User login
Digestive Disease Week (DDW 2014)
Phase III study: Prucalopride eases severe chronic constipation in men
CHICAGO – Prucalopride, a high-affinity serotonin 5-hydroxytryptamine4 receptor agonist, proved effective for the treatment of severe chronic constipation in men in a phase III clinical trial.
In this randomized, 66-site European trial involving 358 subjects on once-daily prucalopride at 2 mg or placebo, 37.9% of the prucalopride group achieved the primary endpoint of a mean of at least three spontaneous complete bowel movements per week over the course of the 12-week treatment period, significantly better than the 17.7% rate in controls, reported Dr. Kevin Etherson of Durham (England) University.
This was no small feat, he noted. Participants had a mean 9.2-year duration of chronic constipation. One-third of controls and 42% of the prucalopride group had experienced less than one spontaneous complete bowel movement per week during the 6 months prior to enrollment.
Prucalopride is already approved in the European Union for the treatment of chronic constipation in women for whom laxatives fail to provide adequate relief. The new phase III data pave the way for an expanded European indication including men. As for when U.S. physicians can expect to be able to prescribe the drug, a Shire spokesperson said the company is "working with the FDA [Food and Drug Administration] to create a regulatory pathway forward."
The new phase III results were consistent with the outcomes seen in the four published pivotal phase III trials conducted with the same endpoints in women, Dr. Etherson said at the annual Digestive Disease Week.
At baseline, 68% of the prucalopride group rated their constipation as "severe" or "very severe." After 12 weeks of active treatment, that was the case for only 22% of the men. At follow-up, 47% of the prucalopride group rated their global treatment efficacy as "quite a bit" or "extremely effective," compared with 30% of placebo-treated controls.
No significant ECG changes occurred during the study. Six men discontinued prucalopride, and seven stopped taking placebo. Gastrointestinal adverse events – mostly diarrhea, nausea, and abdominal pain – were reported by 20% of the men on prucalopride and 14% of the controls. Headaches and dizziness were also more common in the prucalopride group.
The phase III study was sponsored by Shire. Dr. Etherson reported having no financial conflicts of interest.
CHICAGO – Prucalopride, a high-affinity serotonin 5-hydroxytryptamine4 receptor agonist, proved effective for the treatment of severe chronic constipation in men in a phase III clinical trial.
In this randomized, 66-site European trial involving 358 subjects on once-daily prucalopride at 2 mg or placebo, 37.9% of the prucalopride group achieved the primary endpoint of a mean of at least three spontaneous complete bowel movements per week over the course of the 12-week treatment period, significantly better than the 17.7% rate in controls, reported Dr. Kevin Etherson of Durham (England) University.
This was no small feat, he noted. Participants had a mean 9.2-year duration of chronic constipation. One-third of controls and 42% of the prucalopride group had experienced less than one spontaneous complete bowel movement per week during the 6 months prior to enrollment.
Prucalopride is already approved in the European Union for the treatment of chronic constipation in women for whom laxatives fail to provide adequate relief. The new phase III data pave the way for an expanded European indication including men. As for when U.S. physicians can expect to be able to prescribe the drug, a Shire spokesperson said the company is "working with the FDA [Food and Drug Administration] to create a regulatory pathway forward."
The new phase III results were consistent with the outcomes seen in the four published pivotal phase III trials conducted with the same endpoints in women, Dr. Etherson said at the annual Digestive Disease Week.
At baseline, 68% of the prucalopride group rated their constipation as "severe" or "very severe." After 12 weeks of active treatment, that was the case for only 22% of the men. At follow-up, 47% of the prucalopride group rated their global treatment efficacy as "quite a bit" or "extremely effective," compared with 30% of placebo-treated controls.
No significant ECG changes occurred during the study. Six men discontinued prucalopride, and seven stopped taking placebo. Gastrointestinal adverse events – mostly diarrhea, nausea, and abdominal pain – were reported by 20% of the men on prucalopride and 14% of the controls. Headaches and dizziness were also more common in the prucalopride group.
The phase III study was sponsored by Shire. Dr. Etherson reported having no financial conflicts of interest.
CHICAGO – Prucalopride, a high-affinity serotonin 5-hydroxytryptamine4 receptor agonist, proved effective for the treatment of severe chronic constipation in men in a phase III clinical trial.
In this randomized, 66-site European trial involving 358 subjects on once-daily prucalopride at 2 mg or placebo, 37.9% of the prucalopride group achieved the primary endpoint of a mean of at least three spontaneous complete bowel movements per week over the course of the 12-week treatment period, significantly better than the 17.7% rate in controls, reported Dr. Kevin Etherson of Durham (England) University.
This was no small feat, he noted. Participants had a mean 9.2-year duration of chronic constipation. One-third of controls and 42% of the prucalopride group had experienced less than one spontaneous complete bowel movement per week during the 6 months prior to enrollment.
Prucalopride is already approved in the European Union for the treatment of chronic constipation in women for whom laxatives fail to provide adequate relief. The new phase III data pave the way for an expanded European indication including men. As for when U.S. physicians can expect to be able to prescribe the drug, a Shire spokesperson said the company is "working with the FDA [Food and Drug Administration] to create a regulatory pathway forward."
The new phase III results were consistent with the outcomes seen in the four published pivotal phase III trials conducted with the same endpoints in women, Dr. Etherson said at the annual Digestive Disease Week.
At baseline, 68% of the prucalopride group rated their constipation as "severe" or "very severe." After 12 weeks of active treatment, that was the case for only 22% of the men. At follow-up, 47% of the prucalopride group rated their global treatment efficacy as "quite a bit" or "extremely effective," compared with 30% of placebo-treated controls.
No significant ECG changes occurred during the study. Six men discontinued prucalopride, and seven stopped taking placebo. Gastrointestinal adverse events – mostly diarrhea, nausea, and abdominal pain – were reported by 20% of the men on prucalopride and 14% of the controls. Headaches and dizziness were also more common in the prucalopride group.
The phase III study was sponsored by Shire. Dr. Etherson reported having no financial conflicts of interest.
AT DDW 2014
Major finding: Thirty-eight percent of men with longstanding severe chronic constipation achieved a mean of three or more spontaneous complete bowel movements per week while being treated with prucalopride, compared with 17.7% on placebo.
Data source: This was a phase III, randomized, double-blind clinical trial in which 358 evaluable men with chronic constipation received prucalopride at 2 mg once daily or placebo for 12 weeks.
Disclosures: The phase III trial was sponsored by Shire. The presenter reported having no financial conflicts.
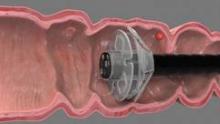
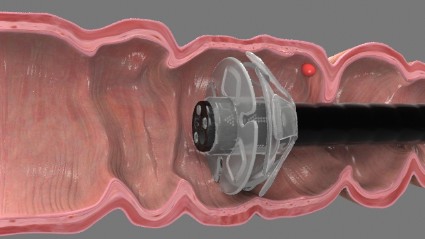
EndoRings-assisted colonoscopy hikes polyp, adenoma detection
CHICAGO – Adding a small rubber tip with flexible, circular rings to the end of a colonoscope dramatically improves polyp and adenoma detection during colonoscopy, according to interim results from a randomized, tandem study.
