User login
American Academy of Neurology (AAN): Annual Meeting
Coming Soon: Highlights from AAN's Annual Meeting
Check back for highlights from this year's meeting in Boston.
Check back for highlights from this year's meeting in Boston.
Check back for highlights from this year's meeting in Boston.
AAN: Finding ways to improve door-to-needle times in stroke treatment
WASHINGTON – A streamlined emergency care service and a low-cost, tablet-based mobile telestroke system are two examples of shortening the time it takes for acute ischemic stroke patients to receive thrombolytic therapy that were presented at the annual meeting of the American Academy of Neurology.
American Heart Association/American Stroke Association guidelines recommend a door-to-needle (DTN) time of 60 minutes or less and set a goal for participating hospitals to administer tissue plasminogen activator (TPA) to at least 50% of their patients with acute ischemic stroke within 60 minutes of arriving at the hospital.
Dr. Judd Jensen described the efforts of Swedish Medical Center, Englewood, Colo. to streamline the emergency care of patients suspected of having an acute ischemic stroke after a task force determined that their previous “sequential, step-by-step process” wasted time. The median DTN time at the hospital’s stroke center had dropped from 46 minutes in 2010 to 39 minutes in 2013, which was better than the national average, “but we felt we could do better,” said Dr. Jensen, a neurologist at the hospital.
The process was modified so that more of the activities take place simultaneously, which includes immediately sending patients for a CT scan before entering the emergency department and administering IV TPA in the CT area to eligible patients, he explained. Previously, these patients were taken to a bed in the ED on arrival, registered, then examined by the emergency physician and neurologist and transported for a CT scan and then transported back to the ED where TPA was administered, if indicated, after several other steps were completed, including interpreting the CT scan, deciding about treatment, acquiring consent, and contacting the pharmacy to mix the TPA.
This process was improved by increasing pre-hospital notification by emergency medical services (EMS) and establishing a “launchpad” area in the back of the ED where the stroke team meets after EMS notification. On arrival, patients are transferred directly to the CT room where they are examined. The pharmacy is instructed to mix the TPA if an ischemic stroke is suspected, and the TPA is brought to the CT room where a stroke neurologist evaluates the CT scan and TPA is administered if indicated.
The impact of the revised process was evaluated in a prospective study of 262 acute ischemic stroke patients who received IV TPA between January 2010 and December 2014 at the hospital. They had a mean age of 73 years, 44% were male, and 84% were white. Their mean initial National Institutes of Health Stroke Scale (NIHSS) score was 12. The median DTN times dropped to a median of 31 minutes in 2014, Dr. Jensen said.
In 2014, almost 50% of the patients received TPA in 30 minutes or less, compared with about 25% in 2011, 2012, and 2013, he added, noting that 11 minutes was the fastest DTN time in 2014. Patients with an excellent discharge modified Rankin Scale (mRS) score (0 or 1) improved from 31% in 2010 and 30% in 2013 to 46% in 2014. During the time period studied, two patients had a symptomatic intracerebral hemorrhage, one in 2010 and another in 2012.
Dr. Jensen described the process as a multidisciplinary team effort, noting that it is important that emergency room physicians feel comfortable with the administration of TPA in the CT scan area, “because it is still their patient being administered a potentially fatal drug outside of the ED.”
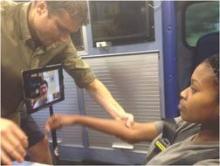
At the meeting, Matthew Padrick, a medical student at the University of Virginia, Charlottesville, presented the results of a pilot study that targeted the EMS transport time as an “untapped treatment window” to improve the time to thrombolytic treatment using a low-cost mobile telestroke system to evaluate patients in the ambulance on their way to the hospital.
Because the catchment area covered by UVA includes a large rural area, transport times to the stroke center can be as long as 30 to 60 minutes, Mr. Padrick said.
In the “Improving Treatment with Rapid Evaluation of Acute Stroke via mobile Telemedicine” (iTREAT) study, he and his associates evaluated the feasibility and reliability of performing acute stroke assessments (with the NIHSS) in the ambulance. The iTREAT system, which includes an Apple iPad with retina display attached to the patient stretcher with an extendable clamp, a secure video conferencing application, a high-speed 4G LTE modem, a magnetic antenna on top of the ambulance, and the regional cellular network, “providing seamless connectivity,” he said. At a total cost of under $2,000, the system is designed so that the neurologist can evaluate the patient remotely, via the iPad.
Acting as patients, three medical students were given two unique stroke scenarios each, with stories and specific instructions; vascular neurologists did a face-to-face assessment and a remote iTREAT assessment from the hospital as the students traveled along the major routes to UVA Medical Center. NIHSS scores in the ambulance with the iTREAT system and with face-to-face assessments correlated well, with an overall intraclass correlation of 0.98, Mr. Padrick reported.
The ratings of audio-video quality during the iTREAT evaluations were judged to be ”good” or “excellent” and the NIHSS correlations and audio-video quality ratings improved with time, he added.
“We currently have IRB approval to move forward with real, live patient encounters and we are currently outfitting and training our local EMS agencies” with the system, Mr. Padrick said in an interview after the meeting.
Mr. Padrick has received research support from the American Heart Association. Dr. Judd had nothing to disclose.
WASHINGTON – A streamlined emergency care service and a low-cost, tablet-based mobile telestroke system are two examples of shortening the time it takes for acute ischemic stroke patients to receive thrombolytic therapy that were presented at the annual meeting of the American Academy of Neurology.
American Heart Association/American Stroke Association guidelines recommend a door-to-needle (DTN) time of 60 minutes or less and set a goal for participating hospitals to administer tissue plasminogen activator (TPA) to at least 50% of their patients with acute ischemic stroke within 60 minutes of arriving at the hospital.
Dr. Judd Jensen described the efforts of Swedish Medical Center, Englewood, Colo. to streamline the emergency care of patients suspected of having an acute ischemic stroke after a task force determined that their previous “sequential, step-by-step process” wasted time. The median DTN time at the hospital’s stroke center had dropped from 46 minutes in 2010 to 39 minutes in 2013, which was better than the national average, “but we felt we could do better,” said Dr. Jensen, a neurologist at the hospital.
The process was modified so that more of the activities take place simultaneously, which includes immediately sending patients for a CT scan before entering the emergency department and administering IV TPA in the CT area to eligible patients, he explained. Previously, these patients were taken to a bed in the ED on arrival, registered, then examined by the emergency physician and neurologist and transported for a CT scan and then transported back to the ED where TPA was administered, if indicated, after several other steps were completed, including interpreting the CT scan, deciding about treatment, acquiring consent, and contacting the pharmacy to mix the TPA.
This process was improved by increasing pre-hospital notification by emergency medical services (EMS) and establishing a “launchpad” area in the back of the ED where the stroke team meets after EMS notification. On arrival, patients are transferred directly to the CT room where they are examined. The pharmacy is instructed to mix the TPA if an ischemic stroke is suspected, and the TPA is brought to the CT room where a stroke neurologist evaluates the CT scan and TPA is administered if indicated.
The impact of the revised process was evaluated in a prospective study of 262 acute ischemic stroke patients who received IV TPA between January 2010 and December 2014 at the hospital. They had a mean age of 73 years, 44% were male, and 84% were white. Their mean initial National Institutes of Health Stroke Scale (NIHSS) score was 12. The median DTN times dropped to a median of 31 minutes in 2014, Dr. Jensen said.
In 2014, almost 50% of the patients received TPA in 30 minutes or less, compared with about 25% in 2011, 2012, and 2013, he added, noting that 11 minutes was the fastest DTN time in 2014. Patients with an excellent discharge modified Rankin Scale (mRS) score (0 or 1) improved from 31% in 2010 and 30% in 2013 to 46% in 2014. During the time period studied, two patients had a symptomatic intracerebral hemorrhage, one in 2010 and another in 2012.
Dr. Jensen described the process as a multidisciplinary team effort, noting that it is important that emergency room physicians feel comfortable with the administration of TPA in the CT scan area, “because it is still their patient being administered a potentially fatal drug outside of the ED.”
At the meeting, Matthew Padrick, a medical student at the University of Virginia, Charlottesville, presented the results of a pilot study that targeted the EMS transport time as an “untapped treatment window” to improve the time to thrombolytic treatment using a low-cost mobile telestroke system to evaluate patients in the ambulance on their way to the hospital.
Because the catchment area covered by UVA includes a large rural area, transport times to the stroke center can be as long as 30 to 60 minutes, Mr. Padrick said.
In the “Improving Treatment with Rapid Evaluation of Acute Stroke via mobile Telemedicine” (iTREAT) study, he and his associates evaluated the feasibility and reliability of performing acute stroke assessments (with the NIHSS) in the ambulance. The iTREAT system, which includes an Apple iPad with retina display attached to the patient stretcher with an extendable clamp, a secure video conferencing application, a high-speed 4G LTE modem, a magnetic antenna on top of the ambulance, and the regional cellular network, “providing seamless connectivity,” he said. At a total cost of under $2,000, the system is designed so that the neurologist can evaluate the patient remotely, via the iPad.
Acting as patients, three medical students were given two unique stroke scenarios each, with stories and specific instructions; vascular neurologists did a face-to-face assessment and a remote iTREAT assessment from the hospital as the students traveled along the major routes to UVA Medical Center. NIHSS scores in the ambulance with the iTREAT system and with face-to-face assessments correlated well, with an overall intraclass correlation of 0.98, Mr. Padrick reported.
The ratings of audio-video quality during the iTREAT evaluations were judged to be ”good” or “excellent” and the NIHSS correlations and audio-video quality ratings improved with time, he added.
“We currently have IRB approval to move forward with real, live patient encounters and we are currently outfitting and training our local EMS agencies” with the system, Mr. Padrick said in an interview after the meeting.
Mr. Padrick has received research support from the American Heart Association. Dr. Judd had nothing to disclose.
WASHINGTON – A streamlined emergency care service and a low-cost, tablet-based mobile telestroke system are two examples of shortening the time it takes for acute ischemic stroke patients to receive thrombolytic therapy that were presented at the annual meeting of the American Academy of Neurology.
American Heart Association/American Stroke Association guidelines recommend a door-to-needle (DTN) time of 60 minutes or less and set a goal for participating hospitals to administer tissue plasminogen activator (TPA) to at least 50% of their patients with acute ischemic stroke within 60 minutes of arriving at the hospital.
Dr. Judd Jensen described the efforts of Swedish Medical Center, Englewood, Colo. to streamline the emergency care of patients suspected of having an acute ischemic stroke after a task force determined that their previous “sequential, step-by-step process” wasted time. The median DTN time at the hospital’s stroke center had dropped from 46 minutes in 2010 to 39 minutes in 2013, which was better than the national average, “but we felt we could do better,” said Dr. Jensen, a neurologist at the hospital.
The process was modified so that more of the activities take place simultaneously, which includes immediately sending patients for a CT scan before entering the emergency department and administering IV TPA in the CT area to eligible patients, he explained. Previously, these patients were taken to a bed in the ED on arrival, registered, then examined by the emergency physician and neurologist and transported for a CT scan and then transported back to the ED where TPA was administered, if indicated, after several other steps were completed, including interpreting the CT scan, deciding about treatment, acquiring consent, and contacting the pharmacy to mix the TPA.
This process was improved by increasing pre-hospital notification by emergency medical services (EMS) and establishing a “launchpad” area in the back of the ED where the stroke team meets after EMS notification. On arrival, patients are transferred directly to the CT room where they are examined. The pharmacy is instructed to mix the TPA if an ischemic stroke is suspected, and the TPA is brought to the CT room where a stroke neurologist evaluates the CT scan and TPA is administered if indicated.
The impact of the revised process was evaluated in a prospective study of 262 acute ischemic stroke patients who received IV TPA between January 2010 and December 2014 at the hospital. They had a mean age of 73 years, 44% were male, and 84% were white. Their mean initial National Institutes of Health Stroke Scale (NIHSS) score was 12. The median DTN times dropped to a median of 31 minutes in 2014, Dr. Jensen said.
In 2014, almost 50% of the patients received TPA in 30 minutes or less, compared with about 25% in 2011, 2012, and 2013, he added, noting that 11 minutes was the fastest DTN time in 2014. Patients with an excellent discharge modified Rankin Scale (mRS) score (0 or 1) improved from 31% in 2010 and 30% in 2013 to 46% in 2014. During the time period studied, two patients had a symptomatic intracerebral hemorrhage, one in 2010 and another in 2012.
