User login
High tobacco use in subpopulations of Americans and rapid uptake in other countries are major concerns
SAN DIEGO – Though progress has been made in reducing the burden of disease, disability, and death caused by tobacco use since the 1964 Surgeon General’s Report on Smoking and Health debuted, much work remains to be done, according to Robert T. Croyle, Ph.D.
According to data from the Department of Health & Human Services, more than 42 million adults and more than 3.5 million middle and high school students continue to smoke tobacco. "We’re in a bizarre situation where we have a legal product on the market, which is responsible for about a half a million deaths," Dr. Croyle, director of division of cancer control and population sciences at the National Cancer Institute (NCI), said during a press briefing at the annual meeting of the American Association for Cancer Research marking the 50th anniversary of the Surgeon General’s Report on Smoking and Health. "In any other circumstance, without this long, strange history, we’d feel there would be a lot more engagement by the scientific community, by clinicians, and by organizations in marshalling every effort to address the problem."
One recurring problem, he said, is the high use of tobacco products by subpopulations of Americans, especially those with low levels of income and education. "This is a challenge, because if you are a college-educated, wealthy individual living in the state of California, you may assume and believe that the tobacco problem is largely one of the past," he said. "But for many of us, we clearly see that across the United States, there are populations where this is still an overwhelming problem that individuals face in their families."
At the same time, Dr. Croyle said, "a rapid uptake" of tobacco use is taking place in many countries around the world. "We have some alarming concerns about the trajectory of tobacco use and the burden of cancer globally," he said. "That’s led us to support more research on global tobacco control and for the NCI to collaborate with international organizations."
In the United States, the National Institutes of Health formed a unique partnership aimed at bringing together expertise from scientists from a variety of disciplines to address the use of tobacco and other addictive substances. According to its website, the goal of Collaborative Research on Addiction at NIH is "to provide a strong collaborative framework to enable the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NCI to integrate resources and expertise to advance substance use, abuse, and addiction research and public health outcomes."
The time is right for such a partnership, Dr. Croyle said, because many current smokers also use alcohol and other drugs. "Unfortunately, even though scientists for many years have been calling for more research on these comorbid risk factors, the research lags behind what we need to inform clinical practice, so we’re paying special attention to issues of mental health," he said. "For example, tobacco use is incredibly high among those with schizophrenia. We’re also concerned about comorbid [tobacco] use and alcohol use, for example, among college students, where we see huge binge drinking problems that co-occur with tobacco use. We also have a concern about the introduction of electronic cigarettes and the fact that we desperately need a lot more evidence in that domain."
Mr. Croyle had no relevant financial conflicts to disclose.
SAN DIEGO – Though progress has been made in reducing the burden of disease, disability, and death caused by tobacco use since the 1964 Surgeon General’s Report on Smoking and Health debuted, much work remains to be done, according to Robert T. Croyle, Ph.D.
According to data from the Department of Health & Human Services, more than 42 million adults and more than 3.5 million middle and high school students continue to smoke tobacco. "We’re in a bizarre situation where we have a legal product on the market, which is responsible for about a half a million deaths," Dr. Croyle, director of division of cancer control and population sciences at the National Cancer Institute (NCI), said during a press briefing at the annual meeting of the American Association for Cancer Research marking the 50th anniversary of the Surgeon General’s Report on Smoking and Health. "In any other circumstance, without this long, strange history, we’d feel there would be a lot more engagement by the scientific community, by clinicians, and by organizations in marshalling every effort to address the problem."
One recurring problem, he said, is the high use of tobacco products by subpopulations of Americans, especially those with low levels of income and education. "This is a challenge, because if you are a college-educated, wealthy individual living in the state of California, you may assume and believe that the tobacco problem is largely one of the past," he said. "But for many of us, we clearly see that across the United States, there are populations where this is still an overwhelming problem that individuals face in their families."
At the same time, Dr. Croyle said, "a rapid uptake" of tobacco use is taking place in many countries around the world. "We have some alarming concerns about the trajectory of tobacco use and the burden of cancer globally," he said. "That’s led us to support more research on global tobacco control and for the NCI to collaborate with international organizations."
In the United States, the National Institutes of Health formed a unique partnership aimed at bringing together expertise from scientists from a variety of disciplines to address the use of tobacco and other addictive substances. According to its website, the goal of Collaborative Research on Addiction at NIH is "to provide a strong collaborative framework to enable the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NCI to integrate resources and expertise to advance substance use, abuse, and addiction research and public health outcomes."
The time is right for such a partnership, Dr. Croyle said, because many current smokers also use alcohol and other drugs. "Unfortunately, even though scientists for many years have been calling for more research on these comorbid risk factors, the research lags behind what we need to inform clinical practice, so we’re paying special attention to issues of mental health," he said. "For example, tobacco use is incredibly high among those with schizophrenia. We’re also concerned about comorbid [tobacco] use and alcohol use, for example, among college students, where we see huge binge drinking problems that co-occur with tobacco use. We also have a concern about the introduction of electronic cigarettes and the fact that we desperately need a lot more evidence in that domain."
Mr. Croyle had no relevant financial conflicts to disclose.
SAN DIEGO – Though progress has been made in reducing the burden of disease, disability, and death caused by tobacco use since the 1964 Surgeon General’s Report on Smoking and Health debuted, much work remains to be done, according to Robert T. Croyle, Ph.D.
According to data from the Department of Health & Human Services, more than 42 million adults and more than 3.5 million middle and high school students continue to smoke tobacco. "We’re in a bizarre situation where we have a legal product on the market, which is responsible for about a half a million deaths," Dr. Croyle, director of division of cancer control and population sciences at the National Cancer Institute (NCI), said during a press briefing at the annual meeting of the American Association for Cancer Research marking the 50th anniversary of the Surgeon General’s Report on Smoking and Health. "In any other circumstance, without this long, strange history, we’d feel there would be a lot more engagement by the scientific community, by clinicians, and by organizations in marshalling every effort to address the problem."
One recurring problem, he said, is the high use of tobacco products by subpopulations of Americans, especially those with low levels of income and education. "This is a challenge, because if you are a college-educated, wealthy individual living in the state of California, you may assume and believe that the tobacco problem is largely one of the past," he said. "But for many of us, we clearly see that across the United States, there are populations where this is still an overwhelming problem that individuals face in their families."
At the same time, Dr. Croyle said, "a rapid uptake" of tobacco use is taking place in many countries around the world. "We have some alarming concerns about the trajectory of tobacco use and the burden of cancer globally," he said. "That’s led us to support more research on global tobacco control and for the NCI to collaborate with international organizations."
In the United States, the National Institutes of Health formed a unique partnership aimed at bringing together expertise from scientists from a variety of disciplines to address the use of tobacco and other addictive substances. According to its website, the goal of Collaborative Research on Addiction at NIH is "to provide a strong collaborative framework to enable the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NCI to integrate resources and expertise to advance substance use, abuse, and addiction research and public health outcomes."
The time is right for such a partnership, Dr. Croyle said, because many current smokers also use alcohol and other drugs. "Unfortunately, even though scientists for many years have been calling for more research on these comorbid risk factors, the research lags behind what we need to inform clinical practice, so we’re paying special attention to issues of mental health," he said. "For example, tobacco use is incredibly high among those with schizophrenia. We’re also concerned about comorbid [tobacco] use and alcohol use, for example, among college students, where we see huge binge drinking problems that co-occur with tobacco use. We also have a concern about the introduction of electronic cigarettes and the fact that we desperately need a lot more evidence in that domain."
Mr. Croyle had no relevant financial conflicts to disclose.
EXPERT ANALYSIS FROM THE AACR ANNUAL MEETING
Pan-HER inhibitor ‘graduates’ from I-SPY 2 trial
SAN DIEGO – Efficacy results from an adaptive trial demonstrated that the investigational pan-HER inhibitor neratinib in combination with standard chemotherapy benefited patients with newly diagnosed hormone receptor–negative, HER2-positive primary breast cancer.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. John W. Park said that neratinib, an investigational agent being developed by Los Angeles–based Puma Biotechnology, "graduated" in the HR-negative/HER-positive signature, with a 79% probability of success in a phase III study of neratinib plus paclitaxel vs. trastuzumab plus paclitaxel. In addition, the Bayesian probability of superiority for the neratinib-containing regimen, compared with standard therapy, is 95% (P = .051).
The findings come from the I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2) trial, a randomized, phase II clinical study of women with newly diagnosed stage II breast cancer with a tumor size of at least 2.5 cm and who are considered to be at high risk for recurrence via MammaPrint test.
I-SPY 2 is designed to investigate whether adding investigational drugs such as neratinib to standard chemotherapy is better than standard therapy alone, said Dr. Park, one of the study investigators who is professor of medicine at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center.
The study’s primary endpoint is pathological complete response (pCR) in the breast and in the lymph nodes at the time of surgery. The trial employs an adaptive design based on Bayesian predictive probability that a regimen will be shown to be statistically superior to standard therapy in an equally randomized 300-patient confirmatory trial. "If at any point in the trial this endpoint is not met, the trial continues to learn from the responses obtained to date, and the randomization is revised to reflect the results that have been obtained thus far," Dr. Park explained. "The trial continues to accrue patients in this weighted randomization fashion until graduation or futility is the result."
Dr. Park presented findings from 115 patients (median age, 51 years) who were assigned to receive paclitaxel plus neratinib (followed by doxorubicin and cyclophosphamide). The rates of pCR in the neratinib arm were compared with those of 78 patients (median age, 48 years) who were concurrently randomized to the control arm containing standard chemotherapy, which consisted of paclitaxel plus trastuzumab (followed by doxorubicin and cyclophosphamide). The researchers also compared 10 biomarker signatures prospectively defined by categories of HR, HER2, and MammaPrint.
Among patients with HR-negative, HER2-positive breast cancer (one of the 10 biomarker signatures), the estimated pCR rate was 56% for the neratinib-containing regimen, compared with 33% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 79%.
Among the 65 patients in the neratinib-containing regimen who were HER2-positive (a separate biomarker signature), the estimated pCR rate was 39% for the neratinib-containing regimen, compared with 23% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 73%.
Neratinib was "largely well tolerated," Dr. Park said, but 39% of patients in the neratinib arm experienced grade 3 or 4 diarrhea, compared with 4% of patients in the control arm. "There was not a particular cardiac safety event that was frequent in either arm, and there are no congestive heart failure events noted to date," he said.
After noticing high rates of diarrhea symptoms, the trial investigators modified the diarrhea supportive care guideline "to include vigorous patient monitoring and early institution of supportive care, which appeared to make a difference in terms of the frequency of diarrhea and amelioration of that side effect," Dr. Park said. "Further to that, there was also the institution of a prophylactic loperamide regimen, which was also instituted later in the trial."
There was no apparent difference in the time to surgery from initiation of treatment in the control versus the neratinib-treated groups (a median of 168 days for both). "Early discontinuation was somewhat more frequently observed with neratinib, primarily due to toxicity, whereas early discontinuation due to progression was observed more frequently in the control group," Dr. Park said.
He concluded his remarks by noting that I-SPY 2 "is a biomarker-rich trial. Additional response predictors and biomarker developments are under investigation, and we anticipate they will be reported in a subsequent forum. Based on these results, neratinib is under consideration for phase III testing in the neoadjuvant population."
In an interview, Dr. Thomas Lynch, director of the Yale Cancer Center in New Haven, Conn., and a member of the American Association for Cancer Research’s annual meeting program committee, praised the novel design of the I-SPY 2 trial, "the fact that it allows rapid evaluation of both biomarkers and new drugs and the interaction between biomarkers and new drugs.
"It’s not a definitive [trial] design; it’s not going to lead to drug approval in and of itself. But it does have a lot of power to sort out which biomarkers and which agents are the most important to combine and look at in putting together treatments for patients," Dr. Lynch said.
He went on to describe the findings regarding neratinib as "intriguing, but I have to see a bigger study to have a sense of magnitude of benefit. What the interaction of the biomarker is, is hard to know."
The study is sponsored by the QuantumLeap Healthcare Collaborative, a 501(3) charitable foundation. Dr. Park said that he had no relevant financial conflicts to disclose. Dr. Lynch disclosed that he is on the board of directors of Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board of Arvinas and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
SAN DIEGO – Efficacy results from an adaptive trial demonstrated that the investigational pan-HER inhibitor neratinib in combination with standard chemotherapy benefited patients with newly diagnosed hormone receptor–negative, HER2-positive primary breast cancer.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. John W. Park said that neratinib, an investigational agent being developed by Los Angeles–based Puma Biotechnology, "graduated" in the HR-negative/HER-positive signature, with a 79% probability of success in a phase III study of neratinib plus paclitaxel vs. trastuzumab plus paclitaxel. In addition, the Bayesian probability of superiority for the neratinib-containing regimen, compared with standard therapy, is 95% (P = .051).
The findings come from the I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2) trial, a randomized, phase II clinical study of women with newly diagnosed stage II breast cancer with a tumor size of at least 2.5 cm and who are considered to be at high risk for recurrence via MammaPrint test.
I-SPY 2 is designed to investigate whether adding investigational drugs such as neratinib to standard chemotherapy is better than standard therapy alone, said Dr. Park, one of the study investigators who is professor of medicine at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center.