In a per lesion analysis, the adenoma miss rate was 13% when patients underwent an EndoRing colonoscopy followed by a standard colonoscopy and 53% when standard colonoscopy was done before the EndoRing colonoscopy (P less than .001).
The miss rates for polyps were 11% and 58% (P less than .001), *Dr. Peter D. Siersema said in a late-breaking abstract session at the annual Digestive Disease Week.
Adenomas are missed in about 25% of tandem studies with standard colonoscopies, and the miss rate may be as high as 40% with new techniques. This is mainly due to inadequate visualization of proximal aspects of folds and inner curves of flexures. The flexible, circular rings of the EndoRings improve visualization by engaging and stretching colonic folds during withdrawal, explained Dr. Siersema of the University Medical Center Utrecht, the Netherlands. The EndoRings device is Food and Drug Administration and CE approved and available in the U.S. and Europe.
From July 2013 to April 2014, investigators in the Netherlands and Israel enrolled 106 patients, aged 40-75 years, with an indication for screening, surveillance, or diagnostic colonoscopy, to undergo same-day, back-to-back colonoscopies by the same endoscopist. Bowel preparation followed standard protocol at the centers (polyethylene glycol or sodium picosulfate). Conscious sedation (midazolam, fentanyl, propofol, or combination) was used in most patients. Polyps found during the first procedure were immediately removed.
The average time to the cecum and withdrawal times were similar in the two groups, although total procedure time was significantly longer with the EndoRings (mean 22 minutes vs. 18 minutes; P less than .001) due to the removal of more polyps, Dr. Siersema said.
Among 49 evaluable patients in the study group, 79 polyps were detected with EndoRings during the first procedure and 10 additional polyps during the subsequent standard colonoscopy.
Among 47 evaluable controls, 25 polyps were detected during the first pass with standard colonoscopy and 34 additional adenomas in the second pass with EndoRings.
There was no difference in the location, size, morphology, and histology of the missed polyps between groups, he said.
In the study group, 46 adenomas were found during the initial procedure with EndoRings and 7 more during the second pass using standard colonoscopy.
Among controls, 17 adenomas were detected during the first pass with standard colonoscopy and 19 more in the second pass with EndoRings.
Surveillance intervals were shortened for eight patients who underwent standard colonoscopy first and two patients who underwent EndoRings colonoscopy first, Dr. Siersema said. There were no adverse events in the study.
During a discussion of the results, Dr. Siersema said the single-use device "was very cheap" to purchase and has an additional advantage of stabilizing the scope in the center of the colon.
Session cochair Dr. Pankaj Pasricha, director of the Johns Hopkins Center for Neurogastroenterology, said some colonoscopes are incorporating expandable tips, and that there are four or five other add-on devices, one with streamers and another with a balloon.
"They are relatively inexpensive, simple approaches to solving a complex problem," he said in an interview. "It is a real problem looking behind folds, missed polyps, and if large-scale studies show that [this is improved], then this could be really something very easily incorporated into your practice, as opposed to buying a new scope for hundreds of thousands of dollars."
Dr. Siersema reported a travel grant to attend the meeting. Three coauthors reported consultancy for and one reported employment with the study sponsor, EndoAid. Dr. Pasricha reported relationships with Pentax, GI Supply, and Apollo Endosurgery.
Correction 5/20/14: A previous version of this story incorrectly identified Dr. Peter D. Siersema in both the photograph and in references throughout the text. This version has been corrected and updated.
CHICAGO – Adding a small rubber tip with flexible, circular rings to the end of a colonoscope dramatically improves polyp and adenoma detection during colonoscopy, according to interim results from a randomized, tandem study.
In a per lesion analysis, the adenoma miss rate was 13% when patients underwent an EndoRing colonoscopy followed by a standard colonoscopy and 53% when standard colonoscopy was done before the EndoRing colonoscopy (P less than .001).
The miss rates for polyps were 11% and 58% (P less than .001), *Dr. Peter D. Siersema said in a late-breaking abstract session at the annual Digestive Disease Week.
Adenomas are missed in about 25% of tandem studies with standard colonoscopies, and the miss rate may be as high as 40% with new techniques. This is mainly due to inadequate visualization of proximal aspects of folds and inner curves of flexures. The flexible, circular rings of the EndoRings improve visualization by engaging and stretching colonic folds during withdrawal, explained Dr. Siersema of the University Medical Center Utrecht, the Netherlands. The EndoRings device is Food and Drug Administration and CE approved and available in the U.S. and Europe.
From July 2013 to April 2014, investigators in the Netherlands and Israel enrolled 106 patients, aged 40-75 years, with an indication for screening, surveillance, or diagnostic colonoscopy, to undergo same-day, back-to-back colonoscopies by the same endoscopist. Bowel preparation followed standard protocol at the centers (polyethylene glycol or sodium picosulfate). Conscious sedation (midazolam, fentanyl, propofol, or combination) was used in most patients. Polyps found during the first procedure were immediately removed.
The average time to the cecum and withdrawal times were similar in the two groups, although total procedure time was significantly longer with the EndoRings (mean 22 minutes vs. 18 minutes; P less than .001) due to the removal of more polyps, Dr. Siersema said.
Among 49 evaluable patients in the study group, 79 polyps were detected with EndoRings during the first procedure and 10 additional polyps during the subsequent standard colonoscopy.
Among 47 evaluable controls, 25 polyps were detected during the first pass with standard colonoscopy and 34 additional adenomas in the second pass with EndoRings.
There was no difference in the location, size, morphology, and histology of the missed polyps between groups, he said.
In the study group, 46 adenomas were found during the initial procedure with EndoRings and 7 more during the second pass using standard colonoscopy.
Among controls, 17 adenomas were detected during the first pass with standard colonoscopy and 19 more in the second pass with EndoRings.
Surveillance intervals were shortened for eight patients who underwent standard colonoscopy first and two patients who underwent EndoRings colonoscopy first, Dr. Siersema said. There were no adverse events in the study.
During a discussion of the results, Dr. Siersema said the single-use device "was very cheap" to purchase and has an additional advantage of stabilizing the scope in the center of the colon.
Session cochair Dr. Pankaj Pasricha, director of the Johns Hopkins Center for Neurogastroenterology, said some colonoscopes are incorporating expandable tips, and that there are four or five other add-on devices, one with streamers and another with a balloon.
"They are relatively inexpensive, simple approaches to solving a complex problem," he said in an interview. "It is a real problem looking behind folds, missed polyps, and if large-scale studies show that [this is improved], then this could be really something very easily incorporated into your practice, as opposed to buying a new scope for hundreds of thousands of dollars."