Dr. Jensen described the process as a multidisciplinary team effort, noting that it is important that emergency room physicians feel comfortable with the administration of TPA in the CT scan area, “because it is still their patient being administered a potentially fatal drug outside of the ED.”
At the meeting, Matthew Padrick, a medical student at the University of Virginia, Charlottesville, presented the results of a pilot study that targeted the EMS transport time as an “untapped treatment window” to improve the time to thrombolytic treatment using a low-cost mobile telestroke system to evaluate patients in the ambulance on their way to the hospital.
Because the catchment area covered by UVA includes a large rural area, transport times to the stroke center can be as long as 30 to 60 minutes, Mr. Padrick said.
In the “Improving Treatment with Rapid Evaluation of Acute Stroke via mobile Telemedicine” (iTREAT) study, he and his associates evaluated the feasibility and reliability of performing acute stroke assessments (with the NIHSS) in the ambulance. The iTREAT system, which includes an Apple iPad with retina display attached to the patient stretcher with an extendable clamp, a secure video conferencing application, a high-speed 4G LTE modem, a magnetic antenna on top of the ambulance, and the regional cellular network, “providing seamless connectivity,” he said. At a total cost of under $2,000, the system is designed so that the neurologist can evaluate the patient remotely, via the iPad.
Acting as patients, three medical students were given two unique stroke scenarios each, with stories and specific instructions; vascular neurologists did a face-to-face assessment and a remote iTREAT assessment from the hospital as the students traveled along the major routes to UVA Medical Center. NIHSS scores in the ambulance with the iTREAT system and with face-to-face assessments correlated well, with an overall intraclass correlation of 0.98, Mr. Padrick reported.
The ratings of audio-video quality during the iTREAT evaluations were judged to be ”good” or “excellent” and the NIHSS correlations and audio-video quality ratings improved with time, he added.
“We currently have IRB approval to move forward with real, live patient encounters and we are currently outfitting and training our local EMS agencies” with the system, Mr. Padrick said in an interview after the meeting.
Mr. Padrick has received research support from the American Heart Association. Dr. Judd had nothing to disclose.
AT THE AAN 2015 ANNUAL MEETING
AAN: Adjunctive perampanel reduces tonic-clonic seizures in refractory patients
WASHINGTON – The antiepileptic drug perampanel cut the frequency of primary generalized tonic-clonic seizures by 50% versus placebo in patients who were already taking a number of other anti-seizure medications in a phase III randomized trial.
Perampanel (Fycompa), a glutamate receptor blocker, was also associated with significantly higher rates of patients being seizure free or having at least a 50% response, compared with placebo, Dr. Jacqueline French reported at the annual meeting of the American Academy of Neurology.
The study randomized 164 patients to either placebo or up to 8 mg daily of perampanel. It was conducted at 78 sites in 16 countries. There was a run-in period of 4-8 weeks, followed by a month of titration, 13 weeks of maintenance therapy, and a 3-year extension phase. Of 164 patients randomized, 162 completed the entire study, said Dr. French, codirector of epilepsy research and epilepsy clinical trials at the NYU Comprehensive Epilepsy Center, New York. She is also chief scientific officer of the Epilepsy Foundation of America.
The patients were all at least 12 years old and taking up to three other antiepileptic drugs without complete seizure control. During the 8-week pre-randomization period, they had to experience at least three primary generalized tonic-clonic (PGTC) seizures.
At the end of follow-up, add-on perampanel significantly outperformed placebo in the primary measure of change in PGTC seizure frequency (median 76% vs. 38%). It also conferred a significant advantage in the percentage of patients with at least a 50% reduction in seizure frequency (64% vs. 39%). Significantly more patients in the perampanel group became seizure-free (31% vs. 12%).
The safety dataset comprised 163 patients. Most patients taking perampanel (83%) experienced some kind of drug-related adverse event. The most common were dizziness (32% vs. 6% placebo), fatigue (15% vs. 6%), and headache (12% vs. 10%). Six serious adverse events occurred in the active group and seven in the placebo group. There were two deaths, one in each group: an accidental drowning in the perampanel group and a case of sudden unexplained death in epilepsy (SUDEP) in the placebo group.
Perampanel is approved as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy aged 12 years and older.
Eisai Inc. sponsored the study. Dr. French has received personal compensation and research support from Eisai, as well as numerous other pharmaceutical companies.
On Twitter @alz_gal
WASHINGTON – The antiepileptic drug perampanel cut the frequency of primary generalized tonic-clonic seizures by 50% versus placebo in patients who were already taking a number of other anti-seizure medications in a phase III randomized trial.
Perampanel (Fycompa), a glutamate receptor blocker, was also associated with significantly higher rates of patients being seizure free or having at least a 50% response, compared with placebo, Dr. Jacqueline French reported at the annual meeting of the American Academy of Neurology.
The study randomized 164 patients to either placebo or up to 8 mg daily of perampanel. It was conducted at 78 sites in 16 countries. There was a run-in period of 4-8 weeks, followed by a month of titration, 13 weeks of maintenance therapy, and a 3-year extension phase. Of 164 patients randomized, 162 completed the entire study, said Dr. French, codirector of epilepsy research and epilepsy clinical trials at the NYU Comprehensive Epilepsy Center, New York. She is also chief scientific officer of the Epilepsy Foundation of America.
The patients were all at least 12 years old and taking up to three other antiepileptic drugs without complete seizure control. During the 8-week pre-randomization period, they had to experience at least three primary generalized tonic-clonic (PGTC) seizures.
At the end of follow-up, add-on perampanel significantly outperformed placebo in the primary measure of change in PGTC seizure frequency (median 76% vs. 38%). It also conferred a significant advantage in the percentage of patients with at least a 50% reduction in seizure frequency (64% vs. 39%). Significantly more patients in the perampanel group became seizure-free (31% vs. 12%).
The safety dataset comprised 163 patients. Most patients taking perampanel (83%) experienced some kind of drug-related adverse event. The most common were dizziness (32% vs. 6% placebo), fatigue (15% vs. 6%), and headache (12% vs. 10%). Six serious adverse events occurred in the active group and seven in the placebo group. There were two deaths, one in each group: an accidental drowning in the perampanel group and a case of sudden unexplained death in epilepsy (SUDEP) in the placebo group.
Perampanel is approved as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy aged 12 years and older.
Eisai Inc. sponsored the study. Dr. French has received personal compensation and research support from Eisai, as well as numerous other pharmaceutical companies.
On Twitter @alz_gal
WASHINGTON – The antiepileptic drug perampanel cut the frequency of primary generalized tonic-clonic seizures by 50% versus placebo in patients who were already taking a number of other anti-seizure medications in a phase III randomized trial.
Perampanel (Fycompa), a glutamate receptor blocker, was also associated with significantly higher rates of patients being seizure free or having at least a 50% response, compared with placebo, Dr. Jacqueline French reported at the annual meeting of the American Academy of Neurology.
The study randomized 164 patients to either placebo or up to 8 mg daily of perampanel. It was conducted at 78 sites in 16 countries. There was a run-in period of 4-8 weeks, followed by a month of titration, 13 weeks of maintenance therapy, and a 3-year extension phase. Of 164 patients randomized, 162 completed the entire study, said Dr. French, codirector of epilepsy research and epilepsy clinical trials at the NYU Comprehensive Epilepsy Center, New York. She is also chief scientific officer of the Epilepsy Foundation of America.
The patients were all at least 12 years old and taking up to three other antiepileptic drugs without complete seizure control. During the 8-week pre-randomization period, they had to experience at least three primary generalized tonic-clonic (PGTC) seizures.
At the end of follow-up, add-on perampanel significantly outperformed placebo in the primary measure of change in PGTC seizure frequency (median 76% vs. 38%). It also conferred a significant advantage in the percentage of patients with at least a 50% reduction in seizure frequency (64% vs. 39%). Significantly more patients in the perampanel group became seizure-free (31% vs. 12%).
The safety dataset comprised 163 patients. Most patients taking perampanel (83%) experienced some kind of drug-related adverse event. The most common were dizziness (32% vs. 6% placebo), fatigue (15% vs. 6%), and headache (12% vs. 10%). Six serious adverse events occurred in the active group and seven in the placebo group. There were two deaths, one in each group: an accidental drowning in the perampanel group and a case of sudden unexplained death in epilepsy (SUDEP) in the placebo group.
Perampanel is approved as an adjunctive therapy for the treatment of partial-onset seizures with or without secondarily generalized seizures in patients with epilepsy aged 12 years and older.
Eisai Inc. sponsored the study. Dr. French has received personal compensation and research support from Eisai, as well as numerous other pharmaceutical companies.
On Twitter @alz_gal
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: The antiepileptic drug perampanel cut the frequency of primary generalized tonic-clonic seizures by 50% versus placebo in patients who were already taking one to three other antiseizure medications.
Major finding: At the end of follow-up, add-on perampanel significantly outperformed placebo in the primary measure of change in PGTC seizure frequency (median 76% vs. 38%).
Data source: The phase III trial randomized 164 patients to either add-on placebo or add-on perampanel.
Disclosures: Eisai Inc. sponsored the study. Dr. French has received personal compensation and research support from Eisai, as well as numerous other pharmaceutical companies.
AAN: Scheduled daily DBS effective in small Tourette syndrome study
WASHINGTON – Scheduled administration of bilateral deep brain stimulation of the centromedian thalamus for less than 2 hours a day resulted in a significant reduction in tics in several patients with Tourette syndrome over 2 years in a proof-of-concept study presented at the annual meeting of the American Academy of Neurology.
Of the four patients who completed the 24-month study, three experienced significant improvements, said Justin Rossi, an MD-PhD candidate at the University of Florida in Gainesville.
Instead of using the standard continuous deep brain stimulation (DBS), Mr. Rossi and colleagues at the university's Center for Movement Disorders and Neurorestoration evaluated a scheduled, personalized stimulation approach, with stimulation of the centromedian thalamus (bilaterally) tailored to the times of the day when patients experienced the most sequelae from the tics, such as when they were driving, exercising, or working, and when the intensity of the tics was the greatest.
The rationale for investigating this approach is that instead of using the “classical continuous approach” to DBS, a tailored approach might be effective in these patients, with the potential benefits of increasing battery life (and delaying another surgical procedure to replace the battery) and reducing side effects associated with stimulation, Mr. Rossi said.
Many studies have found that DBS is effective in “select medication-refractory cases of Tourette syndrome,” he noted. “However, in contrast to Parkinson’s disease, essential tremor, and other movement disorders for which DBS has been commonly used as a therapy, Tourette syndrome is a paroxysmal disorder,” and the frequency of tics can vary from patient to patient, with individual patients reporting that the intensity of tics “waxes and wanes throughout the day, often predictably.”
The study enrolled five patients; responses were evaluated with two rating scales, the Yale Global Tic Severity Scale (YGTSS) and the Modified Rush Video-Based Tic Rating Scale (MRTRS). A patient was considered a responder if there was more than a 40% improvement in the YGTSS or MRTRS from the preoperative baseline level, at 24 months, the primary outcome. (One patient was lost to follow-up after 18 months because the center was too far away.) Patients had the opportunity to modify the schedule at each 6-month visit.
At 24 months, the YGTSS total scores improved by 46%, 58%, and 17% and the MRTRS total scores improved by 79%, 81%, and 44% in the three responders. These patients had a mean stimulation time of 1.85 hours a day, ranging from 47 to 186 minutes per day. The one patient who did not meet the primary endpoint – with a 10% response on the YGTSS and a 21% response in the MRTRS – had the greatest amount of stimulation per day (4 hours a day). At 24 months, the responders had statistically significant improvements from baseline in components of the two scales, including the number of phonic tics per minute, motor tic severity, and phonic tic severity, Mr. Rossi said.
This is a proof-of-concept study and the results and conclusions are preliminary, but the results “warrant larger studies,” he concluded.
More research is needed to understand this mechanism on a more physiological level, which is being pursued at his center, he added. The results shed some light on whether the mechanism of DBS in Tourette syndrome is a cumulative effect of stimulation over time or whether DBS has an effect around the time the tics occur, and these results support the latter explanation, Mr. Rossi speculated.
He had no disclosures. The study was sponsored by the National Institutes of Health.
WASHINGTON – Scheduled administration of bilateral deep brain stimulation of the centromedian thalamus for less than 2 hours a day resulted in a significant reduction in tics in several patients with Tourette syndrome over 2 years in a proof-of-concept study presented at the annual meeting of the American Academy of Neurology.