The study’s primary endpoint is pathological complete response (pCR) in the breast and in the lymph nodes at the time of surgery. The trial employs an adaptive design based on Bayesian predictive probability that a regimen will be shown to be statistically superior to standard therapy in an equally randomized 300-patient confirmatory trial. "If at any point in the trial this endpoint is not met, the trial continues to learn from the responses obtained to date, and the randomization is revised to reflect the results that have been obtained thus far," Dr. Park explained. "The trial continues to accrue patients in this weighted randomization fashion until graduation or futility is the result."
Dr. Park presented findings from 115 patients (median age, 51 years) who were assigned to receive paclitaxel plus neratinib (followed by doxorubicin and cyclophosphamide). The rates of pCR in the neratinib arm were compared with those of 78 patients (median age, 48 years) who were concurrently randomized to the control arm containing standard chemotherapy, which consisted of paclitaxel plus trastuzumab (followed by doxorubicin and cyclophosphamide). The researchers also compared 10 biomarker signatures prospectively defined by categories of HR, HER2, and MammaPrint.
Among patients with HR-negative, HER2-positive breast cancer (one of the 10 biomarker signatures), the estimated pCR rate was 56% for the neratinib-containing regimen, compared with 33% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 79%.
Among the 65 patients in the neratinib-containing regimen who were HER2-positive (a separate biomarker signature), the estimated pCR rate was 39% for the neratinib-containing regimen, compared with 23% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 73%.
Neratinib was "largely well tolerated," Dr. Park said, but 39% of patients in the neratinib arm experienced grade 3 or 4 diarrhea, compared with 4% of patients in the control arm. "There was not a particular cardiac safety event that was frequent in either arm, and there are no congestive heart failure events noted to date," he said.
After noticing high rates of diarrhea symptoms, the trial investigators modified the diarrhea supportive care guideline "to include vigorous patient monitoring and early institution of supportive care, which appeared to make a difference in terms of the frequency of diarrhea and amelioration of that side effect," Dr. Park said. "Further to that, there was also the institution of a prophylactic loperamide regimen, which was also instituted later in the trial."
There was no apparent difference in the time to surgery from initiation of treatment in the control versus the neratinib-treated groups (a median of 168 days for both). "Early discontinuation was somewhat more frequently observed with neratinib, primarily due to toxicity, whereas early discontinuation due to progression was observed more frequently in the control group," Dr. Park said.
He concluded his remarks by noting that I-SPY 2 "is a biomarker-rich trial. Additional response predictors and biomarker developments are under investigation, and we anticipate they will be reported in a subsequent forum. Based on these results, neratinib is under consideration for phase III testing in the neoadjuvant population."
In an interview, Dr. Thomas Lynch, director of the Yale Cancer Center in New Haven, Conn., and a member of the American Association for Cancer Research’s annual meeting program committee, praised the novel design of the I-SPY 2 trial, "the fact that it allows rapid evaluation of both biomarkers and new drugs and the interaction between biomarkers and new drugs.
"It’s not a definitive [trial] design; it’s not going to lead to drug approval in and of itself. But it does have a lot of power to sort out which biomarkers and which agents are the most important to combine and look at in putting together treatments for patients," Dr. Lynch said.
He went on to describe the findings regarding neratinib as "intriguing, but I have to see a bigger study to have a sense of magnitude of benefit. What the interaction of the biomarker is, is hard to know."
The study is sponsored by the QuantumLeap Healthcare Collaborative, a 501(3) charitable foundation. Dr. Park said that he had no relevant financial conflicts to disclose. Dr. Lynch disclosed that he is on the board of directors of Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board of Arvinas and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
SAN DIEGO – Efficacy results from an adaptive trial demonstrated that the investigational pan-HER inhibitor neratinib in combination with standard chemotherapy benefited patients with newly diagnosed hormone receptor–negative, HER2-positive primary breast cancer.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. John W. Park said that neratinib, an investigational agent being developed by Los Angeles–based Puma Biotechnology, "graduated" in the HR-negative/HER-positive signature, with a 79% probability of success in a phase III study of neratinib plus paclitaxel vs. trastuzumab plus paclitaxel. In addition, the Bayesian probability of superiority for the neratinib-containing regimen, compared with standard therapy, is 95% (P = .051).
The findings come from the I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging and Molecular Analysis 2) trial, a randomized, phase II clinical study of women with newly diagnosed stage II breast cancer with a tumor size of at least 2.5 cm and who are considered to be at high risk for recurrence via MammaPrint test.
I-SPY 2 is designed to investigate whether adding investigational drugs such as neratinib to standard chemotherapy is better than standard therapy alone, said Dr. Park, one of the study investigators who is professor of medicine at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center.
The study’s primary endpoint is pathological complete response (pCR) in the breast and in the lymph nodes at the time of surgery. The trial employs an adaptive design based on Bayesian predictive probability that a regimen will be shown to be statistically superior to standard therapy in an equally randomized 300-patient confirmatory trial. "If at any point in the trial this endpoint is not met, the trial continues to learn from the responses obtained to date, and the randomization is revised to reflect the results that have been obtained thus far," Dr. Park explained. "The trial continues to accrue patients in this weighted randomization fashion until graduation or futility is the result."
Dr. Park presented findings from 115 patients (median age, 51 years) who were assigned to receive paclitaxel plus neratinib (followed by doxorubicin and cyclophosphamide). The rates of pCR in the neratinib arm were compared with those of 78 patients (median age, 48 years) who were concurrently randomized to the control arm containing standard chemotherapy, which consisted of paclitaxel plus trastuzumab (followed by doxorubicin and cyclophosphamide). The researchers also compared 10 biomarker signatures prospectively defined by categories of HR, HER2, and MammaPrint.
Among patients with HR-negative, HER2-positive breast cancer (one of the 10 biomarker signatures), the estimated pCR rate was 56% for the neratinib-containing regimen, compared with 33% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 79%.
Among the 65 patients in the neratinib-containing regimen who were HER2-positive (a separate biomarker signature), the estimated pCR rate was 39% for the neratinib-containing regimen, compared with 23% for standard chemotherapy. The Bayesian probability of superiority for the neratinib-containing regimen, compared with standard chemotherapy, is 95%, while the Bayesian probability of success in a phase III trial, compared with standard chemotherapy, is 73%.
Neratinib was "largely well tolerated," Dr. Park said, but 39% of patients in the neratinib arm experienced grade 3 or 4 diarrhea, compared with 4% of patients in the control arm. "There was not a particular cardiac safety event that was frequent in either arm, and there are no congestive heart failure events noted to date," he said.
After noticing high rates of diarrhea symptoms, the trial investigators modified the diarrhea supportive care guideline "to include vigorous patient monitoring and early institution of supportive care, which appeared to make a difference in terms of the frequency of diarrhea and amelioration of that side effect," Dr. Park said. "Further to that, there was also the institution of a prophylactic loperamide regimen, which was also instituted later in the trial."
There was no apparent difference in the time to surgery from initiation of treatment in the control versus the neratinib-treated groups (a median of 168 days for both). "Early discontinuation was somewhat more frequently observed with neratinib, primarily due to toxicity, whereas early discontinuation due to progression was observed more frequently in the control group," Dr. Park said.
He concluded his remarks by noting that I-SPY 2 "is a biomarker-rich trial. Additional response predictors and biomarker developments are under investigation, and we anticipate they will be reported in a subsequent forum. Based on these results, neratinib is under consideration for phase III testing in the neoadjuvant population."
In an interview, Dr. Thomas Lynch, director of the Yale Cancer Center in New Haven, Conn., and a member of the American Association for Cancer Research’s annual meeting program committee, praised the novel design of the I-SPY 2 trial, "the fact that it allows rapid evaluation of both biomarkers and new drugs and the interaction between biomarkers and new drugs.
"It’s not a definitive [trial] design; it’s not going to lead to drug approval in and of itself. But it does have a lot of power to sort out which biomarkers and which agents are the most important to combine and look at in putting together treatments for patients," Dr. Lynch said.
He went on to describe the findings regarding neratinib as "intriguing, but I have to see a bigger study to have a sense of magnitude of benefit. What the interaction of the biomarker is, is hard to know."
The study is sponsored by the QuantumLeap Healthcare Collaborative, a 501(3) charitable foundation. Dr. Park said that he had no relevant financial conflicts to disclose. Dr. Lynch disclosed that he is on the board of directors of Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board of Arvinas and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
AT THE AACR ANNUAL MEETING
Major finding: Among patients with HR-negative, HER2-positive breast cancer, the estimated pathological complete response was 56% for the neratinib-containing regimen, compared with 33% for standard chemotherapy.
Data source: Efficacy data from 115 patients with newly diagnosed stage II or higher breast cancer who were assigned to receive neratinib plus paclitaxel (followed by doxorubicin and cyclophosphamide) and 78 patients who were assigned to receive paclitaxel plus trastuzumab (followed by doxorubicin and cyclophosphamide).
Disclosures: The study is sponsored by the QuantumLeap Healthcare Collaborative, a 501(3) charitable foundation. Dr. Park said that he had no relevant financial conflicts to disclose. Dr. Lynch disclosed that he is on the board of directors of Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board of Arvinas and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
Leukemic breast tumors may cause resistance in AML, ALL
SAN DIEGO—One woman’s curiosity and self-described “aggressive” approach to research have led to some unexpected discoveries about acute leukemias.
Isabel Cunningham, MD, of Columbia University in New York, has found evidence to suggest that treatment resistance in leukemia patients may sometimes result from an interaction between leukemic cells and the breast.
She discovered that leukemic cells in extramedullary niches can adopt a tumor phenotype similar to breast cancer.
And many genes are similarly upregulated in leukemic and epithelial breast tumors.
Her research indicates that a new approach to resistant leukemias that incorporates the principles of solid-tumor treatment—scans to identify any tumors and surgery to remove them—could decrease marrow relapse and death.
Dr Cunningham and her colleagues presented these findings in a poster at the AACR Annual Meeting 2014 (abstract 3996*).
“Chemotherapy resistance is our main problem in treating leukemia,” Dr Cunningham said. “It’s been known for a long time that, occasionally, leukemia forms tumors in an organ, but there’s never been a unified approach to treatment, except for leukemia that occurs in the testis and the meninges.”
Dr Cunningham had encountered many patients with resistant leukemia throughout her career, but her research actually began with a patient she had never met. A case study of a leukemia patient with a breast tumor sparked Dr Cunningham’s interest, and she emailed the study’s author to find out what ultimately became of the patient.
The response she received peaked her curiosity further. So she began seeking more of these cases, contacting authors, and collecting information on this phenomenon.
“I took this on as sort of a hobby,” Dr Cunningham said. “I never had any idea where this was going to lead.”
Eventually, she had amassed information on 235 cases—163 patients with acute myeloid leukemia (AML) and 72 with acute lymphoblastic leukemia (ALL)—who ranged from 1 year to 75 years of age. And an analysis of these cases led to some surprising discoveries.
Clinical findings
Dr Cunningham found these leukemic breast tumors can occur before, during, or after marrow leukemia. And, clinically, they resemble breast cancer. Most tumors were palpable, and some were detected only on routine mammograms.
There were single or multiple nodules that may have involved the entire breast. Sixty percent of cases were unilateral on presentation, but, often, the other breast became involved. Seventy percent of cases exhibited axillary lymphadenopathy that was ipsilateral.
Most tumors grew rapidly, to as large as 12 cm. The tumor behavior was similar in AML and ALL. And the tumors had a metastatic pattern similar to lobular breast cancer—spreading to the contralateral breast, the abdomen or pelvis, the meninges, and culminating in death.
However, some patients did survive. Four percent of patients who were treated only with chemotherapy were alive at 4 years. Twenty-five percent of patients had their tumors excised prior to chemotherapy and were alive anywhere from 3 years to more than 26 years after treatment.
Histology and gene expression
To build upon these findings, Dr Cunningham set her sights on patient samples. She was able to obtain paraffin blocks of leukemic breast tumors from 25 patients and perform immunohistochemical staining.
“It became clear that the leukemic tumors—which are marked by leukemic markers and not breast cancer markers—look, histologically, like breast cancer, specifically, lobular breast cancer,” Dr Cunningham said. “An additional pathologic finding was a specific type of desmoplastic fibrosis seen in all 25 contributed biopsies.”
Dr Cunningham also performed gene expression studies on 3 of the tumors (2 ALL and 1 AML), which were collected 8 months to 22 months after diagnosis, while marrows were in remission. The analyses revealed that a number of genes are significantly upregulated in both leukemic breast tumors and breast cancer.
These include genes involved in adhesion and interactions with the extracellular matrix (ADAM8, COMP, and CDH22), genes involved in the ubiquitin-proteasome pathway (UBE2S, USP32, MDM2, and UBE2C), genes encoding for kinases (MAP4K1, PIM1, and NEK2), and genes involved in RAS signaling (RANBP1 and RAB10).
Conclusions and next steps
“It seems that there’s some kind of crosstalk between the organ microenvironment and leukemic cells that make the leukemic cells have the phenotype of breast cancer,” Dr Cunningham said. “And it may well be that relapse sometimes results from the presence of an undiagnosed collection of these cells.”
Therefore, Dr Cunningham suggests performing scans in treatment-resistant leukemia patients. If a patient relapses, and particularly if lactic dehydrogenase levels are increased, a scan might be in order.
“If we can recognize these tumors and cut them out, the patient could be cured, because we’re successful at treating the bone marrow,” Dr Cunningham said. “We’ve had very good bone marrow drugs for 50 years.”