Dr. Siersema reported a travel grant to attend the meeting. Three coauthors reported consultancy for and one reported employment with the study sponsor, EndoAid. Dr. Pasricha reported relationships with Pentax, GI Supply, and Apollo Endosurgery.
Correction 5/20/14: A previous version of this story incorrectly identified Dr. Peter D. Siersema in both the photograph and in references throughout the text. This version has been corrected and updated.
CHICAGO – Adding a small rubber tip with flexible, circular rings to the end of a colonoscope dramatically improves polyp and adenoma detection during colonoscopy, according to interim results from a randomized, tandem study.
In a per lesion analysis, the adenoma miss rate was 13% when patients underwent an EndoRing colonoscopy followed by a standard colonoscopy and 53% when standard colonoscopy was done before the EndoRing colonoscopy (P less than .001).
The miss rates for polyps were 11% and 58% (P less than .001), *Dr. Peter D. Siersema said in a late-breaking abstract session at the annual Digestive Disease Week.
Adenomas are missed in about 25% of tandem studies with standard colonoscopies, and the miss rate may be as high as 40% with new techniques. This is mainly due to inadequate visualization of proximal aspects of folds and inner curves of flexures. The flexible, circular rings of the EndoRings improve visualization by engaging and stretching colonic folds during withdrawal, explained Dr. Siersema of the University Medical Center Utrecht, the Netherlands. The EndoRings device is Food and Drug Administration and CE approved and available in the U.S. and Europe.
From July 2013 to April 2014, investigators in the Netherlands and Israel enrolled 106 patients, aged 40-75 years, with an indication for screening, surveillance, or diagnostic colonoscopy, to undergo same-day, back-to-back colonoscopies by the same endoscopist. Bowel preparation followed standard protocol at the centers (polyethylene glycol or sodium picosulfate). Conscious sedation (midazolam, fentanyl, propofol, or combination) was used in most patients. Polyps found during the first procedure were immediately removed.
The average time to the cecum and withdrawal times were similar in the two groups, although total procedure time was significantly longer with the EndoRings (mean 22 minutes vs. 18 minutes; P less than .001) due to the removal of more polyps, Dr. Siersema said.
Among 49 evaluable patients in the study group, 79 polyps were detected with EndoRings during the first procedure and 10 additional polyps during the subsequent standard colonoscopy.
Among 47 evaluable controls, 25 polyps were detected during the first pass with standard colonoscopy and 34 additional adenomas in the second pass with EndoRings.
There was no difference in the location, size, morphology, and histology of the missed polyps between groups, he said.
In the study group, 46 adenomas were found during the initial procedure with EndoRings and 7 more during the second pass using standard colonoscopy.
Among controls, 17 adenomas were detected during the first pass with standard colonoscopy and 19 more in the second pass with EndoRings.
Surveillance intervals were shortened for eight patients who underwent standard colonoscopy first and two patients who underwent EndoRings colonoscopy first, Dr. Siersema said. There were no adverse events in the study.
During a discussion of the results, Dr. Siersema said the single-use device "was very cheap" to purchase and has an additional advantage of stabilizing the scope in the center of the colon.
Session cochair Dr. Pankaj Pasricha, director of the Johns Hopkins Center for Neurogastroenterology, said some colonoscopes are incorporating expandable tips, and that there are four or five other add-on devices, one with streamers and another with a balloon.
"They are relatively inexpensive, simple approaches to solving a complex problem," he said in an interview. "It is a real problem looking behind folds, missed polyps, and if large-scale studies show that [this is improved], then this could be really something very easily incorporated into your practice, as opposed to buying a new scope for hundreds of thousands of dollars."
Dr. Siersema reported a travel grant to attend the meeting. Three coauthors reported consultancy for and one reported employment with the study sponsor, EndoAid. Dr. Pasricha reported relationships with Pentax, GI Supply, and Apollo Endosurgery.
Correction 5/20/14: A previous version of this story incorrectly identified Dr. Peter D. Siersema in both the photograph and in references throughout the text. This version has been corrected and updated.
AT DDW 2014
Major finding: In 49 study patients, 79 polyps were found with EndoRings, then 10 more were found with standard follow-up colonoscopy; in 47 control patients 25 polyps were found by standard colonoscopy, then 34 more adenomas were found with EndoRings device.
Data source: A prospective study in 96 patients with back-to-back colonoscopies.
Disclosures: Dr. Dik reported a travel grant to attend the meeting. Three coauthors reported consultancy for and one employment with the study sponsor, EndoAid. Dr. Pasricha reported relationships with Pentax, GI Supply, and Apollo Endosurgery.
What Puts C difficile Patients at Greater Readmission Risk?
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
What puts C. diff patients at greater readmission risk?
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – More and more elderly people are getting infected with Clostridium difficile, and many get readmitted to the hospital after initial treatment for this potentially deadly diarrheal illness. Why?
A large analysis of Medicare data identified risk factors for readmission due to C. difficile in the elderly, Dr. Courtney Collins of the University of Massachusetts, Worcester, and her associates reported at the annual Digestive Disease Week.
The first month after discharge from a C. difficile hospitalization appears to be a crucial time to think about preventing reinfection. Dr. Collins spoke with us about her findings, and how they will change her clinical practice and, perhaps, yours.
Dr. Collins reported having no disclosures.
On Twitter @sherryboschert
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
VIDEO: Vibrating pill may shake up constipation treatment
CHICAGO – A newly developed oral capsule may offer chronic idiopathic constipation patients a novel, nonpharmacologic approach to treatment: It vibrates as it moves through the intestines.
In a video interview at the annual Digestive Disease Week, the study’s lead researcher, Dr. Yishai Ron of Tel Aviv Sourasky Medical Center, explained how the mechanical pill works, what effects it had on users’ spontaneous bowel movements and constipation symptoms, and why such an approach may have promise in treating chronic idiopathic constipation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A newly developed oral capsule may offer chronic idiopathic constipation patients a novel, nonpharmacologic approach to treatment: It vibrates as it moves through the intestines.
In a video interview at the annual Digestive Disease Week, the study’s lead researcher, Dr. Yishai Ron of Tel Aviv Sourasky Medical Center, explained how the mechanical pill works, what effects it had on users’ spontaneous bowel movements and constipation symptoms, and why such an approach may have promise in treating chronic idiopathic constipation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – A newly developed oral capsule may offer chronic idiopathic constipation patients a novel, nonpharmacologic approach to treatment: It vibrates as it moves through the intestines.
In a video interview at the annual Digestive Disease Week, the study’s lead researcher, Dr. Yishai Ron of Tel Aviv Sourasky Medical Center, explained how the mechanical pill works, what effects it had on users’ spontaneous bowel movements and constipation symptoms, and why such an approach may have promise in treating chronic idiopathic constipation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
VIDEO: Acupuncture beats propofol for endoscopy analgesia
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
CHICAGO – Endoscopic ultrasound can be a long, uncomfortable, and even painful procedure. But electroacupuncture starting 45 minutes before the procedure significantly decreased the need for drugs to control pain in a prospective, randomized, double-blind, sham-controlled study of 64 patients in Hong Kong.
