User login
Conference on Retroviruses and Opportunistic Infections (CROI)
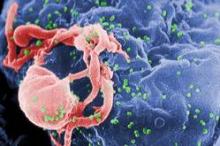
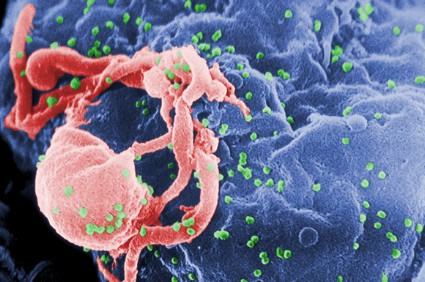
Potential ‘Functional Cure’ for AIDS?
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
FROM CROI 2014
Early study: Potential ‘functional cure’ for AIDS?
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Anthony S. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
By tinkering with a gene involved with the entry of HIV into cells, investigators have developed what they call a potential "functional cure" for HIV/AIDS.
The therapy is designed to keep HIV under control without additional antiretroviral drugs.
The technique involves collection of CD4 T cells from patients infected with HIV, genetically engineering the cells to disable the gene for the HIV coreceptor CCR5, and reinfusing the treated cells after the patients have been conditioned with the chemotherapy drug cyclophosphamide (Cytoxan), which stimulates engraftment of the modified cells, reported Dr. Gary Blick of Circle Care Center in Norwalk, Conn.
"We are seeing what we believe to be dose-dependent marked increases in both absolute CD4 cell counts as well as the engraftment of CCR5 modified T cells with the increasing doses of Cytoxan," he said at the Conference on Retroviruses and Opportunistic Infections 2014.
Results from a phase I proof-of-concept study with the same technology were reported in the March 6 New England Journal of Medicine (2014;370:901-10).
In the phase I/II dose-escalation study reported at CROI, a total of 12 HIV patients with chronic aviremic HIV infection while on highly active antiretroviral therapy (HAART) were enrolled into one of three cohorts. Each patient received one dose of the autologous CD4+ T-cell product in which the gene for CCR5, a coreceptor for HIV entry, is modified via zinc-finger nuclease (ZFN)–mediated genome editing.
Three patients received cyclophosphamide 200 mg as preconditioning, six received cyclophosphamide 500 mg/m2, and three received the drug at a dose of 1 g/m2.
Each patient then received a single infusion (8.2-36 billion cells) of his/her own CD4+ T cells, which had been genetically engineered using an adenoviral vector designed to carry a ZFN, an enzyme targeted at permanently disabling CCR5.
The therapy effectively mimics the CCR5 delta-32 mutation. Patients who are heterozygous for the allele have slower progression of HIV disease, and patients who are homozygous for the deletion are resistant to HIV infection.
The so-called Berlin patient was an HIV-infected man who is HIV free and off of antiretroviral therapy for more than 5 years after receiving a stem cell transplant from a donor who was homozygous for the delta-32 allele.
Dose-escalation study
The primary endpoint of the study was the safety and tolerability of cyclophosphamide. The drug was generally well tolerated, except for low-grade gastrointestinal side effects commonly seen with this agent. The side effects were treated with antiemetics.
The investigators saw a dose-related increase in total CD4 T-cell counts and engraftment of the modified cells at the highest cyclophosphamide dose. The cell counts approached levels found in patients with the CCR5 delta-32 deletion that confers natural resistance to HIV.
In addition, two patients at the 500-mg/m2 dose had an approximate reduction of 0.8-1.1 log10 in viral load after a 16-week treatment interruption, and one patient on the 1-g/m2 dose had a 1.9 log10 decrease after 16 weeks off HAART.
Two patients in the highest cyclophosphamide dose group had stable reduced viral loads and remain on treatment interruption.
The data suggest that cyclophosphamide conditioning may optimize the antiviral and engraftment effects of the adoptive T-cell strategy, and that the drug may play an important role as an immunomodulator in immunotherapy strategies for treating HIV, Dr. Blick said.