For her part, Dr Cunningham is delving further into this phenomenon. She is now conducting gene expression studies on the rest of the 25 leukemic breast tumor samples and comparing these tumors to breast cancer to identify the most significant dysregulated genes in both entities. The long-term goal is to find a way to predict which patients will develop leukemic breast tumors. ![]()
*Information in the abstract differs from that presented at the meeting.
SAN DIEGO—One woman’s curiosity and self-described “aggressive” approach to research have led to some unexpected discoveries about acute leukemias.
Isabel Cunningham, MD, of Columbia University in New York, has found evidence to suggest that treatment resistance in leukemia patients may sometimes result from an interaction between leukemic cells and the breast.
She discovered that leukemic cells in extramedullary niches can adopt a tumor phenotype similar to breast cancer.
And many genes are similarly upregulated in leukemic and epithelial breast tumors.
Her research indicates that a new approach to resistant leukemias that incorporates the principles of solid-tumor treatment—scans to identify any tumors and surgery to remove them—could decrease marrow relapse and death.
Dr Cunningham and her colleagues presented these findings in a poster at the AACR Annual Meeting 2014 (abstract 3996*).
“Chemotherapy resistance is our main problem in treating leukemia,” Dr Cunningham said. “It’s been known for a long time that, occasionally, leukemia forms tumors in an organ, but there’s never been a unified approach to treatment, except for leukemia that occurs in the testis and the meninges.”
Dr Cunningham had encountered many patients with resistant leukemia throughout her career, but her research actually began with a patient she had never met. A case study of a leukemia patient with a breast tumor sparked Dr Cunningham’s interest, and she emailed the study’s author to find out what ultimately became of the patient.
The response she received peaked her curiosity further. So she began seeking more of these cases, contacting authors, and collecting information on this phenomenon.
“I took this on as sort of a hobby,” Dr Cunningham said. “I never had any idea where this was going to lead.”
Eventually, she had amassed information on 235 cases—163 patients with acute myeloid leukemia (AML) and 72 with acute lymphoblastic leukemia (ALL)—who ranged from 1 year to 75 years of age. And an analysis of these cases led to some surprising discoveries.
Clinical findings
Dr Cunningham found these leukemic breast tumors can occur before, during, or after marrow leukemia. And, clinically, they resemble breast cancer. Most tumors were palpable, and some were detected only on routine mammograms.
There were single or multiple nodules that may have involved the entire breast. Sixty percent of cases were unilateral on presentation, but, often, the other breast became involved. Seventy percent of cases exhibited axillary lymphadenopathy that was ipsilateral.
Most tumors grew rapidly, to as large as 12 cm. The tumor behavior was similar in AML and ALL. And the tumors had a metastatic pattern similar to lobular breast cancer—spreading to the contralateral breast, the abdomen or pelvis, the meninges, and culminating in death.
However, some patients did survive. Four percent of patients who were treated only with chemotherapy were alive at 4 years. Twenty-five percent of patients had their tumors excised prior to chemotherapy and were alive anywhere from 3 years to more than 26 years after treatment.
Histology and gene expression
To build upon these findings, Dr Cunningham set her sights on patient samples. She was able to obtain paraffin blocks of leukemic breast tumors from 25 patients and perform immunohistochemical staining.
“It became clear that the leukemic tumors—which are marked by leukemic markers and not breast cancer markers—look, histologically, like breast cancer, specifically, lobular breast cancer,” Dr Cunningham said. “An additional pathologic finding was a specific type of desmoplastic fibrosis seen in all 25 contributed biopsies.”
Dr Cunningham also performed gene expression studies on 3 of the tumors (2 ALL and 1 AML), which were collected 8 months to 22 months after diagnosis, while marrows were in remission. The analyses revealed that a number of genes are significantly upregulated in both leukemic breast tumors and breast cancer.
These include genes involved in adhesion and interactions with the extracellular matrix (ADAM8, COMP, and CDH22), genes involved in the ubiquitin-proteasome pathway (UBE2S, USP32, MDM2, and UBE2C), genes encoding for kinases (MAP4K1, PIM1, and NEK2), and genes involved in RAS signaling (RANBP1 and RAB10).
Conclusions and next steps
“It seems that there’s some kind of crosstalk between the organ microenvironment and leukemic cells that make the leukemic cells have the phenotype of breast cancer,” Dr Cunningham said. “And it may well be that relapse sometimes results from the presence of an undiagnosed collection of these cells.”
Therefore, Dr Cunningham suggests performing scans in treatment-resistant leukemia patients. If a patient relapses, and particularly if lactic dehydrogenase levels are increased, a scan might be in order.
“If we can recognize these tumors and cut them out, the patient could be cured, because we’re successful at treating the bone marrow,” Dr Cunningham said. “We’ve had very good bone marrow drugs for 50 years.”
For her part, Dr Cunningham is delving further into this phenomenon. She is now conducting gene expression studies on the rest of the 25 leukemic breast tumor samples and comparing these tumors to breast cancer to identify the most significant dysregulated genes in both entities. The long-term goal is to find a way to predict which patients will develop leukemic breast tumors. ![]()
*Information in the abstract differs from that presented at the meeting.
SAN DIEGO—One woman’s curiosity and self-described “aggressive” approach to research have led to some unexpected discoveries about acute leukemias.
Isabel Cunningham, MD, of Columbia University in New York, has found evidence to suggest that treatment resistance in leukemia patients may sometimes result from an interaction between leukemic cells and the breast.
She discovered that leukemic cells in extramedullary niches can adopt a tumor phenotype similar to breast cancer.
And many genes are similarly upregulated in leukemic and epithelial breast tumors.
Her research indicates that a new approach to resistant leukemias that incorporates the principles of solid-tumor treatment—scans to identify any tumors and surgery to remove them—could decrease marrow relapse and death.
Dr Cunningham and her colleagues presented these findings in a poster at the AACR Annual Meeting 2014 (abstract 3996*).
“Chemotherapy resistance is our main problem in treating leukemia,” Dr Cunningham said. “It’s been known for a long time that, occasionally, leukemia forms tumors in an organ, but there’s never been a unified approach to treatment, except for leukemia that occurs in the testis and the meninges.”
Dr Cunningham had encountered many patients with resistant leukemia throughout her career, but her research actually began with a patient she had never met. A case study of a leukemia patient with a breast tumor sparked Dr Cunningham’s interest, and she emailed the study’s author to find out what ultimately became of the patient.
The response she received peaked her curiosity further. So she began seeking more of these cases, contacting authors, and collecting information on this phenomenon.
“I took this on as sort of a hobby,” Dr Cunningham said. “I never had any idea where this was going to lead.”
Eventually, she had amassed information on 235 cases—163 patients with acute myeloid leukemia (AML) and 72 with acute lymphoblastic leukemia (ALL)—who ranged from 1 year to 75 years of age. And an analysis of these cases led to some surprising discoveries.
Clinical findings
Dr Cunningham found these leukemic breast tumors can occur before, during, or after marrow leukemia. And, clinically, they resemble breast cancer. Most tumors were palpable, and some were detected only on routine mammograms.
There were single or multiple nodules that may have involved the entire breast. Sixty percent of cases were unilateral on presentation, but, often, the other breast became involved. Seventy percent of cases exhibited axillary lymphadenopathy that was ipsilateral.
Most tumors grew rapidly, to as large as 12 cm. The tumor behavior was similar in AML and ALL. And the tumors had a metastatic pattern similar to lobular breast cancer—spreading to the contralateral breast, the abdomen or pelvis, the meninges, and culminating in death.
However, some patients did survive. Four percent of patients who were treated only with chemotherapy were alive at 4 years. Twenty-five percent of patients had their tumors excised prior to chemotherapy and were alive anywhere from 3 years to more than 26 years after treatment.
Histology and gene expression
To build upon these findings, Dr Cunningham set her sights on patient samples. She was able to obtain paraffin blocks of leukemic breast tumors from 25 patients and perform immunohistochemical staining.
“It became clear that the leukemic tumors—which are marked by leukemic markers and not breast cancer markers—look, histologically, like breast cancer, specifically, lobular breast cancer,” Dr Cunningham said. “An additional pathologic finding was a specific type of desmoplastic fibrosis seen in all 25 contributed biopsies.”
Dr Cunningham also performed gene expression studies on 3 of the tumors (2 ALL and 1 AML), which were collected 8 months to 22 months after diagnosis, while marrows were in remission. The analyses revealed that a number of genes are significantly upregulated in both leukemic breast tumors and breast cancer.
These include genes involved in adhesion and interactions with the extracellular matrix (ADAM8, COMP, and CDH22), genes involved in the ubiquitin-proteasome pathway (UBE2S, USP32, MDM2, and UBE2C), genes encoding for kinases (MAP4K1, PIM1, and NEK2), and genes involved in RAS signaling (RANBP1 and RAB10).
Conclusions and next steps
“It seems that there’s some kind of crosstalk between the organ microenvironment and leukemic cells that make the leukemic cells have the phenotype of breast cancer,” Dr Cunningham said. “And it may well be that relapse sometimes results from the presence of an undiagnosed collection of these cells.”
Therefore, Dr Cunningham suggests performing scans in treatment-resistant leukemia patients. If a patient relapses, and particularly if lactic dehydrogenase levels are increased, a scan might be in order.
“If we can recognize these tumors and cut them out, the patient could be cured, because we’re successful at treating the bone marrow,” Dr Cunningham said. “We’ve had very good bone marrow drugs for 50 years.”
For her part, Dr Cunningham is delving further into this phenomenon. She is now conducting gene expression studies on the rest of the 25 leukemic breast tumor samples and comparing these tumors to breast cancer to identify the most significant dysregulated genes in both entities. The long-term goal is to find a way to predict which patients will develop leukemic breast tumors. ![]()
*Information in the abstract differs from that presented at the meeting.
Team identifies potential treatment for FLT3-ITD AML

Credit: Eric Smith
Researchers have presented evidence to support the use of a BET protein antagonist in FLT3-ITD-mutated acute myeloid leukemia (AML).
The group’s experiments showed the antagonist, JQ1, was active against FLT3-ITD-expressing AML cells in vitro and in vivo.
The agent also demonstrated synergy with the tyrosine kinase inhibitor (TKI) AC220 and the histone deacetylase (HDAC) inhibitor panobinostat.
In fact, JQ1 and panobinostat in combination induced apoptosis in a TKI-resistant cell line.
Melissa Rodriguez, MD, PhD, of the Houston Methodist Research Institute in Texas, and her colleagues presented these findings at the AACR Annual Meeting 2014 as abstract 1721.
The BET protein family members, including BRD4, bind to acetylated lysines on histone proteins, help assemble transcriptional regulators at the target gene promoters and enhancers, and regulate the expression of oncogenes such as MYC and BCL-2.
JQ1 interferes with BRD4 binding to acetylated lysines on histone proteins, resulting in the displacement of the BET proteins. This, in turn, disrupts transcription initiation and elongation factors situated on the chromatin, thereby inhibiting expressions of c-MYC and BCL-2 and their target genes. And this leads to growth arrest and the induction of apoptosis in AML cells.
Dr Rodriguez and her colleagues found that JQ1 alone induced apoptosis in cultured mouse lymphoid cells such as Ba/F3/FLT3-ITD but also Ba/F3/FLT3-ITD that expressed the FLT3-TKI-resistant mutations F691L and D835V.
JQ1 also attenuated the expression of c-MYC, BCL2, and CDK6 oncogenes; induced the expression of p21, p27, and BIM; and cleaved PARP levels.
Furthermore, JQ1 dose-dependently induced apoptosis in MOLM13 and MV4-11 cell lines, as well as in primary AML cells that all expressed FLT3-ITD but had not become resistant to TKIs.
In SCID mice that received non-TKI-treated MOLM13 xenografts, JQ1 alone significantly improved survival compared to vehicle controls. And the researchers observed no toxicity in the treated mice.
JQ1 plus AC220 or panobinostat synergistically induced apoptosis in MV4-11 cells, MOLM13 cells, and primary AML cells expressing FLT3-ITD.
In testing MOLM13/TKIR cells, which had a greater than 50-fold resistance to AC220 over the other cell lines tested, the researchers discovered these cells express higher levels of BRD4, c-MYC, and class I HDACs. They were also significantly more sensitive to JQ1-induced apoptosis.
In this AC220-resistant cell line, JQ1 and panobinostat synergistically induced apoptosis. But, as expected, the same effect did not occur when JQ1 was administered with AC220.
The synergistic apoptotic response of panobinostat and JQ1 was associated with the down-regulation of c-MYC and demonstrated JQ1’s ability to overcome AC220-induced TKI resistance in FLT3-ITD-expressing cells.
The researchers said these findings support future in vivo testing of BRD4 antagonists such as JQ1 in combination with TKIs such as AC220 or HDAC inhibitors such as panobinostat against FLT3-TKI-sensitive cell lines. The research also supports using BRD4 antagonists in combination with panobinostat against TKI-resistant, FLT3-ITD-mutated AML. ![]()

Credit: Eric Smith
Researchers have presented evidence to support the use of a BET protein antagonist in FLT3-ITD-mutated acute myeloid leukemia (AML).
The group’s experiments showed the antagonist, JQ1, was active against FLT3-ITD-expressing AML cells in vitro and in vivo.
The agent also demonstrated synergy with the tyrosine kinase inhibitor (TKI) AC220 and the histone deacetylase (HDAC) inhibitor panobinostat.