Of the four patients who completed the 24-month study, three experienced significant improvements, said Justin Rossi, an MD-PhD candidate at the University of Florida in Gainesville.
Instead of using the standard continuous deep brain stimulation (DBS), Mr. Rossi and colleagues at the university's Center for Movement Disorders and Neurorestoration evaluated a scheduled, personalized stimulation approach, with stimulation of the centromedian thalamus (bilaterally) tailored to the times of the day when patients experienced the most sequelae from the tics, such as when they were driving, exercising, or working, and when the intensity of the tics was the greatest.
The rationale for investigating this approach is that instead of using the “classical continuous approach” to DBS, a tailored approach might be effective in these patients, with the potential benefits of increasing battery life (and delaying another surgical procedure to replace the battery) and reducing side effects associated with stimulation, Mr. Rossi said.
Many studies have found that DBS is effective in “select medication-refractory cases of Tourette syndrome,” he noted. “However, in contrast to Parkinson’s disease, essential tremor, and other movement disorders for which DBS has been commonly used as a therapy, Tourette syndrome is a paroxysmal disorder,” and the frequency of tics can vary from patient to patient, with individual patients reporting that the intensity of tics “waxes and wanes throughout the day, often predictably.”
The study enrolled five patients; responses were evaluated with two rating scales, the Yale Global Tic Severity Scale (YGTSS) and the Modified Rush Video-Based Tic Rating Scale (MRTRS). A patient was considered a responder if there was more than a 40% improvement in the YGTSS or MRTRS from the preoperative baseline level, at 24 months, the primary outcome. (One patient was lost to follow-up after 18 months because the center was too far away.) Patients had the opportunity to modify the schedule at each 6-month visit.
At 24 months, the YGTSS total scores improved by 46%, 58%, and 17% and the MRTRS total scores improved by 79%, 81%, and 44% in the three responders. These patients had a mean stimulation time of 1.85 hours a day, ranging from 47 to 186 minutes per day. The one patient who did not meet the primary endpoint – with a 10% response on the YGTSS and a 21% response in the MRTRS – had the greatest amount of stimulation per day (4 hours a day). At 24 months, the responders had statistically significant improvements from baseline in components of the two scales, including the number of phonic tics per minute, motor tic severity, and phonic tic severity, Mr. Rossi said.
This is a proof-of-concept study and the results and conclusions are preliminary, but the results “warrant larger studies,” he concluded.
More research is needed to understand this mechanism on a more physiological level, which is being pursued at his center, he added. The results shed some light on whether the mechanism of DBS in Tourette syndrome is a cumulative effect of stimulation over time or whether DBS has an effect around the time the tics occur, and these results support the latter explanation, Mr. Rossi speculated.
He had no disclosures. The study was sponsored by the National Institutes of Health.
WASHINGTON – Scheduled administration of bilateral deep brain stimulation of the centromedian thalamus for less than 2 hours a day resulted in a significant reduction in tics in several patients with Tourette syndrome over 2 years in a proof-of-concept study presented at the annual meeting of the American Academy of Neurology.
Of the four patients who completed the 24-month study, three experienced significant improvements, said Justin Rossi, an MD-PhD candidate at the University of Florida in Gainesville.
Instead of using the standard continuous deep brain stimulation (DBS), Mr. Rossi and colleagues at the university's Center for Movement Disorders and Neurorestoration evaluated a scheduled, personalized stimulation approach, with stimulation of the centromedian thalamus (bilaterally) tailored to the times of the day when patients experienced the most sequelae from the tics, such as when they were driving, exercising, or working, and when the intensity of the tics was the greatest.
The rationale for investigating this approach is that instead of using the “classical continuous approach” to DBS, a tailored approach might be effective in these patients, with the potential benefits of increasing battery life (and delaying another surgical procedure to replace the battery) and reducing side effects associated with stimulation, Mr. Rossi said.
Many studies have found that DBS is effective in “select medication-refractory cases of Tourette syndrome,” he noted. “However, in contrast to Parkinson’s disease, essential tremor, and other movement disorders for which DBS has been commonly used as a therapy, Tourette syndrome is a paroxysmal disorder,” and the frequency of tics can vary from patient to patient, with individual patients reporting that the intensity of tics “waxes and wanes throughout the day, often predictably.”
The study enrolled five patients; responses were evaluated with two rating scales, the Yale Global Tic Severity Scale (YGTSS) and the Modified Rush Video-Based Tic Rating Scale (MRTRS). A patient was considered a responder if there was more than a 40% improvement in the YGTSS or MRTRS from the preoperative baseline level, at 24 months, the primary outcome. (One patient was lost to follow-up after 18 months because the center was too far away.) Patients had the opportunity to modify the schedule at each 6-month visit.
At 24 months, the YGTSS total scores improved by 46%, 58%, and 17% and the MRTRS total scores improved by 79%, 81%, and 44% in the three responders. These patients had a mean stimulation time of 1.85 hours a day, ranging from 47 to 186 minutes per day. The one patient who did not meet the primary endpoint – with a 10% response on the YGTSS and a 21% response in the MRTRS – had the greatest amount of stimulation per day (4 hours a day). At 24 months, the responders had statistically significant improvements from baseline in components of the two scales, including the number of phonic tics per minute, motor tic severity, and phonic tic severity, Mr. Rossi said.
This is a proof-of-concept study and the results and conclusions are preliminary, but the results “warrant larger studies,” he concluded.
More research is needed to understand this mechanism on a more physiological level, which is being pursued at his center, he added. The results shed some light on whether the mechanism of DBS in Tourette syndrome is a cumulative effect of stimulation over time or whether DBS has an effect around the time the tics occur, and these results support the latter explanation, Mr. Rossi speculated.
He had no disclosures. The study was sponsored by the National Institutes of Health.
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: Promising results of a tailored approach to deep brain stimulation in three patients with Tourette syndrome merits a larger trial.
Major finding: In three of the four patients who completed the study, DBS of the centromedian thalamus for less than 2 hours a day resulted in significant improvements over 24 months.
Data source: A proof-of-concept study in five patients with Tourette syndrome, evaluating DBS of the centromedian thalamus, scheduled for times when tics interfered with activities or were most intense.
Disclosures: The National Institutes of Health sponsored the study. Mr. Rossi had no disclosures.
AAN: Largest neuroleptic malignant syndrome study finds predictors of poor outcome
WASHINGTON – Older age was a significant predictor of a poor outcome associated with neuroleptic malignant syndrome in a retrospective review of more than 1,700 inpatient cases in the United States over a recent 12-year period.
Other factors that were significant positive predictors of a poor outcome were acute kidney injury, respiratory failure, and seizures, Dr. Sumul Modi said at the annual meeting of the American Academy of Neurology. The study also showed that inpatient mortality due to neuroleptic malignant syndrome (NMS) has significantly dropped since it was first described in 1960. Because NMS is rare, it is difficult to study a large sample of patients, and large real-world studies of NMS are lacking, especially in the last few decades, he said, noting that most of the literature on NMS is from the 20th century.
The aim of this study was to identify predictors of poor outcome associated with NMS, using the National Inpatient Sample, a large, publicly available, all-payer, inpatient health care database that covers about 20% of all admissions to nonfederal hospitals in the United States. ICD-9 diagnostic and procedure were used to identify major complications and procedures. Univariate and multivariate logistic regression analyses were used to identify predictors.
From 2000 through 2011, Dr. Modi, a neurology resident at Henry Ford Hospital, Detroit, and his coauthor identified 1,725 cases in the database with a primary diagnosis of NMS in patients aged 16 and older, making this the largest study of NMS to date. (Cases with a secondary diagnosis that can affect the diagnosis of NMS, including serotonin syndrome and substance abuse, alcohol, or drug withdrawal, were excluded.)
Of these patients, 183 – almost 11% – had a poor outcome, defined as in-hospital death or having undergone a feeding tube placement. Of the total, 99 patients – almost 6% – died while hospitalized.
The most common complication associated with NMS was rhabdomyolysis, in 24%, which was not directly associated with a poor outcome. But other complications – seizures (12.9% of the total), acute kidney injury (15.7%), respiratory failure (11.4%), and cardiac dysrhythmia (8.8%) – were also significantly associated with poor outcome, Dr. Modi said.
Medical complications significantly associated with a poor outcome included pneumonia (reported in 8.5% of the patients with NMS), urinary tract infection (18%), sepsis (6.3%), and acute liver failure (1.3%).
A multivariate analysis, with adjusted odds ratios, determined that the following were predictors of poor outcome: age (OR, 1.5), acute kidney injury (OR, 2.1), acute respiratory failure (OR, 10.7), and seizures (OR, 1.7).
Every calendar year increase was a “small but significant” negative predictor of poor outcome (OR, 0.9). During the period studied, the highest mortality rate – about 9% – was in 2002, dropping to about 2.5% in 2009 and increasing to about 5% in 2010 and 2011. The marked drop in mortality since NMS was first described in 1960 could be due to improved intensive care, but could also be related to the various treatment approaches used, such as dantrolene and dopamine agonists, Dr. Modi said.
Increasing use of atypical antipsychotics may also play a role, but the investigators were not able to determine if patients had been treated with an atypical or typical antipsychotic and what treatments were used to manage NMS, which are limitations of the study, he said. They were also not able to determine if the time to diagnosis played a role in the outcome or whether patients were treated in a tertiary medical center.
Dr. Modi and his coauthor had no disclosures.
WASHINGTON – Older age was a significant predictor of a poor outcome associated with neuroleptic malignant syndrome in a retrospective review of more than 1,700 inpatient cases in the United States over a recent 12-year period.
Other factors that were significant positive predictors of a poor outcome were acute kidney injury, respiratory failure, and seizures, Dr. Sumul Modi said at the annual meeting of the American Academy of Neurology. The study also showed that inpatient mortality due to neuroleptic malignant syndrome (NMS) has significantly dropped since it was first described in 1960. Because NMS is rare, it is difficult to study a large sample of patients, and large real-world studies of NMS are lacking, especially in the last few decades, he said, noting that most of the literature on NMS is from the 20th century.
The aim of this study was to identify predictors of poor outcome associated with NMS, using the National Inpatient Sample, a large, publicly available, all-payer, inpatient health care database that covers about 20% of all admissions to nonfederal hospitals in the United States. ICD-9 diagnostic and procedure were used to identify major complications and procedures. Univariate and multivariate logistic regression analyses were used to identify predictors.
From 2000 through 2011, Dr. Modi, a neurology resident at Henry Ford Hospital, Detroit, and his coauthor identified 1,725 cases in the database with a primary diagnosis of NMS in patients aged 16 and older, making this the largest study of NMS to date. (Cases with a secondary diagnosis that can affect the diagnosis of NMS, including serotonin syndrome and substance abuse, alcohol, or drug withdrawal, were excluded.)
Of these patients, 183 – almost 11% – had a poor outcome, defined as in-hospital death or having undergone a feeding tube placement. Of the total, 99 patients – almost 6% – died while hospitalized.
The most common complication associated with NMS was rhabdomyolysis, in 24%, which was not directly associated with a poor outcome. But other complications – seizures (12.9% of the total), acute kidney injury (15.7%), respiratory failure (11.4%), and cardiac dysrhythmia (8.8%) – were also significantly associated with poor outcome, Dr. Modi said.
Medical complications significantly associated with a poor outcome included pneumonia (reported in 8.5% of the patients with NMS), urinary tract infection (18%), sepsis (6.3%), and acute liver failure (1.3%).
A multivariate analysis, with adjusted odds ratios, determined that the following were predictors of poor outcome: age (OR, 1.5), acute kidney injury (OR, 2.1), acute respiratory failure (OR, 10.7), and seizures (OR, 1.7).
Every calendar year increase was a “small but significant” negative predictor of poor outcome (OR, 0.9). During the period studied, the highest mortality rate – about 9% – was in 2002, dropping to about 2.5% in 2009 and increasing to about 5% in 2010 and 2011. The marked drop in mortality since NMS was first described in 1960 could be due to improved intensive care, but could also be related to the various treatment approaches used, such as dantrolene and dopamine agonists, Dr. Modi said.
Increasing use of atypical antipsychotics may also play a role, but the investigators were not able to determine if patients had been treated with an atypical or typical antipsychotic and what treatments were used to manage NMS, which are limitations of the study, he said. They were also not able to determine if the time to diagnosis played a role in the outcome or whether patients were treated in a tertiary medical center.
Dr. Modi and his coauthor had no disclosures.
