User login
Applications expand for vorapaxar in cardiovascular prevention
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
AT ACC 15
Key clinical point: Vorapaxar reduced the composite outcome of cardiovascular death, MI, or stroke by 29% compared with placebo in patients with a history of MI and/or peripheral arterial disease and prior coronary artery bypass surgery.
Major finding: The novel antiplatelet agent also reduced acute limb ischemia events by 42% in patients with symptomatic peripheral arterial disease.
Data source: These were findings from post hoc, exploratory analyses of a 26,449-patient, randomized, double-blind pivotal trial of vorapaxar for secondary cardiovascular prevention.
Disclosures: The TRA 2°P TIMI-50 trial was sponsored by Merck. The presenters reported having no financial conflicts.
Ischemic preconditioning fails to shift CABG outcomes
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
SAN DIEGO – Remote ischemic preconditioning failed to improve long-term clinical outcomes in higher-risk patients undergoing coronary artery bypass surgery in the ERICCA trial.
At 1 year, there were no differences between patients receiving remote ischemic conditioning (RIC) or a sham procedure in the combined primary endpoint of cardiovascular death, MI, stroke, and coronary revascularization (27% vs. 28%) or its individual components.
Only the extent of perioperative myocardial injury, measured as area under the curve troponin T levels, at 72 hours was significantly lower with RIC (median 30.1 ng.h/mL vs. 35.7 ng.h/mL), principal investigator Dr. Derek Hausenloy reported at the annual meeting of the American College of Cardiology.
The simple, low-cost intervention consisted of four 5-minute blood pressure cuff inflations to 200 mm Hg and deflations immediately before patients went on bypass.
Multiple proof-of-concept studies have shown that brief, reversible episodes of ischemia followed by reperfusion reduces the extent of perioperative myocardial injury in patients undergoing elective coronary artery stenting or bypass grafting.
“In the setting of cardiac bypass surgery, the cardioprotective effect presented by RIC, or remote ischemic conditioning, may be affected by factors during surgery,” said Dr. Hausenloy of University College, London.
There are multiple causes of injury in patients undergoing bypass that include not only myocardial reperfusion injury, but also coronary microembolization, inflammation as the patient is taken on and off bypass, and direct injury to the heart, he noted.
ERICCA (Effect of Remote Ischemic Preconditioning on Clinical Outcomes in Patients Undergoing Coronary Artery Bypass Graft Surgery) also focused on a higher-risk aged population (76 years) with high rates of comorbidities like diabetes (25%) and hypertension (75%) that have been shown to impact RIC and other conditioning strategies.
Discussant Dr. Richard Fogel of St. Vincent Heart Center in Indianapolis, suggested RIC may not have worked because of a dose-response issue and questioned whether the results would have been different had the investigators, for example, done six inflations for 10 minutes each or performed RIC the day before.
Discussant Dr. Eric Bates of the University of Michigan in Ann Arbor, suggested that as long as patients are anesthetized, prolonged conditioning immediately before and after surgery might be considered.
“The RIC protocol has not been very well characterized, although most of the prior studies used three or four cycles,” Dr. Hausenloy said. “Whether this is the optimal stimulus is not known or clear.”
ERICCA enrolled 1,612 patients with an additive Euroscore of at least 5 who underwent CABG using blood cardioplegia at 29 centers in the United Kingdom. Of these, 801 received RIC and 811 received sham, simulated BP cuff inflations/deflations.
One year after surgery, the RIC and control groups had similar rates of major adverse cardiac and cerebral events, at 26.7% and 27.7%, respectively; cardiovascular death, at 5.9% and 3.9%; MI, at 21.8% and 23.7%; stroke, at 2.1% and 2.0%; and revascularization, at 0.2% and 0.4%.
“It’s interesting that we show a modest effect on reducing perioperative myocardial injury, but we didn’t see any associated improvement in clinical outcome,” he said. “This may question the use of perioperative myocardial injury, as measured by serum biomarkers, as a surrogate marker of cardioprotection. However, the caveat is that we only have a complete dataset for this conclusion in half the patients.”
The potential effect of RIC remains to be investigated in other settings of ischemia and reperfusion injury such as patients with ST-segment elevation MI or undergoing organ transplantation, Dr. Hausenloy said.
“Clearly in these settings of STEMI and organ transplantation, the contribution of ischemia reperfusion injury is greater, and one may speculate that the effect of RIC may be greater,” he added.
AT ACC 2015
Key clinical point: Remote ischemic conditioning prior to CABG did not improve outcomes at 1 year.
Major finding: Cardiovascular death, MI, stroke, and coronary revascularization rates at 1 year were similar with and without RIC (27% vs. 28%).
Data source: ERICCA, a double-blind, randomized, controlled trial in 1,612 patients undergoing CABG.
Disclosures: The study was funded by the National Institute for Health Research, Medical Research Council, and British Heart Foundation. Dr. Hausenloy and Dr. Fogel reported having no disclosures. Dr. Bates reported consulting fees/honoraria from Merck and Astra Zeneca.
Testosterone therapy has neutral cardiovascular effects
SAN DIEGO– Testosterone therapy for men with low serum testosterone had no impact one way or another on cardiovascular risk in a large, community-based study.
“Testosterone therapy is not associated with any deleterious or beneficial effect on cardiovascular outcomes. But our study does reinforce that it’s very important to go back to the basics and try to manage the traditional risk factors that are known predictors of adverse outcomes,” Dr. Zuber Ali said at the annual meeting of the American College of Cardiology.
The cardiovascular impact of testosterone therapy in men with low serum levels is a controversial subject. Early studies reported cardioprotective benefits; however, at least three more recent studies found adverse effects, and the Food and Drug Administration recently issued a request that manufacturers of approved testosterone products revise their product labeling to include a new black box warning of “a possible increased cardiovascular risk associated with testosterone use.”
“Given these conflicting reports, we wanted to look at our experience in a large, community-based health care system,” explained Dr. Ali of the Aurora University of Wisconsin Medical Group in Milwaukee.
He presented a retrospective cohort study of 7,245 men, mean age 54 years, with a low baseline total testosterone level below 300 ng/dL who were followed during 2011-2014.
The combined rate of the composite outcome comprising all-cause mortality, acute MI, and stroke was 5.5% in men who received testosterone therapy, compared with 6.7% in those who did not. This translated into a statistically significant unadjusted 29% relative risk reduction in favor of testosterone therapy. However, upon adjusting for differences in baseline cardiovascular risk factors, the relative risk advantage for testosterone therapy dropped from 29% to 20%, which was no longer statistically significant.
This cardiovascular-neutral result was confirmed in a separate analysis in which 3,155 of the men on testosterone therapy were paired one to one with an equal number of controls who were propensity-matched on the basis of demographics and cardiovascular risk factors, including diabetes, atrial fibrillation, and chronic kidney disease. During a mean 1.8 years of follow-up, the composite outcome occurred in 1.7% of the testosterone therapy group and 2.2% of matched controls, a nonsignificant difference.
In a multivariate analysis, the significant predictors of cardiovascular outcomes proved to be dyslipidemia, age, length of follow-up, a history of acute MI or stroke/TIA, and smoking. Testosterone therapy didn’t make the list, according to Dr. Ali.
One audience member commented that while these data are reassuring up to a point, 1.8 years of follow-up is not very long from a cardiovascular risk perspective. Dr. Ali agreed, adding that he and his coinvestigators are continuing to follow these men and will be gathering longer-term data. But so far, at least, there is no signal of increased cardiovascular risk.
In issuing its request for a label change, the FDA also required manufacturers of approved testosterone products to conduct a well-designed clinical trial, either separately or in collaboration, to specifically address the question of possible cardiovascular risk. While Dr. Ali welcomed this development because it should finally provide definitive answers, he noted that no such study has begun, and long-term outcome data are years away. In the interim, he added, data such as he presented are probably the best that can be expected in terms of providing guidance on risks and benefits for physician/patient treatment decision making.
Dr. Ali reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO– Testosterone therapy for men with low serum testosterone had no impact one way or another on cardiovascular risk in a large, community-based study.
“Testosterone therapy is not associated with any deleterious or beneficial effect on cardiovascular outcomes. But our study does reinforce that it’s very important to go back to the basics and try to manage the traditional risk factors that are known predictors of adverse outcomes,” Dr. Zuber Ali said at the annual meeting of the American College of Cardiology.
The cardiovascular impact of testosterone therapy in men with low serum levels is a controversial subject. Early studies reported cardioprotective benefits; however, at least three more recent studies found adverse effects, and the Food and Drug Administration recently issued a request that manufacturers of approved testosterone products revise their product labeling to include a new black box warning of “a possible increased cardiovascular risk associated with testosterone use.”
“Given these conflicting reports, we wanted to look at our experience in a large, community-based health care system,” explained Dr. Ali of the Aurora University of Wisconsin Medical Group in Milwaukee.
He presented a retrospective cohort study of 7,245 men, mean age 54 years, with a low baseline total testosterone level below 300 ng/dL who were followed during 2011-2014.
The combined rate of the composite outcome comprising all-cause mortality, acute MI, and stroke was 5.5% in men who received testosterone therapy, compared with 6.7% in those who did not. This translated into a statistically significant unadjusted 29% relative risk reduction in favor of testosterone therapy. However, upon adjusting for differences in baseline cardiovascular risk factors, the relative risk advantage for testosterone therapy dropped from 29% to 20%, which was no longer statistically significant.
This cardiovascular-neutral result was confirmed in a separate analysis in which 3,155 of the men on testosterone therapy were paired one to one with an equal number of controls who were propensity-matched on the basis of demographics and cardiovascular risk factors, including diabetes, atrial fibrillation, and chronic kidney disease. During a mean 1.8 years of follow-up, the composite outcome occurred in 1.7% of the testosterone therapy group and 2.2% of matched controls, a nonsignificant difference.
In a multivariate analysis, the significant predictors of cardiovascular outcomes proved to be dyslipidemia, age, length of follow-up, a history of acute MI or stroke/TIA, and smoking. Testosterone therapy didn’t make the list, according to Dr. Ali.
One audience member commented that while these data are reassuring up to a point, 1.8 years of follow-up is not very long from a cardiovascular risk perspective. Dr. Ali agreed, adding that he and his coinvestigators are continuing to follow these men and will be gathering longer-term data. But so far, at least, there is no signal of increased cardiovascular risk.