Dr. Anthony Y. Teoh, who did the endoscopies, and Dr. Wing Wa Leung, the acupuncturist, showed us how they did it in an interview at the annual Digestive Disease Week. Both are of the department of surgery at Chinese University of Hong Kong.
The 32 patients in the electroacupuncture group took a median of only two hits of patient-controlled analgesia with a mean of 0.22 mg/kg propofol, compared with 10 hits for the 32 patients in the control group with a mean of 0.71 mg/kg propofol. Patients rated their pain significantly lower and their satisfaction with the procedure significantly higher in the electroacupuncture group than in the control group, while it made no difference for the endoscopist, whose satisfaction scores were similar between groups. Fifteen patients with acupuncture said they’d be willing to repeat the procedure, compared with eight control patients.
Does that mean electroacupuncture will be coming to your endoscopy suite? Hear what Dr. Teoh and Dr. Leung have to say.
They reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT DDW 2014
GERD may boost risk of MI
CHICAGO – Gastroesophageal reflux disease may constitute a heretofore unrecognized risk factor for coronary heart disease.
In a nationwide case-control study of prodigious proportions, endoscopically confirmed GERD in patients without known coronary or peripheral artery disease at baseline was independently associated with a 57% increased risk of having a first acute MI within the next 5 years, Dr. Ravi K. Prakash reported at the annual Digestive Disease Week.
If this novel finding is confirmed in other databases, the clinical implications would be profound. GERD is after all an extremely common problem, affecting 30%-40% of the U.S. population, noted Dr. Prakash, a gastroenterology fellow at MetroHealth Medical Center and Case Western Reserve University, Cleveland.
The good news: When Dr. Prakash and his coinvestigators divided the 316,390 study participants with GERD into those who were on proton pump inhibitor therapy and those who weren’t, they found that the PPI users weren’t at increased MI risk, compared with the more than 13.6 million similarly aged control subjects who didn’t have GERD, had never undergone an upper endoscopy, and were free of known atherosclerotic disease at baseline.
"Effective treatment of GERD appears to protect against MI," he concluded.
The study population was drawn from Explorys, a private, cloud-based health database containing the electronic health records of 35 million U.S. patients as provided by more than 200,000 physicians in 300-plus health care systems.
A first MI occurred during follow-up in 18,860 GERD patients, or 5.96%, compared with 144,140 controls, or 1.05%. This translates into an unadjusted sixfold increased risk of acute MI in patients with rigorously diagnosed GERD, compared with GERD-free controls.
The investigators then performed a multivariate logistic regression analysis adjusted for six major cardiovascular risk factors: obesity, hypertension, diabetes, tobacco use, hyperlipidemia, and sex. Many of these risk factors were substantially more prevalent in the GERD population. As a result, the unadjusted sixfold increase in MI risk associated with GERD was attenuated in this adjusted risk model; however, the adjusted 57% increased risk remained highly significant.
Moreover, the increased relative risk of GERD seen in the multivariate analysis was comparable with the risks posed by many of the established risk factors. Obesity, for example, showed a 49% increase in MI risk, smoking a 90% increase, and hypertension a 10% increased relative risk, compared with normotension. Only hyperlipidemia and diabetes were in another league, with relative risks of 9.5- and 2.2-fold, respectively, Dr. Prakash continued.
He observed that during the past decade, the research focus has shifted away from GERD as a caustic disease process causing local injury to GERD as a systemic inflammatory disease. Among the proinflammatory cytokines shown to be elevated both in the esophagus and circulation of GERD patients are interleukins-6, -8, and -1B as well as platelet-activating factor. These cytokines are well established as important players in the formation of atherosclerotic plaque in arteries. It was the shared elevations in proinflammatory cytokines that led Dr. Prakash and coworkers to hypothesize that patients with GERD might have an increased incidence of MI.
It’s only relatively recently that several other common chronic diseases have been reconceptualized as systemic inflammatory diseases associated with increased cardiovascular risk. Diabetes, for example, has been repositioned from a straightforward endocrine disease to new status as a coronary heart disease equivalent. And strong bodies of evidence link psoriasis and periodontitis to increased cardiovascular risk, Dr. Prakash noted.
Audience member Dr. Nimish B. Vakil, a Milwaukee gastroenterologist, rose to caution about the possibility of residual confounding by other factors not accounted for in the multivariate analysis, and thus the need for confirmatory studies. That being said, he added that he finds the notion of a GERD/acute MI link mechanistically quite plausible. Acid exposure from GERD might very well trigger exaggerated firing of the esophagocardiac reflex, with resultant episodes of coronary hypoperfusion. Effective PPI therapy for GERD would be expected to reduce such events.
Dr. Prakash said his literature search suggested another possible mechanism for the apparent protective effect of PPIs against MI: effective treatment of GERD, whether using PPIs or via a fundoplication procedure, has been reported to reduce levels of the inflammatory cytokines present in GERD.
Dr. Prakash reported having no financial conflicts with regard to his study, which was supported by institutional funds.
CHICAGO – Gastroesophageal reflux disease may constitute a heretofore unrecognized risk factor for coronary heart disease.
In a nationwide case-control study of prodigious proportions, endoscopically confirmed GERD in patients without known coronary or peripheral artery disease at baseline was independently associated with a 57% increased risk of having a first acute MI within the next 5 years, Dr. Ravi K. Prakash reported at the annual Digestive Disease Week.
If this novel finding is confirmed in other databases, the clinical implications would be profound. GERD is after all an extremely common problem, affecting 30%-40% of the U.S. population, noted Dr. Prakash, a gastroenterology fellow at MetroHealth Medical Center and Case Western Reserve University, Cleveland.
The good news: When Dr. Prakash and his coinvestigators divided the 316,390 study participants with GERD into those who were on proton pump inhibitor therapy and those who weren’t, they found that the PPI users weren’t at increased MI risk, compared with the more than 13.6 million similarly aged control subjects who didn’t have GERD, had never undergone an upper endoscopy, and were free of known atherosclerotic disease at baseline.
"Effective treatment of GERD appears to protect against MI," he concluded.
The study population was drawn from Explorys, a private, cloud-based health database containing the electronic health records of 35 million U.S. patients as provided by more than 200,000 physicians in 300-plus health care systems.
A first MI occurred during follow-up in 18,860 GERD patients, or 5.96%, compared with 144,140 controls, or 1.05%. This translates into an unadjusted sixfold increased risk of acute MI in patients with rigorously diagnosed GERD, compared with GERD-free controls.
The investigators then performed a multivariate logistic regression analysis adjusted for six major cardiovascular risk factors: obesity, hypertension, diabetes, tobacco use, hyperlipidemia, and sex. Many of these risk factors were substantially more prevalent in the GERD population. As a result, the unadjusted sixfold increase in MI risk associated with GERD was attenuated in this adjusted risk model; however, the adjusted 57% increased risk remained highly significant.