WASHINGTON – Older age was a significant predictor of a poor outcome associated with neuroleptic malignant syndrome in a retrospective review of more than 1,700 inpatient cases in the United States over a recent 12-year period.
Other factors that were significant positive predictors of a poor outcome were acute kidney injury, respiratory failure, and seizures, Dr. Sumul Modi said at the annual meeting of the American Academy of Neurology. The study also showed that inpatient mortality due to neuroleptic malignant syndrome (NMS) has significantly dropped since it was first described in 1960. Because NMS is rare, it is difficult to study a large sample of patients, and large real-world studies of NMS are lacking, especially in the last few decades, he said, noting that most of the literature on NMS is from the 20th century.
The aim of this study was to identify predictors of poor outcome associated with NMS, using the National Inpatient Sample, a large, publicly available, all-payer, inpatient health care database that covers about 20% of all admissions to nonfederal hospitals in the United States. ICD-9 diagnostic and procedure were used to identify major complications and procedures. Univariate and multivariate logistic regression analyses were used to identify predictors.
From 2000 through 2011, Dr. Modi, a neurology resident at Henry Ford Hospital, Detroit, and his coauthor identified 1,725 cases in the database with a primary diagnosis of NMS in patients aged 16 and older, making this the largest study of NMS to date. (Cases with a secondary diagnosis that can affect the diagnosis of NMS, including serotonin syndrome and substance abuse, alcohol, or drug withdrawal, were excluded.)
Of these patients, 183 – almost 11% – had a poor outcome, defined as in-hospital death or having undergone a feeding tube placement. Of the total, 99 patients – almost 6% – died while hospitalized.
The most common complication associated with NMS was rhabdomyolysis, in 24%, which was not directly associated with a poor outcome. But other complications – seizures (12.9% of the total), acute kidney injury (15.7%), respiratory failure (11.4%), and cardiac dysrhythmia (8.8%) – were also significantly associated with poor outcome, Dr. Modi said.
Medical complications significantly associated with a poor outcome included pneumonia (reported in 8.5% of the patients with NMS), urinary tract infection (18%), sepsis (6.3%), and acute liver failure (1.3%).
A multivariate analysis, with adjusted odds ratios, determined that the following were predictors of poor outcome: age (OR, 1.5), acute kidney injury (OR, 2.1), acute respiratory failure (OR, 10.7), and seizures (OR, 1.7).
Every calendar year increase was a “small but significant” negative predictor of poor outcome (OR, 0.9). During the period studied, the highest mortality rate – about 9% – was in 2002, dropping to about 2.5% in 2009 and increasing to about 5% in 2010 and 2011. The marked drop in mortality since NMS was first described in 1960 could be due to improved intensive care, but could also be related to the various treatment approaches used, such as dantrolene and dopamine agonists, Dr. Modi said.
Increasing use of atypical antipsychotics may also play a role, but the investigators were not able to determine if patients had been treated with an atypical or typical antipsychotic and what treatments were used to manage NMS, which are limitations of the study, he said. They were also not able to determine if the time to diagnosis played a role in the outcome or whether patients were treated in a tertiary medical center.
Dr. Modi and his coauthor had no disclosures.
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: Patients diagnosed with neuroleptic malignant syndrome may be at increased risk of a poor outcome if they are older and have seizures, respiratory failure, or acute kidney injury while hospitalized.
Major finding: A poor outcome was independently predicted by age (OR, 1.5), acute kidney injury (OR, 2.1), acute respiratory failure (OR, 10.7), and seizures (OR, 1.7).
Data source: The retrospective study evaluated 1,725 NMS cases in the National Inpatient Sample.
Disclosures: Dr. Modi and his coauthor had no disclosures.
AAN: Study supports safety of fingolimod for MS
WASHINGTON – An analysis of long-term safety data of fingolimod in patients with relapsing multiple sclerosis treated for as long as 7 years has not identified new safety issues or increases in any known adverse events identified in the core studies conducted before approval, Dr. Jeffrey Cohen reported at the annual meeting of the American Academy of Neurology.
The results, based on data from the ongoing LONGTERMS open-label extension study following the safety and tolerability of fingolimod in patients enrolled in phase II and III trials, confirm that the treatment is “suitable” for long-term therapy for patients with relapsing MS, said Dr. Cohen, director of the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
Fingolimod, a sphingosine 1-phosphate receptor modulator marketed as Gilenya, was approved by the Food and Drug Administration in 2010 for relapsing forms of MS at a dose of 0.5 mg taken orally once a day. As of November 2014, more than 114,000 people worldwide have been treated with fingolimod, representing more than 195,000 patient-years of exposure, according to Dr. Cohen, who cited data from Novartis, the manufacturer.
He presented the results of the analysis of adverse events (AEs), serious adverse events (SAEs), and AEs of “special interest,” among 1,655 people with relapsing-remitting MS enrolled in LONGTERMS who were treated with fingolimod (0.5 mg a day) for a mean of 4 years (839 were on treatment for at least 7 years). They included 1,212 people treated with fingolimod in the core studies, as well as people in those studies who were on placebo or a comparator treatment and were rerandomized to treatment with fingolimod. At enrollment in the original study, the mean age was 38 years, and 70% of the patients were females. The patients had had MS for a mean of 5 years, and had a mean score of 2.31 on the Expanded Disability Status Scale (EDSS).
The analysis compared the safety profile in the extension and core studies by calculating the incidence rate (IR) for the events in each database, and calculating the incidence rate ratio (IRR) by dividing the IR for the longterm study cohort by the IR for the core cohort. (The IRs were expressed as the number of AEs “per 100 patient-years of the at-risk population.”) AEs of special interest were infections, hypertension, respiratory conditions, lymphopenia, macular edema, bradyarrhythmia after the first dose, reproductive toxicity, liver transaminase elevation, skin cancer, other malignant neoplasms, and thromboembolic events.
The only SAE with an IRR that exceeded 1 was herpes zoster infections, but the odds ratio was broad and overlapped one, Dr. Cohen said. The results for other SAEs included an IRR of 0.47 for bradyarrhythmia after the first dose, and an IRR of 0.45 for macular edema.
In the analysis of AEs, leucopenia and lymphopenia “seem to be increased” with more time on fingolimod, he noted. In the long-term cohort, the IR for this adverse event was 6.2, compared with 4.6 for the core cohort, for an IRR of 1.36. However, this could have been a result of increased reporting resulting from investigators’ awareness of lymphocyte counts, since it is an open-label study, he said, pointing out that in the core studies, lymphocyte counts were unblinded only if they reached 0.2 x 109/L. There was no evidence of a progressive decline in lymphocyte counts, with no significant differences in trends over time with mean lymphocyte counts between those in the core and extension studies, although “we can’t eliminate” this possibility, he added.
The overall rate of infections did not appear to increase over time, with an IRR of 0.72.
There were more serious infections with longer treatment among patients with sustained low levels of peripheral lymphocyte counts, defined as absolute lymphocyte counts below 0.4 x 109/L for at least 60% of the records during the corresponding time interval. There were 28 cases of serious infection among those on treatment for more than 2 years and 14 cases among those on treatment for 2 years or less. The occurrence rate for the number of serious infections, which could be more than once for an individual patient, was 1.4 among those treated for more than 2 years vs. 1.0 for those treated for 2 years or less, for an occurrence rate ratio of 1.47.
Pointing out that the number of cases was small and the confidence intervals for this finding were wide, Dr. Cohen concluded that the LONGTERMS data do not support “an overall increased risk of infections with long-term exposure or with sustained low peripheral lymphocyte counts.”
Dr. Cohen disclosed ties with Novartis, which funded the study.
WASHINGTON – An analysis of long-term safety data of fingolimod in patients with relapsing multiple sclerosis treated for as long as 7 years has not identified new safety issues or increases in any known adverse events identified in the core studies conducted before approval, Dr. Jeffrey Cohen reported at the annual meeting of the American Academy of Neurology.
The results, based on data from the ongoing LONGTERMS open-label extension study following the safety and tolerability of fingolimod in patients enrolled in phase II and III trials, confirm that the treatment is “suitable” for long-term therapy for patients with relapsing MS, said Dr. Cohen, director of the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
Fingolimod, a sphingosine 1-phosphate receptor modulator marketed as Gilenya, was approved by the Food and Drug Administration in 2010 for relapsing forms of MS at a dose of 0.5 mg taken orally once a day. As of November 2014, more than 114,000 people worldwide have been treated with fingolimod, representing more than 195,000 patient-years of exposure, according to Dr. Cohen, who cited data from Novartis, the manufacturer.
He presented the results of the analysis of adverse events (AEs), serious adverse events (SAEs), and AEs of “special interest,” among 1,655 people with relapsing-remitting MS enrolled in LONGTERMS who were treated with fingolimod (0.5 mg a day) for a mean of 4 years (839 were on treatment for at least 7 years). They included 1,212 people treated with fingolimod in the core studies, as well as people in those studies who were on placebo or a comparator treatment and were rerandomized to treatment with fingolimod. At enrollment in the original study, the mean age was 38 years, and 70% of the patients were females. The patients had had MS for a mean of 5 years, and had a mean score of 2.31 on the Expanded Disability Status Scale (EDSS).
The analysis compared the safety profile in the extension and core studies by calculating the incidence rate (IR) for the events in each database, and calculating the incidence rate ratio (IRR) by dividing the IR for the longterm study cohort by the IR for the core cohort. (The IRs were expressed as the number of AEs “per 100 patient-years of the at-risk population.”) AEs of special interest were infections, hypertension, respiratory conditions, lymphopenia, macular edema, bradyarrhythmia after the first dose, reproductive toxicity, liver transaminase elevation, skin cancer, other malignant neoplasms, and thromboembolic events.
The only SAE with an IRR that exceeded 1 was herpes zoster infections, but the odds ratio was broad and overlapped one, Dr. Cohen said. The results for other SAEs included an IRR of 0.47 for bradyarrhythmia after the first dose, and an IRR of 0.45 for macular edema.
In the analysis of AEs, leucopenia and lymphopenia “seem to be increased” with more time on fingolimod, he noted. In the long-term cohort, the IR for this adverse event was 6.2, compared with 4.6 for the core cohort, for an IRR of 1.36. However, this could have been a result of increased reporting resulting from investigators’ awareness of lymphocyte counts, since it is an open-label study, he said, pointing out that in the core studies, lymphocyte counts were unblinded only if they reached 0.2 x 109/L. There was no evidence of a progressive decline in lymphocyte counts, with no significant differences in trends over time with mean lymphocyte counts between those in the core and extension studies, although “we can’t eliminate” this possibility, he added.
The overall rate of infections did not appear to increase over time, with an IRR of 0.72.
There were more serious infections with longer treatment among patients with sustained low levels of peripheral lymphocyte counts, defined as absolute lymphocyte counts below 0.4 x 109/L for at least 60% of the records during the corresponding time interval. There were 28 cases of serious infection among those on treatment for more than 2 years and 14 cases among those on treatment for 2 years or less. The occurrence rate for the number of serious infections, which could be more than once for an individual patient, was 1.4 among those treated for more than 2 years vs. 1.0 for those treated for 2 years or less, for an occurrence rate ratio of 1.47.
Pointing out that the number of cases was small and the confidence intervals for this finding were wide, Dr. Cohen concluded that the LONGTERMS data do not support “an overall increased risk of infections with long-term exposure or with sustained low peripheral lymphocyte counts.”
Dr. Cohen disclosed ties with Novartis, which funded the study.
WASHINGTON – An analysis of long-term safety data of fingolimod in patients with relapsing multiple sclerosis treated for as long as 7 years has not identified new safety issues or increases in any known adverse events identified in the core studies conducted before approval, Dr. Jeffrey Cohen reported at the annual meeting of the American Academy of Neurology.
The results, based on data from the ongoing LONGTERMS open-label extension study following the safety and tolerability of fingolimod in patients enrolled in phase II and III trials, confirm that the treatment is “suitable” for long-term therapy for patients with relapsing MS, said Dr. Cohen, director of the Mellen Center for MS Treatment and Research at the Cleveland Clinic.
Fingolimod, a sphingosine 1-phosphate receptor modulator marketed as Gilenya, was approved by the Food and Drug Administration in 2010 for relapsing forms of MS at a dose of 0.5 mg taken orally once a day. As of November 2014, more than 114,000 people worldwide have been treated with fingolimod, representing more than 195,000 patient-years of exposure, according to Dr. Cohen, who cited data from Novartis, the manufacturer.