"The potential future of gene knockout by ZFNs and other techniques is not restricted to HIV infection. There are now methods that can be used not only to inactivate a gene but also to make specific nucleotide changes in a specific site in the genome and gene addition. These methods will be useful in fixing genes that contain harmful mutations and in supplying therapeutic proteins. Through repeated trips from bedside to bench and back again, it is likely that these approaches represent a basis for effective future therapeutic interventions," commented Dr. Mark A Kay of Stanford (Calif.) University and Dr. Bruce D. Walker of the Ragon Institute of MGH, MIT, and Harvard, in Cambridge, Mass., in an editorial accompanying the study published in the New England Journal of Medicine.
The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay, and Dr. Walker reported having no relevant financial disclosures.
Commentary – ‘Elegant science’ with uncertain future
Dr. Anthony S. Fauci comments: The only way this is really going to be useful is if they in fact completely replace normal T cells ultimately, because as long as you have normal cells getting infected and spitting out virus, you’ve got a problem, and you’ve got a problem because normal cells making virus give you that immune activation that’s a very deleterious part of HIV disease – the aberrant activation associated with virus replication.
Is this elegant science? Yes. Is it an important step in the right direction? Yes. Will this turn out to be a successful approach? I can’t tell you that right now. It might not be.
Dr. Fauci is director of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
FROM CROI 2014
Major finding: Three of 12 HIV-positive patients treated with modified autologous T cells had viral load reductions between 0.8 and 1.9 log10 after 16 weeks of antiretroviral therapy interruption.
Data source: Phase I/II dose-escalation study in 12 patients.
Disclosures: The study was supported by Sangamo Biosciences. Dr. Blick, Dr.Kay and Dr. Walker reported having no relevant financial disclosures.
Early treatment appears to clear virus in second HIV-infected newborn
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
An HIV-infected newborn infant started on combined antiretroviral drug therapy just 4 hours after birth had no detectable viral load by 11 days of age and at 9.5 months of treatment appears to be HIV free, investigators have reported.
The case of the unidentified child, born in Los Angeles (L.A.) County, Calif., to a mother with untreated HIV infection, echoes that of the so-called Mississippi child. The latter case made headlines last year when investigators reported that, at the age of 26 months, a girl born with perinatal HIV infection had no detectable virus 10 months after stopping therapy with zidovudine (AZT), zidovudine (3TC), and nevirapine.
Dr. Deborah Persaud from Johns Hopkins Children’s Center in Baltimore, who presented the case of the Mississippi child at the Conference on Retroviruses and Opportunistic Infections (CROI) last year, reported an update on that case and described early results from the aforementioned L.A. child at this year’s CROI meeting.
As she reported in 2013, the Mississippi child was born to an HIV-infected mother with no evidence of antiretroviral therapy or prophylaxis during labor to prevent transmission to the infant.
The child was started on antiretroviral therapy at 31 hours of age with AZT, 3TC, and nevirapine given twice daily at 2 mg/kg per dose.
This regimen differed from the prophylactic regimen used in high transmission–risk situations, in which nevirapine is given in three doses at 0, 48, and 96 hours, Dr. Persaud noted. Investigators are still uncertain as to the optimal nevirapine dose for children under 2 weeks of age, she said.
At the most recent follow-up, the child was 41 months old and had been off combined antiretroviral therapy (cART) for 23 months.
"With respect to the Mississippi child, we can say that this child remains in remission. There’s no replication-competent T-cell reservoir to date, and it supports our hypothesis that very early treatment may prevent formation of critical reservoirs that currently preclude cure," she said.
Despite the apparent clearance of HIV in the Mississippi child, investigators continue to detect HIV proviral DNA in the child’s peripheral blood mononuclear cells.
"We’re not sure whether this is a real signal or really at the assay limits of detection, and it certainly requires continued follow-up and study. It’s important to point out that the clinical relevance of this detection remains unclear, but to date it does not signify impending rebound viremia," she said.
The investigators hypothesized that the residual microviral DNA seen in the child might be explained by maternal microchimerism, that is, residual maternal cells with HIV proviral DNA, but at 40-month follow-up, there was no evidence of maternal chimerism, Dr. Persaud said.
L.A. story
The L.A. child, whose sex was not disclosed, was born to a mother with untreated HIV infection and a high viral load (138,711 copies/mL) and low CD4 count (70 cells/mm3). At 4 hours of age, the child had a blood sample positive for HIV DNA, and at 36 hours, showed HIV RNA at 217 copies/mL.