Moreover, the increased relative risk of GERD seen in the multivariate analysis was comparable with the risks posed by many of the established risk factors. Obesity, for example, showed a 49% increase in MI risk, smoking a 90% increase, and hypertension a 10% increased relative risk, compared with normotension. Only hyperlipidemia and diabetes were in another league, with relative risks of 9.5- and 2.2-fold, respectively, Dr. Prakash continued.
He observed that during the past decade, the research focus has shifted away from GERD as a caustic disease process causing local injury to GERD as a systemic inflammatory disease. Among the proinflammatory cytokines shown to be elevated both in the esophagus and circulation of GERD patients are interleukins-6, -8, and -1B as well as platelet-activating factor. These cytokines are well established as important players in the formation of atherosclerotic plaque in arteries. It was the shared elevations in proinflammatory cytokines that led Dr. Prakash and coworkers to hypothesize that patients with GERD might have an increased incidence of MI.
It’s only relatively recently that several other common chronic diseases have been reconceptualized as systemic inflammatory diseases associated with increased cardiovascular risk. Diabetes, for example, has been repositioned from a straightforward endocrine disease to new status as a coronary heart disease equivalent. And strong bodies of evidence link psoriasis and periodontitis to increased cardiovascular risk, Dr. Prakash noted.
Audience member Dr. Nimish B. Vakil, a Milwaukee gastroenterologist, rose to caution about the possibility of residual confounding by other factors not accounted for in the multivariate analysis, and thus the need for confirmatory studies. That being said, he added that he finds the notion of a GERD/acute MI link mechanistically quite plausible. Acid exposure from GERD might very well trigger exaggerated firing of the esophagocardiac reflex, with resultant episodes of coronary hypoperfusion. Effective PPI therapy for GERD would be expected to reduce such events.
Dr. Prakash said his literature search suggested another possible mechanism for the apparent protective effect of PPIs against MI: effective treatment of GERD, whether using PPIs or via a fundoplication procedure, has been reported to reduce levels of the inflammatory cytokines present in GERD.
Dr. Prakash reported having no financial conflicts with regard to his study, which was supported by institutional funds.
CHICAGO – Gastroesophageal reflux disease may constitute a heretofore unrecognized risk factor for coronary heart disease.
In a nationwide case-control study of prodigious proportions, endoscopically confirmed GERD in patients without known coronary or peripheral artery disease at baseline was independently associated with a 57% increased risk of having a first acute MI within the next 5 years, Dr. Ravi K. Prakash reported at the annual Digestive Disease Week.
If this novel finding is confirmed in other databases, the clinical implications would be profound. GERD is after all an extremely common problem, affecting 30%-40% of the U.S. population, noted Dr. Prakash, a gastroenterology fellow at MetroHealth Medical Center and Case Western Reserve University, Cleveland.
The good news: When Dr. Prakash and his coinvestigators divided the 316,390 study participants with GERD into those who were on proton pump inhibitor therapy and those who weren’t, they found that the PPI users weren’t at increased MI risk, compared with the more than 13.6 million similarly aged control subjects who didn’t have GERD, had never undergone an upper endoscopy, and were free of known atherosclerotic disease at baseline.
"Effective treatment of GERD appears to protect against MI," he concluded.
The study population was drawn from Explorys, a private, cloud-based health database containing the electronic health records of 35 million U.S. patients as provided by more than 200,000 physicians in 300-plus health care systems.
A first MI occurred during follow-up in 18,860 GERD patients, or 5.96%, compared with 144,140 controls, or 1.05%. This translates into an unadjusted sixfold increased risk of acute MI in patients with rigorously diagnosed GERD, compared with GERD-free controls.
The investigators then performed a multivariate logistic regression analysis adjusted for six major cardiovascular risk factors: obesity, hypertension, diabetes, tobacco use, hyperlipidemia, and sex. Many of these risk factors were substantially more prevalent in the GERD population. As a result, the unadjusted sixfold increase in MI risk associated with GERD was attenuated in this adjusted risk model; however, the adjusted 57% increased risk remained highly significant.
Moreover, the increased relative risk of GERD seen in the multivariate analysis was comparable with the risks posed by many of the established risk factors. Obesity, for example, showed a 49% increase in MI risk, smoking a 90% increase, and hypertension a 10% increased relative risk, compared with normotension. Only hyperlipidemia and diabetes were in another league, with relative risks of 9.5- and 2.2-fold, respectively, Dr. Prakash continued.
He observed that during the past decade, the research focus has shifted away from GERD as a caustic disease process causing local injury to GERD as a systemic inflammatory disease. Among the proinflammatory cytokines shown to be elevated both in the esophagus and circulation of GERD patients are interleukins-6, -8, and -1B as well as platelet-activating factor. These cytokines are well established as important players in the formation of atherosclerotic plaque in arteries. It was the shared elevations in proinflammatory cytokines that led Dr. Prakash and coworkers to hypothesize that patients with GERD might have an increased incidence of MI.
It’s only relatively recently that several other common chronic diseases have been reconceptualized as systemic inflammatory diseases associated with increased cardiovascular risk. Diabetes, for example, has been repositioned from a straightforward endocrine disease to new status as a coronary heart disease equivalent. And strong bodies of evidence link psoriasis and periodontitis to increased cardiovascular risk, Dr. Prakash noted.
Audience member Dr. Nimish B. Vakil, a Milwaukee gastroenterologist, rose to caution about the possibility of residual confounding by other factors not accounted for in the multivariate analysis, and thus the need for confirmatory studies. That being said, he added that he finds the notion of a GERD/acute MI link mechanistically quite plausible. Acid exposure from GERD might very well trigger exaggerated firing of the esophagocardiac reflex, with resultant episodes of coronary hypoperfusion. Effective PPI therapy for GERD would be expected to reduce such events.
Dr. Prakash said his literature search suggested another possible mechanism for the apparent protective effect of PPIs against MI: effective treatment of GERD, whether using PPIs or via a fundoplication procedure, has been reported to reduce levels of the inflammatory cytokines present in GERD.
Dr. Prakash reported having no financial conflicts with regard to his study, which was supported by institutional funds.
AT DDW 2014
Major finding: GERD was found to be independently associated with a 57% increased risk of acute MI.
Data source: This was a case-control study that harnessed the huge national Explorys database to compare first MI rates in 316,390 subjects with confirmed GERD to more than 13.6 million controls without GERD who’d never had an upper endoscopy.
Disclosures: This study was supported by institutional funds. The presenter reported having no financial conflicts.
VIDEO: PCPs fuzzy on HCC surveillance in cirrhosis
CHICAGO – Several medical societies recommend that patients with cirrhosis undergo ultrasound surveillance for hepatocellular carcinoma every 6 months, but studies suggest less than 20% receive surveillance. The most common reason for failure to get the screening is lack of provider recommendation.