He presented the results of the analysis of adverse events (AEs), serious adverse events (SAEs), and AEs of “special interest,” among 1,655 people with relapsing-remitting MS enrolled in LONGTERMS who were treated with fingolimod (0.5 mg a day) for a mean of 4 years (839 were on treatment for at least 7 years). They included 1,212 people treated with fingolimod in the core studies, as well as people in those studies who were on placebo or a comparator treatment and were rerandomized to treatment with fingolimod. At enrollment in the original study, the mean age was 38 years, and 70% of the patients were females. The patients had had MS for a mean of 5 years, and had a mean score of 2.31 on the Expanded Disability Status Scale (EDSS).
The analysis compared the safety profile in the extension and core studies by calculating the incidence rate (IR) for the events in each database, and calculating the incidence rate ratio (IRR) by dividing the IR for the longterm study cohort by the IR for the core cohort. (The IRs were expressed as the number of AEs “per 100 patient-years of the at-risk population.”) AEs of special interest were infections, hypertension, respiratory conditions, lymphopenia, macular edema, bradyarrhythmia after the first dose, reproductive toxicity, liver transaminase elevation, skin cancer, other malignant neoplasms, and thromboembolic events.
The only SAE with an IRR that exceeded 1 was herpes zoster infections, but the odds ratio was broad and overlapped one, Dr. Cohen said. The results for other SAEs included an IRR of 0.47 for bradyarrhythmia after the first dose, and an IRR of 0.45 for macular edema.
In the analysis of AEs, leucopenia and lymphopenia “seem to be increased” with more time on fingolimod, he noted. In the long-term cohort, the IR for this adverse event was 6.2, compared with 4.6 for the core cohort, for an IRR of 1.36. However, this could have been a result of increased reporting resulting from investigators’ awareness of lymphocyte counts, since it is an open-label study, he said, pointing out that in the core studies, lymphocyte counts were unblinded only if they reached 0.2 x 109/L. There was no evidence of a progressive decline in lymphocyte counts, with no significant differences in trends over time with mean lymphocyte counts between those in the core and extension studies, although “we can’t eliminate” this possibility, he added.
The overall rate of infections did not appear to increase over time, with an IRR of 0.72.
There were more serious infections with longer treatment among patients with sustained low levels of peripheral lymphocyte counts, defined as absolute lymphocyte counts below 0.4 x 109/L for at least 60% of the records during the corresponding time interval. There were 28 cases of serious infection among those on treatment for more than 2 years and 14 cases among those on treatment for 2 years or less. The occurrence rate for the number of serious infections, which could be more than once for an individual patient, was 1.4 among those treated for more than 2 years vs. 1.0 for those treated for 2 years or less, for an occurrence rate ratio of 1.47.
Pointing out that the number of cases was small and the confidence intervals for this finding were wide, Dr. Cohen concluded that the LONGTERMS data do not support “an overall increased risk of infections with long-term exposure or with sustained low peripheral lymphocyte counts.”
Dr. Cohen disclosed ties with Novartis, which funded the study.
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: Results of an ongoing study support the safety of fingolimod for long-term treatment of patients with relapsing-remitting multiple sclerosis.
Major finding: A comparison between preapproval studies and a large long-term extension study of patients with MS did not identify any increase in known safety issues or new safety issues associated with fingolimod.
Data source: Study of more than 1,655 people with relapsing-remitting MS who have been treated with fingolimod for a mean of 4 years.
Disclosures: Dr. Cohen disclosed ties with Novartis, which funded the study.
Peginterferon beta-1a MS data show positive early and long-term results
WASHINGTON – Beneficial effects of peginterferon beta-1a administered every 2 weeks were evident as early as 12 weeks after starting treatment, in the pivotal phase III study comparing peginterferon beta-1a to placebo in patients with relapsing remitting multiple sclerosis, Dr. Scott Newsome, said at the annual meeting of the American Academy of Neurology.
At 12 weeks, treatment with peginterferon beta-1a was associated with a 37% reduction in the adjusted annualized relapse rate (ARR) compared with placebo, in the ADVANCE study, said Dr. Newsome of the department of neurology at Johns Hopkins University, Baltimore.
ADVANCE compared 125 mcg of peginterferon beta-1a administered every 2 or 4 weeks to placebo in patients with relapsing remitting MS. The efficacy of peginterferon beta-1a was maintained over 2 years. Greater efficacy was seen with administration every 2 weeks (Lancet Neurol. 2014;13:657-65). In August 2014, the Food and Drug Administration approved peginterferon beta-1a, at a dose of 125 mcg administered subcutaneously every 14 days, for relapsing forms of multiple sclerosis; it is marketed as Plegridy by Biogen Idec.
Interim results of the follow-up study of patients enrolled in ADVANCE – the ATTAIN trial – have shown that over 3 years of treatment, no new safety issues or concerns have emerged, and the clinical effects of treatment are maintained, according to Dr. Marcelo Kremenchutzky of Western University, London, Ont., who presented those results at the meeting.
Dr. Newsome presented the 12-week data among 512 patients randomized to treatment every 2 weeks and 500 randomized to placebo in the ADVANCE study; their mean age was 36-37 years, almost two-thirds were female, and 81%-82% were white. They were enrolled for a mean of 6-7 years from their first MS symptoms and had had a mean of 2.6 relapses within the previous 3 years. Their mean Expanded Disability Status Scale (EDSS) scores were 2.44-2.47.
At 12 weeks, the ARR, the primary endpoint, among those on peginterferon beta-1a was 0.259 vs. 0.414 among those on placebo, a 37.4% reduction over placebo (P = .045), Dr. Newsome said. The ARR was adjusted for baseline EDSS, relapse rate, and age. (At 48 weeks, the ARR among those on peginterferon beta-1a every 2 weeks was 0.26, compared with 0.40 among those on placebo, a significantly reduced risk of 36%, with a P value of .0007.)
The proportion of patients who relapsed at 12 weeks – 6% of those on peginterferon beta-1a vs. 9.5% of those on placebo – was reduced by 36.8% (P = .041), with a “clear separation” between the treatment and control arms at 12 weeks, he noted.
In addition, the estimated proportion of patients with an onset of 24-week confirmed disability progression (CDP) was 0 among those on peginterferon beta-1a versus 1.0% among those on placebo (P = .026). This result was not as robust as the relapse results, but there was a clear separation between the arms with statistical significance, said Dr. Newsome, director of outpatient neurology services at Johns Hopkins.
In the ADVANCE study, PEG administered every 2 or 4 weeks was well tolerated with a favorable safety profile in the first year, and a similar safety profile between the two dosing regimens, he added.
Dr. Kremenchutzky, director of the Multiple Sclerosis Clinic in London, Ont., presented the 1-year data in the ongoing extension study that is evaluating long-term MS outcomes, safety, and tolerability of peginterferon beta-1a among patients enrolled in ADVANCE, including those treated with peginterferon beta-1a every 2 weeks (376) or every 4 weeks (354).
Also included are 344 patients who were on placebo but were rerandomized to peginterferon beta-1a every 2 or 4 weeks (the delayed treatment group) after 1 year in the ADVANCE study; they were included in the analyses after they had been on treatment for 2 years.
After 1 year in the ATTAIN study, injection-site reactions and flu-like symptoms remained the most frequent adverse events associated with peginterferon beta-1a, and most were mild to moderate. Treatment has also been associated with “low levels” of laboratory abnormalities and immunogenicity, and clinical efficacy has been maintained for 3 years, Dr. Kremenchutzky said at the meeting.
Adverse events were “very similar” in the 4-week and 2-week treatment groups. Over 3 years, 96% of patients in both groups had experienced an adverse event, and the rates of treatment-related adverse events (91%), and severe adverse events (24%) were the same in both groups. The rates of treatment discontinuations due to an adverse event (7%-8%) and withdrawal from the study due to an adverse event (8%-9%) were similar.
The rates of flu-like symptoms over 3 years were also similar (62%-64%) in the two groups, and influenza-like illness was reported in 55% of those dosed every 2 weeks and in 45% of those dosed every 4 weeks. In addition to influenza-like illness, pyrexia was the most common flu-like symptom reported over 3 years (43%-45%).
About 65%-69% of those in the two groups reported injection-site reactions, with erythema the most common (62%-64%), followed by pain (17%-18%) and pruritus (12%-16%).
Lab abnormalities were comparable with both dosing schedules: Over 3 years, white blood cell counts were reduced in 6% of those on the 2-week schedule, compared with 8% among those on the 4-week schedule. Lymphocyte counts were reduced in 12% and 9% of those on the 2-week and 4-week schedules, respectively. In addition, 8% of those on the 2-week dose and 6% of those on the 4-week dose had low hemoglobin (100 g/L or less) over the 3 years.
Rates of anti-IFN and anti-PEG antibodies associated with both schedules were low, Dr. Kremenchutzkysaid. Over 3 years, 7%-9% tested positive for IFN binding antibody at least once, and 4%-6% of the total were “persistent positive.” Another 9% of those on the 4-week schedule and 6% of those on the 2-week schedule were anti-PEG antibody positive at least once, and 3%-5% of the patients in total had persistent levels. Titers were high in less than 1% of the total.
In ATTAIN, efficacy endpoints were secondary, and included ARR, which was reduced by 25.1% over 3 years among those receiving the dose every 2 weeks, compared with every 4 weeks (P = .0199). By 144 weeks, 30.5% of those on the 2-week schedule and 41.1% of those on the 4-week schedule had experienced a relapse, a significant difference (P = .0070), Dr. Kremenchutzky said.
The proportion of those with CDP by 144 weeks was 15.5% among those on the 4-week schedule, vs. 9.2% among those on the 2-week schedule (P = .0098), he added.
The studies were funded by Biogen Idec. Dr. Newsome and Dr. Kremenchutzky both disclosed having been compensated for activities with Biogen Idec and other companies that market MS drugs.
WASHINGTON – Beneficial effects of peginterferon beta-1a administered every 2 weeks were evident as early as 12 weeks after starting treatment, in the pivotal phase III study comparing peginterferon beta-1a to placebo in patients with relapsing remitting multiple sclerosis, Dr. Scott Newsome, said at the annual meeting of the American Academy of Neurology.
At 12 weeks, treatment with peginterferon beta-1a was associated with a 37% reduction in the adjusted annualized relapse rate (ARR) compared with placebo, in the ADVANCE study, said Dr. Newsome of the department of neurology at Johns Hopkins University, Baltimore.
ADVANCE compared 125 mcg of peginterferon beta-1a administered every 2 or 4 weeks to placebo in patients with relapsing remitting MS. The efficacy of peginterferon beta-1a was maintained over 2 years. Greater efficacy was seen with administration every 2 weeks (Lancet Neurol. 2014;13:657-65). In August 2014, the Food and Drug Administration approved peginterferon beta-1a, at a dose of 125 mcg administered subcutaneously every 14 days, for relapsing forms of multiple sclerosis; it is marketed as Plegridy by Biogen Idec.
Interim results of the follow-up study of patients enrolled in ADVANCE – the ATTAIN trial – have shown that over 3 years of treatment, no new safety issues or concerns have emerged, and the clinical effects of treatment are maintained, according to Dr. Marcelo Kremenchutzky of Western University, London, Ont., who presented those results at the meeting.
Dr. Newsome presented the 12-week data among 512 patients randomized to treatment every 2 weeks and 500 randomized to placebo in the ADVANCE study; their mean age was 36-37 years, almost two-thirds were female, and 81%-82% were white. They were enrolled for a mean of 6-7 years from their first MS symptoms and had had a mean of 2.6 relapses within the previous 3 years. Their mean Expanded Disability Status Scale (EDSS) scores were 2.44-2.47.
At 12 weeks, the ARR, the primary endpoint, among those on peginterferon beta-1a was 0.259 vs. 0.414 among those on placebo, a 37.4% reduction over placebo (P = .045), Dr. Newsome said. The ARR was adjusted for baseline EDSS, relapse rate, and age. (At 48 weeks, the ARR among those on peginterferon beta-1a every 2 weeks was 0.26, compared with 0.40 among those on placebo, a significantly reduced risk of 36%, with a P value of .0007.)
The proportion of patients who relapsed at 12 weeks – 6% of those on peginterferon beta-1a vs. 9.5% of those on placebo – was reduced by 36.8% (P = .041), with a “clear separation” between the treatment and control arms at 12 weeks, he noted.
In addition, the estimated proportion of patients with an onset of 24-week confirmed disability progression (CDP) was 0 among those on peginterferon beta-1a versus 1.0% among those on placebo (P = .026). This result was not as robust as the relapse results, but there was a clear separation between the arms with statistical significance, said Dr. Newsome, director of outpatient neurology services at Johns Hopkins.