In fact, JQ1 and panobinostat in combination induced apoptosis in a TKI-resistant cell line.
Melissa Rodriguez, MD, PhD, of the Houston Methodist Research Institute in Texas, and her colleagues presented these findings at the AACR Annual Meeting 2014 as abstract 1721.
The BET protein family members, including BRD4, bind to acetylated lysines on histone proteins, help assemble transcriptional regulators at the target gene promoters and enhancers, and regulate the expression of oncogenes such as MYC and BCL-2.
JQ1 interferes with BRD4 binding to acetylated lysines on histone proteins, resulting in the displacement of the BET proteins. This, in turn, disrupts transcription initiation and elongation factors situated on the chromatin, thereby inhibiting expressions of c-MYC and BCL-2 and their target genes. And this leads to growth arrest and the induction of apoptosis in AML cells.
Dr Rodriguez and her colleagues found that JQ1 alone induced apoptosis in cultured mouse lymphoid cells such as Ba/F3/FLT3-ITD but also Ba/F3/FLT3-ITD that expressed the FLT3-TKI-resistant mutations F691L and D835V.
JQ1 also attenuated the expression of c-MYC, BCL2, and CDK6 oncogenes; induced the expression of p21, p27, and BIM; and cleaved PARP levels.
Furthermore, JQ1 dose-dependently induced apoptosis in MOLM13 and MV4-11 cell lines, as well as in primary AML cells that all expressed FLT3-ITD but had not become resistant to TKIs.
In SCID mice that received non-TKI-treated MOLM13 xenografts, JQ1 alone significantly improved survival compared to vehicle controls. And the researchers observed no toxicity in the treated mice.
JQ1 plus AC220 or panobinostat synergistically induced apoptosis in MV4-11 cells, MOLM13 cells, and primary AML cells expressing FLT3-ITD.
In testing MOLM13/TKIR cells, which had a greater than 50-fold resistance to AC220 over the other cell lines tested, the researchers discovered these cells express higher levels of BRD4, c-MYC, and class I HDACs. They were also significantly more sensitive to JQ1-induced apoptosis.
In this AC220-resistant cell line, JQ1 and panobinostat synergistically induced apoptosis. But, as expected, the same effect did not occur when JQ1 was administered with AC220.
The synergistic apoptotic response of panobinostat and JQ1 was associated with the down-regulation of c-MYC and demonstrated JQ1’s ability to overcome AC220-induced TKI resistance in FLT3-ITD-expressing cells.
The researchers said these findings support future in vivo testing of BRD4 antagonists such as JQ1 in combination with TKIs such as AC220 or HDAC inhibitors such as panobinostat against FLT3-TKI-sensitive cell lines. The research also supports using BRD4 antagonists in combination with panobinostat against TKI-resistant, FLT3-ITD-mutated AML. ![]()

Credit: Eric Smith
Researchers have presented evidence to support the use of a BET protein antagonist in FLT3-ITD-mutated acute myeloid leukemia (AML).
The group’s experiments showed the antagonist, JQ1, was active against FLT3-ITD-expressing AML cells in vitro and in vivo.
The agent also demonstrated synergy with the tyrosine kinase inhibitor (TKI) AC220 and the histone deacetylase (HDAC) inhibitor panobinostat.
In fact, JQ1 and panobinostat in combination induced apoptosis in a TKI-resistant cell line.
Melissa Rodriguez, MD, PhD, of the Houston Methodist Research Institute in Texas, and her colleagues presented these findings at the AACR Annual Meeting 2014 as abstract 1721.
The BET protein family members, including BRD4, bind to acetylated lysines on histone proteins, help assemble transcriptional regulators at the target gene promoters and enhancers, and regulate the expression of oncogenes such as MYC and BCL-2.
JQ1 interferes with BRD4 binding to acetylated lysines on histone proteins, resulting in the displacement of the BET proteins. This, in turn, disrupts transcription initiation and elongation factors situated on the chromatin, thereby inhibiting expressions of c-MYC and BCL-2 and their target genes. And this leads to growth arrest and the induction of apoptosis in AML cells.
Dr Rodriguez and her colleagues found that JQ1 alone induced apoptosis in cultured mouse lymphoid cells such as Ba/F3/FLT3-ITD but also Ba/F3/FLT3-ITD that expressed the FLT3-TKI-resistant mutations F691L and D835V.
JQ1 also attenuated the expression of c-MYC, BCL2, and CDK6 oncogenes; induced the expression of p21, p27, and BIM; and cleaved PARP levels.
Furthermore, JQ1 dose-dependently induced apoptosis in MOLM13 and MV4-11 cell lines, as well as in primary AML cells that all expressed FLT3-ITD but had not become resistant to TKIs.
In SCID mice that received non-TKI-treated MOLM13 xenografts, JQ1 alone significantly improved survival compared to vehicle controls. And the researchers observed no toxicity in the treated mice.
JQ1 plus AC220 or panobinostat synergistically induced apoptosis in MV4-11 cells, MOLM13 cells, and primary AML cells expressing FLT3-ITD.
In testing MOLM13/TKIR cells, which had a greater than 50-fold resistance to AC220 over the other cell lines tested, the researchers discovered these cells express higher levels of BRD4, c-MYC, and class I HDACs. They were also significantly more sensitive to JQ1-induced apoptosis.
In this AC220-resistant cell line, JQ1 and panobinostat synergistically induced apoptosis. But, as expected, the same effect did not occur when JQ1 was administered with AC220.
The synergistic apoptotic response of panobinostat and JQ1 was associated with the down-regulation of c-MYC and demonstrated JQ1’s ability to overcome AC220-induced TKI resistance in FLT3-ITD-expressing cells.
The researchers said these findings support future in vivo testing of BRD4 antagonists such as JQ1 in combination with TKIs such as AC220 or HDAC inhibitors such as panobinostat against FLT3-TKI-sensitive cell lines. The research also supports using BRD4 antagonists in combination with panobinostat against TKI-resistant, FLT3-ITD-mutated AML. ![]()
Two investigational immunotherapies show promise in advanced melanoma
SAN DIEGO – DEDN6526A, a new anti–endothelin B receptor antibody-drug conjugate, demonstrated safety, tolerability, and hints of clinical efficacy against different types of metastatic or unresectable melanoma, results from a phase I trial showed.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. Jeffrey R. Infante said that anti–endothelin B receptor (ETBR), a G-protein–coupled receptor that can activate RAF/MEK signaling, is overexpressed in metastatic melanoma, compared with normal skin. Developed by Genentech using Seattle Genetics antibody-drug conjugate (ADC) technology, DEDN6526A is an ADC with the anti-mitotic agent monomethyl auristatin E (MMAE) linked to the humanized IgG1 anti-ETBR antibody and represents a targeted chemotherapy to melanoma. ETBR "regulates migration and proliferation of melanocyte precursors from the neural crest during embryonic development," explained Dr. Infante, director of the drug development program at Sarah Cannon Research Institute in Nashville, Tenn. "It is associated with malignant transformation of melanocytes and with potentiation of metastatic spread."
A prototype ETBR assay is being developed as a potential comparison diagnostic in melanoma. The assay is used on formalin-fixed, paraffin-embedded melanoma.
In an effort to determine the maximum tolerated dose of DEDN6526A, 28 patients with metastatic or unresectable cutaneous, mucosal, or ocular melanoma received the intravenous agent every 3 weeks. The researchers collected pharmacokinetic samples and assessed tumor tissue for ETBR expression by immunohistochemistry. Clinical activity was evaluated per RECIST v1.1. (Response Evaluation Criteria in Solid Tumors version 1.1).
Dr. Infante reported that more than half of study participants had more than three prior therapies and 70% had two or more prior therapies. Eight of the patients had ocular melanoma and three of them had mucosal melanoma. Dose escalation started at 0.3 mg/kg and patients received a median of six doses of the agent. The maximum tolerated dose was determined to be 2.4 mg/kg, which is currently being tested in the expansion phase of the trial.
The most common adverse event of any grade was fatigue (57%), followed by chills (39%), alopecia (32%), diarrhea (32%), nausea (29%), decreased appetite (25%), headache (25%), and infusion-related reaction (25%). Neutropenia was the most frequent grade 3 or 4 event. "It often did not require a dose reduction, so it was manageable," Dr. Infante said.
Complete radiographic data were available on 24 patients. Among these, clinical benefit was observed in 12 of the 19 patients who were assigned to a dosing regimen of 1.8 g/kg of DEDN6526A or more. Four of the 12 patients achieved complete response (two were cases of cutaneous melanoma and two were cases of mucosal melanoma). "It did not seem to depend on whether they had a BRAF mutation or had prior treatment with ipilimumab," Dr. Infante said. The other eight patients had stable disease for at least 6 months. "If you can stay on drug for 6 months, that’s probably meaningful benefit in an early phase I trial," he commented.
Dr. Thomas Lynch, director of the Yale Cancer Center and physician-in-chief of Smilow Cancer Hospital at Yale University, New Haven, Conn., who moderated the press briefing, noted that while antibody-drug conjugates such as DEDN6526A "look very promising," the biomarker used in the trial is not fully developed yet. "It may well be that with a mature biomarker, it turns out to have good predictive value," he said.
In an unrelated presentation at the meeting, Dr. Mark Middleton presented findings from a phase I trial of IMCgp100, an investigational agent being developed by Immunocore that is comprised of an affinity-enhanced T-cell receptor specific for the HLA-A2 restricted melanoma gp100 peptide fused to an anti-CD3 antibody fragment. "What’s important about this method of targeting is that it’s very different from a lot of immunotherapies that have been reported," said Dr. Middleton, the study’s principal investigator who is professor of experimental cancer medicine at the University of Oxford (England). "Rather than targeting cell surface proteins, this is targeting the peptide HLA complex, and therefore brings in intracellular protein targets. The premise is that high affinity binding of the T-cell receptor portions of the cancer cell then leads to recruitment of T cells, formation of an immune synapse, and the release of lytic granules leading to apoptotic cell death of the cancer cell."
He and his associates conducted a phase I dose-escalation study using a standard 3+3 cohort design in melanoma patients who had disease in a variety of sites, including the lung, liver, lymphatic system, and various soft tissues. All patients received a single, 5 ng/kg dose of IMCgp100 followed by 30 days of observation. After this 30-day observation period patients could go on to have six weekly infusions followed by 4 weeks of rest if they continued to derive clinical benefit. "On establishment of the maximum tolerated dose, we specified that we would conduct an expansion cohort to explore pharmacodynamics, clinical efficacy, and safety in more detail," Dr. Middleton said, noting that a total of eight cohorts were studied. Patients had to have stage 4 or unresectable stage 3 melanoma, had to be HLA-A2 positive and have an Eastern Cooperative Oncology Group Performance Status of 0 or 1.
The researchers administered IMCgp100 as a 4-hour infusion followed by 48 hours of in-patient observation. Over that 48-hour period they conducted extensive safety evaluations and pharmacokinetic and pharmacodynamic analysis, including detailed audiometric and ophthalmic review. "Because we started with such a low dose we said that we would triple the dose in the absence of toxicity, moving to smaller increments according to safety and pharmacokinetic profile," he said.
Dose-limiting toxicities were defined as drug-related toxicities that occurred within 8 days of drug administration. Anything grade 3 or higher would be deemed as a dose-limiting toxicity.
Among the 40 patients in the trial, the mean age was 59 years and 23 were male. The researchers started to see toxicity at a dose of 45 ng/kg, "which would manifest as a rash that would endure for a few hours, perhaps into the next day," Dr. Middleton said. "At a dose of 135 ng/kg this became more widespread, albeit transient, and we therefore slowed the rate of increase between cohorts." One patient in the 405 ng/kg cohort and two patients in the 900 ng/kg cohort developed grade 3 hypotension 10-12 hours after drug administration. This in part led the researchers to declare a nontolerated dose of 900 ng/kg and a maximum tolerated dose of 600 ng/kg.
Among the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg, the most common adverse event was an itchy rash, "which is often widespread, and associated with edema, which can be periorbital and more widespread," Dr. Middleton said. "This is transient; it usually settles down within 48-72 hours and is not as severe with subsequent administration of the drug." One case of hypotension occurred. The patient recovered after intensive supportive care. Evaluation of pharmacokinetics showed evidence of lymphocyte trafficking, neutrophilia, and a rise in C-reactive protein.
Efficacy results from 10 of the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg showed evidence of "significant and durable clinical responses, particularly in two patients who were treated in the dose escalation phase," he said. Those two patients met the RECIST criteria for response.
Dr. Middleton and his associates are continuing study expansion at the current dose level, with mandated tumor biopsies before treatment, and testing a weekly dosing arm. The goal is to identify the optimal dosing regimen for IMCgp100. The trial is expected to be complete in 2015.
The study was funded by Immunocore. Dr. Middleton disclosed that he is a consultant for Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, GSK, Millenium, and Roche.
The study of DEDN6526A was funded by Genentech. Dr. Infante said that he had no relevant financial conflicts to disclose.
Dr. Lynch disclosed that he is on the board of directors for Bristol Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and he receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation–testing patent and receives royalties.