In a video interview with us at the annual Digestive Disease Week, Dr. Eimile Dalton-Fitzgerald of the University of Texas Southwestern Medical Center at Dallas delves into primary care provider (PCP) practice patterns, knowledge, and attitudes regarding hepatocellular carcinoma (HCC) surveillance gleaned from a survey of 77 PCPs in a large safety-net hospital in Dallas County, Texas.
Though most PCPs surveyed believe HCC surveillance is their responsibility (90%) and is effective for early tumor detection in cirrhosis (85%), many had misconceptions about the appropriate surveillance test choice.
PCPs also report several barriers to HCC surveillance, including not being up to date with surveillance guidelines (68%), difficulty with patient communication (56%), and having more important issues to manage in the clinic (52%).
A full 87% also said they’re influenced by a lack of recommendations from the U.S. Preventive Services Task Force, which has yet to weigh in on the issue.
Dr. Dalton-Fitzgerald reported no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Several medical societies recommend that patients with cirrhosis undergo ultrasound surveillance for hepatocellular carcinoma every 6 months, but studies suggest less than 20% receive surveillance. The most common reason for failure to get the screening is lack of provider recommendation.
In a video interview with us at the annual Digestive Disease Week, Dr. Eimile Dalton-Fitzgerald of the University of Texas Southwestern Medical Center at Dallas delves into primary care provider (PCP) practice patterns, knowledge, and attitudes regarding hepatocellular carcinoma (HCC) surveillance gleaned from a survey of 77 PCPs in a large safety-net hospital in Dallas County, Texas.
Though most PCPs surveyed believe HCC surveillance is their responsibility (90%) and is effective for early tumor detection in cirrhosis (85%), many had misconceptions about the appropriate surveillance test choice.
PCPs also report several barriers to HCC surveillance, including not being up to date with surveillance guidelines (68%), difficulty with patient communication (56%), and having more important issues to manage in the clinic (52%).
A full 87% also said they’re influenced by a lack of recommendations from the U.S. Preventive Services Task Force, which has yet to weigh in on the issue.
Dr. Dalton-Fitzgerald reported no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Several medical societies recommend that patients with cirrhosis undergo ultrasound surveillance for hepatocellular carcinoma every 6 months, but studies suggest less than 20% receive surveillance. The most common reason for failure to get the screening is lack of provider recommendation.
In a video interview with us at the annual Digestive Disease Week, Dr. Eimile Dalton-Fitzgerald of the University of Texas Southwestern Medical Center at Dallas delves into primary care provider (PCP) practice patterns, knowledge, and attitudes regarding hepatocellular carcinoma (HCC) surveillance gleaned from a survey of 77 PCPs in a large safety-net hospital in Dallas County, Texas.
Though most PCPs surveyed believe HCC surveillance is their responsibility (90%) and is effective for early tumor detection in cirrhosis (85%), many had misconceptions about the appropriate surveillance test choice.
PCPs also report several barriers to HCC surveillance, including not being up to date with surveillance guidelines (68%), difficulty with patient communication (56%), and having more important issues to manage in the clinic (52%).
A full 87% also said they’re influenced by a lack of recommendations from the U.S. Preventive Services Task Force, which has yet to weigh in on the issue.
Dr. Dalton-Fitzgerald reported no conflicting interests.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT DDW 2014
RM-131 makes headway against diabetic gastroparesis
CHICAGO – The investigational ghrelin agonist RM-131 significantly improved gastric emptying and vomiting in patients with diabetic gastroparesis in a phase II, double-blind study.
Gastric emptying improved by an average of 23 minutes from baseline after 4 weeks of twice-daily subcutaneous injections of RM-131 10 mcg (P less than .001). This compares with nonsignificant improvements of 7.5 minutes for placebo and 5.9 minutes for once-daily RM-131 10 mcg, Dr. Anthony Lembo reported in a late-breaking abstract session at the annual Digestive Disease Week.
Twice-daily RM-131 also reduced weekly vomiting episodes by 63% (P = .033) and vomiting severity by 58% (P = .005), compared with placebo.
RM-131, also known as relamorelin, has been granted fast-track review status by the Food and Drug Administration for the treatment of diabetic gastroparesis. The novel ghrelin pentapeptide agonist is 15- to 130-fold more potent than ghrelin, a hormone produced in the stomach that stimulates gastrointestinal activity, according to Dr. Lembo, with Beth Israel Deaconess Medical Center in Boston.
The study enrolled 204 patients with type 1 or type 2 diabetes and a hemoglobin A1c value of less than 11%; a history of ongoing gastroparesis symptoms; nausea and/or vomiting at least once in the 2 weeks prior to enrollment; and a screening gastric emptying time of more than 79 minutes. Their mean age was 55 years.
A post hoc analysis on patients with baseline vomiting showed that twice-daily RM-131 was effective on all endpoints, including four subjective patient-reported symptoms – nausea, abdominal pain, bloating, and early satiety, Dr. Lembo said.
Gastric emptying, as demonstrated by the Gastric Emptying Breath Test, improved by 30.6 minutes from baseline (P = .02), weekly vomiting episodes by 63% (P = .041), and vomiting severity by 59% (P = .006).
The four-symptom composite score improved numerically, but not significantly, in the overall population, whereas there was a clear and significant improvement in 1 week in the subgroup with vomiting (6.36 points; P less than .043), he said.
The subgroup with vomiting comprised about 60% of the study population, and had 5.6 to 6.4 baseline vomiting episodes per week versus 3.2 to 3.5 per week in the overall population. Vomiting is the most bothersome symptom in diabetic gastroparesis and often brings patients to the hospital for treatment, Dr. Lembo observed.
The subset with vomiting also had more severe overall gastric emptying symptoms at baseline. Initial analyses, however, suggest a weak correlation between improvement in gastric emptying and symptom improvement, he said.
Session cochair Dr. John Inadomi, professor of medicine and head of gastroenterology, University of Washington, Seattle, said in an interview that the weak correlation "is not surprising given what we know about gastroparesis and the pathogenesis of symptoms, but it does raise questions about where the most effective therapy is going to come from. Is it going to come from trying to accelerate gastric emptying or trying to go after some other way to relieve symptoms? Nevertheless, this is the first study of a so-called pure prokinetic that has had at least some clinical benefit, but again, it was in the subset of patients with vomiting."
Treatment-emergent adverse events occurred in 30 of 69 patients (43.5%) on placebo, 32 of 67 patients (47.8%) on once-daily RM-131, and 25 of 68 patients (36.8%) on twice-daily dosing. No adverse event was more common with RM-131 and likewise, there were no concerns about labs, glucose, weight, ECGs, physical exam, or injection-site reactions, Dr. Lembo said.
Rhythm Pharmaceuticals sponsored the study. Dr. Lembo reported relationships with Salix Pharmaceuticals, AstraZeneca, and other companies. Dr. Inadomi reported relationships with Given Imaging, ChemImage, and other companies.