In the ADVANCE study, PEG administered every 2 or 4 weeks was well tolerated with a favorable safety profile in the first year, and a similar safety profile between the two dosing regimens, he added.
Dr. Kremenchutzky, director of the Multiple Sclerosis Clinic in London, Ont., presented the 1-year data in the ongoing extension study that is evaluating long-term MS outcomes, safety, and tolerability of peginterferon beta-1a among patients enrolled in ADVANCE, including those treated with peginterferon beta-1a every 2 weeks (376) or every 4 weeks (354).
Also included are 344 patients who were on placebo but were rerandomized to peginterferon beta-1a every 2 or 4 weeks (the delayed treatment group) after 1 year in the ADVANCE study; they were included in the analyses after they had been on treatment for 2 years.
After 1 year in the ATTAIN study, injection-site reactions and flu-like symptoms remained the most frequent adverse events associated with peginterferon beta-1a, and most were mild to moderate. Treatment has also been associated with “low levels” of laboratory abnormalities and immunogenicity, and clinical efficacy has been maintained for 3 years, Dr. Kremenchutzky said at the meeting.
Adverse events were “very similar” in the 4-week and 2-week treatment groups. Over 3 years, 96% of patients in both groups had experienced an adverse event, and the rates of treatment-related adverse events (91%), and severe adverse events (24%) were the same in both groups. The rates of treatment discontinuations due to an adverse event (7%-8%) and withdrawal from the study due to an adverse event (8%-9%) were similar.
The rates of flu-like symptoms over 3 years were also similar (62%-64%) in the two groups, and influenza-like illness was reported in 55% of those dosed every 2 weeks and in 45% of those dosed every 4 weeks. In addition to influenza-like illness, pyrexia was the most common flu-like symptom reported over 3 years (43%-45%).
About 65%-69% of those in the two groups reported injection-site reactions, with erythema the most common (62%-64%), followed by pain (17%-18%) and pruritus (12%-16%).
Lab abnormalities were comparable with both dosing schedules: Over 3 years, white blood cell counts were reduced in 6% of those on the 2-week schedule, compared with 8% among those on the 4-week schedule. Lymphocyte counts were reduced in 12% and 9% of those on the 2-week and 4-week schedules, respectively. In addition, 8% of those on the 2-week dose and 6% of those on the 4-week dose had low hemoglobin (100 g/L or less) over the 3 years.
Rates of anti-IFN and anti-PEG antibodies associated with both schedules were low, Dr. Kremenchutzkysaid. Over 3 years, 7%-9% tested positive for IFN binding antibody at least once, and 4%-6% of the total were “persistent positive.” Another 9% of those on the 4-week schedule and 6% of those on the 2-week schedule were anti-PEG antibody positive at least once, and 3%-5% of the patients in total had persistent levels. Titers were high in less than 1% of the total.
In ATTAIN, efficacy endpoints were secondary, and included ARR, which was reduced by 25.1% over 3 years among those receiving the dose every 2 weeks, compared with every 4 weeks (P = .0199). By 144 weeks, 30.5% of those on the 2-week schedule and 41.1% of those on the 4-week schedule had experienced a relapse, a significant difference (P = .0070), Dr. Kremenchutzky said.
The proportion of those with CDP by 144 weeks was 15.5% among those on the 4-week schedule, vs. 9.2% among those on the 2-week schedule (P = .0098), he added.
The studies were funded by Biogen Idec. Dr. Newsome and Dr. Kremenchutzky both disclosed having been compensated for activities with Biogen Idec and other companies that market MS drugs.
WASHINGTON – Beneficial effects of peginterferon beta-1a administered every 2 weeks were evident as early as 12 weeks after starting treatment, in the pivotal phase III study comparing peginterferon beta-1a to placebo in patients with relapsing remitting multiple sclerosis, Dr. Scott Newsome, said at the annual meeting of the American Academy of Neurology.
At 12 weeks, treatment with peginterferon beta-1a was associated with a 37% reduction in the adjusted annualized relapse rate (ARR) compared with placebo, in the ADVANCE study, said Dr. Newsome of the department of neurology at Johns Hopkins University, Baltimore.
ADVANCE compared 125 mcg of peginterferon beta-1a administered every 2 or 4 weeks to placebo in patients with relapsing remitting MS. The efficacy of peginterferon beta-1a was maintained over 2 years. Greater efficacy was seen with administration every 2 weeks (Lancet Neurol. 2014;13:657-65). In August 2014, the Food and Drug Administration approved peginterferon beta-1a, at a dose of 125 mcg administered subcutaneously every 14 days, for relapsing forms of multiple sclerosis; it is marketed as Plegridy by Biogen Idec.
Interim results of the follow-up study of patients enrolled in ADVANCE – the ATTAIN trial – have shown that over 3 years of treatment, no new safety issues or concerns have emerged, and the clinical effects of treatment are maintained, according to Dr. Marcelo Kremenchutzky of Western University, London, Ont., who presented those results at the meeting.
Dr. Newsome presented the 12-week data among 512 patients randomized to treatment every 2 weeks and 500 randomized to placebo in the ADVANCE study; their mean age was 36-37 years, almost two-thirds were female, and 81%-82% were white. They were enrolled for a mean of 6-7 years from their first MS symptoms and had had a mean of 2.6 relapses within the previous 3 years. Their mean Expanded Disability Status Scale (EDSS) scores were 2.44-2.47.
At 12 weeks, the ARR, the primary endpoint, among those on peginterferon beta-1a was 0.259 vs. 0.414 among those on placebo, a 37.4% reduction over placebo (P = .045), Dr. Newsome said. The ARR was adjusted for baseline EDSS, relapse rate, and age. (At 48 weeks, the ARR among those on peginterferon beta-1a every 2 weeks was 0.26, compared with 0.40 among those on placebo, a significantly reduced risk of 36%, with a P value of .0007.)
The proportion of patients who relapsed at 12 weeks – 6% of those on peginterferon beta-1a vs. 9.5% of those on placebo – was reduced by 36.8% (P = .041), with a “clear separation” between the treatment and control arms at 12 weeks, he noted.
In addition, the estimated proportion of patients with an onset of 24-week confirmed disability progression (CDP) was 0 among those on peginterferon beta-1a versus 1.0% among those on placebo (P = .026). This result was not as robust as the relapse results, but there was a clear separation between the arms with statistical significance, said Dr. Newsome, director of outpatient neurology services at Johns Hopkins.
In the ADVANCE study, PEG administered every 2 or 4 weeks was well tolerated with a favorable safety profile in the first year, and a similar safety profile between the two dosing regimens, he added.
Dr. Kremenchutzky, director of the Multiple Sclerosis Clinic in London, Ont., presented the 1-year data in the ongoing extension study that is evaluating long-term MS outcomes, safety, and tolerability of peginterferon beta-1a among patients enrolled in ADVANCE, including those treated with peginterferon beta-1a every 2 weeks (376) or every 4 weeks (354).
Also included are 344 patients who were on placebo but were rerandomized to peginterferon beta-1a every 2 or 4 weeks (the delayed treatment group) after 1 year in the ADVANCE study; they were included in the analyses after they had been on treatment for 2 years.
After 1 year in the ATTAIN study, injection-site reactions and flu-like symptoms remained the most frequent adverse events associated with peginterferon beta-1a, and most were mild to moderate. Treatment has also been associated with “low levels” of laboratory abnormalities and immunogenicity, and clinical efficacy has been maintained for 3 years, Dr. Kremenchutzky said at the meeting.
Adverse events were “very similar” in the 4-week and 2-week treatment groups. Over 3 years, 96% of patients in both groups had experienced an adverse event, and the rates of treatment-related adverse events (91%), and severe adverse events (24%) were the same in both groups. The rates of treatment discontinuations due to an adverse event (7%-8%) and withdrawal from the study due to an adverse event (8%-9%) were similar.
The rates of flu-like symptoms over 3 years were also similar (62%-64%) in the two groups, and influenza-like illness was reported in 55% of those dosed every 2 weeks and in 45% of those dosed every 4 weeks. In addition to influenza-like illness, pyrexia was the most common flu-like symptom reported over 3 years (43%-45%).
About 65%-69% of those in the two groups reported injection-site reactions, with erythema the most common (62%-64%), followed by pain (17%-18%) and pruritus (12%-16%).
Lab abnormalities were comparable with both dosing schedules: Over 3 years, white blood cell counts were reduced in 6% of those on the 2-week schedule, compared with 8% among those on the 4-week schedule. Lymphocyte counts were reduced in 12% and 9% of those on the 2-week and 4-week schedules, respectively. In addition, 8% of those on the 2-week dose and 6% of those on the 4-week dose had low hemoglobin (100 g/L or less) over the 3 years.
Rates of anti-IFN and anti-PEG antibodies associated with both schedules were low, Dr. Kremenchutzkysaid. Over 3 years, 7%-9% tested positive for IFN binding antibody at least once, and 4%-6% of the total were “persistent positive.” Another 9% of those on the 4-week schedule and 6% of those on the 2-week schedule were anti-PEG antibody positive at least once, and 3%-5% of the patients in total had persistent levels. Titers were high in less than 1% of the total.
In ATTAIN, efficacy endpoints were secondary, and included ARR, which was reduced by 25.1% over 3 years among those receiving the dose every 2 weeks, compared with every 4 weeks (P = .0199). By 144 weeks, 30.5% of those on the 2-week schedule and 41.1% of those on the 4-week schedule had experienced a relapse, a significant difference (P = .0070), Dr. Kremenchutzky said.
The proportion of those with CDP by 144 weeks was 15.5% among those on the 4-week schedule, vs. 9.2% among those on the 2-week schedule (P = .0098), he added.
The studies were funded by Biogen Idec. Dr. Newsome and Dr. Kremenchutzky both disclosed having been compensated for activities with Biogen Idec and other companies that market MS drugs.
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: Benefits from peginterferon beta-1a, recently approved in the United States, may start early in the course of treatment and persist for at least 3 years.
Major finding: Peginterferon beta-1a shows beneficial effects as early as 12 weeks after starting treatment, and for up to 3 years of treatment, in the pivotal and extension studies.
Data source: Phase III pivotal study in 1,012 patients treated with 125 mcg of peginterferon beta-1a every 2 weeks, or placebo. Longer follow-up data are from the 2-year extension study, which is following patients treated with peginterferon beta-1a in the pivotal study and those randomized to treatment every 2 or 4 weeks after 1 year on placebo.
Disclosures: The ADVANCE and ATTAIN studies were funded by Biogen Idec. Dr. Newsome and Dr. Kremenchutzky disclosed having been compensated for activities with Biogen Idec and other companies that market MS drugs.
AAN: Facial nerve stimulator relieves cluster headaches
WASHINGTON – An implantable device that stimulates the sphenopalatine ganglion nerve bundle either reduced or eliminated pain in 68% of more than 5,000 cluster headaches, a 3-year study has determined.
The device, which is approved in Europe, was more effective in attacks of moderate severity, with a 78% rate of pain reduction or elimination, Dr. Jose Miguel Lainez reported at the annual meeting of the American Academy of Neurology.
The Pulsante System, manufactured by Autonomic Technologiesof Redwood City, Calif., consists of a neurostimulator about the size of an almond, and a lead with six electrodes. It’s inserted under local anesthetic via a small incision in the upper gum on the side in which the patient experiences symptoms. The electrodes are positioned along the sphenopalatine ganglion (SPG) nerve and the neurostimulator is affixed to the zygomatic process.
A hand-held remote controller placed against the cheek activates the device and controls the intensity of stimulation, which is thought to work by blocking signals to the postganglionic parasympathetic fibers. Those fibers innervate facial structures and the cerebral and meningeal blood vessels and are implicated in the pain and accompanying autonomic symptoms of a cluster headache attack.
Dr. Lainez, professor of neurology at Catholic University of Valencia (Spain), presented 3-year follow-up data from Pathway CH-1, a randomized, sham-controlled trial of 43 patients with cluster headache. Of these, 33 completed the 3-year follow-up period. Of the remaining 10, 1 was lost from observation, 5 violated protocol, 1 had the device implanted incorrectly, and 3 had the device explanted because of incorrect placement or lead migration.
