirin dosed according to body weight.
In patients with genotype 1a, 97% who got the regimen plus ribavirin and 90.2% of those who got the regimen plus placebo achieved a sustained virologic response (SVR) 12 weeks after treatment ended (defined as a hepatitis C virus RNA level of less than 25 IU/ml). The difference between those two groups was statistically significant, and the treatment without ribavirin was inferior to the regimen with ribavirin in genotype 1a patients. These response rates were superior, however, to rates in the medical literature for treatment-naive patients who got conventional treatment with telaprevir plus peginterferon-ribavirin, Dr. Peter Ferenci and his associates reported.
Virologic failure was significantly more likely in patients with genotype 1a who got the regimen plus placebo in the current study, compared with those who got ribavirin – 7.8% vs. 2%, respectively, reported Dr. Ferenci of the Medical University of Vienna. Only 2 of the 18 patients with genotype 1a who developed virologic failure received ribavirin.
The findings were published online by the New England Journal of Medicine (N. Engl. J. Med. 2014 May 4 [doi:10.1056/NEJMoa1402338]).
Previous data on treatment with telaprevir plus peginterferon-ribavirin showed sustained virologic response rates of 60%-65%, coinvestigator Dr. David Bernstein said in an interview. With the experimental regimen, "the take-home message is that there are extraordinarily high cure rates for patients with hepatitis C genotype 1," said Dr. Bernstein, chief of hepatology at the Center for Liver Disease, North Shore University Hospital, Manhasset, N.Y. He reported results of the PEARL IV study in a poster presentation at the annual Digestive Disease Week.
Genotype 1b is the most prevalent form of hepatitis C, especially in Europe and East Asia, but genotype 1a is more prevalent in North America.
For patients with genotype 1b infection in the study, 99.5% who received the regimen with ribavirin and 99% who got placebo achieved SVR at 12 weeks.
ABT-450/4, ombitasvir, and dasabuvir are not yet approved for treatment, but approval is expected later this year, Dr. Bernstein said.
Fewer than 1% of patients discontinued treatment due to adverse events. "The second take-home message is that it’s a 12-week course of therapy and the overall side effect profile is minimal," he said. "We will be using these therapies once they become available."
ABT-450 inhibits the hepatitis C virus nonstructural 3/4A protease and is given with ritonavir to increase ABT-450 plasma levels and half-life. Ombitasvir inhibits the hepatitis C virus NS5A replication complex. Dasabuvir is a nonnucleoside NS5B polymerase inhibitor.
AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
On Twitter @sherryboschert
AT DDW 2014
Major finding: For patients with genotype 1a infection, SVR occurred in 97% with ribavirin and 90.2% without ribavirin. For patients with genotype 1b infection, rates were 99.5% and 99%, respectively.
Data source: Two randomized, phase III trials of previously untreated patients with hepatitis C and no cirrhosis who were treated with 12 weeks of ABT-450/r, ombitasvir, and dasabuvir with or without ribavirin.
Disclosures: AbbVie, which is developing the new drugs, funded the study. Dr. Ferenci reported financial associations with 10 companies and his coinvestigators reported financial associations with dozens of companies.
When to Screen for Celiac Disease in Hypothyroidism
CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington. That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.

CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington. That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.

CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington. That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.

At DDW 2014
VIDEO: When to screen for celiac disease in hypothyroidism
CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington.That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.
CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington.That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.
CHICAGO – Patients with hypothyroidism who needed higher doses of levothyroxine were more likely to have celiac disease, according to a study released at the annual Digestive Disease Week.
In a study of 400 patients with hypothyroidism, about 5% of those who needed at least 125 mcg/day of levothyroxine also had celiac disease, according to Dr. Richard Zubarik of the University of Vermont, Burlington.That prevalence rate tops even the rate among patients with iron deficiency anemia, for whom celiac disease screening is recommended.
In a video interview, Dr. Zubarik explains which patients with hypothyroidism should be screened for celiac disease, and he discusses what mechanisms might link the need for higher levels of levothyroxine with an increased risk of celiac disease.
At DDW 2014
VIDEO: Low FODMAP diet reduced kids’ IBS pain
CHICAGO – A diet that restricts wheat, beans, dairy, and fruit juice in children with irritable bowel syndrome reduced the frequency and severity of abdominal pain, according to a study presented at the annual Digestive Disease Week.
In a double-blind, randomized, crossover trial of 55 children, those who followed a low FODMAP (fermentable oligo-di-monosaccharides and polyols) diet decreased abdominal pain frequency 20%-30%, explained Dr. Bruno Chumpitazi of Texas Children’s Hospital, Houston.
In a video interview, Dr. Chumpitazi discusses the study's implications for the nearly 20% of school-age children and adolescents who may have IBS, and whether the low FODMAP diet could be a first-line therapy for those children.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A diet that restricts wheat, beans, dairy, and fruit juice in children with irritable bowel syndrome reduced the frequency and severity of abdominal pain, according to a study presented at the annual Digestive Disease Week.
In a double-blind, randomized, crossover trial of 55 children, those who followed a low FODMAP (fermentable oligo-di-monosaccharides and polyols) diet decreased abdominal pain frequency 20%-30%, explained Dr. Bruno Chumpitazi of Texas Children’s Hospital, Houston.
In a video interview, Dr. Chumpitazi discusses the study's implications for the nearly 20% of school-age children and adolescents who may have IBS, and whether the low FODMAP diet could be a first-line therapy for those children.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A diet that restricts wheat, beans, dairy, and fruit juice in children with irritable bowel syndrome reduced the frequency and severity of abdominal pain, according to a study presented at the annual Digestive Disease Week.
In a double-blind, randomized, crossover trial of 55 children, those who followed a low FODMAP (fermentable oligo-di-monosaccharides and polyols) diet decreased abdominal pain frequency 20%-30%, explained Dr. Bruno Chumpitazi of Texas Children’s Hospital, Houston.
In a video interview, Dr. Chumpitazi discusses the study's implications for the nearly 20% of school-age children and adolescents who may have IBS, and whether the low FODMAP diet could be a first-line therapy for those children.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At DDW 2014