SAN DIEGO – DEDN6526A, a new anti–endothelin B receptor antibody-drug conjugate, demonstrated safety, tolerability, and hints of clinical efficacy against different types of metastatic or unresectable melanoma, results from a phase I trial showed.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. Jeffrey R. Infante said that anti–endothelin B receptor (ETBR), a G-protein–coupled receptor that can activate RAF/MEK signaling, is overexpressed in metastatic melanoma, compared with normal skin. Developed by Genentech using Seattle Genetics antibody-drug conjugate (ADC) technology, DEDN6526A is an ADC with the anti-mitotic agent monomethyl auristatin E (MMAE) linked to the humanized IgG1 anti-ETBR antibody and represents a targeted chemotherapy to melanoma. ETBR "regulates migration and proliferation of melanocyte precursors from the neural crest during embryonic development," explained Dr. Infante, director of the drug development program at Sarah Cannon Research Institute in Nashville, Tenn. "It is associated with malignant transformation of melanocytes and with potentiation of metastatic spread."
A prototype ETBR assay is being developed as a potential comparison diagnostic in melanoma. The assay is used on formalin-fixed, paraffin-embedded melanoma.
In an effort to determine the maximum tolerated dose of DEDN6526A, 28 patients with metastatic or unresectable cutaneous, mucosal, or ocular melanoma received the intravenous agent every 3 weeks. The researchers collected pharmacokinetic samples and assessed tumor tissue for ETBR expression by immunohistochemistry. Clinical activity was evaluated per RECIST v1.1. (Response Evaluation Criteria in Solid Tumors version 1.1).
Dr. Infante reported that more than half of study participants had more than three prior therapies and 70% had two or more prior therapies. Eight of the patients had ocular melanoma and three of them had mucosal melanoma. Dose escalation started at 0.3 mg/kg and patients received a median of six doses of the agent. The maximum tolerated dose was determined to be 2.4 mg/kg, which is currently being tested in the expansion phase of the trial.
The most common adverse event of any grade was fatigue (57%), followed by chills (39%), alopecia (32%), diarrhea (32%), nausea (29%), decreased appetite (25%), headache (25%), and infusion-related reaction (25%). Neutropenia was the most frequent grade 3 or 4 event. "It often did not require a dose reduction, so it was manageable," Dr. Infante said.
Complete radiographic data were available on 24 patients. Among these, clinical benefit was observed in 12 of the 19 patients who were assigned to a dosing regimen of 1.8 g/kg of DEDN6526A or more. Four of the 12 patients achieved complete response (two were cases of cutaneous melanoma and two were cases of mucosal melanoma). "It did not seem to depend on whether they had a BRAF mutation or had prior treatment with ipilimumab," Dr. Infante said. The other eight patients had stable disease for at least 6 months. "If you can stay on drug for 6 months, that’s probably meaningful benefit in an early phase I trial," he commented.
Dr. Thomas Lynch, director of the Yale Cancer Center and physician-in-chief of Smilow Cancer Hospital at Yale University, New Haven, Conn., who moderated the press briefing, noted that while antibody-drug conjugates such as DEDN6526A "look very promising," the biomarker used in the trial is not fully developed yet. "It may well be that with a mature biomarker, it turns out to have good predictive value," he said.
In an unrelated presentation at the meeting, Dr. Mark Middleton presented findings from a phase I trial of IMCgp100, an investigational agent being developed by Immunocore that is comprised of an affinity-enhanced T-cell receptor specific for the HLA-A2 restricted melanoma gp100 peptide fused to an anti-CD3 antibody fragment. "What’s important about this method of targeting is that it’s very different from a lot of immunotherapies that have been reported," said Dr. Middleton, the study’s principal investigator who is professor of experimental cancer medicine at the University of Oxford (England). "Rather than targeting cell surface proteins, this is targeting the peptide HLA complex, and therefore brings in intracellular protein targets. The premise is that high affinity binding of the T-cell receptor portions of the cancer cell then leads to recruitment of T cells, formation of an immune synapse, and the release of lytic granules leading to apoptotic cell death of the cancer cell."
He and his associates conducted a phase I dose-escalation study using a standard 3+3 cohort design in melanoma patients who had disease in a variety of sites, including the lung, liver, lymphatic system, and various soft tissues. All patients received a single, 5 ng/kg dose of IMCgp100 followed by 30 days of observation. After this 30-day observation period patients could go on to have six weekly infusions followed by 4 weeks of rest if they continued to derive clinical benefit. "On establishment of the maximum tolerated dose, we specified that we would conduct an expansion cohort to explore pharmacodynamics, clinical efficacy, and safety in more detail," Dr. Middleton said, noting that a total of eight cohorts were studied. Patients had to have stage 4 or unresectable stage 3 melanoma, had to be HLA-A2 positive and have an Eastern Cooperative Oncology Group Performance Status of 0 or 1.
The researchers administered IMCgp100 as a 4-hour infusion followed by 48 hours of in-patient observation. Over that 48-hour period they conducted extensive safety evaluations and pharmacokinetic and pharmacodynamic analysis, including detailed audiometric and ophthalmic review. "Because we started with such a low dose we said that we would triple the dose in the absence of toxicity, moving to smaller increments according to safety and pharmacokinetic profile," he said.
Dose-limiting toxicities were defined as drug-related toxicities that occurred within 8 days of drug administration. Anything grade 3 or higher would be deemed as a dose-limiting toxicity.
Among the 40 patients in the trial, the mean age was 59 years and 23 were male. The researchers started to see toxicity at a dose of 45 ng/kg, "which would manifest as a rash that would endure for a few hours, perhaps into the next day," Dr. Middleton said. "At a dose of 135 ng/kg this became more widespread, albeit transient, and we therefore slowed the rate of increase between cohorts." One patient in the 405 ng/kg cohort and two patients in the 900 ng/kg cohort developed grade 3 hypotension 10-12 hours after drug administration. This in part led the researchers to declare a nontolerated dose of 900 ng/kg and a maximum tolerated dose of 600 ng/kg.
Among the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg, the most common adverse event was an itchy rash, "which is often widespread, and associated with edema, which can be periorbital and more widespread," Dr. Middleton said. "This is transient; it usually settles down within 48-72 hours and is not as severe with subsequent administration of the drug." One case of hypotension occurred. The patient recovered after intensive supportive care. Evaluation of pharmacokinetics showed evidence of lymphocyte trafficking, neutrophilia, and a rise in C-reactive protein.
Efficacy results from 10 of the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg showed evidence of "significant and durable clinical responses, particularly in two patients who were treated in the dose escalation phase," he said. Those two patients met the RECIST criteria for response.
Dr. Middleton and his associates are continuing study expansion at the current dose level, with mandated tumor biopsies before treatment, and testing a weekly dosing arm. The goal is to identify the optimal dosing regimen for IMCgp100. The trial is expected to be complete in 2015.
The study was funded by Immunocore. Dr. Middleton disclosed that he is a consultant for Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, GSK, Millenium, and Roche.
The study of DEDN6526A was funded by Genentech. Dr. Infante said that he had no relevant financial conflicts to disclose.
Dr. Lynch disclosed that he is on the board of directors for Bristol Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and he receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation–testing patent and receives royalties.
SAN DIEGO – DEDN6526A, a new anti–endothelin B receptor antibody-drug conjugate, demonstrated safety, tolerability, and hints of clinical efficacy against different types of metastatic or unresectable melanoma, results from a phase I trial showed.
During a press briefing at the annual meeting of the American Association for Cancer Research, Dr. Jeffrey R. Infante said that anti–endothelin B receptor (ETBR), a G-protein–coupled receptor that can activate RAF/MEK signaling, is overexpressed in metastatic melanoma, compared with normal skin. Developed by Genentech using Seattle Genetics antibody-drug conjugate (ADC) technology, DEDN6526A is an ADC with the anti-mitotic agent monomethyl auristatin E (MMAE) linked to the humanized IgG1 anti-ETBR antibody and represents a targeted chemotherapy to melanoma. ETBR "regulates migration and proliferation of melanocyte precursors from the neural crest during embryonic development," explained Dr. Infante, director of the drug development program at Sarah Cannon Research Institute in Nashville, Tenn. "It is associated with malignant transformation of melanocytes and with potentiation of metastatic spread."
A prototype ETBR assay is being developed as a potential comparison diagnostic in melanoma. The assay is used on formalin-fixed, paraffin-embedded melanoma.
In an effort to determine the maximum tolerated dose of DEDN6526A, 28 patients with metastatic or unresectable cutaneous, mucosal, or ocular melanoma received the intravenous agent every 3 weeks. The researchers collected pharmacokinetic samples and assessed tumor tissue for ETBR expression by immunohistochemistry. Clinical activity was evaluated per RECIST v1.1. (Response Evaluation Criteria in Solid Tumors version 1.1).
Dr. Infante reported that more than half of study participants had more than three prior therapies and 70% had two or more prior therapies. Eight of the patients had ocular melanoma and three of them had mucosal melanoma. Dose escalation started at 0.3 mg/kg and patients received a median of six doses of the agent. The maximum tolerated dose was determined to be 2.4 mg/kg, which is currently being tested in the expansion phase of the trial.
The most common adverse event of any grade was fatigue (57%), followed by chills (39%), alopecia (32%), diarrhea (32%), nausea (29%), decreased appetite (25%), headache (25%), and infusion-related reaction (25%). Neutropenia was the most frequent grade 3 or 4 event. "It often did not require a dose reduction, so it was manageable," Dr. Infante said.
Complete radiographic data were available on 24 patients. Among these, clinical benefit was observed in 12 of the 19 patients who were assigned to a dosing regimen of 1.8 g/kg of DEDN6526A or more. Four of the 12 patients achieved complete response (two were cases of cutaneous melanoma and two were cases of mucosal melanoma). "It did not seem to depend on whether they had a BRAF mutation or had prior treatment with ipilimumab," Dr. Infante said. The other eight patients had stable disease for at least 6 months. "If you can stay on drug for 6 months, that’s probably meaningful benefit in an early phase I trial," he commented.
Dr. Thomas Lynch, director of the Yale Cancer Center and physician-in-chief of Smilow Cancer Hospital at Yale University, New Haven, Conn., who moderated the press briefing, noted that while antibody-drug conjugates such as DEDN6526A "look very promising," the biomarker used in the trial is not fully developed yet. "It may well be that with a mature biomarker, it turns out to have good predictive value," he said.
In an unrelated presentation at the meeting, Dr. Mark Middleton presented findings from a phase I trial of IMCgp100, an investigational agent being developed by Immunocore that is comprised of an affinity-enhanced T-cell receptor specific for the HLA-A2 restricted melanoma gp100 peptide fused to an anti-CD3 antibody fragment. "What’s important about this method of targeting is that it’s very different from a lot of immunotherapies that have been reported," said Dr. Middleton, the study’s principal investigator who is professor of experimental cancer medicine at the University of Oxford (England). "Rather than targeting cell surface proteins, this is targeting the peptide HLA complex, and therefore brings in intracellular protein targets. The premise is that high affinity binding of the T-cell receptor portions of the cancer cell then leads to recruitment of T cells, formation of an immune synapse, and the release of lytic granules leading to apoptotic cell death of the cancer cell."
He and his associates conducted a phase I dose-escalation study using a standard 3+3 cohort design in melanoma patients who had disease in a variety of sites, including the lung, liver, lymphatic system, and various soft tissues. All patients received a single, 5 ng/kg dose of IMCgp100 followed by 30 days of observation. After this 30-day observation period patients could go on to have six weekly infusions followed by 4 weeks of rest if they continued to derive clinical benefit. "On establishment of the maximum tolerated dose, we specified that we would conduct an expansion cohort to explore pharmacodynamics, clinical efficacy, and safety in more detail," Dr. Middleton said, noting that a total of eight cohorts were studied. Patients had to have stage 4 or unresectable stage 3 melanoma, had to be HLA-A2 positive and have an Eastern Cooperative Oncology Group Performance Status of 0 or 1.
The researchers administered IMCgp100 as a 4-hour infusion followed by 48 hours of in-patient observation. Over that 48-hour period they conducted extensive safety evaluations and pharmacokinetic and pharmacodynamic analysis, including detailed audiometric and ophthalmic review. "Because we started with such a low dose we said that we would triple the dose in the absence of toxicity, moving to smaller increments according to safety and pharmacokinetic profile," he said.
Dose-limiting toxicities were defined as drug-related toxicities that occurred within 8 days of drug administration. Anything grade 3 or higher would be deemed as a dose-limiting toxicity.
Among the 40 patients in the trial, the mean age was 59 years and 23 were male. The researchers started to see toxicity at a dose of 45 ng/kg, "which would manifest as a rash that would endure for a few hours, perhaps into the next day," Dr. Middleton said. "At a dose of 135 ng/kg this became more widespread, albeit transient, and we therefore slowed the rate of increase between cohorts." One patient in the 405 ng/kg cohort and two patients in the 900 ng/kg cohort developed grade 3 hypotension 10-12 hours after drug administration. This in part led the researchers to declare a nontolerated dose of 900 ng/kg and a maximum tolerated dose of 600 ng/kg.
Among the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg, the most common adverse event was an itchy rash, "which is often widespread, and associated with edema, which can be periorbital and more widespread," Dr. Middleton said. "This is transient; it usually settles down within 48-72 hours and is not as severe with subsequent administration of the drug." One case of hypotension occurred. The patient recovered after intensive supportive care. Evaluation of pharmacokinetics showed evidence of lymphocyte trafficking, neutrophilia, and a rise in C-reactive protein.