In issuing its request for a label change, the FDA also required manufacturers of approved testosterone products to conduct a well-designed clinical trial, either separately or in collaboration, to specifically address the question of possible cardiovascular risk. While Dr. Ali welcomed this development because it should finally provide definitive answers, he noted that no such study has begun, and long-term outcome data are years away. In the interim, he added, data such as he presented are probably the best that can be expected in terms of providing guidance on risks and benefits for physician/patient treatment decision making.
Dr. Ali reported having no financial conflicts regarding this study, which was conducted free of commercial support.
SAN DIEGO– Testosterone therapy for men with low serum testosterone had no impact one way or another on cardiovascular risk in a large, community-based study.
“Testosterone therapy is not associated with any deleterious or beneficial effect on cardiovascular outcomes. But our study does reinforce that it’s very important to go back to the basics and try to manage the traditional risk factors that are known predictors of adverse outcomes,” Dr. Zuber Ali said at the annual meeting of the American College of Cardiology.
The cardiovascular impact of testosterone therapy in men with low serum levels is a controversial subject. Early studies reported cardioprotective benefits; however, at least three more recent studies found adverse effects, and the Food and Drug Administration recently issued a request that manufacturers of approved testosterone products revise their product labeling to include a new black box warning of “a possible increased cardiovascular risk associated with testosterone use.”
“Given these conflicting reports, we wanted to look at our experience in a large, community-based health care system,” explained Dr. Ali of the Aurora University of Wisconsin Medical Group in Milwaukee.
He presented a retrospective cohort study of 7,245 men, mean age 54 years, with a low baseline total testosterone level below 300 ng/dL who were followed during 2011-2014.
The combined rate of the composite outcome comprising all-cause mortality, acute MI, and stroke was 5.5% in men who received testosterone therapy, compared with 6.7% in those who did not. This translated into a statistically significant unadjusted 29% relative risk reduction in favor of testosterone therapy. However, upon adjusting for differences in baseline cardiovascular risk factors, the relative risk advantage for testosterone therapy dropped from 29% to 20%, which was no longer statistically significant.
This cardiovascular-neutral result was confirmed in a separate analysis in which 3,155 of the men on testosterone therapy were paired one to one with an equal number of controls who were propensity-matched on the basis of demographics and cardiovascular risk factors, including diabetes, atrial fibrillation, and chronic kidney disease. During a mean 1.8 years of follow-up, the composite outcome occurred in 1.7% of the testosterone therapy group and 2.2% of matched controls, a nonsignificant difference.
In a multivariate analysis, the significant predictors of cardiovascular outcomes proved to be dyslipidemia, age, length of follow-up, a history of acute MI or stroke/TIA, and smoking. Testosterone therapy didn’t make the list, according to Dr. Ali.
One audience member commented that while these data are reassuring up to a point, 1.8 years of follow-up is not very long from a cardiovascular risk perspective. Dr. Ali agreed, adding that he and his coinvestigators are continuing to follow these men and will be gathering longer-term data. But so far, at least, there is no signal of increased cardiovascular risk.
In issuing its request for a label change, the FDA also required manufacturers of approved testosterone products to conduct a well-designed clinical trial, either separately or in collaboration, to specifically address the question of possible cardiovascular risk. While Dr. Ali welcomed this development because it should finally provide definitive answers, he noted that no such study has begun, and long-term outcome data are years away. In the interim, he added, data such as he presented are probably the best that can be expected in terms of providing guidance on risks and benefits for physician/patient treatment decision making.
Dr. Ali reported having no financial conflicts regarding this study, which was conducted free of commercial support.
AT ACC 15
Key clinical point: Testosterone therapy for men with low serum testosterone had no significant impact on their cardiovascular event rate.
Major finding: The incidence of the composite outcome of all-cause mortality, acute MI, or stroke during a mean 1.8 years of follow-up was 1.7% in men on testosterone therapy and 2.2% in propensity-matched controls, a nonsignificant difference.
Data source: A retrospective cohort study in 7,245 men with low serum testosterone.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial support.
Experience equalizes transulnar and transradial approaches to heart procedures
SAN DIEGO – Surgical experience puts transulnar access on a par with transradial access for percutaneous coronary procedures, the randomized AJULAR trial showed.
The primary composite outcome of major adverse cardiac events or major vascular events during hospitalization was 14.6% for transulnar access and 14.4% for transradial access, meeting the noninferiority criteria.
“If used as a default strategy, [transulnar access] is noninferior to the transradial approach when performed by an experienced operator,” study author Dr. Rajendra Gokhroo reported at the annual meeting of the American College of Cardiology.
The use of radial artery cannulation is growing in the United States as an approach for coronary access because of obvious safety advantages over femoral access, but has its own limitations such as frequent vasospasm, small caliber, and unsuitability as a graft for coronary artery bypass grafting after cannulation.
Transulnar access is used by some as an alternative, but was found inferior to transradial access in the AURA of ARTEMIS study because of significantly more large hematomas and a high crossover rate in the transulnar group (Circ. Cardiovasc. Interv. 2013;6:252-261).
However, the study used inexperienced ulnar operators and attempted to cannulate even nearly absent ulnar arteries, said Dr. Gokhroo, a pioneer in ulnar intervention at Jawaharlal Nehru Medical College, Ajmer, India, and president of the Indian Society of Cardiology. Based on their observations, the risk of such events is threefold higher during the first 50 procedures, but is no longer statistically significant after 51-100 operations or after 100 operations.
For the prospective, single-center AJULAR (Ajmer Ulnar Artery) trial, all operators were required to have a minimum experience of 50 transulnar cannulations, and cannulation was attempted only if the ulnar artery was easily palpable and the anatomy favorable.
“Excessive calcification, tortuosity, and low-volume pulse were the additional features where the ulnar was avoided; otherwise no other patient characteristics or demographic features compelled us to avoid ulnar access,” Dr. Gokhroo said in an interview.
Inability to palpate the radial artery was also an exclusion criterion.
A total of 2,532 patients scheduled to undergo elective coronary angiography and ad hoc percutaneous transluminal coronary angioplasty were evenly randomized to transulnar access or transradial access. The mean age for the 1,270 ulnar patients was 67 years and 63 years for the 1,262 radial patients.
There were no significant differences in an intention-to-treat analysis between the transulnar and transradial groups with respect to the individual components of the primary outcome: major adverse cardiac events (2.9% vs. 3.2%), large hematoma (1% vs. 0.9%), or occlusion (6.1% vs. 6.6%), Dr. Gokhroo reported.
The secondary endpoints of crossover rate (4.4% vs. 3.8%) and vessel spasm (6.9% vs. 8.7%) were also similar.
“Our study definitely says that ulnar access is as good as radial access,” he said.
An analysis of 48 transradial crossover events revealed that, had ulnar cannulation been considered, the need for crossover to femoral artery access would have been reduced from 38 patients to 2 patients, with the remaining 10 patients accessed via the contralateral radial artery.
“If you have expertise in ulnar cannulation, 75% of femoral artery cannulations can be avoided,” Dr. Gokhroo said. “Transulnar cannulation is also an easy, safe, and comfortable procedure.”
Dr. Gokhroo and his associates said they had no relevant financial disclosures.
SAN DIEGO – Surgical experience puts transulnar access on a par with transradial access for percutaneous coronary procedures, the randomized AJULAR trial showed.
The primary composite outcome of major adverse cardiac events or major vascular events during hospitalization was 14.6% for transulnar access and 14.4% for transradial access, meeting the noninferiority criteria.
“If used as a default strategy, [transulnar access] is noninferior to the transradial approach when performed by an experienced operator,” study author Dr. Rajendra Gokhroo reported at the annual meeting of the American College of Cardiology.
The use of radial artery cannulation is growing in the United States as an approach for coronary access because of obvious safety advantages over femoral access, but has its own limitations such as frequent vasospasm, small caliber, and unsuitability as a graft for coronary artery bypass grafting after cannulation.
Transulnar access is used by some as an alternative, but was found inferior to transradial access in the AURA of ARTEMIS study because of significantly more large hematomas and a high crossover rate in the transulnar group (Circ. Cardiovasc. Interv. 2013;6:252-261).
However, the study used inexperienced ulnar operators and attempted to cannulate even nearly absent ulnar arteries, said Dr. Gokhroo, a pioneer in ulnar intervention at Jawaharlal Nehru Medical College, Ajmer, India, and president of the Indian Society of Cardiology. Based on their observations, the risk of such events is threefold higher during the first 50 procedures, but is no longer statistically significant after 51-100 operations or after 100 operations.
For the prospective, single-center AJULAR (Ajmer Ulnar Artery) trial, all operators were required to have a minimum experience of 50 transulnar cannulations, and cannulation was attempted only if the ulnar artery was easily palpable and the anatomy favorable.
“Excessive calcification, tortuosity, and low-volume pulse were the additional features where the ulnar was avoided; otherwise no other patient characteristics or demographic features compelled us to avoid ulnar access,” Dr. Gokhroo said in an interview.
Inability to palpate the radial artery was also an exclusion criterion.
A total of 2,532 patients scheduled to undergo elective coronary angiography and ad hoc percutaneous transluminal coronary angioplasty were evenly randomized to transulnar access or transradial access. The mean age for the 1,270 ulnar patients was 67 years and 63 years for the 1,262 radial patients.
There were no significant differences in an intention-to-treat analysis between the transulnar and transradial groups with respect to the individual components of the primary outcome: major adverse cardiac events (2.9% vs. 3.2%), large hematoma (1% vs. 0.9%), or occlusion (6.1% vs. 6.6%), Dr. Gokhroo reported.
The secondary endpoints of crossover rate (4.4% vs. 3.8%) and vessel spasm (6.9% vs. 8.7%) were also similar.
“Our study definitely says that ulnar access is as good as radial access,” he said.
An analysis of 48 transradial crossover events revealed that, had ulnar cannulation been considered, the need for crossover to femoral artery access would have been reduced from 38 patients to 2 patients, with the remaining 10 patients accessed via the contralateral radial artery.
“If you have expertise in ulnar cannulation, 75% of femoral artery cannulations can be avoided,” Dr. Gokhroo said. “Transulnar cannulation is also an easy, safe, and comfortable procedure.”
Dr. Gokhroo and his associates said they had no relevant financial disclosures.