Increased public awareness and the recent release of updated clinical guidelines have made the diagnosis of gastroparesis easier for clinicians. The treatment of gastroparesis, however, remains problematic for both patients and providers for a number of reasons. One, gastroparesis is not a single disorder; rather, the pathophysiology of gastroparesis is multifactorial, and patients with identical symptoms often have different underlying pathophysiologic processes responsible for symptom generation. Two, treating the underlying pathophysiology (i.e., delayed gastric emptying) does not necessarily translate into an improvement in symptoms. Three, metoclopramide is the only medication currently approved by the Food and Drug Administration for the treatment of diabetic gastroparesis, although it is not recommended for long-term use and may cause side effects in up to 30% of diabetic patients. Finally, distinguishing idiopathic gastroparesis from functional dyspepsia can be difficult, further complicating treatment.
 |
| Dr. Brian E. Lacy |
The authors also reported that RM-131 improved gastric emptying at the end of the 4-week study, measured by a breath test. Other individual symptoms of gastroparesis were not improved in the total group, although in a subgroup of patients with more severe vomiting symptoms, RM-131 appeared to provide the greatest improvement in symptoms. As in other studies in gastroparetic patients, improvement in gastric emptying did not predict an improvement in symptoms.
Although the precise mechanism by which RM-131 improves symptoms, especially in the subset of patients with predominant vomiting, is unknown, the current study is important, as it signals interest in an entirely new class of therapeutic agents for diabetic gastroparesis. Larger prospective studies, subtyped based on predominant symptoms and the extent of delayed gastric emptying, are eagerly awaited.
Dr. Brian E. Lacy is professor of medicine, Geisel School of Medicine at Dartmouth, and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center, Hanover, N.H. He is on the scientific advisory boards of Ironwood, Takeda, Prometheus, and Salix.
Increased public awareness and the recent release of updated clinical guidelines have made the diagnosis of gastroparesis easier for clinicians. The treatment of gastroparesis, however, remains problematic for both patients and providers for a number of reasons. One, gastroparesis is not a single disorder; rather, the pathophysiology of gastroparesis is multifactorial, and patients with identical symptoms often have different underlying pathophysiologic processes responsible for symptom generation. Two, treating the underlying pathophysiology (i.e., delayed gastric emptying) does not necessarily translate into an improvement in symptoms. Three, metoclopramide is the only medication currently approved by the Food and Drug Administration for the treatment of diabetic gastroparesis, although it is not recommended for long-term use and may cause side effects in up to 30% of diabetic patients. Finally, distinguishing idiopathic gastroparesis from functional dyspepsia can be difficult, further complicating treatment.
 |
| Dr. Brian E. Lacy |
The authors also reported that RM-131 improved gastric emptying at the end of the 4-week study, measured by a breath test. Other individual symptoms of gastroparesis were not improved in the total group, although in a subgroup of patients with more severe vomiting symptoms, RM-131 appeared to provide the greatest improvement in symptoms. As in other studies in gastroparetic patients, improvement in gastric emptying did not predict an improvement in symptoms.
Although the precise mechanism by which RM-131 improves symptoms, especially in the subset of patients with predominant vomiting, is unknown, the current study is important, as it signals interest in an entirely new class of therapeutic agents for diabetic gastroparesis. Larger prospective studies, subtyped based on predominant symptoms and the extent of delayed gastric emptying, are eagerly awaited.
Dr. Brian E. Lacy is professor of medicine, Geisel School of Medicine at Dartmouth, and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center, Hanover, N.H. He is on the scientific advisory boards of Ironwood, Takeda, Prometheus, and Salix.
Increased public awareness and the recent release of updated clinical guidelines have made the diagnosis of gastroparesis easier for clinicians. The treatment of gastroparesis, however, remains problematic for both patients and providers for a number of reasons. One, gastroparesis is not a single disorder; rather, the pathophysiology of gastroparesis is multifactorial, and patients with identical symptoms often have different underlying pathophysiologic processes responsible for symptom generation. Two, treating the underlying pathophysiology (i.e., delayed gastric emptying) does not necessarily translate into an improvement in symptoms. Three, metoclopramide is the only medication currently approved by the Food and Drug Administration for the treatment of diabetic gastroparesis, although it is not recommended for long-term use and may cause side effects in up to 30% of diabetic patients. Finally, distinguishing idiopathic gastroparesis from functional dyspepsia can be difficult, further complicating treatment.
 |
| Dr. Brian E. Lacy |
The authors also reported that RM-131 improved gastric emptying at the end of the 4-week study, measured by a breath test. Other individual symptoms of gastroparesis were not improved in the total group, although in a subgroup of patients with more severe vomiting symptoms, RM-131 appeared to provide the greatest improvement in symptoms. As in other studies in gastroparetic patients, improvement in gastric emptying did not predict an improvement in symptoms.
Although the precise mechanism by which RM-131 improves symptoms, especially in the subset of patients with predominant vomiting, is unknown, the current study is important, as it signals interest in an entirely new class of therapeutic agents for diabetic gastroparesis. Larger prospective studies, subtyped based on predominant symptoms and the extent of delayed gastric emptying, are eagerly awaited.
Dr. Brian E. Lacy is professor of medicine, Geisel School of Medicine at Dartmouth, and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center, Hanover, N.H. He is on the scientific advisory boards of Ironwood, Takeda, Prometheus, and Salix.
CHICAGO – The investigational ghrelin agonist RM-131 significantly improved gastric emptying and vomiting in patients with diabetic gastroparesis in a phase II, double-blind study.
Gastric emptying improved by an average of 23 minutes from baseline after 4 weeks of twice-daily subcutaneous injections of RM-131 10 mcg (P less than .001). This compares with nonsignificant improvements of 7.5 minutes for placebo and 5.9 minutes for once-daily RM-131 10 mcg, Dr. Anthony Lembo reported in a late-breaking abstract session at the annual Digestive Disease Week.
Twice-daily RM-131 also reduced weekly vomiting episodes by 63% (P = .033) and vomiting severity by 58% (P = .005), compared with placebo.
RM-131, also known as relamorelin, has been granted fast-track review status by the Food and Drug Administration for the treatment of diabetic gastroparesis. The novel ghrelin pentapeptide agonist is 15- to 130-fold more potent than ghrelin, a hormone produced in the stomach that stimulates gastrointestinal activity, according to Dr. Lembo, with Beth Israel Deaconess Medical Center in Boston.
The study enrolled 204 patients with type 1 or type 2 diabetes and a hemoglobin A1c value of less than 11%; a history of ongoing gastroparesis symptoms; nausea and/or vomiting at least once in the 2 weeks prior to enrollment; and a screening gastric emptying time of more than 79 minutes. Their mean age was 55 years.
A post hoc analysis on patients with baseline vomiting showed that twice-daily RM-131 was effective on all endpoints, including four subjective patient-reported symptoms – nausea, abdominal pain, bloating, and early satiety, Dr. Lembo said.