Efficacy results from 10 of the first 15 patients to be treated with the maximum tolerated dose of 600 ng/kg showed evidence of "significant and durable clinical responses, particularly in two patients who were treated in the dose escalation phase," he said. Those two patients met the RECIST criteria for response.
Dr. Middleton and his associates are continuing study expansion at the current dose level, with mandated tumor biopsies before treatment, and testing a weekly dosing arm. The goal is to identify the optimal dosing regimen for IMCgp100. The trial is expected to be complete in 2015.
The study was funded by Immunocore. Dr. Middleton disclosed that he is a consultant for Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, GSK, Millenium, and Roche.
The study of DEDN6526A was funded by Genentech. Dr. Infante said that he had no relevant financial conflicts to disclose.
Dr. Lynch disclosed that he is on the board of directors for Bristol Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and he receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation–testing patent and receives royalties.
AT THE AACR ANNUAL MEETING
Major finding: Two investigational immunotherapies for advanced melanoma, one an antibody-drug conjugate, the other directing patients’ immune responses toward tumor cell killing, demonstrated safety and signs of clinical efficacy.
Data source: Two phase I studies, one of DEDN6526A, a new anti-endothelin B receptor antibody-drug conjugate, and one of IMCgp100, composed of an affinity-enhanced T-cell receptor specific for the HLA-A2 restricted melanoma gp100 peptide fused to an anti-CD3 antibody fragment.
Disclosures: The first study was funded by Genentech. Dr. Infante said that he had no relevant financial conflicts to disclose. The second study was funded by Immunocore. Dr. Middleton disclosed that he is a consultant for Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, GSK, Millenium and Roche.
Molecule shows preclinical activity in leukemias, lymphomas

SAN DIEGO—A small molecule that has previously proven effective against solid tumors exhibits activity against leukemias and lymphomas, preclinical research suggests.
The molecule, LOR-253, showed antiproliferative activity in a range of leukemia and lymphoma cell lines, induced apoptosis in acute myeloid leukemia (AML) in vitro, and demonstrated synergy with chemotherapeutic agents.
Ronnie Lum, PhD, and colleagues at Lorus Therapeutics, Inc., the Toronto, Canada-based company developing LOR-253, presented these results at the AACR Annual Meeting 2014 (abstract 4544).
LOR-253 acts through induction of the innate tumor suppressor KLF4. Recent research has suggested that upregulation of the transcription factor CDX2 drives the development or progression of leukemic disease. And CDX2 has been shown to silence KLF4, which is reported to be a critical oncogenic event in AML.
Wih this in mind, the researchers decided to test LOR-253’s activity against AML and other hematologic malignancies in vitro.
Experiments revealed that LOR-253 exerts antiproliferative activity against a range of leukemia and lymphoma cell lines, including Ramos, Raji, K-562, Jurkat, MOLT-4, CCRF-CEM, HEL92.1.7, MOLM-13, THP-1, MV411, NB4, HL-60, KG-1, NOMO-1, SKM-1, OCI-AML-2, EOL-1, and Kasumi-1.
IC50 values were substantially lower in these cell lines than in melanoma cell lines, as well as lines of lung, bladder, colon, prostate, and breast cancers.
The researchers also found that LOR-253 induces KLF4 mRNA expression in the AML cell lines HL60 and THP1. This prompts increased expression of p21, a cyclin-dependent kinase inhibitor that is transcriptionally regulated by KLF4.
Consistent with these results, LOR-253 induced cell-cycle arrest and apoptosis in the AML cell lines, which suggests the molecule acted through its intended mechanism of action.
LOR-253 also showed “strong anticancer synergy” in HL60 cells when delivered in combination with daunorubicin, azacitidine, decitabine, or cytarabine.
When LOR-253 was delivered concurrently with chemotherapy, cell viability decreased the most with cytarabine, followed by decitabine, azacitidine, and daunorubicin. With sequential treatment, cell viability decreased the most when LOR-253 was delivered with decitabine, followed by azacitidine, cytarabine, and daunorubicin.
The researchers said these results suggest LOR-253 could provide a new approach to treat AML and, possibly, other hematologic malignancies.
They are now conducting studies to further characterize the pathway that mediates KLF4 induction by LOR-253, to characterize the effects of LOR-253 in combination with approved chemotherapies for AML, and to assess the efficacy of LOR-253 in animal models of AML.
Lorus Therapeutics is also planning a dose-escalating, phase 1b trial of LOR-253 as monotherapy in AML, myelodysplastic syndromes, and other hematologic malignancies. The company expects to begin the trial this summer. ![]()

SAN DIEGO—A small molecule that has previously proven effective against solid tumors exhibits activity against leukemias and lymphomas, preclinical research suggests.
The molecule, LOR-253, showed antiproliferative activity in a range of leukemia and lymphoma cell lines, induced apoptosis in acute myeloid leukemia (AML) in vitro, and demonstrated synergy with chemotherapeutic agents.
Ronnie Lum, PhD, and colleagues at Lorus Therapeutics, Inc., the Toronto, Canada-based company developing LOR-253, presented these results at the AACR Annual Meeting 2014 (abstract 4544).
LOR-253 acts through induction of the innate tumor suppressor KLF4. Recent research has suggested that upregulation of the transcription factor CDX2 drives the development or progression of leukemic disease. And CDX2 has been shown to silence KLF4, which is reported to be a critical oncogenic event in AML.
Wih this in mind, the researchers decided to test LOR-253’s activity against AML and other hematologic malignancies in vitro.
Experiments revealed that LOR-253 exerts antiproliferative activity against a range of leukemia and lymphoma cell lines, including Ramos, Raji, K-562, Jurkat, MOLT-4, CCRF-CEM, HEL92.1.7, MOLM-13, THP-1, MV411, NB4, HL-60, KG-1, NOMO-1, SKM-1, OCI-AML-2, EOL-1, and Kasumi-1.
IC50 values were substantially lower in these cell lines than in melanoma cell lines, as well as lines of lung, bladder, colon, prostate, and breast cancers.
The researchers also found that LOR-253 induces KLF4 mRNA expression in the AML cell lines HL60 and THP1. This prompts increased expression of p21, a cyclin-dependent kinase inhibitor that is transcriptionally regulated by KLF4.
Consistent with these results, LOR-253 induced cell-cycle arrest and apoptosis in the AML cell lines, which suggests the molecule acted through its intended mechanism of action.
LOR-253 also showed “strong anticancer synergy” in HL60 cells when delivered in combination with daunorubicin, azacitidine, decitabine, or cytarabine.
When LOR-253 was delivered concurrently with chemotherapy, cell viability decreased the most with cytarabine, followed by decitabine, azacitidine, and daunorubicin. With sequential treatment, cell viability decreased the most when LOR-253 was delivered with decitabine, followed by azacitidine, cytarabine, and daunorubicin.
The researchers said these results suggest LOR-253 could provide a new approach to treat AML and, possibly, other hematologic malignancies.
They are now conducting studies to further characterize the pathway that mediates KLF4 induction by LOR-253, to characterize the effects of LOR-253 in combination with approved chemotherapies for AML, and to assess the efficacy of LOR-253 in animal models of AML.
Lorus Therapeutics is also planning a dose-escalating, phase 1b trial of LOR-253 as monotherapy in AML, myelodysplastic syndromes, and other hematologic malignancies. The company expects to begin the trial this summer. ![]()

SAN DIEGO—A small molecule that has previously proven effective against solid tumors exhibits activity against leukemias and lymphomas, preclinical research suggests.
The molecule, LOR-253, showed antiproliferative activity in a range of leukemia and lymphoma cell lines, induced apoptosis in acute myeloid leukemia (AML) in vitro, and demonstrated synergy with chemotherapeutic agents.
Ronnie Lum, PhD, and colleagues at Lorus Therapeutics, Inc., the Toronto, Canada-based company developing LOR-253, presented these results at the AACR Annual Meeting 2014 (abstract 4544).
LOR-253 acts through induction of the innate tumor suppressor KLF4. Recent research has suggested that upregulation of the transcription factor CDX2 drives the development or progression of leukemic disease. And CDX2 has been shown to silence KLF4, which is reported to be a critical oncogenic event in AML.
Wih this in mind, the researchers decided to test LOR-253’s activity against AML and other hematologic malignancies in vitro.
Experiments revealed that LOR-253 exerts antiproliferative activity against a range of leukemia and lymphoma cell lines, including Ramos, Raji, K-562, Jurkat, MOLT-4, CCRF-CEM, HEL92.1.7, MOLM-13, THP-1, MV411, NB4, HL-60, KG-1, NOMO-1, SKM-1, OCI-AML-2, EOL-1, and Kasumi-1.
IC50 values were substantially lower in these cell lines than in melanoma cell lines, as well as lines of lung, bladder, colon, prostate, and breast cancers.
The researchers also found that LOR-253 induces KLF4 mRNA expression in the AML cell lines HL60 and THP1. This prompts increased expression of p21, a cyclin-dependent kinase inhibitor that is transcriptionally regulated by KLF4.
Consistent with these results, LOR-253 induced cell-cycle arrest and apoptosis in the AML cell lines, which suggests the molecule acted through its intended mechanism of action.
LOR-253 also showed “strong anticancer synergy” in HL60 cells when delivered in combination with daunorubicin, azacitidine, decitabine, or cytarabine.
When LOR-253 was delivered concurrently with chemotherapy, cell viability decreased the most with cytarabine, followed by decitabine, azacitidine, and daunorubicin. With sequential treatment, cell viability decreased the most when LOR-253 was delivered with decitabine, followed by azacitidine, cytarabine, and daunorubicin.
The researchers said these results suggest LOR-253 could provide a new approach to treat AML and, possibly, other hematologic malignancies.
They are now conducting studies to further characterize the pathway that mediates KLF4 induction by LOR-253, to characterize the effects of LOR-253 in combination with approved chemotherapies for AML, and to assess the efficacy of LOR-253 in animal models of AML.
Lorus Therapeutics is also planning a dose-escalating, phase 1b trial of LOR-253 as monotherapy in AML, myelodysplastic syndromes, and other hematologic malignancies. The company expects to begin the trial this summer. ![]()
Compound targets mutated DLBCL, WM cells
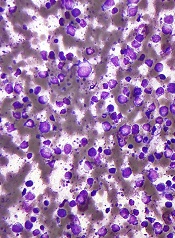
SAN DIEGO—A Toll-like receptor (TLR) antagonist can target B-cell lymphoma cells harboring the MYD88 L265P mutation, preclinical research suggests.
The compound, IMO-8400, decreased the viability of mutated diffuse large B-cell lymphoma (DLBCL) cells and Waldenström’s macroglobulinemia (WM) cells in vitro.
IMO-8400 also decreased tumor growth and prolonged survival in mice with MYD88 L265P-positive DLBCL.
Lakshmi Bhagat, PhD, and colleagues from the Cambridge, Massachusetts-based Idera Pharmaceuticals, Inc.—the company developing IMO-8400—presented these results at the AACR Annual Meeting 2014 (abstract 2570).
The researchers said their data provide additional evidence that the MYD88 L265P mutation results in over-activation of TLR7- and TLR9-mediated signaling, and blocking these TLRs leads to tumor cell death. IMO-8400 is an oligonucleotide-based antagonist of TLRs 7, 8, and 9.
In experiments with OCI‐Ly10 cells (DLBCL cells harboring the MYD88 L265P mutation), IMO-8400 prompted cell death and decreased proliferative cell signaling. But the compound did not produce these effects in SU-DHL-6 cells (DLBCL cells without the MYD88 L265P mutation).
In OCI‐Ly10 cells, IMO-8400 inhibited the IRAK-1, IRAK-4, BTK, STAT-3, Ik-Ba, and NF-κB pathways. The compound did not affect signaling pathways in SU-DHL-6 cells.
IMO-8400 also inhibited tumor growth in a mouse model of MYD88 L265P-positive, activated B-cell-like DLBCL. This inhibition was linked to the suppression of tumor-associated cytokines, including human IL-10, IL-2R, IP-10, and MIG.
Treated mice had significantly longer survival than controls, and the effect was dose-dependent. When IMO-8400 was given at 12.5 mg/kg, the P value was 0.0002. At 25 mg/kg, the P value was less than 0.0002. And at 50 mg/kg, the P value was less than 0.0001.
The researchers also found that IMO‐8400 inhibited cell viability, cytokine production, and signaling pathways in MYD88 L265P-positive WM cells. They observed these effects in the MWCL‐1 cell line and in cells from WM patients.
The team said these results provide a “strong foundation” for accelerating the clinical development of IMO-8400 in patients with B-cell lymphomas harboring the MYD88 L265P mutation.
To that end, Idera has opened enrollment in a phase 1/2 trial of IMO-8400 in WM patients who are refractory to prior therapies. The company has also submitted a protocol to the US Food and Drug Administration to conduct a phase 1/2 trial in patients with MYD88 L265P-positive DLBCL. ![]()

SAN DIEGO—A Toll-like receptor (TLR) antagonist can target B-cell lymphoma cells harboring the MYD88 L265P mutation, preclinical research suggests.
The compound, IMO-8400, decreased the viability of mutated diffuse large B-cell lymphoma (DLBCL) cells and Waldenström’s macroglobulinemia (WM) cells in vitro.
IMO-8400 also decreased tumor growth and prolonged survival in mice with MYD88 L265P-positive DLBCL.