SAN DIEGO – Surgical experience puts transulnar access on a par with transradial access for percutaneous coronary procedures, the randomized AJULAR trial showed.
The primary composite outcome of major adverse cardiac events or major vascular events during hospitalization was 14.6% for transulnar access and 14.4% for transradial access, meeting the noninferiority criteria.
“If used as a default strategy, [transulnar access] is noninferior to the transradial approach when performed by an experienced operator,” study author Dr. Rajendra Gokhroo reported at the annual meeting of the American College of Cardiology.
The use of radial artery cannulation is growing in the United States as an approach for coronary access because of obvious safety advantages over femoral access, but has its own limitations such as frequent vasospasm, small caliber, and unsuitability as a graft for coronary artery bypass grafting after cannulation.
Transulnar access is used by some as an alternative, but was found inferior to transradial access in the AURA of ARTEMIS study because of significantly more large hematomas and a high crossover rate in the transulnar group (Circ. Cardiovasc. Interv. 2013;6:252-261).
However, the study used inexperienced ulnar operators and attempted to cannulate even nearly absent ulnar arteries, said Dr. Gokhroo, a pioneer in ulnar intervention at Jawaharlal Nehru Medical College, Ajmer, India, and president of the Indian Society of Cardiology. Based on their observations, the risk of such events is threefold higher during the first 50 procedures, but is no longer statistically significant after 51-100 operations or after 100 operations.
For the prospective, single-center AJULAR (Ajmer Ulnar Artery) trial, all operators were required to have a minimum experience of 50 transulnar cannulations, and cannulation was attempted only if the ulnar artery was easily palpable and the anatomy favorable.
“Excessive calcification, tortuosity, and low-volume pulse were the additional features where the ulnar was avoided; otherwise no other patient characteristics or demographic features compelled us to avoid ulnar access,” Dr. Gokhroo said in an interview.
Inability to palpate the radial artery was also an exclusion criterion.
A total of 2,532 patients scheduled to undergo elective coronary angiography and ad hoc percutaneous transluminal coronary angioplasty were evenly randomized to transulnar access or transradial access. The mean age for the 1,270 ulnar patients was 67 years and 63 years for the 1,262 radial patients.
There were no significant differences in an intention-to-treat analysis between the transulnar and transradial groups with respect to the individual components of the primary outcome: major adverse cardiac events (2.9% vs. 3.2%), large hematoma (1% vs. 0.9%), or occlusion (6.1% vs. 6.6%), Dr. Gokhroo reported.
The secondary endpoints of crossover rate (4.4% vs. 3.8%) and vessel spasm (6.9% vs. 8.7%) were also similar.
“Our study definitely says that ulnar access is as good as radial access,” he said.
An analysis of 48 transradial crossover events revealed that, had ulnar cannulation been considered, the need for crossover to femoral artery access would have been reduced from 38 patients to 2 patients, with the remaining 10 patients accessed via the contralateral radial artery.
“If you have expertise in ulnar cannulation, 75% of femoral artery cannulations can be avoided,” Dr. Gokhroo said. “Transulnar cannulation is also an easy, safe, and comfortable procedure.”
Dr. Gokhroo and his associates said they had no relevant financial disclosures.
AT ACC 2015
Key clinical point: In experienced hands, transulnar access is a good alternative default approach for coronary angiography or angioplasty.
Major finding: The primary outcome of major adverse cardiac events and major vascular events occurred in 14.6% with transulnar access and 14.4% with transradial access.
Data source: A randomized, parallel group noninferiority trial in 2,532 patients.
Disclosures: Dr. Gokhroo and his associates reported having no relevant financial disclosures.
Novel agent lowers LDL more than ezetimibe
SAN DIEGO– ETC-1002, an oral LDL-lowering drug with a novel mechanism of action, decreased LDL by up to 30% more than did ezetimibe in a large phase IIb study.
The 348-patient trial also showed that ETC-1002 and ezetimibe are additive in their LDL-lowering effects, with the combination reducing LDL levels by up to 48%, compared with baseline, Dr. Paul Thompson reported at the annual meeting of the American College of Cardiology.
Importantly, the investigational drug and ezetimibe, which is marketed in the United States as Zetia, showed similarly favorable safety and tolerability profiles. And ETC-1002 proved equally effective at LDL-lowering in statin-intolerant and -tolerant patients, noted Dr. Thompson, director of cardiology at Hartford (Conn.) Hospital and professor of medicine at the University of Connecticut, Storrs.
ETC-1002 is a first-in-class oral modulator of the enzymes adenosine triphosphate citrate lyase and adenosine monophosphate–activated protein kinase. These dual mechanisms of action cause the liver to take up LDL cholesterol from the blood.
Asked how he sees the drug potentially fitting into the dyslipidemia treatment landscape, which could soon include the eagerly anticipated, super-potent LDL-lowering proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, Dr. Thompson pointed out that there have been no cardiovascular outcome studies of ETC-1002.
“Without outcomes data in terms of survival, I’d say ETC-1002 will be an additional drug in our armamentarium for treating high cholesterol levels. And so far, we’ve generally seen that reductions in LDL cholesterol result in reductions in clinical events. I would think that this drug would be a way to treat statin-intolerant or -tolerant patients in combination with ezetimibe or alone. And where it fits in will depend somewhat on the cost of the medication,” he said.
The double-blind, phase IIb study randomized 177 hypercholesterolemic patients with muscle-related statin intolerance and 171 without statin intolerance to 12 weeks of once-daily ETC-1002 at 120 mg or 180 mg, ezetimibe at 10 mg, the combination of ETC-1002 at 120 mg plus ezetimibe 10 mg, or ETC-1002 at 180 and ezetimibe 10 mg.
ETC-1002 at the lower dose reduced LDL by a mean of 27% from a baseline of 165 mg/dL. The 180-mg dose decreased LDL by 30%. In contrast, ezetimibe monotherapy lowered LDL by 21%, a significantly lesser effect than with either dose of ETC-1002. The effects of combination therapy were additive: ETC-1002 at 120 mg plus ezetimibe reduced LDL by 43%, compared with baseline, while ETC-1002 at 180 mg plus ezetimibe 10 mg lowered LDL by 48%.
ETC-1002 also improved other atherogenic lipids and markers of systemic inflammation. For example, apolipoprotein B decreased by 30% with ETC-1002 at 120 mg, 40% with ETC-1002 at 180 mg, and 10% with ezetimibe at 10 mg. Similarly, C-reactive protein levels fell by 30% and 40% with low- and higher-dose ETC-1002, compared with a 10% reduction with ezetimibe.
Muscle-related complaints were several-fold more common in the group with a history of statin intolerance as defined by the Food and Drug Administration – namely, muscle-related intolerance to at least two statins, including one at the lowest approved dose. However, rates of discontinuation from drug-related adverse events were similarly low at 3%-8% across the five treatment arms.
“I think the take-home message is that the safety profile of the drug looks good,” the cardiologist said.
No significant changes were seen in body weight, blood glucose levels, or blood pressure during the 12-week study.
Dr. Thompson reported receiving a research grant from Esperion Therapeutics, the study sponsor. He serves as a consultant to AstraZeneca, Merck, and Sanofi-Aventis.
SAN DIEGO– ETC-1002, an oral LDL-lowering drug with a novel mechanism of action, decreased LDL by up to 30% more than did ezetimibe in a large phase IIb study.
The 348-patient trial also showed that ETC-1002 and ezetimibe are additive in their LDL-lowering effects, with the combination reducing LDL levels by up to 48%, compared with baseline, Dr. Paul Thompson reported at the annual meeting of the American College of Cardiology.
Importantly, the investigational drug and ezetimibe, which is marketed in the United States as Zetia, showed similarly favorable safety and tolerability profiles. And ETC-1002 proved equally effective at LDL-lowering in statin-intolerant and -tolerant patients, noted Dr. Thompson, director of cardiology at Hartford (Conn.) Hospital and professor of medicine at the University of Connecticut, Storrs.
ETC-1002 is a first-in-class oral modulator of the enzymes adenosine triphosphate citrate lyase and adenosine monophosphate–activated protein kinase. These dual mechanisms of action cause the liver to take up LDL cholesterol from the blood.
Asked how he sees the drug potentially fitting into the dyslipidemia treatment landscape, which could soon include the eagerly anticipated, super-potent LDL-lowering proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, Dr. Thompson pointed out that there have been no cardiovascular outcome studies of ETC-1002.
“Without outcomes data in terms of survival, I’d say ETC-1002 will be an additional drug in our armamentarium for treating high cholesterol levels. And so far, we’ve generally seen that reductions in LDL cholesterol result in reductions in clinical events. I would think that this drug would be a way to treat statin-intolerant or -tolerant patients in combination with ezetimibe or alone. And where it fits in will depend somewhat on the cost of the medication,” he said.
The double-blind, phase IIb study randomized 177 hypercholesterolemic patients with muscle-related statin intolerance and 171 without statin intolerance to 12 weeks of once-daily ETC-1002 at 120 mg or 180 mg, ezetimibe at 10 mg, the combination of ETC-1002 at 120 mg plus ezetimibe 10 mg, or ETC-1002 at 180 and ezetimibe 10 mg.
ETC-1002 at the lower dose reduced LDL by a mean of 27% from a baseline of 165 mg/dL. The 180-mg dose decreased LDL by 30%. In contrast, ezetimibe monotherapy lowered LDL by 21%, a significantly lesser effect than with either dose of ETC-1002. The effects of combination therapy were additive: ETC-1002 at 120 mg plus ezetimibe reduced LDL by 43%, compared with baseline, while ETC-1002 at 180 mg plus ezetimibe 10 mg lowered LDL by 48%.
ETC-1002 also improved other atherogenic lipids and markers of systemic inflammation. For example, apolipoprotein B decreased by 30% with ETC-1002 at 120 mg, 40% with ETC-1002 at 180 mg, and 10% with ezetimibe at 10 mg. Similarly, C-reactive protein levels fell by 30% and 40% with low- and higher-dose ETC-1002, compared with a 10% reduction with ezetimibe.
Muscle-related complaints were several-fold more common in the group with a history of statin intolerance as defined by the Food and Drug Administration – namely, muscle-related intolerance to at least two statins, including one at the lowest approved dose. However, rates of discontinuation from drug-related adverse events were similarly low at 3%-8% across the five treatment arms.