A cerebrospinal fluid sample taken at 6 days to rule out sepsis showed HIV RNA at 32 copies/mL.
At age 4 hour, the child was started on a regimen of AZT, 3TC, and nevirapine, with the addition of lopinavir/ritonavir (Kaletra) at 2 weeks of age and was continued on this combination for 3.4 months. The child has since been maintained on the initial three-drug regimen.
"What we did find in this case is that by using clinical monitoring the viral load was undetectable by 11 days of age. Sequential samples collected through 9 months of age show undetectable plasma viral load," Dr. Persaud said.
The clinical assay used to diagnose infection found no detectable viral levels by 60 days of age, and the investigators have been unable to recover infectious virus from the child’s resting CD4 T cells at 1, 3, or 9 months.
However, when they looked at the noninduced proviral genome in a culture obtained at 1 month of age, the investigators detected HIV DNA at both days 7 and 14 of culturing, "but not at a level that we could amplify and sequence, so it’s unclear what those signals mean," she said.
Subsequent culture data have shown no detection of non-induced proviral genomes. The proviral DNA remains low. At last testing at 9 months of age, proviral DNA was less than 2 copies/mL in peripheral blood mononuclear cells, and the child had seroreverted and become HIV negative.
"I think we’ve shown that very early treatment, certainly in the Mississippi child, has led to sustained HIV remission now for up to 23 months off cART, and now with the second initiating treatment at 4 hours of life, this has led to rapid clearance of replicating virus and proviral DNA, supporting restriction of HIV spread with very early cART," Dr. Persaud said.
The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
FROM CROI 2014
Major finding: Combined antiretroviral therapy in the first hours of life appears to clear HIV from perinatally infected infants.
Data source: Case reports of two children born with HIV infection to untreated mothers.
Disclosures: The work was supported by the Center for AIDS Research at Johns Hopkins University, the Foundation for AIDS Research (amfAR), and the National Institute of Allergy and Infectious Diseases.
Risk of condomless sex low if HIV-positive partner is well controlled on ART
Public health experts do not condone the practice, but it appears that the risk of transmission from an HIV-positive person to an uninfected sex partner during condomless sex is low if the infected partner has good viral control on antiretroviral therapy.
That’s the tentative conclusion investigators in the PARTNER study have drawn from an interim analysis looking at HIV transmission among serodifferent couples in which the positive partner is taking antiretroviral therapy (ART) and has a plasma viral load below 200 copies/mL, said Dr. Alison Rodger from the Research Department of Infection & Population Health at University College London.
"Overall, we had no linked transmissions during eligible follow-up, giving a transmission rate of zero," she reported at the Conference on Retroviruses and Opportunistic Infections.
However, the investigators cannot say with certainty that the actual risk is zero, as the upper limit of the 95% confidence interval was 0.4 per 100 couple-years, translating into a 10-year risk of 4%, Dr. Rodger said.
Although HIV transmissions did occur during the study, the investigators were able to rule out linked transmissions – that is, transmissions that are phylogenetically confirmed to be the same viral strain.
"The uncertainty is particularly advanced for anal sex, where for the moment we do not have sufficient data to exclude that 1 in 10 [HIV-negative partners] would be infected over a 10-year period," commented coinvestigator Dr. Jens Lundgren from the Centre for Viral Diseases and the Copenhagen HIV Programme at the University of Copenhagen.
The investigators plan to continue the study and conduct new analyses each time an additional 1,000 couple-years have been reached, he said at a briefing following the presentation.
The investigators enrolled serodifferent heterosexual and men having sex with men (MSM) couples who reported having had condomless penetrative anal or vaginal sex in the month prior to study entry, and did not use pre- or postexposure HIV prophylaxis.
Every 6 months during follow-up, each partner fills out a sexual behaviors questionnaire, and the seronegative partner is tested for HIV.
The informed consent for the study included information on the need for consistent condom use and explicit reference to the fact that the HIV-negative partners knew that their partner was positive and that there was risk for HIV transmission.