Lakshmi Bhagat, PhD, and colleagues from the Cambridge, Massachusetts-based Idera Pharmaceuticals, Inc.—the company developing IMO-8400—presented these results at the AACR Annual Meeting 2014 (abstract 2570).
The researchers said their data provide additional evidence that the MYD88 L265P mutation results in over-activation of TLR7- and TLR9-mediated signaling, and blocking these TLRs leads to tumor cell death. IMO-8400 is an oligonucleotide-based antagonist of TLRs 7, 8, and 9.
In experiments with OCI‐Ly10 cells (DLBCL cells harboring the MYD88 L265P mutation), IMO-8400 prompted cell death and decreased proliferative cell signaling. But the compound did not produce these effects in SU-DHL-6 cells (DLBCL cells without the MYD88 L265P mutation).
In OCI‐Ly10 cells, IMO-8400 inhibited the IRAK-1, IRAK-4, BTK, STAT-3, Ik-Ba, and NF-κB pathways. The compound did not affect signaling pathways in SU-DHL-6 cells.
IMO-8400 also inhibited tumor growth in a mouse model of MYD88 L265P-positive, activated B-cell-like DLBCL. This inhibition was linked to the suppression of tumor-associated cytokines, including human IL-10, IL-2R, IP-10, and MIG.
Treated mice had significantly longer survival than controls, and the effect was dose-dependent. When IMO-8400 was given at 12.5 mg/kg, the P value was 0.0002. At 25 mg/kg, the P value was less than 0.0002. And at 50 mg/kg, the P value was less than 0.0001.
The researchers also found that IMO‐8400 inhibited cell viability, cytokine production, and signaling pathways in MYD88 L265P-positive WM cells. They observed these effects in the MWCL‐1 cell line and in cells from WM patients.
The team said these results provide a “strong foundation” for accelerating the clinical development of IMO-8400 in patients with B-cell lymphomas harboring the MYD88 L265P mutation.
To that end, Idera has opened enrollment in a phase 1/2 trial of IMO-8400 in WM patients who are refractory to prior therapies. The company has also submitted a protocol to the US Food and Drug Administration to conduct a phase 1/2 trial in patients with MYD88 L265P-positive DLBCL. ![]()

SAN DIEGO—A Toll-like receptor (TLR) antagonist can target B-cell lymphoma cells harboring the MYD88 L265P mutation, preclinical research suggests.
The compound, IMO-8400, decreased the viability of mutated diffuse large B-cell lymphoma (DLBCL) cells and Waldenström’s macroglobulinemia (WM) cells in vitro.
IMO-8400 also decreased tumor growth and prolonged survival in mice with MYD88 L265P-positive DLBCL.
Lakshmi Bhagat, PhD, and colleagues from the Cambridge, Massachusetts-based Idera Pharmaceuticals, Inc.—the company developing IMO-8400—presented these results at the AACR Annual Meeting 2014 (abstract 2570).
The researchers said their data provide additional evidence that the MYD88 L265P mutation results in over-activation of TLR7- and TLR9-mediated signaling, and blocking these TLRs leads to tumor cell death. IMO-8400 is an oligonucleotide-based antagonist of TLRs 7, 8, and 9.
In experiments with OCI‐Ly10 cells (DLBCL cells harboring the MYD88 L265P mutation), IMO-8400 prompted cell death and decreased proliferative cell signaling. But the compound did not produce these effects in SU-DHL-6 cells (DLBCL cells without the MYD88 L265P mutation).
In OCI‐Ly10 cells, IMO-8400 inhibited the IRAK-1, IRAK-4, BTK, STAT-3, Ik-Ba, and NF-κB pathways. The compound did not affect signaling pathways in SU-DHL-6 cells.
IMO-8400 also inhibited tumor growth in a mouse model of MYD88 L265P-positive, activated B-cell-like DLBCL. This inhibition was linked to the suppression of tumor-associated cytokines, including human IL-10, IL-2R, IP-10, and MIG.
Treated mice had significantly longer survival than controls, and the effect was dose-dependent. When IMO-8400 was given at 12.5 mg/kg, the P value was 0.0002. At 25 mg/kg, the P value was less than 0.0002. And at 50 mg/kg, the P value was less than 0.0001.
The researchers also found that IMO‐8400 inhibited cell viability, cytokine production, and signaling pathways in MYD88 L265P-positive WM cells. They observed these effects in the MWCL‐1 cell line and in cells from WM patients.
The team said these results provide a “strong foundation” for accelerating the clinical development of IMO-8400 in patients with B-cell lymphomas harboring the MYD88 L265P mutation.
To that end, Idera has opened enrollment in a phase 1/2 trial of IMO-8400 in WM patients who are refractory to prior therapies. The company has also submitted a protocol to the US Food and Drug Administration to conduct a phase 1/2 trial in patients with MYD88 L265P-positive DLBCL. ![]()
Novel epigenetic treatment showed activity in hematologic cancers
SAN DIEGO – OTX015, an investigational agent that blocks the activity of bromodomain and extraterminal bromodomain proteins, demonstrated activity against a variety of hematologic cancers, preliminary results from a phase I trial demonstrated.
The finding is believed to be the first clinical proof that BET bromodomain inhibitors, a new class of anticancer agents that intervene with the down-regulation of the critical oncogene MYC may play a role in the treatment of human malignancies.
"We are seeing things that we did not expect to see," Dr. Esteban Cvitkovic, chief scientific officer of Lausanne, Switzerland–based OncoEthix, said during a press briefing at the annual meeting of the American Association for Cancer Research. OncoEthix is developing OTX015, an orally available molecule.
Dr. Cvitkovic presented findings from 42 patients with hematologic malignancies who were enrolled in a trial designed to determine the recommended dose and schedule of OTX015 to be given orally as a single agent. Of the 42 patients, 21 had acute leukemia – primarily acute myelogenous leukemia – and the remaining 21 had other hematologic malignancies, including diffuse large B-cell lymphoma and multiple myeloma. The researchers assigned study participants to a single dose of 10, 20, 40, or 80 mg of OTX015 daily, or to two 40-mg doses of OTX015 daily. Each dosing regimen included three to six patients with acute leukemia and three to six patients with another hematologic malignancy. The drug was administered in 21-day cycles, with a 14 days on, 7 days off regimen for acute leukemia patients and a continuous dosing regimen for patients with other hematologic malignancies.
The mean age of patients was 69 years and 60% were male. Complete data were available on 38 patients. Among these, the researchers observed significant clinical activity in seven patients: four with acute myelogenous leukemia (including one case of complete remission) and three with lymphoma. Of the seven patients, four received a single dose of 80 mg OTX015 daily, one received 10 mg daily, and the other two received 40 mg daily.
"The drug was very well tolerated, and half of the patients had at least three cycles [of the drug]," Dr. Cvitkovic said. Among acute leukemia patients, no dose-limiting toxicity was observed; the maximum tolerated dose was not reached at the 80-mg everyday or 40-mg b.i.d. doses.
Among patients with other hematologic malignancies, thrombocytopenia is emerging as a dose-related toxicity. "Thrombocytopenia is very peculiar, because as with this and any other adverse events, we stopped the drug and within 3 or 4 days everything went back to normal," Dr. Cvitkovic said. "Every adverse event is reversible upon discontinuation of the drug." Enrollment in the trial is continuing, and dose escalation with single doses of 120 mg OTX015 daily is being explored.
Other adverse events reported to date included increased blood glucose levels in previously diabetic patients, mild to moderate digestive symptoms, and declining platelet counts. No cumulative toxicity has been observed, Dr. Cvitkovic said.
"You look at cancer right now, and you look at tumors that are driven by c-Myc, RAS, RB, and p53," Dr. Thomas Lynch, director of the Yale Cancer Center, New Haven, Conn., said at the briefing. "Those are the four big actors in cancer, and we can do [nothing] for all of those major genetic abnormalities. If this approach works, and this is a way of targeting C-Myc or related genes, this would be a major breakthrough. If it works, it offers a [treatment for a] huge unmet medical need."
The study was supported by OncoEthix. Dr. Cvitkovic is chief scientific officer of the company. Dr. Lynch disclosed that he is on the board of directors for Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
SAN DIEGO – OTX015, an investigational agent that blocks the activity of bromodomain and extraterminal bromodomain proteins, demonstrated activity against a variety of hematologic cancers, preliminary results from a phase I trial demonstrated.
The finding is believed to be the first clinical proof that BET bromodomain inhibitors, a new class of anticancer agents that intervene with the down-regulation of the critical oncogene MYC may play a role in the treatment of human malignancies.
"We are seeing things that we did not expect to see," Dr. Esteban Cvitkovic, chief scientific officer of Lausanne, Switzerland–based OncoEthix, said during a press briefing at the annual meeting of the American Association for Cancer Research. OncoEthix is developing OTX015, an orally available molecule.
Dr. Cvitkovic presented findings from 42 patients with hematologic malignancies who were enrolled in a trial designed to determine the recommended dose and schedule of OTX015 to be given orally as a single agent. Of the 42 patients, 21 had acute leukemia – primarily acute myelogenous leukemia – and the remaining 21 had other hematologic malignancies, including diffuse large B-cell lymphoma and multiple myeloma. The researchers assigned study participants to a single dose of 10, 20, 40, or 80 mg of OTX015 daily, or to two 40-mg doses of OTX015 daily. Each dosing regimen included three to six patients with acute leukemia and three to six patients with another hematologic malignancy. The drug was administered in 21-day cycles, with a 14 days on, 7 days off regimen for acute leukemia patients and a continuous dosing regimen for patients with other hematologic malignancies.
The mean age of patients was 69 years and 60% were male. Complete data were available on 38 patients. Among these, the researchers observed significant clinical activity in seven patients: four with acute myelogenous leukemia (including one case of complete remission) and three with lymphoma. Of the seven patients, four received a single dose of 80 mg OTX015 daily, one received 10 mg daily, and the other two received 40 mg daily.
"The drug was very well tolerated, and half of the patients had at least three cycles [of the drug]," Dr. Cvitkovic said. Among acute leukemia patients, no dose-limiting toxicity was observed; the maximum tolerated dose was not reached at the 80-mg everyday or 40-mg b.i.d. doses.
Among patients with other hematologic malignancies, thrombocytopenia is emerging as a dose-related toxicity. "Thrombocytopenia is very peculiar, because as with this and any other adverse events, we stopped the drug and within 3 or 4 days everything went back to normal," Dr. Cvitkovic said. "Every adverse event is reversible upon discontinuation of the drug." Enrollment in the trial is continuing, and dose escalation with single doses of 120 mg OTX015 daily is being explored.
Other adverse events reported to date included increased blood glucose levels in previously diabetic patients, mild to moderate digestive symptoms, and declining platelet counts. No cumulative toxicity has been observed, Dr. Cvitkovic said.
"You look at cancer right now, and you look at tumors that are driven by c-Myc, RAS, RB, and p53," Dr. Thomas Lynch, director of the Yale Cancer Center, New Haven, Conn., said at the briefing. "Those are the four big actors in cancer, and we can do [nothing] for all of those major genetic abnormalities. If this approach works, and this is a way of targeting C-Myc or related genes, this would be a major breakthrough. If it works, it offers a [treatment for a] huge unmet medical need."
The study was supported by OncoEthix. Dr. Cvitkovic is chief scientific officer of the company. Dr. Lynch disclosed that he is on the board of directors for Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
SAN DIEGO – OTX015, an investigational agent that blocks the activity of bromodomain and extraterminal bromodomain proteins, demonstrated activity against a variety of hematologic cancers, preliminary results from a phase I trial demonstrated.
The finding is believed to be the first clinical proof that BET bromodomain inhibitors, a new class of anticancer agents that intervene with the down-regulation of the critical oncogene MYC may play a role in the treatment of human malignancies.
"We are seeing things that we did not expect to see," Dr. Esteban Cvitkovic, chief scientific officer of Lausanne, Switzerland–based OncoEthix, said during a press briefing at the annual meeting of the American Association for Cancer Research. OncoEthix is developing OTX015, an orally available molecule.
Dr. Cvitkovic presented findings from 42 patients with hematologic malignancies who were enrolled in a trial designed to determine the recommended dose and schedule of OTX015 to be given orally as a single agent. Of the 42 patients, 21 had acute leukemia – primarily acute myelogenous leukemia – and the remaining 21 had other hematologic malignancies, including diffuse large B-cell lymphoma and multiple myeloma. The researchers assigned study participants to a single dose of 10, 20, 40, or 80 mg of OTX015 daily, or to two 40-mg doses of OTX015 daily. Each dosing regimen included three to six patients with acute leukemia and three to six patients with another hematologic malignancy. The drug was administered in 21-day cycles, with a 14 days on, 7 days off regimen for acute leukemia patients and a continuous dosing regimen for patients with other hematologic malignancies.
The mean age of patients was 69 years and 60% were male. Complete data were available on 38 patients. Among these, the researchers observed significant clinical activity in seven patients: four with acute myelogenous leukemia (including one case of complete remission) and three with lymphoma. Of the seven patients, four received a single dose of 80 mg OTX015 daily, one received 10 mg daily, and the other two received 40 mg daily.
"The drug was very well tolerated, and half of the patients had at least three cycles [of the drug]," Dr. Cvitkovic said. Among acute leukemia patients, no dose-limiting toxicity was observed; the maximum tolerated dose was not reached at the 80-mg everyday or 40-mg b.i.d. doses.