“I think the take-home message is that the safety profile of the drug looks good,” the cardiologist said.
No significant changes were seen in body weight, blood glucose levels, or blood pressure during the 12-week study.
Dr. Thompson reported receiving a research grant from Esperion Therapeutics, the study sponsor. He serves as a consultant to AstraZeneca, Merck, and Sanofi-Aventis.
SAN DIEGO– ETC-1002, an oral LDL-lowering drug with a novel mechanism of action, decreased LDL by up to 30% more than did ezetimibe in a large phase IIb study.
The 348-patient trial also showed that ETC-1002 and ezetimibe are additive in their LDL-lowering effects, with the combination reducing LDL levels by up to 48%, compared with baseline, Dr. Paul Thompson reported at the annual meeting of the American College of Cardiology.
Importantly, the investigational drug and ezetimibe, which is marketed in the United States as Zetia, showed similarly favorable safety and tolerability profiles. And ETC-1002 proved equally effective at LDL-lowering in statin-intolerant and -tolerant patients, noted Dr. Thompson, director of cardiology at Hartford (Conn.) Hospital and professor of medicine at the University of Connecticut, Storrs.
ETC-1002 is a first-in-class oral modulator of the enzymes adenosine triphosphate citrate lyase and adenosine monophosphate–activated protein kinase. These dual mechanisms of action cause the liver to take up LDL cholesterol from the blood.
Asked how he sees the drug potentially fitting into the dyslipidemia treatment landscape, which could soon include the eagerly anticipated, super-potent LDL-lowering proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, Dr. Thompson pointed out that there have been no cardiovascular outcome studies of ETC-1002.
“Without outcomes data in terms of survival, I’d say ETC-1002 will be an additional drug in our armamentarium for treating high cholesterol levels. And so far, we’ve generally seen that reductions in LDL cholesterol result in reductions in clinical events. I would think that this drug would be a way to treat statin-intolerant or -tolerant patients in combination with ezetimibe or alone. And where it fits in will depend somewhat on the cost of the medication,” he said.
The double-blind, phase IIb study randomized 177 hypercholesterolemic patients with muscle-related statin intolerance and 171 without statin intolerance to 12 weeks of once-daily ETC-1002 at 120 mg or 180 mg, ezetimibe at 10 mg, the combination of ETC-1002 at 120 mg plus ezetimibe 10 mg, or ETC-1002 at 180 and ezetimibe 10 mg.
ETC-1002 at the lower dose reduced LDL by a mean of 27% from a baseline of 165 mg/dL. The 180-mg dose decreased LDL by 30%. In contrast, ezetimibe monotherapy lowered LDL by 21%, a significantly lesser effect than with either dose of ETC-1002. The effects of combination therapy were additive: ETC-1002 at 120 mg plus ezetimibe reduced LDL by 43%, compared with baseline, while ETC-1002 at 180 mg plus ezetimibe 10 mg lowered LDL by 48%.
ETC-1002 also improved other atherogenic lipids and markers of systemic inflammation. For example, apolipoprotein B decreased by 30% with ETC-1002 at 120 mg, 40% with ETC-1002 at 180 mg, and 10% with ezetimibe at 10 mg. Similarly, C-reactive protein levels fell by 30% and 40% with low- and higher-dose ETC-1002, compared with a 10% reduction with ezetimibe.
Muscle-related complaints were several-fold more common in the group with a history of statin intolerance as defined by the Food and Drug Administration – namely, muscle-related intolerance to at least two statins, including one at the lowest approved dose. However, rates of discontinuation from drug-related adverse events were similarly low at 3%-8% across the five treatment arms.
“I think the take-home message is that the safety profile of the drug looks good,” the cardiologist said.
No significant changes were seen in body weight, blood glucose levels, or blood pressure during the 12-week study.
Dr. Thompson reported receiving a research grant from Esperion Therapeutics, the study sponsor. He serves as a consultant to AstraZeneca, Merck, and Sanofi-Aventis.
AT ACC 15
Key clinical point: A novel investigational LDL cholesterol–lowering agent showed greater lipid lowering than did ezetimibe along with similar safety and tolerability.
Major finding: ETC-1002 at 180 mg once daily lowered LDL by a mean of 30%, while ezetimibe at 10 mg/day decreased LDL by 21%.
Data source: This phase IIb, double-blind, randomized, 12-week trial included 348 hypercholesterolemic patients, roughly half with a history of statin intolerance.
Disclosures: The study presenter received a research grant from Esperion Therapeutics, which sponsored the trial.
How to forestall heart failure by 15 years
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
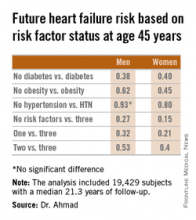
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
SAN DIEGO– Men and women who are able to prevent or delay onset of hypertension, obesity, and diabetes beyond age 45 years can expect to reap a major benefit: living for 11-15 years longer without heart failure, according to a novel study featuring more than 500,000 person-years of follow-up.
“We’re interested in thinking about risk in a different way. Traditionally, risk has been thought of in terms of how different risk factors lead to increased chances for heart failure. Instead, we’re interested in thinking about how preventing the development of risk factors leads to increased longevity and extension of heart failure–free survival. It’s a much more powerful message when you’re talking to patients in their 30s or 40s to say that they’ll be able to live 11-15 years longer without heart failure if they can avoid developing these three risk factors,” Dr. Faraz S. Ahmad explained at the annual meeting of the American College of Cardiology.
He presented an analysis of pooled data from four large studies with adjudicated heart failure outcomes. The analysis, conducted as part of the Cardiovascular Lifetime Risk Pooling Project, included a total of 19,429 subjects with a median 21.3 years of follow-up, during which 1,677 participants were diagnosed with incident heart failure.
This analysis quantified the association between prevalent hypertension, diabetes, and/or obesity with heart failure–free and overall survival, beginning at age 45 years and with 50 years of subsequent follow-up, noted Dr. Ahmad, a cardiology fellow at Northwestern University, Chicago.
Among men with none of the three key risk factors at age 45 years, the multivariate-adjusted risk of subsequently developing heart failure was reduced by 73%, compared with that of men with all three risk factors present. Women with none of the three risk factors enjoyed an 85% relative risk reduction.
Among men who developed heart failure, those with diabetes by age 45 years were diagnosed with heart failure 8.6 years earlier than were those without diabetes at age 45 years. Among women with diabetes at age 45 years, heart failure was diagnosed when they were 10.6 years younger than in those without diabetes were.
These data take on added weight in light of projections regarding the future of heart failure in the United States. At present, there are an estimated 825,000 new cases of heart failure per year. The disease prevalence is 5.1 million. The annual cost is $31 billion and is expected to climb by 126% over the next 20 years, Dr. Ahmad said.
The four studies upon which this lifetime risk analysis was based were the Framingham Heart Study, the Framingham Offspring Study, the Atherosclerosis Risk in Communities study, and the Chicago Heart Association Detection Project in Industry. Together they included 509,650 person-years of follow-up. All four studies were funded by the National Heart, Lung, and Blood Institute, as was this analysis. Dr. Ahmad reported having no financial conflicts.
AT ACC 15
Key clinical point: Individuals who remain free of hypertension, obesity, and diabetes at age 45 years can expect to enjoy an extra 11-15 years of heart failure–free survival.
Major finding: The lifetime risk of developing heart failure in men without hypertension, obesity, and diabetes at age 45 years was reduced by 73%, compared with the risk in men having all three risk factors at that age. In women free of the three risk factors at age 45 years, the relative risk reduction was 85%.
Data source: This pooled analysis of data from four major studies included 19,429 subjects with 509,650 person-years of follow-up, during which 1,677 participants were newly diagnosed with heart failure.
Disclosures: This analysis was supported by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
Screen for acute stress disorder in ACS patients
SAN DIEGO – Screening for acute stress disorder with depression upon hospital admission for acute coronary syndrome enables physicians to identify a subgroup of ACS patients at increased risk for in-hospital mortality, Dr. Edina Cenko reported at the annual meeting of the American College of Cardiology.
“It’s a simple, quick test that’s easy to do in clinical practice, and it identifies a group of ACS patients who warrant special attention. It’s important for physicians to do because most deaths in ACS patients occur before arrival at the hospital or in-hospital,” said Dr. Cenko of the University of Bologna (Italy).
She presented a study of 5,880 patients enrolled in the International Survey of Acute Coronary Syndromes in Transitional Countries, all of whom had survived day 1 following admission for ACS at seven Serbian hospitals during 2009-2014. They were screened for acute stress disorder (ASD) and depression upon admission.
Patients who met the inclusion criteria for ASD due to a traumatic event occurring within 4 weeks prior to their ACS and who also had at least mild depression upon admission as indicated by a score of 8 or more out of a possible 27 points on the Patient Health Questionnaire-9 had a significantly higher subsequent in-hospital mortality: 10.7% compared with 8.1%.
The prevalence of ASD with depression was 51% in patients with ST-elevation MI compared with 67% in those with non–ST-elevation ACS. Upon statistical adjustment for these and other clinical and demographic factors, patients with ASD plus depression were 68% more likely to die in-hospital. Upon further adjustment for the use of guideline-recommended medications and percutaneous coronary intervention, the ASD/depression group remained at 35% increased risk of in-hospital mortality.
Dr. Cenko explained that this study was conducted in Serbia because the nation’s turbulent history in recent decades made for an enriched population of individuals with ASD.
Acute stress disorder was introduced as a new diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The diagnosis was created for patients who met most of the criteria for posttraumatic stress disorder except their duration of symptoms was insufficient because the traumatic event had taken place too recently to qualify for PTSD. The diagnosis of ASD is included in DSM-5 as well.
The physician-investigators in the international registry identified ACS patients as having ASD if they had experienced a traumatic event within 4 weeks preceding the ACS and about which they were experiencing intrusive thoughts, memories, or nightmares, avoidance of situations that reminded them about the traumatic event, and increased arousal as typically manifest in insomnia and/or irritability.
Dr. Cenko reported having no financial conflicts regarding her study.
SAN DIEGO – Screening for acute stress disorder with depression upon hospital admission for acute coronary syndrome enables physicians to identify a subgroup of ACS patients at increased risk for in-hospital mortality, Dr. Edina Cenko reported at the annual meeting of the American College of Cardiology.