A total of 767 couples have contributed a total of 894 eligible couple follow-up years (586 in heterosexual couples and 308 in MSM) as of November 2013.
Median ART duration at baseline was 4.8 years, and the median duration of condomless sex reported was 2 years.
Although the risk of transmission across all categories of penetrative anal or vaginal sex was zero, as noted before, the upper limit of the confidence interval for receptive anal sex with ejaculation – the type of sex that carries the highest risk of HIV transmission – extends to 4, which translates into 32% risk of transmission at 10 years, Dr. Rodger said.
"Additional follow-up in MSM is needed through PARTNER phase 2, which will extend through 2017 in gay men only to provide more precise estimates for transmission risk to inform policy, and also to involve individual choice on whether to use condoms or not," Dr. Rodger said.
The PARTNER study is supported by the U.K. National Institute for Health Research. Dr. Rodger and Dr. Lundgren reported having no financial conflicts of interest.
Public health experts do not condone the practice, but it appears that the risk of transmission from an HIV-positive person to an uninfected sex partner during condomless sex is low if the infected partner has good viral control on antiretroviral therapy.
That’s the tentative conclusion investigators in the PARTNER study have drawn from an interim analysis looking at HIV transmission among serodifferent couples in which the positive partner is taking antiretroviral therapy (ART) and has a plasma viral load below 200 copies/mL, said Dr. Alison Rodger from the Research Department of Infection & Population Health at University College London.
"Overall, we had no linked transmissions during eligible follow-up, giving a transmission rate of zero," she reported at the Conference on Retroviruses and Opportunistic Infections.
However, the investigators cannot say with certainty that the actual risk is zero, as the upper limit of the 95% confidence interval was 0.4 per 100 couple-years, translating into a 10-year risk of 4%, Dr. Rodger said.
Although HIV transmissions did occur during the study, the investigators were able to rule out linked transmissions – that is, transmissions that are phylogenetically confirmed to be the same viral strain.
"The uncertainty is particularly advanced for anal sex, where for the moment we do not have sufficient data to exclude that 1 in 10 [HIV-negative partners] would be infected over a 10-year period," commented coinvestigator Dr. Jens Lundgren from the Centre for Viral Diseases and the Copenhagen HIV Programme at the University of Copenhagen.
The investigators plan to continue the study and conduct new analyses each time an additional 1,000 couple-years have been reached, he said at a briefing following the presentation.
The investigators enrolled serodifferent heterosexual and men having sex with men (MSM) couples who reported having had condomless penetrative anal or vaginal sex in the month prior to study entry, and did not use pre- or postexposure HIV prophylaxis.
Every 6 months during follow-up, each partner fills out a sexual behaviors questionnaire, and the seronegative partner is tested for HIV.
The informed consent for the study included information on the need for consistent condom use and explicit reference to the fact that the HIV-negative partners knew that their partner was positive and that there was risk for HIV transmission.
A total of 767 couples have contributed a total of 894 eligible couple follow-up years (586 in heterosexual couples and 308 in MSM) as of November 2013.
Median ART duration at baseline was 4.8 years, and the median duration of condomless sex reported was 2 years.
Although the risk of transmission across all categories of penetrative anal or vaginal sex was zero, as noted before, the upper limit of the confidence interval for receptive anal sex with ejaculation – the type of sex that carries the highest risk of HIV transmission – extends to 4, which translates into 32% risk of transmission at 10 years, Dr. Rodger said.
"Additional follow-up in MSM is needed through PARTNER phase 2, which will extend through 2017 in gay men only to provide more precise estimates for transmission risk to inform policy, and also to involve individual choice on whether to use condoms or not," Dr. Rodger said.
The PARTNER study is supported by the U.K. National Institute for Health Research. Dr. Rodger and Dr. Lundgren reported having no financial conflicts of interest.
Public health experts do not condone the practice, but it appears that the risk of transmission from an HIV-positive person to an uninfected sex partner during condomless sex is low if the infected partner has good viral control on antiretroviral therapy.
That’s the tentative conclusion investigators in the PARTNER study have drawn from an interim analysis looking at HIV transmission among serodifferent couples in which the positive partner is taking antiretroviral therapy (ART) and has a plasma viral load below 200 copies/mL, said Dr. Alison Rodger from the Research Department of Infection & Population Health at University College London.