Most of the patients were male. Mean age was 41 years. They had a mean disease duration of 10 years and averaged 17 cluster headaches per week but ranged from 4 to 70 attacks per week. Over the 3 years, 5,130 attacks were treated; the mean stimulation duration for these was 14 minutes with a mean response time of 11 minutes. Therapy was considered effective in 65% (3,354) of these attacks based on a clinically meaningful reduction in pain or pain elimination.
Dr. Lainez did not parse these results. However, in the initial 28-week phase of the Pathway CH-1 study, pain was reduced in 68% of attacks treated with the device and 7% of those treated with the sham control. Pain freedom by 15 minutes was achieved in 34% of attacks with full stimulation, compared with 1.5% of those treated with sham.
In the follow-up study, the device seemed most effective in attacks of moderate severity (78% response rate of pain reduction or elimination). The response rate was 59% in mild attacks and 51% in severe attacks. Most attacks treated with the device (77%) did not involve the use of abortive therapy.
Dr. Lainez did not mention adverse events related to the device. However, in the 28-week study, there were 92, including parasthesias and numbness; facial and tooth pain; and swelling. Others were considered mild and included dry eye, nose bleed, and facial asymmetry.
The device is currently being investigated in a U.S. study. The open-label Pathway-CH2 study aims to recruit 120 patients. For information on Pathway CH-2, contact Anthony Caparso.
The trial was sponsored by Autonomic Technologies Inc. Dr. Lainez had no financial ties with the company.
On Twitter @alz_gal
WASHINGTON – An implantable device that stimulates the sphenopalatine ganglion nerve bundle either reduced or eliminated pain in 68% of more than 5,000 cluster headaches, a 3-year study has determined.
The device, which is approved in Europe, was more effective in attacks of moderate severity, with a 78% rate of pain reduction or elimination, Dr. Jose Miguel Lainez reported at the annual meeting of the American Academy of Neurology.
The Pulsante System, manufactured by Autonomic Technologiesof Redwood City, Calif., consists of a neurostimulator about the size of an almond, and a lead with six electrodes. It’s inserted under local anesthetic via a small incision in the upper gum on the side in which the patient experiences symptoms. The electrodes are positioned along the sphenopalatine ganglion (SPG) nerve and the neurostimulator is affixed to the zygomatic process.
A hand-held remote controller placed against the cheek activates the device and controls the intensity of stimulation, which is thought to work by blocking signals to the postganglionic parasympathetic fibers. Those fibers innervate facial structures and the cerebral and meningeal blood vessels and are implicated in the pain and accompanying autonomic symptoms of a cluster headache attack.
Dr. Lainez, professor of neurology at Catholic University of Valencia (Spain), presented 3-year follow-up data from Pathway CH-1, a randomized, sham-controlled trial of 43 patients with cluster headache. Of these, 33 completed the 3-year follow-up period. Of the remaining 10, 1 was lost from observation, 5 violated protocol, 1 had the device implanted incorrectly, and 3 had the device explanted because of incorrect placement or lead migration.
Most of the patients were male. Mean age was 41 years. They had a mean disease duration of 10 years and averaged 17 cluster headaches per week but ranged from 4 to 70 attacks per week. Over the 3 years, 5,130 attacks were treated; the mean stimulation duration for these was 14 minutes with a mean response time of 11 minutes. Therapy was considered effective in 65% (3,354) of these attacks based on a clinically meaningful reduction in pain or pain elimination.
Dr. Lainez did not parse these results. However, in the initial 28-week phase of the Pathway CH-1 study, pain was reduced in 68% of attacks treated with the device and 7% of those treated with the sham control. Pain freedom by 15 minutes was achieved in 34% of attacks with full stimulation, compared with 1.5% of those treated with sham.
In the follow-up study, the device seemed most effective in attacks of moderate severity (78% response rate of pain reduction or elimination). The response rate was 59% in mild attacks and 51% in severe attacks. Most attacks treated with the device (77%) did not involve the use of abortive therapy.
Dr. Lainez did not mention adverse events related to the device. However, in the 28-week study, there were 92, including parasthesias and numbness; facial and tooth pain; and swelling. Others were considered mild and included dry eye, nose bleed, and facial asymmetry.
The device is currently being investigated in a U.S. study. The open-label Pathway-CH2 study aims to recruit 120 patients. For information on Pathway CH-2, contact Anthony Caparso.
The trial was sponsored by Autonomic Technologies Inc. Dr. Lainez had no financial ties with the company.
On Twitter @alz_gal
WASHINGTON – An implantable device that stimulates the sphenopalatine ganglion nerve bundle either reduced or eliminated pain in 68% of more than 5,000 cluster headaches, a 3-year study has determined.
The device, which is approved in Europe, was more effective in attacks of moderate severity, with a 78% rate of pain reduction or elimination, Dr. Jose Miguel Lainez reported at the annual meeting of the American Academy of Neurology.
The Pulsante System, manufactured by Autonomic Technologiesof Redwood City, Calif., consists of a neurostimulator about the size of an almond, and a lead with six electrodes. It’s inserted under local anesthetic via a small incision in the upper gum on the side in which the patient experiences symptoms. The electrodes are positioned along the sphenopalatine ganglion (SPG) nerve and the neurostimulator is affixed to the zygomatic process.
A hand-held remote controller placed against the cheek activates the device and controls the intensity of stimulation, which is thought to work by blocking signals to the postganglionic parasympathetic fibers. Those fibers innervate facial structures and the cerebral and meningeal blood vessels and are implicated in the pain and accompanying autonomic symptoms of a cluster headache attack.
Dr. Lainez, professor of neurology at Catholic University of Valencia (Spain), presented 3-year follow-up data from Pathway CH-1, a randomized, sham-controlled trial of 43 patients with cluster headache. Of these, 33 completed the 3-year follow-up period. Of the remaining 10, 1 was lost from observation, 5 violated protocol, 1 had the device implanted incorrectly, and 3 had the device explanted because of incorrect placement or lead migration.
Most of the patients were male. Mean age was 41 years. They had a mean disease duration of 10 years and averaged 17 cluster headaches per week but ranged from 4 to 70 attacks per week. Over the 3 years, 5,130 attacks were treated; the mean stimulation duration for these was 14 minutes with a mean response time of 11 minutes. Therapy was considered effective in 65% (3,354) of these attacks based on a clinically meaningful reduction in pain or pain elimination.
Dr. Lainez did not parse these results. However, in the initial 28-week phase of the Pathway CH-1 study, pain was reduced in 68% of attacks treated with the device and 7% of those treated with the sham control. Pain freedom by 15 minutes was achieved in 34% of attacks with full stimulation, compared with 1.5% of those treated with sham.
In the follow-up study, the device seemed most effective in attacks of moderate severity (78% response rate of pain reduction or elimination). The response rate was 59% in mild attacks and 51% in severe attacks. Most attacks treated with the device (77%) did not involve the use of abortive therapy.
Dr. Lainez did not mention adverse events related to the device. However, in the 28-week study, there were 92, including parasthesias and numbness; facial and tooth pain; and swelling. Others were considered mild and included dry eye, nose bleed, and facial asymmetry.
The device is currently being investigated in a U.S. study. The open-label Pathway-CH2 study aims to recruit 120 patients. For information on Pathway CH-2, contact Anthony Caparso.
The trial was sponsored by Autonomic Technologies Inc. Dr. Lainez had no financial ties with the company.
On Twitter @alz_gal
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: An implantable device that stimulates the sphenopalatine ganglion nerve provided pain relief in cluster headaches.
Major finding: The device reduced or eliminated pain in 68% of more than 5,000 cluster headaches.
Data source: A 3-year follow-up study that examined response in more than 5,000 cluster headaches.
Disclosures: The trial was sponsored by Autonomic Technologies Inc., which makes the Pulsante System. Dr. Lainez had no financial disclosures.
AAN: OnabotulinumtoxinA injections reduce migraine hospitalizations
WASHINGTON – OnabotulinumtoxinA injections significantly decreased both emergency department visits and hospitalizations for chronic migraine for up to 1 year among patients who had previously been admitted for the condition.
By 6 months after the first injection, ED visits were down 32% and hospitalizations were down 52%, Dr. Noah Rosen said at the annual meeting of the American Academy of Neurology.
Dr. Rosen of the Hofstra North Shore Headache Center in Great Neck, N.Y., based his findings on data from the MarketScan health resource utilization database, which captures about 66 million patient visits per year. The initial cohort included 3,840 patients who had a new diagnosis of chronic migraine with at least one subsequent onabotulinumtoxinA treatment within 6 months of diagnosis. These were followed for 1 year before the diagnosis and for 1 year after treatment began. The cohort comprised 1,831 patients at 6 months and 936 patients at 12 months.
Most (80%) of the population already had a diagnosis of migraine before the chronic migraine diagnosis; about half also had another headache disorder. They were a mean of 46 years old and were predominantly women. Depression was present in 20% and anxiety in about 11%. They also were taking one to three other headache medications, including triptans, opioids, nonsteroidal anti-inflammatory drugs, muscle relaxants, benzodiazepines, barbiturates, and ergots. The time from diagnosis to first injection was about a month, with subsequent injections 90 days apart.
The 6-month improvements in ED visits and hospitalizations remained steady, Dr. Rosen said. The reductions at 9 months in ED visits and hospitalizations were identical to those at 6 months. At 12 months, they had declined 25% and 47%, respectively, from baseline.
“This shows that the immediate benefits, which have been reported before, are maintained for at least a year,” he noted.
Dr. Rosen is on the speakers’ board and has been an advisor for Allergan and Zogenix. He also has received remuneration from Curelator.
On Twitter @alz_gal
WASHINGTON – OnabotulinumtoxinA injections significantly decreased both emergency department visits and hospitalizations for chronic migraine for up to 1 year among patients who had previously been admitted for the condition.
By 6 months after the first injection, ED visits were down 32% and hospitalizations were down 52%, Dr. Noah Rosen said at the annual meeting of the American Academy of Neurology.
Dr. Rosen of the Hofstra North Shore Headache Center in Great Neck, N.Y., based his findings on data from the MarketScan health resource utilization database, which captures about 66 million patient visits per year. The initial cohort included 3,840 patients who had a new diagnosis of chronic migraine with at least one subsequent onabotulinumtoxinA treatment within 6 months of diagnosis. These were followed for 1 year before the diagnosis and for 1 year after treatment began. The cohort comprised 1,831 patients at 6 months and 936 patients at 12 months.
Most (80%) of the population already had a diagnosis of migraine before the chronic migraine diagnosis; about half also had another headache disorder. They were a mean of 46 years old and were predominantly women. Depression was present in 20% and anxiety in about 11%. They also were taking one to three other headache medications, including triptans, opioids, nonsteroidal anti-inflammatory drugs, muscle relaxants, benzodiazepines, barbiturates, and ergots. The time from diagnosis to first injection was about a month, with subsequent injections 90 days apart.
The 6-month improvements in ED visits and hospitalizations remained steady, Dr. Rosen said. The reductions at 9 months in ED visits and hospitalizations were identical to those at 6 months. At 12 months, they had declined 25% and 47%, respectively, from baseline.
“This shows that the immediate benefits, which have been reported before, are maintained for at least a year,” he noted.
Dr. Rosen is on the speakers’ board and has been an advisor for Allergan and Zogenix. He also has received remuneration from Curelator.
On Twitter @alz_gal
WASHINGTON – OnabotulinumtoxinA injections significantly decreased both emergency department visits and hospitalizations for chronic migraine for up to 1 year among patients who had previously been admitted for the condition.
By 6 months after the first injection, ED visits were down 32% and hospitalizations were down 52%, Dr. Noah Rosen said at the annual meeting of the American Academy of Neurology.
Dr. Rosen of the Hofstra North Shore Headache Center in Great Neck, N.Y., based his findings on data from the MarketScan health resource utilization database, which captures about 66 million patient visits per year. The initial cohort included 3,840 patients who had a new diagnosis of chronic migraine with at least one subsequent onabotulinumtoxinA treatment within 6 months of diagnosis. These were followed for 1 year before the diagnosis and for 1 year after treatment began. The cohort comprised 1,831 patients at 6 months and 936 patients at 12 months.
Most (80%) of the population already had a diagnosis of migraine before the chronic migraine diagnosis; about half also had another headache disorder. They were a mean of 46 years old and were predominantly women. Depression was present in 20% and anxiety in about 11%. They also were taking one to three other headache medications, including triptans, opioids, nonsteroidal anti-inflammatory drugs, muscle relaxants, benzodiazepines, barbiturates, and ergots. The time from diagnosis to first injection was about a month, with subsequent injections 90 days apart.