“It’s a simple, quick test that’s easy to do in clinical practice, and it identifies a group of ACS patients who warrant special attention. It’s important for physicians to do because most deaths in ACS patients occur before arrival at the hospital or in-hospital,” said Dr. Cenko of the University of Bologna (Italy).
She presented a study of 5,880 patients enrolled in the International Survey of Acute Coronary Syndromes in Transitional Countries, all of whom had survived day 1 following admission for ACS at seven Serbian hospitals during 2009-2014. They were screened for acute stress disorder (ASD) and depression upon admission.
Patients who met the inclusion criteria for ASD due to a traumatic event occurring within 4 weeks prior to their ACS and who also had at least mild depression upon admission as indicated by a score of 8 or more out of a possible 27 points on the Patient Health Questionnaire-9 had a significantly higher subsequent in-hospital mortality: 10.7% compared with 8.1%.
The prevalence of ASD with depression was 51% in patients with ST-elevation MI compared with 67% in those with non–ST-elevation ACS. Upon statistical adjustment for these and other clinical and demographic factors, patients with ASD plus depression were 68% more likely to die in-hospital. Upon further adjustment for the use of guideline-recommended medications and percutaneous coronary intervention, the ASD/depression group remained at 35% increased risk of in-hospital mortality.
Dr. Cenko explained that this study was conducted in Serbia because the nation’s turbulent history in recent decades made for an enriched population of individuals with ASD.
Acute stress disorder was introduced as a new diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The diagnosis was created for patients who met most of the criteria for posttraumatic stress disorder except their duration of symptoms was insufficient because the traumatic event had taken place too recently to qualify for PTSD. The diagnosis of ASD is included in DSM-5 as well.
The physician-investigators in the international registry identified ACS patients as having ASD if they had experienced a traumatic event within 4 weeks preceding the ACS and about which they were experiencing intrusive thoughts, memories, or nightmares, avoidance of situations that reminded them about the traumatic event, and increased arousal as typically manifest in insomnia and/or irritability.
Dr. Cenko reported having no financial conflicts regarding her study.
SAN DIEGO – Screening for acute stress disorder with depression upon hospital admission for acute coronary syndrome enables physicians to identify a subgroup of ACS patients at increased risk for in-hospital mortality, Dr. Edina Cenko reported at the annual meeting of the American College of Cardiology.
“It’s a simple, quick test that’s easy to do in clinical practice, and it identifies a group of ACS patients who warrant special attention. It’s important for physicians to do because most deaths in ACS patients occur before arrival at the hospital or in-hospital,” said Dr. Cenko of the University of Bologna (Italy).
She presented a study of 5,880 patients enrolled in the International Survey of Acute Coronary Syndromes in Transitional Countries, all of whom had survived day 1 following admission for ACS at seven Serbian hospitals during 2009-2014. They were screened for acute stress disorder (ASD) and depression upon admission.
Patients who met the inclusion criteria for ASD due to a traumatic event occurring within 4 weeks prior to their ACS and who also had at least mild depression upon admission as indicated by a score of 8 or more out of a possible 27 points on the Patient Health Questionnaire-9 had a significantly higher subsequent in-hospital mortality: 10.7% compared with 8.1%.
The prevalence of ASD with depression was 51% in patients with ST-elevation MI compared with 67% in those with non–ST-elevation ACS. Upon statistical adjustment for these and other clinical and demographic factors, patients with ASD plus depression were 68% more likely to die in-hospital. Upon further adjustment for the use of guideline-recommended medications and percutaneous coronary intervention, the ASD/depression group remained at 35% increased risk of in-hospital mortality.
Dr. Cenko explained that this study was conducted in Serbia because the nation’s turbulent history in recent decades made for an enriched population of individuals with ASD.
Acute stress disorder was introduced as a new diagnosis in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. The diagnosis was created for patients who met most of the criteria for posttraumatic stress disorder except their duration of symptoms was insufficient because the traumatic event had taken place too recently to qualify for PTSD. The diagnosis of ASD is included in DSM-5 as well.
The physician-investigators in the international registry identified ACS patients as having ASD if they had experienced a traumatic event within 4 weeks preceding the ACS and about which they were experiencing intrusive thoughts, memories, or nightmares, avoidance of situations that reminded them about the traumatic event, and increased arousal as typically manifest in insomnia and/or irritability.
Dr. Cenko reported having no financial conflicts regarding her study.
AT ACC 15
Key clinical point: Acute coronary syndrome patients who meet criteria for acute stress disorder along with at least mild depression are at increased risk for in-hospital mortality.
Major finding: In-hospital mortality occurred in 10.7% of ACS patients with acute stress disorder plus depression compared with 8.1% of unaffected ACS patients.
Data source: A study of 5,880 participants in the International Survey of Acute Coronary Syndromes in Transitional Countries.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was conducted free of commercial funding.
Novel oral anticoagulants best warfarin for AF in heart failure
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
SAN DIEGO – The novel oral anticoagulants clearly outperformed warfarin for stroke prevention and safety endpoints in patients with atrial fibrillation and comorbid heart failure in a meta-analysis of four recent landmark Phase 3 clinical trials.
Collectively the four novel oral anticoagulants (NOACs) approved for stroke prophylaxis in nonvalvular atrial fibrillation (AF) reduced the risk of stroke and systemic embolism by 14%, compared with patients randomized to warfarin. Moreover, the NOACs decreased the risks of major bleeding and intracranial bleeding by 23% and 45%, respectively, Dr. Gianluigi Savarese reported at the annual meeting of the American College of Cardiology.
“NOACs represent a valuable therapeutic option in patients with nonvalvular atrial fibrillation and heart failure,” concluded Dr. Savarese of Federico II University, Naples.
There has never been a randomized trial comparing a NOAC to warfarin specifically in patients with these dual diagnoses. In the absence of such a definitive study, the next best thing is a meta-analysis of the pivotal Phase 3 trials in which warfarin was compared to dabigatran (Pradaxa, the RE-LY study), apixaban (Eliquis, ARISTOTLE), rivaroxaban (Xarelto, ROCKET AF), and edoxaban (Savaysa, ENGAGE AF-TIMI 48).
The meta-analysis focused on a subset population of 26,384 randomized patients with AF and heart failure. It’s important to know how the NOACs stack up against warfarin in this population because symptomatic heart failure is common: indeed, it’s present in 30% of patients with AF. Patients with AF and comorbid heart failure are generally older, frailer, have more comorbidities, and are at higher risk of both stroke and bleeding, compared with AF patients without heart failure. Since heart failure is a recognized risk factor for reduced time in the therapeutic international normalized ratio (INR) range for patients on warfarin, it’s likely that warfarin-treated dual diagnosis patients would be exposed to further increased risks of stroke and bleeding, according to Dr. Savarese.
In the meta-analysis, in addition to the NOAC-treated patients’ significantly reduced risks of stroke, major bleeding, and intracranial bleeding, they showed a 12% decrease in total bleeding and an 8% reduction in cardiovascular death, compared with warfarin-treated controls, although neither of those latter two favorable trends achieved statistical significance.
The four NOACs didn’t differ significantly on any of the prespecified outcomes in the meta-analysis.
One audience member noted that while the relative risk reductions for stroke and major bleeding seen with the NOACs in the meta-analysis were large and impressive, the absolute risk reductions were actually quite small. For example, warfarin-treated controls in RE-LY, the first of the major trials, had a stroke/systemic embolism rate of 1.69%/year and a major bleeding rate of 3.4%/year (N. Engl. J. Med. 2009;361:1139-51), while controls in ENGAGE AF-TIMI 48 had annualized stroke and major bleeding rates of 1.5% and 3.4%, respectively (N. Engl. J. Med. 2013;369:2093-2104).
Dr. Savarese replied that he and his coinvestigators consider those absolute risk reductions to be clinically meaningful, especially in light of the enormous and rapidly growing number of patients with both AF and heart failure.
He reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
AT ACC 15
Key clinical point: Patients with nonvalvular atrial fibrillation and heart failure clearly fare better on any of the novel oral anticoagulants than with warfarin for stroke prophylaxis.
Major finding: Dual diagnosis patients randomized to a novel oral anticoagulant had a 14% reduction in stroke/systemic embolism and a 23% decrease in major bleeding compared with those on warfarin.
Data source: This was a meta-analysis of the 26,384 patients with both atrial fibrillation and heart failure who were included in four pivotal Phase 3 clinical trials that led to approval of dabigatran, apixaban, rivaroxaban, and edoxaban.
Disclosures: The presenter reported having no financial conflicts regarding this meta-analysis, which was carried out free of commercial support.
Multiple systems showcase TAVR’s advances
SAN DIEGO – Three separate reports of pivotal trial results from three different systems for performing transcatheter aortic valve replacement performed during three distinct periods highlighted the rapid advances of this intervention that have now put it on the cusp of being the preferred, default strategy for replacing stenosed aortic valves in older patients.
First SAPIEN 3 U.S. outcomes reported
The most recent technologic advance in transcatheter aortic valve replacement (TAVR), showcased in one of the three presentations at the annual meeting of the American College of Cardiology, was the SAPIEN 3 System, the lowest-profile TAVR system so far to undergo U.S. testing with a 14F delivery system for all valve sizes except the largest, 29 mm valves, which require a 16F system. SAPIEN 3 produced 30-day outcome results so good that one TAVR operator expressed shock at the dramatic, short-term success.
After 30 days follow-up, SAPIEN 3 produced a 2% mortality rate and a 2% stroke rate in 583 “high-risk” patients treated during 2013 and 2014 who were an average of 83 years old and had an average Society of Thoracic Surgeons (STS) risk score of 8.6%, Dr. Susheel K. Kodali reported at the meeting. By contrast, in the pivotal trial of the first-generation SAPIEN TAVR system, run during 2007-2009, in 348 “high-risk” patients (who averaged 84 years old and had an average STS risk score of 11.8%) the 30-day mortality ran 5% and 30-day strokes affected 6%, Dr. Kodali noted.
The new trial also enrolled 1,076 “intermediate”-risk patients, who averaged 82 years old with an average STS score of 5.3%. Their 30-day mortality rate was 1% and their total stroke rate was 3%, with a disabling stroke rate (modified Rankin scale score of 2 or more) of 1%.