Gastric emptying, as demonstrated by the Gastric Emptying Breath Test, improved by 30.6 minutes from baseline (P = .02), weekly vomiting episodes by 63% (P = .041), and vomiting severity by 59% (P = .006).
The four-symptom composite score improved numerically, but not significantly, in the overall population, whereas there was a clear and significant improvement in 1 week in the subgroup with vomiting (6.36 points; P less than .043), he said.
The subgroup with vomiting comprised about 60% of the study population, and had 5.6 to 6.4 baseline vomiting episodes per week versus 3.2 to 3.5 per week in the overall population. Vomiting is the most bothersome symptom in diabetic gastroparesis and often brings patients to the hospital for treatment, Dr. Lembo observed.
The subset with vomiting also had more severe overall gastric emptying symptoms at baseline. Initial analyses, however, suggest a weak correlation between improvement in gastric emptying and symptom improvement, he said.
Session cochair Dr. John Inadomi, professor of medicine and head of gastroenterology, University of Washington, Seattle, said in an interview that the weak correlation "is not surprising given what we know about gastroparesis and the pathogenesis of symptoms, but it does raise questions about where the most effective therapy is going to come from. Is it going to come from trying to accelerate gastric emptying or trying to go after some other way to relieve symptoms? Nevertheless, this is the first study of a so-called pure prokinetic that has had at least some clinical benefit, but again, it was in the subset of patients with vomiting."
Treatment-emergent adverse events occurred in 30 of 69 patients (43.5%) on placebo, 32 of 67 patients (47.8%) on once-daily RM-131, and 25 of 68 patients (36.8%) on twice-daily dosing. No adverse event was more common with RM-131 and likewise, there were no concerns about labs, glucose, weight, ECGs, physical exam, or injection-site reactions, Dr. Lembo said.
Rhythm Pharmaceuticals sponsored the study. Dr. Lembo reported relationships with Salix Pharmaceuticals, AstraZeneca, and other companies. Dr. Inadomi reported relationships with Given Imaging, ChemImage, and other companies.
CHICAGO – The investigational ghrelin agonist RM-131 significantly improved gastric emptying and vomiting in patients with diabetic gastroparesis in a phase II, double-blind study.
Gastric emptying improved by an average of 23 minutes from baseline after 4 weeks of twice-daily subcutaneous injections of RM-131 10 mcg (P less than .001). This compares with nonsignificant improvements of 7.5 minutes for placebo and 5.9 minutes for once-daily RM-131 10 mcg, Dr. Anthony Lembo reported in a late-breaking abstract session at the annual Digestive Disease Week.
Twice-daily RM-131 also reduced weekly vomiting episodes by 63% (P = .033) and vomiting severity by 58% (P = .005), compared with placebo.
RM-131, also known as relamorelin, has been granted fast-track review status by the Food and Drug Administration for the treatment of diabetic gastroparesis. The novel ghrelin pentapeptide agonist is 15- to 130-fold more potent than ghrelin, a hormone produced in the stomach that stimulates gastrointestinal activity, according to Dr. Lembo, with Beth Israel Deaconess Medical Center in Boston.
The study enrolled 204 patients with type 1 or type 2 diabetes and a hemoglobin A1c value of less than 11%; a history of ongoing gastroparesis symptoms; nausea and/or vomiting at least once in the 2 weeks prior to enrollment; and a screening gastric emptying time of more than 79 minutes. Their mean age was 55 years.
A post hoc analysis on patients with baseline vomiting showed that twice-daily RM-131 was effective on all endpoints, including four subjective patient-reported symptoms – nausea, abdominal pain, bloating, and early satiety, Dr. Lembo said.
Gastric emptying, as demonstrated by the Gastric Emptying Breath Test, improved by 30.6 minutes from baseline (P = .02), weekly vomiting episodes by 63% (P = .041), and vomiting severity by 59% (P = .006).
The four-symptom composite score improved numerically, but not significantly, in the overall population, whereas there was a clear and significant improvement in 1 week in the subgroup with vomiting (6.36 points; P less than .043), he said.
The subgroup with vomiting comprised about 60% of the study population, and had 5.6 to 6.4 baseline vomiting episodes per week versus 3.2 to 3.5 per week in the overall population. Vomiting is the most bothersome symptom in diabetic gastroparesis and often brings patients to the hospital for treatment, Dr. Lembo observed.
The subset with vomiting also had more severe overall gastric emptying symptoms at baseline. Initial analyses, however, suggest a weak correlation between improvement in gastric emptying and symptom improvement, he said.
Session cochair Dr. John Inadomi, professor of medicine and head of gastroenterology, University of Washington, Seattle, said in an interview that the weak correlation "is not surprising given what we know about gastroparesis and the pathogenesis of symptoms, but it does raise questions about where the most effective therapy is going to come from. Is it going to come from trying to accelerate gastric emptying or trying to go after some other way to relieve symptoms? Nevertheless, this is the first study of a so-called pure prokinetic that has had at least some clinical benefit, but again, it was in the subset of patients with vomiting."
Treatment-emergent adverse events occurred in 30 of 69 patients (43.5%) on placebo, 32 of 67 patients (47.8%) on once-daily RM-131, and 25 of 68 patients (36.8%) on twice-daily dosing. No adverse event was more common with RM-131 and likewise, there were no concerns about labs, glucose, weight, ECGs, physical exam, or injection-site reactions, Dr. Lembo said.
Rhythm Pharmaceuticals sponsored the study. Dr. Lembo reported relationships with Salix Pharmaceuticals, AstraZeneca, and other companies. Dr. Inadomi reported relationships with Given Imaging, ChemImage, and other companies.
AT DDW 2014
Major finding: Twice-daily RM-131 reduced overall weekly vomiting episodes by 63% (P = .033) and vomiting severity by 58% (P = .005).
Data source: A phase II, double-blind, randomized study in 204 diabetic gastroparesis patients.
Disclosures: Rhythm Pharmaceuticals sponsored the study. Dr. Lembo reported relationships with Salix Pharmaceuticals, AstraZeneca, and other companies.
VIDEO: Oral Drugs Replacing Interferon for Hepatitis C
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Two old workhorses in the treatment of hepatitis C are being put out to pasture – interferon and ribavirin.
Newer, less toxic oral regimens can cure more patients with fewer side effects, Dr. Bruce R. Bacon said in this interview at the annual Digestive Disease Week.
Ribavirin is helpful in some patients, but not needed in all. As for interferon: "I used interferon for 30 years," Dr. Bacon said. "I’m glad it’s gone."
Well, almost gone. Some of the oral therapies have yet to be approved, and cost is an issue.
Dr. Bacon is the James F. King Endowed Chair in Gastroenterology and a professor of medicine at Saint Louis University. He was not involved in the study. He reported financial associations with AbbVie, Gilead Sciences, and Janssen Pharmaceuticals.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM DDW 2014