"Overall, we had no linked transmissions during eligible follow-up, giving a transmission rate of zero," she reported at the Conference on Retroviruses and Opportunistic Infections.
However, the investigators cannot say with certainty that the actual risk is zero, as the upper limit of the 95% confidence interval was 0.4 per 100 couple-years, translating into a 10-year risk of 4%, Dr. Rodger said.
Although HIV transmissions did occur during the study, the investigators were able to rule out linked transmissions – that is, transmissions that are phylogenetically confirmed to be the same viral strain.
"The uncertainty is particularly advanced for anal sex, where for the moment we do not have sufficient data to exclude that 1 in 10 [HIV-negative partners] would be infected over a 10-year period," commented coinvestigator Dr. Jens Lundgren from the Centre for Viral Diseases and the Copenhagen HIV Programme at the University of Copenhagen.
The investigators plan to continue the study and conduct new analyses each time an additional 1,000 couple-years have been reached, he said at a briefing following the presentation.
The investigators enrolled serodifferent heterosexual and men having sex with men (MSM) couples who reported having had condomless penetrative anal or vaginal sex in the month prior to study entry, and did not use pre- or postexposure HIV prophylaxis.
Every 6 months during follow-up, each partner fills out a sexual behaviors questionnaire, and the seronegative partner is tested for HIV.
The informed consent for the study included information on the need for consistent condom use and explicit reference to the fact that the HIV-negative partners knew that their partner was positive and that there was risk for HIV transmission.
A total of 767 couples have contributed a total of 894 eligible couple follow-up years (586 in heterosexual couples and 308 in MSM) as of November 2013.
Median ART duration at baseline was 4.8 years, and the median duration of condomless sex reported was 2 years.
Although the risk of transmission across all categories of penetrative anal or vaginal sex was zero, as noted before, the upper limit of the confidence interval for receptive anal sex with ejaculation – the type of sex that carries the highest risk of HIV transmission – extends to 4, which translates into 32% risk of transmission at 10 years, Dr. Rodger said.
"Additional follow-up in MSM is needed through PARTNER phase 2, which will extend through 2017 in gay men only to provide more precise estimates for transmission risk to inform policy, and also to involve individual choice on whether to use condoms or not," Dr. Rodger said.
The PARTNER study is supported by the U.K. National Institute for Health Research. Dr. Rodger and Dr. Lundgren reported having no financial conflicts of interest.
FROM CROI 2014
Major finding: When HIV-infected individuals were well controlled on antiretroviral therapy, the risk of HIV transmission to uninfected sex partners was zero, but confidence intervals indicate that there may still be some transmission risk.
Data source: An observational cohort study in which 767 couples reported 894 couple-years at 75 sites across Europe.
Disclosures: The PARTNER study is supported by the U.K. National Institute for Health Research. Dr. Rodger and Dr. Lundgren reported having no financial conflicts of interest.
HIV-1 has gained virulence over the course of the epidemic
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
Even as antiretroviral therapies began making inroads into the AIDS epidemic, the virulence of HIV-1 increased and the time from seroconversion to a significant CD4-cell drop was cut in half, investigators reported at the Conference on Retroviruses and Opportunistic Infections.
"These results have important public health implications, as higher viremial levels are associated with higher risk of transmission. Based on published formulas, our estimated increase of 0.4 log10 copies/mL in viral set point corresponds to a potential 44% increase in transmissibility," reported Dr. Giota Touloumi, of Athens University, in a briefing.
"We all know that the HIV-1 is characterized by huge genetic diversity, and different strains of the virus may differ in virulence," said coinvestigator and lead author Dr. Nikos Pantazis, also from Athens University. Dr. Pantazis presented the data in a plenary session.
In the era before antiretroviral therapy (ART), HIV virulence was measured directly by time to the development of AIDS and death. In the ART era, however, clinicians must rely on marker-based proxies of virulence, such as CD4 seroconversion, CD4 slope (i.e., rate of decline), and viral load set point.
"The picture we have from published research is mixed, with conflicting results, and [some] studies suggest that the HIV virulence has increased, others declare it stable, and others say the virulence is even decreasing," he said.