These excellent results prompted Edwards, the company developing the SAPIEN 3 System, to apply for Food and Drug Administration approval for the device in high-risk patients, Dr. Kodali noted. In March, Edwards released a statement in which it said it expects SAPIEN 3 to receive U.S. marketing approval within a year. While Dr. Kodali said longer-term follow-up is needed for the intermediate-risk patients, the outcome results he has seen make him rethink the TAVR’s role, compared with conventional aortic valve replacement by open surgery.
“With surgery, these [intermediate-risk] patients would have a 5% mortality rate. The conversation now may need to change,” he said. “We have always said we use TAVR when surgery is not a good option, but based on the recent, 2-year findings with the CoreValve and our data, with a 1% mortality and a 1% disabling stroke rate [in intermediate-risk patients] maybe TAVR is now the preferred option, at least for 80-year-olds, said Dr. Kodali, codirector of the Heart Valve Center at NewYork-Presbyterian/Columbia University Medical Center.
Commenting on the SAPIEN 3 results as well as excellent 2-year results from the CoreValve TAVR System pivotal trial, Dr. Jeffrey J. Popma attributed the success to three factors: improved patient selection, refined and routinely used imaging methods for sizing the aortic annulus to better match the TAVR valve size to the annulus size, and improved TAVR techniques based on what is now about an 8-year clinical experience using TAVR.
“All this now puts us in a place where the bar has been set very high for surgery. How does surgery compete?” asked Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “The results are so clean at 30 days, what more information do we really need” for device approval? Dr. Popma, one of the discussants for the meeting report by Dr. Kodali, asked.
“I’m shocked the SAPIEN 3 data were so good,” said Dr. Stephen Ramee, an interventional cardiologist and medical director of the Structural and Valvular Heart Center at the Ochsner Medical Center in New Orleans. “We’ll need to wait to do intermediate-risk patients routinely, but the handwriting is on the wall with these data and the CoreValve results. I think TAVR will be preferred for all high-risk patients and for intermediate-risk patients who are at least 80 years old,” Dr. Ramee said in an interview.
Commenting on whether the FDA should approve SAPIEN 3 based on 30-day outcomes in high-risk patients, Dr. Popma noted that SAPIEN 3 is “an iteration of what has been demonstrated” in long-term results with the first-generation SAPIEN device. “Do we really need a randomized clinical trail with several thousand patients after the [SAPIEN] platform was already established?” he asked.
“We don’t demand that for iterations of surgical valves,” agreed Dr. Kodali.
CoreValve looks good longer-term
While SAPIEN 3’s performance turned heads, 2-year results from the CoreValve’s pivotal trial reported at the meeting further deepened the impression that TAVR offered substantial advantages, compared with surgical aortic valve replacement in high-risk patients. The 2-year outcomes followed the trial’s primary endpoint reported last year, the 1-year results, which had shown a statistically significant advantage in survival, compared with surgery (N. Engl. J. Med. 2014;370:1790-8).
The 2-year follow-up showed this advantage was “sustainable, durable, and widening,” reported Dr. Michael Reardon, professor of cardiothoracic surgery at the Methodist Hospital in Houston.
For example, 2-year all-cause mortality stood at 29% in patients who underwent surgical valve replacement and 22% in those who received TAVR with CoreValve, a statistically significant widening of the between-group gap, compared with the respective 19% and 14% mortality rates after 1 year. The rate of all-cause death or major stroke after 2 years ran 33% and 24% with surgical valve replacement or TAVR, respectively, compared with rates of 23% and 16% after 1 year.
Concern about excess strokes with TAVR, “one of our early worries, seems to have been put to bed,” Dr. Reardon said. The results also showed CoreValve substantially surpassed open surgery for valvular blood flow metrics across the full range of follow-up time points.
Several factors might have produced the widening outcomes between years 1 and 2 following a different one-time intervention, Dr. Reardon said, such as significant differences in the incident rate following intervention for disabling or life-threatening bleeding, atrial fibrillation, and acute kidney injury. All three complications occurred significantly more often in the surgery patients, compared with those treated with TAVR, and while the bulk of the difference in incidence showed up within the first month after intervention, each of these complications could have important long-term effects on survival and stroke rates, Dr. Reardon explained. On the other side of the ledger, open surgery when compared with CoreValve resulted in significantly fewer acute and long-term episodes of vascular complications and fewer new pacemakers, but these complications likely have less impact on stroke and mortality than the three that occur more often with surgery.
Overall, the widening gap in outcomes between surgery and TAVR “suggests that TAVR with a self-expanding valve [CoreValve] should be considered the preferred treatment,” compared with surgery in high-risk patients, Dr. Reardon said during his presentation at the meeting, a similar conclusion to what Dr. Kodali said about SAPIEN 3.
“These results really do move the needle forward,” said Dr. Popma, a CoreValve trial coinvestigator. “We see for the first time that [TAVR] may indeed be superior, and although durability remains a long-term question we have 2-year data that CoreValve has held up.”
First-generation SAPIEN TAVR shows 5-year stability
The third piece of the TAVR trilogy reported at the meeting was 5-year follow-up results from the first U.S. TAVR pivotal trial (PARTNER 1) using the first-generation SAPIEN device, which is no longer available in the United States. Those results from patients treated during 2007-2009 showed continuation of the statistical overlap between surgical valve replacement and SAPIEN TAVR for all-cause mortality, stroke, and other outcomes (Lancet 2015 [doi:10.1016/S0140-6736(15)60290-2]). Importantly, the results also showed no suggestions of deteriorating function in the TAVR valve after 5 years, reported Dr. Michael J. Mack at the meeting.
“It’s very encouraging, but we’d all like to go out to 10-12 years, and then we’d feel a lot better about it,” commented Dr. Reardon, who did not participate in the PARTNER trial. But given 5 years of apparently reliable function from a TAVR valve, the issue of long-term durability of these valves, an open question at the start of the SAPIEN trial, may be now coming to resolution, he suggested, at least for octogenarians, who are emerging as the prime demographic for TAVR.
“Tissue valves we place surgically deteriorate at different rates depending on a patient’s age when you put them in. In 30-year-olds many valves deteriorate after 10 years; in 50-year-olds maybe 15% will deteriorate, and in 70-year-olds almost none,” Dr. Reardon explained. “No one knows why.” Because many TAVR patients are at least 80 years old “it may take 10 years to see a signal of deterioration, or we might never see a signal,” he said.
SAPIEN versus CoreValve?
Although SAPIEN 3 may be on the U.S. market within the next year, for the time being routine U.S. TAVR practice is limited to two options, approved for high-risk or inoperable patients but not patients at intermediate risk: the CoreValve, which has now clearly bested surgery for all major endpoints over at least the midterm, and the SAPIEN XT model, the second-generation SAPIEN TAVR system that sits between the first generation and SAPIEN 3 and showed documented performance that roughly tracked with the original SAPIEN model. When approving the SAPIEN XT for U.S. marketing in June 2014 for operable high-risk patients as well as inoperable patients, the agency said the evidence indicated that XT was “noninferior” to SAPIEN in high-risk patients.
Several experts at the meeting agreed that despite this difference in performance between CoreValve and SAPIEN XT when each was compared with open surgery, the CoreValve experience better reflected more contemporary performance expectations for TAVR as a class, regardless of device. They cited improvements in valve sizing with imaging, better patient selection, and better TAVR technique as critical in boosting more favorable outcomes, factors that are not valve specific.
They also cited technical factors that may favor the SAPIEN XT device, notably more accurate valve placement using SAPIEN’s balloon-expandable format, compared with the self-expanding CoreValve.
Dr. Ramee said that outside of trials he’s now using SAPIEN XT for about 60% of his cases because of its “more predictable deployment. With CoreValve, you never know exactly where it will end up.” On the other hand, CoreValve works best for a more calcified aortic annulus because self-expanding placement is gentler and less likely to rupture a fragile annulus. That advantage gives CoreValve the edge for about 40% of his patients, Dr. Ramee said in an interview.
“There has been a general forward movement of the TAVR field” in which the focus has been TAVR versus surgery rather than CoreValve versus SAPIEN, Dr. Reardon said in an interview. Some anatomic features favor balloon expandable, others favor self-expanding. New TAVR systems in development by other manufacturers are mostly self-expanding models because of their ability to allow for repositioning, he noted.
Dr. Kodali has an equity interest in Thubrikar Aortic Valve; he has received honoraria from St. Jude; he has served on the steering committee for trials sponsored by Edwards, which markets the SAPIEN systems, Claret Medical, and Meril; and he has received research support from Edwards. Dr. Popma has been a consultant to Abbott, Abiomed, Boston Scientific, Cordis, and Abbott Vascular, and he has received research grants from Medtronic, which markets the CoreValve, and from five other companies. Dr. Reardon has served on an advisory board for Medtronic, Dr. Ramee has received honoraria from Edwards and from Medtronic and has a financial interest in several other companies developing TAVR systems.
On Twitter @mitchelzoler
SAN DIEGO – Three separate reports of pivotal trial results from three different systems for performing transcatheter aortic valve replacement performed during three distinct periods highlighted the rapid advances of this intervention that have now put it on the cusp of being the preferred, default strategy for replacing stenosed aortic valves in older patients.
First SAPIEN 3 U.S. outcomes reported
The most recent technologic advance in transcatheter aortic valve replacement (TAVR), showcased in one of the three presentations at the annual meeting of the American College of Cardiology, was the SAPIEN 3 System, the lowest-profile TAVR system so far to undergo U.S. testing with a 14F delivery system for all valve sizes except the largest, 29 mm valves, which require a 16F system. SAPIEN 3 produced 30-day outcome results so good that one TAVR operator expressed shock at the dramatic, short-term success.
After 30 days follow-up, SAPIEN 3 produced a 2% mortality rate and a 2% stroke rate in 583 “high-risk” patients treated during 2013 and 2014 who were an average of 83 years old and had an average Society of Thoracic Surgeons (STS) risk score of 8.6%, Dr. Susheel K. Kodali reported at the meeting. By contrast, in the pivotal trial of the first-generation SAPIEN TAVR system, run during 2007-2009, in 348 “high-risk” patients (who averaged 84 years old and had an average STS risk score of 11.8%) the 30-day mortality ran 5% and 30-day strokes affected 6%, Dr. Kodali noted.
The new trial also enrolled 1,076 “intermediate”-risk patients, who averaged 82 years old with an average STS score of 5.3%. Their 30-day mortality rate was 1% and their total stroke rate was 3%, with a disabling stroke rate (modified Rankin scale score of 2 or more) of 1%.