The 6-month improvements in ED visits and hospitalizations remained steady, Dr. Rosen said. The reductions at 9 months in ED visits and hospitalizations were identical to those at 6 months. At 12 months, they had declined 25% and 47%, respectively, from baseline.
“This shows that the immediate benefits, which have been reported before, are maintained for at least a year,” he noted.
Dr. Rosen is on the speakers’ board and has been an advisor for Allergan and Zogenix. He also has received remuneration from Curelator.
On Twitter @alz_gal
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: OnabotulinumtoxinA injections reduced hospital usage for chronic migraine for up to 1 year.
Major finding: By 6 months after the first injection, emergency dept. visits were down 32% and hospitalizations were down 52%. At 12 months, the reductions were 25% and 47% from baseline, respectively.
Data source: The retrospective database study comprised 3,840 patients.
Disclosures: Dr. Rosen is on the speakers’ board and has been an advisor for Allergan and Zogenix. He also has received remuneration from Curelator.
AAN: Intranasal sumatriptan powder works faster than tablet form
WASHINGTON – A low-dose intranasal sumatriptan powder worked faster than the 100-mg oral formulation on acute migraine, with a significantly lower rate of triptan-related chest tightening.
Additionally, the 22-mg powder formulation reduced or eliminated pain just as long as the 100-mg tablet did, with pain relief reported by 60% of each group and pain freedom by 50% at 48 hours, Dr. Stewart Tepper said at the annual meeting of the American Academy of Neurology.
“Oral was not superior to the powder in any of the efficacy outcomes” in the COMPASS trial, a phase III, placebo-controlled, crossover study, said Dr. Tepper of the Cleveland Clinic in Ohio.
Nor was the powder associated with the “foul” taste patients often complain of when they use a liquid sumatriptan nasal spray, he added.
The investigational drug, AVP-825 (Avanir Pharmaceuticals) is packaged in a unique intranasal delivery system. The breath-powered device holds a single-drug cartridge. Two cartridges equal one 22-mg dose; of this, about 16 mg are actually delivered.
Patients put the nasal piece into one nostril, seal their lips around a mouthpiece, and blow into it. “This elevates and closes the soft palate, so the powder doesn’t enter the oropharynx,” Dr. Tepper said. “The powder is delivered high into the nose. The top of the septum is open, so the breath comes out the other side.”
The trial enrolled 185 patients and assessed 1,531 migraines over 24 weeks. Patients were randomized to active powder or placebo tablet for 12 weeks or five migraines, and then crossed to placebo powder or active tablet. The primary endpoint was the change in SPID-30 (sum of pain intensity differences from baseline-30 minutes).
AVP-825 significantly outperformed oral sumatriptan on the SPID-30 measure, with a mean 11-point decrease on the pain scale, compared with a mean 7-point decrease.
Secondary endpoints were pain relief – from moderate or severe to none or mild – and pain freedom. These were measured every 15 minutes for the first hour and then again at 60, 90, and 120 minutes. Pain was also assessed 24 and 48 hours post dose.
AVP-825 showed its greatest advantage during the first 60 minutes, reducing pain in significantly more migraine attacks than did the oral form. By 30 minutes, response rates were 54% vs. 39%; by 1 hour, they were 72% vs. 63%. Thereafter, both forms were equally effective. There were no differences in 24- and 48-hour outcomes, with about 60% of each group reporting sustained pain relief.
The findings were similar for the outcome of pain freedom, although – as could be expected – response rates were much lower. Again, AVP-825 showed an early advantage, with 7% of migraines completely abating by 15 minutes. At 30 minutes, response rates were 18% vs. 11%. AVP-825 continued to show about a 10% advantage over oral through 90 minutes. By 2 hours, the response rate was about 60% for both treatments. At 24 and 48 hours about half of the migraines attacks were still completely abated.
The investigational drug was well tolerated. Although 54% of patients experienced some sort of treatment-related adverse event, most were mild (44%); a burning sensation in the nose was the most commonly reported.
Systemic adverse events occurred more frequently in those taking the oral medication. Triptan-related sensations – tightening in the chest and neck – occurred in 5% of those taking the tablet and 2% of those who used AVP-825.
Last fall, the Food and Drug Administration issued a Complete Response Letter to Avanir’s New Drug Application, requesting that the company address device use errors seen in some of the trials. The agency did not, however, find any clinical or nonclinical safety or efficacy issues or request that additional clinical trials be conducted prior to approval.
The study was cofunded by OptiNose U.S. and Avanir Pharmaceuticals. Dr. Tepper has received research funds from, serves as a consultant to, or sits the advisory board of Allergan, ATI, Impax, and a number of other pharmaceutical companies.
On Twitter @alz_gal
WASHINGTON – A low-dose intranasal sumatriptan powder worked faster than the 100-mg oral formulation on acute migraine, with a significantly lower rate of triptan-related chest tightening.
Additionally, the 22-mg powder formulation reduced or eliminated pain just as long as the 100-mg tablet did, with pain relief reported by 60% of each group and pain freedom by 50% at 48 hours, Dr. Stewart Tepper said at the annual meeting of the American Academy of Neurology.
“Oral was not superior to the powder in any of the efficacy outcomes” in the COMPASS trial, a phase III, placebo-controlled, crossover study, said Dr. Tepper of the Cleveland Clinic in Ohio.
Nor was the powder associated with the “foul” taste patients often complain of when they use a liquid sumatriptan nasal spray, he added.
The investigational drug, AVP-825 (Avanir Pharmaceuticals) is packaged in a unique intranasal delivery system. The breath-powered device holds a single-drug cartridge. Two cartridges equal one 22-mg dose; of this, about 16 mg are actually delivered.
Patients put the nasal piece into one nostril, seal their lips around a mouthpiece, and blow into it. “This elevates and closes the soft palate, so the powder doesn’t enter the oropharynx,” Dr. Tepper said. “The powder is delivered high into the nose. The top of the septum is open, so the breath comes out the other side.”
The trial enrolled 185 patients and assessed 1,531 migraines over 24 weeks. Patients were randomized to active powder or placebo tablet for 12 weeks or five migraines, and then crossed to placebo powder or active tablet. The primary endpoint was the change in SPID-30 (sum of pain intensity differences from baseline-30 minutes).
AVP-825 significantly outperformed oral sumatriptan on the SPID-30 measure, with a mean 11-point decrease on the pain scale, compared with a mean 7-point decrease.
Secondary endpoints were pain relief – from moderate or severe to none or mild – and pain freedom. These were measured every 15 minutes for the first hour and then again at 60, 90, and 120 minutes. Pain was also assessed 24 and 48 hours post dose.
AVP-825 showed its greatest advantage during the first 60 minutes, reducing pain in significantly more migraine attacks than did the oral form. By 30 minutes, response rates were 54% vs. 39%; by 1 hour, they were 72% vs. 63%. Thereafter, both forms were equally effective. There were no differences in 24- and 48-hour outcomes, with about 60% of each group reporting sustained pain relief.
The findings were similar for the outcome of pain freedom, although – as could be expected – response rates were much lower. Again, AVP-825 showed an early advantage, with 7% of migraines completely abating by 15 minutes. At 30 minutes, response rates were 18% vs. 11%. AVP-825 continued to show about a 10% advantage over oral through 90 minutes. By 2 hours, the response rate was about 60% for both treatments. At 24 and 48 hours about half of the migraines attacks were still completely abated.
The investigational drug was well tolerated. Although 54% of patients experienced some sort of treatment-related adverse event, most were mild (44%); a burning sensation in the nose was the most commonly reported.
Systemic adverse events occurred more frequently in those taking the oral medication. Triptan-related sensations – tightening in the chest and neck – occurred in 5% of those taking the tablet and 2% of those who used AVP-825.
Last fall, the Food and Drug Administration issued a Complete Response Letter to Avanir’s New Drug Application, requesting that the company address device use errors seen in some of the trials. The agency did not, however, find any clinical or nonclinical safety or efficacy issues or request that additional clinical trials be conducted prior to approval.
The study was cofunded by OptiNose U.S. and Avanir Pharmaceuticals. Dr. Tepper has received research funds from, serves as a consultant to, or sits the advisory board of Allergan, ATI, Impax, and a number of other pharmaceutical companies.
On Twitter @alz_gal
WASHINGTON – A low-dose intranasal sumatriptan powder worked faster than the 100-mg oral formulation on acute migraine, with a significantly lower rate of triptan-related chest tightening.
Additionally, the 22-mg powder formulation reduced or eliminated pain just as long as the 100-mg tablet did, with pain relief reported by 60% of each group and pain freedom by 50% at 48 hours, Dr. Stewart Tepper said at the annual meeting of the American Academy of Neurology.
“Oral was not superior to the powder in any of the efficacy outcomes” in the COMPASS trial, a phase III, placebo-controlled, crossover study, said Dr. Tepper of the Cleveland Clinic in Ohio.
Nor was the powder associated with the “foul” taste patients often complain of when they use a liquid sumatriptan nasal spray, he added.
The investigational drug, AVP-825 (Avanir Pharmaceuticals) is packaged in a unique intranasal delivery system. The breath-powered device holds a single-drug cartridge. Two cartridges equal one 22-mg dose; of this, about 16 mg are actually delivered.
Patients put the nasal piece into one nostril, seal their lips around a mouthpiece, and blow into it. “This elevates and closes the soft palate, so the powder doesn’t enter the oropharynx,” Dr. Tepper said. “The powder is delivered high into the nose. The top of the septum is open, so the breath comes out the other side.”
The trial enrolled 185 patients and assessed 1,531 migraines over 24 weeks. Patients were randomized to active powder or placebo tablet for 12 weeks or five migraines, and then crossed to placebo powder or active tablet. The primary endpoint was the change in SPID-30 (sum of pain intensity differences from baseline-30 minutes).
AVP-825 significantly outperformed oral sumatriptan on the SPID-30 measure, with a mean 11-point decrease on the pain scale, compared with a mean 7-point decrease.
Secondary endpoints were pain relief – from moderate or severe to none or mild – and pain freedom. These were measured every 15 minutes for the first hour and then again at 60, 90, and 120 minutes. Pain was also assessed 24 and 48 hours post dose.
AVP-825 showed its greatest advantage during the first 60 minutes, reducing pain in significantly more migraine attacks than did the oral form. By 30 minutes, response rates were 54% vs. 39%; by 1 hour, they were 72% vs. 63%. Thereafter, both forms were equally effective. There were no differences in 24- and 48-hour outcomes, with about 60% of each group reporting sustained pain relief.
The findings were similar for the outcome of pain freedom, although – as could be expected – response rates were much lower. Again, AVP-825 showed an early advantage, with 7% of migraines completely abating by 15 minutes. At 30 minutes, response rates were 18% vs. 11%. AVP-825 continued to show about a 10% advantage over oral through 90 minutes. By 2 hours, the response rate was about 60% for both treatments. At 24 and 48 hours about half of the migraines attacks were still completely abated.
The investigational drug was well tolerated. Although 54% of patients experienced some sort of treatment-related adverse event, most were mild (44%); a burning sensation in the nose was the most commonly reported.
Systemic adverse events occurred more frequently in those taking the oral medication. Triptan-related sensations – tightening in the chest and neck – occurred in 5% of those taking the tablet and 2% of those who used AVP-825.
Last fall, the Food and Drug Administration issued a Complete Response Letter to Avanir’s New Drug Application, requesting that the company address device use errors seen in some of the trials. The agency did not, however, find any clinical or nonclinical safety or efficacy issues or request that additional clinical trials be conducted prior to approval.
The study was cofunded by OptiNose U.S. and Avanir Pharmaceuticals. Dr. Tepper has received research funds from, serves as a consultant to, or sits the advisory board of Allergan, ATI, Impax, and a number of other pharmaceutical companies.
On Twitter @alz_gal
AT THE AAN 2015 ANNUAL MEETING
Key clinical point: An intranasal powdered sumatriptan worked fast and more effectively on acute migraine attacks than did the oral form of the drug.
Major finding: By 30 minutes, response rates were 54% for the inhaled powder vs. 39% for the tablet. By 1 hour, they were 72% vs. 63%. Thereafter, both forms were equally effective.
Data source: The placebo-controlled crossover trial involved 185 patients who experienced more than 1,500 migraines during 24 weeks.
Disclosures: The study was cofunded by OptiNose U.S. and Avanir Pharmaceuticals. Dr. Tepper has received research funds from, serves as a consultant to, or sits on the advisory board of Allergan, ATI, Impax, and a number of other pharmaceutical companies.