To see whether the virulence of HIV-1 has changed over the course of the epidemic, the investigators reviewed data from CASCADE, a collaboration between the investigators of 26 cohorts of persons with well-estimated dates of HIV seroconversion. They did not include data on African cohorts, patients with seroconversion from 2009 on, or children.
They censored follow-up either at the time of ART initiation or at the onset of clinical AIDS. A total of 15,875 cohort members met the study criteria.
The authors estimated the CD4 counts at seroconversion declined from about 770/mcL in the early 1980s to about 570/mcL after 2000. The virulence appeared to plateau after the turn of the millennium, they noted.
The rate of CD4-cell loss (CD4 slope) was relatively stable up to 1996, but accelerated from 1996 through 2004. In addition, the estimated HIV set point (viral load) increased from 4.05 log10 copies/mL in 1980 to 4.50 in 2002, suggesting a more virulent virus.
The results are compatible with both a 2012 meta-analysis (AIDS 2012;26:193-205 [doi: 10.1097/QAD.0b013e32834db418]) and a 2007 evolutionary hypothesis (Proc. Natl. Acad. Sci. USA 2007;104:17441-6), Dr. Pantazis said.
The combined findings of a drop in CD4 cell count of about 200 cells/mcL over 30 years and an apparent increase in CD4 slope translated into an estimated 50% decrease in the time from seroconversion to 350 CD4 cells/mcL, from 7 years in a 1980 seroconverter to 3.4 years in a 2004 seroconverter, he said.
Although the study was subject to residual confounding bias, such as changes in methods for viral load and CD4 cell count assays and quantification, and difficulties in pinpointing the time of seroconversion, a wide range of sensitivity analyses furnished qualitatively similar results, Dr. Pantazis said.
The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
FROM CROI 2014
Major finding: An increase in the HIV-1 viral set point from 1980 through 2000 translates into an estimated 44% increase in viral transmissibility.
Data source: Retrospective study of 15,875 HIV-positive patients in 1 of 26 cohorts.
Disclosures: The study was supported by a grant from the European Union. Dr. Pantazis, Dr. Touloumi, and their colleagues reported having no financial disclosures.
New Drugs Trump Interferon in HCV Therapy
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
FROM CROI 2014
New drugs trump interferon in HCV therapy
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
The era of interferon and ribavirin in the treatment of hepatitis C viral infections appears to be drawing to a close, and few clinicians will mourn the passing of the effective but highly toxic combination, investigators said at the Conference on Retroviruses and Opportunistic Infections.
In patients with hepatitis C virus infection alone or HCV with HIV coinfection, a host of new interferon-free drugs and new combinations are transforming therapy, reported Dr. Jean-Michel Pawlotsky, professor of medicine at the University of Paris-Est.
"Hepatitis C is living a real therapeutic revolution. Everything is changing very fast. We’re now getting infection cure rates higher than 90% with the classes of drugs we have," he said at a briefing.
STARTVerso4
With all of the drugs, the sustained virologic response (SVR) rates "are exactly the same in coinfected patients as they are in monoinfected patients," said Dr. Douglas Dieterich of Mt. Sinai Medical Center, New York.
For example, a combination of the protease inhibitor faldaprevir with pegylated interferon alfa-2a plus ribavirin (PR) produced SVR rates at week 4 of follow-up (SVR4) of 74% in HCV/HIV coinfected patients, said Dr. Dietrich, a principal investigator for the STARTVerso4 trial.
In this phase III open-label trial, 308 patients with HCV/HIV coinfection who were treatment naive or relapsed after prior interferon-based therapy were randomly assigned to receive faldaprevir 120 mg daily for 24 weeks or 240 mg for 12 or 24 weeks according to on-treatment response. In both arms, faldaprevir was given on a PR backbone, with duration guided by response to therapy.
For the primary endpoint of SVR12, the investigators saw a 72% rate, with no significant difference between the two dose groups, compared with approximately 80% in monoinfected patients in other phase III studies. There was also no difference in efficacy between patients with or without cirrhosis, he said.
Adverse events included mild hyperbilirubinemia in some patients and interferon side effects.