These excellent results prompted Edwards, the company developing the SAPIEN 3 System, to apply for Food and Drug Administration approval for the device in high-risk patients, Dr. Kodali noted. In March, Edwards released a statement in which it said it expects SAPIEN 3 to receive U.S. marketing approval within a year. While Dr. Kodali said longer-term follow-up is needed for the intermediate-risk patients, the outcome results he has seen make him rethink the TAVR’s role, compared with conventional aortic valve replacement by open surgery.
“With surgery, these [intermediate-risk] patients would have a 5% mortality rate. The conversation now may need to change,” he said. “We have always said we use TAVR when surgery is not a good option, but based on the recent, 2-year findings with the CoreValve and our data, with a 1% mortality and a 1% disabling stroke rate [in intermediate-risk patients] maybe TAVR is now the preferred option, at least for 80-year-olds, said Dr. Kodali, codirector of the Heart Valve Center at NewYork-Presbyterian/Columbia University Medical Center.
Commenting on the SAPIEN 3 results as well as excellent 2-year results from the CoreValve TAVR System pivotal trial, Dr. Jeffrey J. Popma attributed the success to three factors: improved patient selection, refined and routinely used imaging methods for sizing the aortic annulus to better match the TAVR valve size to the annulus size, and improved TAVR techniques based on what is now about an 8-year clinical experience using TAVR.
“All this now puts us in a place where the bar has been set very high for surgery. How does surgery compete?” asked Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “The results are so clean at 30 days, what more information do we really need” for device approval? Dr. Popma, one of the discussants for the meeting report by Dr. Kodali, asked.
“I’m shocked the SAPIEN 3 data were so good,” said Dr. Stephen Ramee, an interventional cardiologist and medical director of the Structural and Valvular Heart Center at the Ochsner Medical Center in New Orleans. “We’ll need to wait to do intermediate-risk patients routinely, but the handwriting is on the wall with these data and the CoreValve results. I think TAVR will be preferred for all high-risk patients and for intermediate-risk patients who are at least 80 years old,” Dr. Ramee said in an interview.
Commenting on whether the FDA should approve SAPIEN 3 based on 30-day outcomes in high-risk patients, Dr. Popma noted that SAPIEN 3 is “an iteration of what has been demonstrated” in long-term results with the first-generation SAPIEN device. “Do we really need a randomized clinical trail with several thousand patients after the [SAPIEN] platform was already established?” he asked.
“We don’t demand that for iterations of surgical valves,” agreed Dr. Kodali.
CoreValve looks good longer-term
While SAPIEN 3’s performance turned heads, 2-year results from the CoreValve’s pivotal trial reported at the meeting further deepened the impression that TAVR offered substantial advantages, compared with surgical aortic valve replacement in high-risk patients. The 2-year outcomes followed the trial’s primary endpoint reported last year, the 1-year results, which had shown a statistically significant advantage in survival, compared with surgery (N. Engl. J. Med. 2014;370:1790-8).
The 2-year follow-up showed this advantage was “sustainable, durable, and widening,” reported Dr. Michael Reardon, professor of cardiothoracic surgery at the Methodist Hospital in Houston.
For example, 2-year all-cause mortality stood at 29% in patients who underwent surgical valve replacement and 22% in those who received TAVR with CoreValve, a statistically significant widening of the between-group gap, compared with the respective 19% and 14% mortality rates after 1 year. The rate of all-cause death or major stroke after 2 years ran 33% and 24% with surgical valve replacement or TAVR, respectively, compared with rates of 23% and 16% after 1 year.
Concern about excess strokes with TAVR, “one of our early worries, seems to have been put to bed,” Dr. Reardon said. The results also showed CoreValve substantially surpassed open surgery for valvular blood flow metrics across the full range of follow-up time points.
Several factors might have produced the widening outcomes between years 1 and 2 following a different one-time intervention, Dr. Reardon said, such as significant differences in the incident rate following intervention for disabling or life-threatening bleeding, atrial fibrillation, and acute kidney injury. All three complications occurred significantly more often in the surgery patients, compared with those treated with TAVR, and while the bulk of the difference in incidence showed up within the first month after intervention, each of these complications could have important long-term effects on survival and stroke rates, Dr. Reardon explained. On the other side of the ledger, open surgery when compared with CoreValve resulted in significantly fewer acute and long-term episodes of vascular complications and fewer new pacemakers, but these complications likely have less impact on stroke and mortality than the three that occur more often with surgery.
Overall, the widening gap in outcomes between surgery and TAVR “suggests that TAVR with a self-expanding valve [CoreValve] should be considered the preferred treatment,” compared with surgery in high-risk patients, Dr. Reardon said during his presentation at the meeting, a similar conclusion to what Dr. Kodali said about SAPIEN 3.
“These results really do move the needle forward,” said Dr. Popma, a CoreValve trial coinvestigator. “We see for the first time that [TAVR] may indeed be superior, and although durability remains a long-term question we have 2-year data that CoreValve has held up.”
First-generation SAPIEN TAVR shows 5-year stability
The third piece of the TAVR trilogy reported at the meeting was 5-year follow-up results from the first U.S. TAVR pivotal trial (PARTNER 1) using the first-generation SAPIEN device, which is no longer available in the United States. Those results from patients treated during 2007-2009 showed continuation of the statistical overlap between surgical valve replacement and SAPIEN TAVR for all-cause mortality, stroke, and other outcomes (Lancet 2015 [doi:10.1016/S0140-6736(15)60290-2]). Importantly, the results also showed no suggestions of deteriorating function in the TAVR valve after 5 years, reported Dr. Michael J. Mack at the meeting.
“It’s very encouraging, but we’d all like to go out to 10-12 years, and then we’d feel a lot better about it,” commented Dr. Reardon, who did not participate in the PARTNER trial. But given 5 years of apparently reliable function from a TAVR valve, the issue of long-term durability of these valves, an open question at the start of the SAPIEN trial, may be now coming to resolution, he suggested, at least for octogenarians, who are emerging as the prime demographic for TAVR.
“Tissue valves we place surgically deteriorate at different rates depending on a patient’s age when you put them in. In 30-year-olds many valves deteriorate after 10 years; in 50-year-olds maybe 15% will deteriorate, and in 70-year-olds almost none,” Dr. Reardon explained. “No one knows why.” Because many TAVR patients are at least 80 years old “it may take 10 years to see a signal of deterioration, or we might never see a signal,” he said.
SAPIEN versus CoreValve?
Although SAPIEN 3 may be on the U.S. market within the next year, for the time being routine U.S. TAVR practice is limited to two options, approved for high-risk or inoperable patients but not patients at intermediate risk: the CoreValve, which has now clearly bested surgery for all major endpoints over at least the midterm, and the SAPIEN XT model, the second-generation SAPIEN TAVR system that sits between the first generation and SAPIEN 3 and showed documented performance that roughly tracked with the original SAPIEN model. When approving the SAPIEN XT for U.S. marketing in June 2014 for operable high-risk patients as well as inoperable patients, the agency said the evidence indicated that XT was “noninferior” to SAPIEN in high-risk patients.
Several experts at the meeting agreed that despite this difference in performance between CoreValve and SAPIEN XT when each was compared with open surgery, the CoreValve experience better reflected more contemporary performance expectations for TAVR as a class, regardless of device. They cited improvements in valve sizing with imaging, better patient selection, and better TAVR technique as critical in boosting more favorable outcomes, factors that are not valve specific.
They also cited technical factors that may favor the SAPIEN XT device, notably more accurate valve placement using SAPIEN’s balloon-expandable format, compared with the self-expanding CoreValve.
Dr. Ramee said that outside of trials he’s now using SAPIEN XT for about 60% of his cases because of its “more predictable deployment. With CoreValve, you never know exactly where it will end up.” On the other hand, CoreValve works best for a more calcified aortic annulus because self-expanding placement is gentler and less likely to rupture a fragile annulus. That advantage gives CoreValve the edge for about 40% of his patients, Dr. Ramee said in an interview.
“There has been a general forward movement of the TAVR field” in which the focus has been TAVR versus surgery rather than CoreValve versus SAPIEN, Dr. Reardon said in an interview. Some anatomic features favor balloon expandable, others favor self-expanding. New TAVR systems in development by other manufacturers are mostly self-expanding models because of their ability to allow for repositioning, he noted.
Dr. Kodali has an equity interest in Thubrikar Aortic Valve; he has received honoraria from St. Jude; he has served on the steering committee for trials sponsored by Edwards, which markets the SAPIEN systems, Claret Medical, and Meril; and he has received research support from Edwards. Dr. Popma has been a consultant to Abbott, Abiomed, Boston Scientific, Cordis, and Abbott Vascular, and he has received research grants from Medtronic, which markets the CoreValve, and from five other companies. Dr. Reardon has served on an advisory board for Medtronic, Dr. Ramee has received honoraria from Edwards and from Medtronic and has a financial interest in several other companies developing TAVR systems.
On Twitter @mitchelzoler
SAN DIEGO – Three separate reports of pivotal trial results from three different systems for performing transcatheter aortic valve replacement performed during three distinct periods highlighted the rapid advances of this intervention that have now put it on the cusp of being the preferred, default strategy for replacing stenosed aortic valves in older patients.
First SAPIEN 3 U.S. outcomes reported
The most recent technologic advance in transcatheter aortic valve replacement (TAVR), showcased in one of the three presentations at the annual meeting of the American College of Cardiology, was the SAPIEN 3 System, the lowest-profile TAVR system so far to undergo U.S. testing with a 14F delivery system for all valve sizes except the largest, 29 mm valves, which require a 16F system. SAPIEN 3 produced 30-day outcome results so good that one TAVR operator expressed shock at the dramatic, short-term success.
After 30 days follow-up, SAPIEN 3 produced a 2% mortality rate and a 2% stroke rate in 583 “high-risk” patients treated during 2013 and 2014 who were an average of 83 years old and had an average Society of Thoracic Surgeons (STS) risk score of 8.6%, Dr. Susheel K. Kodali reported at the meeting. By contrast, in the pivotal trial of the first-generation SAPIEN TAVR system, run during 2007-2009, in 348 “high-risk” patients (who averaged 84 years old and had an average STS risk score of 11.8%) the 30-day mortality ran 5% and 30-day strokes affected 6%, Dr. Kodali noted.
The new trial also enrolled 1,076 “intermediate”-risk patients, who averaged 82 years old with an average STS score of 5.3%. Their 30-day mortality rate was 1% and their total stroke rate was 3%, with a disabling stroke rate (modified Rankin scale score of 2 or more) of 1%.
These excellent results prompted Edwards, the company developing the SAPIEN 3 System, to apply for Food and Drug Administration approval for the device in high-risk patients, Dr. Kodali noted. In March, Edwards released a statement in which it said it expects SAPIEN 3 to receive U.S. marketing approval within a year. While Dr. Kodali said longer-term follow-up is needed for the intermediate-risk patients, the outcome results he has seen make him rethink the TAVR’s role, compared with conventional aortic valve replacement by open surgery.
“With surgery, these [intermediate-risk] patients would have a 5% mortality rate. The conversation now may need to change,” he said. “We have always said we use TAVR when surgery is not a good option, but based on the recent, 2-year findings with the CoreValve and our data, with a 1% mortality and a 1% disabling stroke rate [in intermediate-risk patients] maybe TAVR is now the preferred option, at least for 80-year-olds, said Dr. Kodali, codirector of the Heart Valve Center at NewYork-Presbyterian/Columbia University Medical Center.
Commenting on the SAPIEN 3 results as well as excellent 2-year results from the CoreValve TAVR System pivotal trial, Dr. Jeffrey J. Popma attributed the success to three factors: improved patient selection, refined and routinely used imaging methods for sizing the aortic annulus to better match the TAVR valve size to the annulus size, and improved TAVR techniques based on what is now about an 8-year clinical experience using TAVR.
“All this now puts us in a place where the bar has been set very high for surgery. How does surgery compete?” asked Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “The results are so clean at 30 days, what more information do we really need” for device approval? Dr. Popma, one of the discussants for the meeting report by Dr. Kodali, asked.
“I’m shocked the SAPIEN 3 data were so good,” said Dr. Stephen Ramee, an interventional cardiologist and medical director of the Structural and Valvular Heart Center at the Ochsner Medical Center in New Orleans. “We’ll need to wait to do intermediate-risk patients routinely, but the handwriting is on the wall with these data and the CoreValve results. I think TAVR will be preferred for all high-risk patients and for intermediate-risk patients who are at least 80 years old,” Dr. Ramee said in an interview.
Commenting on whether the FDA should approve SAPIEN 3 based on 30-day outcomes in high-risk patients, Dr. Popma noted that SAPIEN 3 is “an iteration of what has been demonstrated” in long-term results with the first-generation SAPIEN device. “Do we really need a randomized clinical trail with several thousand patients after the [SAPIEN] platform was already established?” he asked.
“We don’t demand that for iterations of surgical valves,” agreed Dr. Kodali.
CoreValve looks good longer-term
While SAPIEN 3’s performance turned heads, 2-year results from the CoreValve’s pivotal trial reported at the meeting further deepened the impression that TAVR offered substantial advantages, compared with surgical aortic valve replacement in high-risk patients. The 2-year outcomes followed the trial’s primary endpoint reported last year, the 1-year results, which had shown a statistically significant advantage in survival, compared with surgery (N. Engl. J. Med. 2014;370:1790-8).
The 2-year follow-up showed this advantage was “sustainable, durable, and widening,” reported Dr. Michael Reardon, professor of cardiothoracic surgery at the Methodist Hospital in Houston.
For example, 2-year all-cause mortality stood at 29% in patients who underwent surgical valve replacement and 22% in those who received TAVR with CoreValve, a statistically significant widening of the between-group gap, compared with the respective 19% and 14% mortality rates after 1 year. The rate of all-cause death or major stroke after 2 years ran 33% and 24% with surgical valve replacement or TAVR, respectively, compared with rates of 23% and 16% after 1 year.
Concern about excess strokes with TAVR, “one of our early worries, seems to have been put to bed,” Dr. Reardon said. The results also showed CoreValve substantially surpassed open surgery for valvular blood flow metrics across the full range of follow-up time points.
Several factors might have produced the widening outcomes between years 1 and 2 following a different one-time intervention, Dr. Reardon said, such as significant differences in the incident rate following intervention for disabling or life-threatening bleeding, atrial fibrillation, and acute kidney injury. All three complications occurred significantly more often in the surgery patients, compared with those treated with TAVR, and while the bulk of the difference in incidence showed up within the first month after intervention, each of these complications could have important long-term effects on survival and stroke rates, Dr. Reardon explained. On the other side of the ledger, open surgery when compared with CoreValve resulted in significantly fewer acute and long-term episodes of vascular complications and fewer new pacemakers, but these complications likely have less impact on stroke and mortality than the three that occur more often with surgery.
Overall, the widening gap in outcomes between surgery and TAVR “suggests that TAVR with a self-expanding valve [CoreValve] should be considered the preferred treatment,” compared with surgery in high-risk patients, Dr. Reardon said during his presentation at the meeting, a similar conclusion to what Dr. Kodali said about SAPIEN 3.
“These results really do move the needle forward,” said Dr. Popma, a CoreValve trial coinvestigator. “We see for the first time that [TAVR] may indeed be superior, and although durability remains a long-term question we have 2-year data that CoreValve has held up.”
First-generation SAPIEN TAVR shows 5-year stability
The third piece of the TAVR trilogy reported at the meeting was 5-year follow-up results from the first U.S. TAVR pivotal trial (PARTNER 1) using the first-generation SAPIEN device, which is no longer available in the United States. Those results from patients treated during 2007-2009 showed continuation of the statistical overlap between surgical valve replacement and SAPIEN TAVR for all-cause mortality, stroke, and other outcomes (Lancet 2015 [doi:10.1016/S0140-6736(15)60290-2]). Importantly, the results also showed no suggestions of deteriorating function in the TAVR valve after 5 years, reported Dr. Michael J. Mack at the meeting.
“It’s very encouraging, but we’d all like to go out to 10-12 years, and then we’d feel a lot better about it,” commented Dr. Reardon, who did not participate in the PARTNER trial. But given 5 years of apparently reliable function from a TAVR valve, the issue of long-term durability of these valves, an open question at the start of the SAPIEN trial, may be now coming to resolution, he suggested, at least for octogenarians, who are emerging as the prime demographic for TAVR.
“Tissue valves we place surgically deteriorate at different rates depending on a patient’s age when you put them in. In 30-year-olds many valves deteriorate after 10 years; in 50-year-olds maybe 15% will deteriorate, and in 70-year-olds almost none,” Dr. Reardon explained. “No one knows why.” Because many TAVR patients are at least 80 years old “it may take 10 years to see a signal of deterioration, or we might never see a signal,” he said.
SAPIEN versus CoreValve?
Although SAPIEN 3 may be on the U.S. market within the next year, for the time being routine U.S. TAVR practice is limited to two options, approved for high-risk or inoperable patients but not patients at intermediate risk: the CoreValve, which has now clearly bested surgery for all major endpoints over at least the midterm, and the SAPIEN XT model, the second-generation SAPIEN TAVR system that sits between the first generation and SAPIEN 3 and showed documented performance that roughly tracked with the original SAPIEN model. When approving the SAPIEN XT for U.S. marketing in June 2014 for operable high-risk patients as well as inoperable patients, the agency said the evidence indicated that XT was “noninferior” to SAPIEN in high-risk patients.
Several experts at the meeting agreed that despite this difference in performance between CoreValve and SAPIEN XT when each was compared with open surgery, the CoreValve experience better reflected more contemporary performance expectations for TAVR as a class, regardless of device. They cited improvements in valve sizing with imaging, better patient selection, and better TAVR technique as critical in boosting more favorable outcomes, factors that are not valve specific.
They also cited technical factors that may favor the SAPIEN XT device, notably more accurate valve placement using SAPIEN’s balloon-expandable format, compared with the self-expanding CoreValve.
Dr. Ramee said that outside of trials he’s now using SAPIEN XT for about 60% of his cases because of its “more predictable deployment. With CoreValve, you never know exactly where it will end up.” On the other hand, CoreValve works best for a more calcified aortic annulus because self-expanding placement is gentler and less likely to rupture a fragile annulus. That advantage gives CoreValve the edge for about 40% of his patients, Dr. Ramee said in an interview.
“There has been a general forward movement of the TAVR field” in which the focus has been TAVR versus surgery rather than CoreValve versus SAPIEN, Dr. Reardon said in an interview. Some anatomic features favor balloon expandable, others favor self-expanding. New TAVR systems in development by other manufacturers are mostly self-expanding models because of their ability to allow for repositioning, he noted.
Dr. Kodali has an equity interest in Thubrikar Aortic Valve; he has received honoraria from St. Jude; he has served on the steering committee for trials sponsored by Edwards, which markets the SAPIEN systems, Claret Medical, and Meril; and he has received research support from Edwards. Dr. Popma has been a consultant to Abbott, Abiomed, Boston Scientific, Cordis, and Abbott Vascular, and he has received research grants from Medtronic, which markets the CoreValve, and from five other companies. Dr. Reardon has served on an advisory board for Medtronic, Dr. Ramee has received honoraria from Edwards and from Medtronic and has a financial interest in several other companies developing TAVR systems.
On Twitter @mitchelzoler
AT ACC 2015
VIDEO: Treating Heart Failure Congestion Improved Hyperglycemia
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Congestion secondary to advanced heart failure appears to cause type 2 diabetes in a significant subgroup of patients, based on suggestive findings from a series of recently reported studies, Dr. Maya Guglin said during an interview at the annual meeting of the American College of Cardiology.
Dr. Guglin reviewed convergent findings from several groups of patients who received left ventricular assist devices to treat advanced heart failure. The analyses all showed that many, though not a majority, of those patients also had type 2 diabetes. Soon after patients received an assist device, the type 2 diabetes uniformly improved – and in many cases, glycemic control normalized.
Dr. Guglin, who has named this condition “cardiogenic diabetes,” said that reducing congestion with an assist device or with diuretic treatment seems the best way to both reduce congestion and improve or resolve the diabetes.
Those treatments will “improve quality of life, reduce hospital admissions for heart failure, and also improve the course of diabetes,” said Dr. Guglin, professor of medicine and medical director of the ventricular assist device program at the University of Kentucky in Lexington.
Dr. Guglin had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ACC 15