Simeprevir in coinfection
Dr. Dietrich was also the lead on study C212, which looked at simeprevir (Olysio) on a PR backbone in coinfected patients. The results were similar to those seen with faldaprevir (73.6% overall SVR12).
Interestingly, the presence of the simeprevir-resistant q80K polymorphism did not make a difference in response rates, he said. Among monoinfected patients in prior studies, those with q80k polymorphism had significantly lower SVR rates. The adverse events were also similar to those seen in patients with monoinfection.
PHOTON-1
The PHOTON-1 trial evaluated the first interferon-free regimen (sofosbuvir plus ribavirin) in patients with HCV genotypes 1-3 and HIV.
In this study, patients with HCV and stable HIV infection received sofosbuvir 400 mg and ribavirin 1,000-1,200 mg daily. Treatment-naive patients with HCV genotype 1 and treatment-experienced patients with genotypes 2 or 3 received treatment for 24 weeks, while treatment-naive genotype 2/3 patients received 12 weeks of treatment. Patients on multiple antiretroviral (ART) regimens and those with compensated cirrhosis were included in the study.
The primary efficacy endpoint, SVR12, was achieved in 88% of treatment-naive genotype 2 patients and 67% of genotype 3 patients. In genotype 1 patients, the SVR was approximately 70%, Dr. Dietrich said.
Adverse events were general and limited to anemias, headache, and other symptoms commonly seen with HCV therapies, he added.
SYNERGY trial
Dr. Anita Kohli presented final results from the SYNERGY trial, which looked at combination oral HCV therapy for 6 or 12 weeks (SVR4 results from this trial were presented at the 2013 Liver Meeting).
In this phase II prospective cohort study, 60 treatment-naive patients with HCV genotype 1 were enrolled into one of three arms to receive either sofosbuvir 400 mg with ledipasvir 90 mg once daily in a fixed-dose combination for 12 weeks (arm A); the same fixed-dose combination plus the non-nucleoside NS5B inhibitor GS-9669 500 mg/day for 6 weeks (arm B); or the fixed-dose combination plus the NS3 protease inhibitor GS-9451 80 mg/day for 6 weeks.
The SVR12 rate among the patients on sofosbuvir/ledipasvir alone (arm A) was 100%, the rate in arm B was 95%, and the rate in arm C was 100%.
"We find these results very promising," said Dr. Kohli of the National Institutes of Health.
She noted that all patients in the trial were treatment naïve, and that all stages of liver disease were included in the 12-week treatment arm, but cirrhotic patients were excluded from the 6-week arms.
"These regimens are very simple. They’re one, two, or three pills a day," she noted. In addition, "our patient population is one that has been historically very difficult to treat, that is, predominantly African American," she noted.
Most of the patients had genotype 1a with a high viral load, and 25%-30% of patients in all treatment arms had advanced-stage liver disease, she added.
PEARL-III
The PEARL III trial looked at 419 treatment-naive, noncirrhotic patients with HCV genotype 1b, who were randomly assigned to receive either a ritonavir-boosted protease inhibitor (ABT-450) with ABT-267, which is an inhibitor of HCV NS5A, coformulated into a single pill; or ABT-333, a non-nucleoside polymerase inhibitor, with or without ribavirin.
In the ribavirin-containing arm, SVR12 was 99.5%, compared with 99% among controls. There was only one virologic failure in the study, and two patients who did not achieve SVR4 were lost to follow-up at week 12, noted Dr. Daniel Cohen of AbbVie Pharmaceuticals.
Adverse events included predominantly mild headache and fatigue in about 25% of patients, with slightly more events seen in the ribavirin combination arm.
STARTVerso4 was sponsored by Boehringer Ingelheim. C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.
FROM CROI 2014
Major finding: In a phase II study 100% of treatment naive patients with hepatitis C genotype 1 had an SVR12 with once-daily combination pill.
Data source: Five clinical trials presented in oral abstract sessions.
Disclosures: STARTVerso4 was sponsored by Boehringer Ingelheim. Study C212 was sponsored by Janssen. SYNERGY was supported by the National Institutes of Health and Gilead Sciences. Dr. Cohen is employed by AbbVie, which sponsored PEARL III.