Among patients with other hematologic malignancies, thrombocytopenia is emerging as a dose-related toxicity. "Thrombocytopenia is very peculiar, because as with this and any other adverse events, we stopped the drug and within 3 or 4 days everything went back to normal," Dr. Cvitkovic said. "Every adverse event is reversible upon discontinuation of the drug." Enrollment in the trial is continuing, and dose escalation with single doses of 120 mg OTX015 daily is being explored.
Other adverse events reported to date included increased blood glucose levels in previously diabetic patients, mild to moderate digestive symptoms, and declining platelet counts. No cumulative toxicity has been observed, Dr. Cvitkovic said.
"You look at cancer right now, and you look at tumors that are driven by c-Myc, RAS, RB, and p53," Dr. Thomas Lynch, director of the Yale Cancer Center, New Haven, Conn., said at the briefing. "Those are the four big actors in cancer, and we can do [nothing] for all of those major genetic abnormalities. If this approach works, and this is a way of targeting C-Myc or related genes, this would be a major breakthrough. If it works, it offers a [treatment for a] huge unmet medical need."
The study was supported by OncoEthix. Dr. Cvitkovic is chief scientific officer of the company. Dr. Lynch disclosed that he is on the board of directors for Bristol-Myers Squibb and Infinity Pharmaceuticals, and that he receives honoraria and stock from both companies. He is also on the scientific advisory board for Arvinas, and receives honoraria and stock from that company. In addition, Dr. Lynch is a patent holder with Partners Healthcare for an EGFR mutation testing patent and receives royalties.
AT THE AACR ANNUAL MEETING
Major Finding: Among 38 patients with a variety of hematologic cancers who were treated with the novel bromodomain and extraterminal bromodomain inhibitor OTX015, significant clinical activity was observed in 7 patients, including 1 case of complete remission.
Data Source: An ongoing study in which patients with acute leukemia and patients with other hematologic malignancies were assigned, in 21-day cycles, to a single dose of 10, 20, 40, or 80 mg of OTX015 daily, or to two 40-mg doses of OTX015 daily.
Disclosures: The study was supported by OncoEthix. Dr. Cvitkovic is the company’s chief scientific officer.
Reovirus shows synergy with agents used to treat MM
SAN DIEGO—Reovirus can induce cell death in a range of multiple myeloma (MM) cell lines, and it is synergistic with drugs used to treat MM, according to research presented at the AACR Annual Meeting 2014.
Six of the 7 MM cell lines tested were at least moderately sensitive to reovirus, and introducing the virus in combination with the proteasome inhibitor carfilzomib or the Akt inhibitor perifosine increased antimyeloma activity.
Chandini M. Thirukkumaran, PhD, of The University of Calgary in Canada, and her colleagues presented these results at the meeting as abstract 1709. The study was funded by the Cancer Research Society of Canada.
“Our university, about 15 years ago, found that reovirus can infect all cells, but it will specifically kill cancer cells and not harm normal cells,” Dr Thirukkumaran said. “This is because cancer cells have aberrant Ras signaling pathways, and the virus utilizes that aberrant signaling for its replication.”
“Reovirus kills a lot of myeloma cell lines and cells from patients too, but you always find that therapy-resistant population. So we wondered if we could combine reovirus with common myeloma drugs, like carfilzomib and perifosine, and see whether we could get synergy.”
Dr Thirukkumaran and her colleagues first wanted to quantify the effect of reovirus alone on MM cell lines. So they incubated the cell lines RPMI 8226, MM1.S, NCIH929, U266, INA-6, KMS11, and OPM2 with live reovirus or UV-inactivated reovirus and assessed cell death.
They found that RPMI8226 was highly sensitive to reovirus, and OPM2 was resistant to it. The remaining cell lines were somewhat sensitive to the virus.
The researchers chose RPMI8226, KMS-11, and OPM2 to test reovirus in combination with either carfilzomib or perifosine. They tested the drugs at various concentrations to determine effective dose 50% (ED50) values. They combined ED50 values for each drug and reovirus at various concentrations but with consistent ratios and determined toxicity.
The team used software to generate combination index (CI) values and determine synergism per the Chou-Talalay method. A CI equal to 1 suggested an additive effect, a CI greater than 1 suggested an antagonistic effect, and a CI less than 1 suggested a synergistic effect.
Results showed that reovirus synergized with both carfilzomib and perifosine. Furthermore, the greater a cell line’s resistance to reovirus, the greater the synergy.
For instance, with reovirus and carfilzomib in combination, the ED50 was 1.06 + 0.15 in the reovirus-sensitive RPMI8226 cell line, 0.78 + 0.14 in the moderately sensitive KMS11 cell line, and 0.57 + 0.05 in the resistant OPM2 cell line.
When reovirus and perifosine were combined, the ED50 was 0.97 + 0.19 in the RMI8226 cell line, 0.26 + 0.11 in the KMS11 cell line, and 0.88 + 0.22 in the OPM2 cell line.
The researchers said these results highlight the significance of preclinical studies in evaluating reovirus-drug combinations that could be extrapolated to a clinical setting.
Two phase 2 trials evaluating reovirus in combination therapy for MM are now underway. Meanwhile, Dr Thirukkumaran and her colleagues are hoping to gain more insight into how reovirus-drug combinations work by testing them in mouse models of MM. ![]()
SAN DIEGO—Reovirus can induce cell death in a range of multiple myeloma (MM) cell lines, and it is synergistic with drugs used to treat MM, according to research presented at the AACR Annual Meeting 2014.
Six of the 7 MM cell lines tested were at least moderately sensitive to reovirus, and introducing the virus in combination with the proteasome inhibitor carfilzomib or the Akt inhibitor perifosine increased antimyeloma activity.
Chandini M. Thirukkumaran, PhD, of The University of Calgary in Canada, and her colleagues presented these results at the meeting as abstract 1709. The study was funded by the Cancer Research Society of Canada.
“Our university, about 15 years ago, found that reovirus can infect all cells, but it will specifically kill cancer cells and not harm normal cells,” Dr Thirukkumaran said. “This is because cancer cells have aberrant Ras signaling pathways, and the virus utilizes that aberrant signaling for its replication.”
“Reovirus kills a lot of myeloma cell lines and cells from patients too, but you always find that therapy-resistant population. So we wondered if we could combine reovirus with common myeloma drugs, like carfilzomib and perifosine, and see whether we could get synergy.”
Dr Thirukkumaran and her colleagues first wanted to quantify the effect of reovirus alone on MM cell lines. So they incubated the cell lines RPMI 8226, MM1.S, NCIH929, U266, INA-6, KMS11, and OPM2 with live reovirus or UV-inactivated reovirus and assessed cell death.
They found that RPMI8226 was highly sensitive to reovirus, and OPM2 was resistant to it. The remaining cell lines were somewhat sensitive to the virus.
The researchers chose RPMI8226, KMS-11, and OPM2 to test reovirus in combination with either carfilzomib or perifosine. They tested the drugs at various concentrations to determine effective dose 50% (ED50) values. They combined ED50 values for each drug and reovirus at various concentrations but with consistent ratios and determined toxicity.
The team used software to generate combination index (CI) values and determine synergism per the Chou-Talalay method. A CI equal to 1 suggested an additive effect, a CI greater than 1 suggested an antagonistic effect, and a CI less than 1 suggested a synergistic effect.
Results showed that reovirus synergized with both carfilzomib and perifosine. Furthermore, the greater a cell line’s resistance to reovirus, the greater the synergy.
For instance, with reovirus and carfilzomib in combination, the ED50 was 1.06 + 0.15 in the reovirus-sensitive RPMI8226 cell line, 0.78 + 0.14 in the moderately sensitive KMS11 cell line, and 0.57 + 0.05 in the resistant OPM2 cell line.
When reovirus and perifosine were combined, the ED50 was 0.97 + 0.19 in the RMI8226 cell line, 0.26 + 0.11 in the KMS11 cell line, and 0.88 + 0.22 in the OPM2 cell line.
The researchers said these results highlight the significance of preclinical studies in evaluating reovirus-drug combinations that could be extrapolated to a clinical setting.
Two phase 2 trials evaluating reovirus in combination therapy for MM are now underway. Meanwhile, Dr Thirukkumaran and her colleagues are hoping to gain more insight into how reovirus-drug combinations work by testing them in mouse models of MM. ![]()
SAN DIEGO—Reovirus can induce cell death in a range of multiple myeloma (MM) cell lines, and it is synergistic with drugs used to treat MM, according to research presented at the AACR Annual Meeting 2014.
Six of the 7 MM cell lines tested were at least moderately sensitive to reovirus, and introducing the virus in combination with the proteasome inhibitor carfilzomib or the Akt inhibitor perifosine increased antimyeloma activity.
Chandini M. Thirukkumaran, PhD, of The University of Calgary in Canada, and her colleagues presented these results at the meeting as abstract 1709. The study was funded by the Cancer Research Society of Canada.
“Our university, about 15 years ago, found that reovirus can infect all cells, but it will specifically kill cancer cells and not harm normal cells,” Dr Thirukkumaran said. “This is because cancer cells have aberrant Ras signaling pathways, and the virus utilizes that aberrant signaling for its replication.”
“Reovirus kills a lot of myeloma cell lines and cells from patients too, but you always find that therapy-resistant population. So we wondered if we could combine reovirus with common myeloma drugs, like carfilzomib and perifosine, and see whether we could get synergy.”
Dr Thirukkumaran and her colleagues first wanted to quantify the effect of reovirus alone on MM cell lines. So they incubated the cell lines RPMI 8226, MM1.S, NCIH929, U266, INA-6, KMS11, and OPM2 with live reovirus or UV-inactivated reovirus and assessed cell death.
They found that RPMI8226 was highly sensitive to reovirus, and OPM2 was resistant to it. The remaining cell lines were somewhat sensitive to the virus.
The researchers chose RPMI8226, KMS-11, and OPM2 to test reovirus in combination with either carfilzomib or perifosine. They tested the drugs at various concentrations to determine effective dose 50% (ED50) values. They combined ED50 values for each drug and reovirus at various concentrations but with consistent ratios and determined toxicity.
The team used software to generate combination index (CI) values and determine synergism per the Chou-Talalay method. A CI equal to 1 suggested an additive effect, a CI greater than 1 suggested an antagonistic effect, and a CI less than 1 suggested a synergistic effect.
Results showed that reovirus synergized with both carfilzomib and perifosine. Furthermore, the greater a cell line’s resistance to reovirus, the greater the synergy.
For instance, with reovirus and carfilzomib in combination, the ED50 was 1.06 + 0.15 in the reovirus-sensitive RPMI8226 cell line, 0.78 + 0.14 in the moderately sensitive KMS11 cell line, and 0.57 + 0.05 in the resistant OPM2 cell line.
When reovirus and perifosine were combined, the ED50 was 0.97 + 0.19 in the RMI8226 cell line, 0.26 + 0.11 in the KMS11 cell line, and 0.88 + 0.22 in the OPM2 cell line.
The researchers said these results highlight the significance of preclinical studies in evaluating reovirus-drug combinations that could be extrapolated to a clinical setting.
Two phase 2 trials evaluating reovirus in combination therapy for MM are now underway. Meanwhile, Dr Thirukkumaran and her colleagues are hoping to gain more insight into how reovirus-drug combinations work by testing them in mouse models of MM. ![]()
Palbociclib shows promise in advanced ER-positive breast cancer patients
SAN DIEGO – Palbociclib, a first-in-class cyclin-dependent kinase 4/6 inhibitor, in combination with letrozole, significantly improved median progression-free survival in patients with advanced estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer in the first-line setting.
In preclinical studies, palbociclib, previously known as PD 0332991, "has been shown to block the transition from G1 to S in the cell cycle," Dr. Richard S. Finn said during a press briefing at the annual meeting of the American Association for Cancer Research. "That’s a place where CDK 4 and 6 play a critical role in regulating cell metabolism. What is important to note is that palbociclib, unlike prior CDK inhibitors, is very specific for CDK 4 and 6. Earlier [nonspecific] CDK inhibitors had trouble in clinical development."
In a study supported by Pfizer Inc., which is developing palbociclib, Dr. Finn of the University of California, Los Angeles, and his associates conducted an open-label, randomized, phase II study of palbociclib in combination with letrozole vs. letrozole alone for first-line treatment of ER-positive, HER2-negative advanced breast cancer.
The study, known as PALOMA-1, included 165 patients and was conducted in two parts. Part one included 66 postmenopausal women with ER-positive/HER2-negative breast cancer, while part two included 99 postmenopausal women with the same cancer subtype who were additionally screened for CCND1 amplification and/or loss of p16. Women in both study arms were randomized 1:1 open label to letrozole vs. palbociclib 125 mg on a 3-week-on, 3-week-off course and were followed for tumor assessments every 2 months. The primary endpoint was investigator-assessed progression-free survival defined as the time from randomization to objective progression or death.
The median age of the patients was 64 years, and about one-third had received prior hormone replacement therapy in the adjuvant setting. The median progression-free survival was 10.2 months among patients who received letrozole alone, compared with 20.2 months among patients who received letrozole plus palbociclib. This difference reached statistical significance with a hazard ratio of 0.488, or a 51% decrease in the risk of progression with the addition of palbociclib (P = .0004), Dr. Finn reported.
In secondary measures, the researchers observed a benefit of letrozole plus palbociclib, compared with letrozole only, in terms of the objective response rate among all randomized patients (43% vs. 33%, respectively), the objective response rate among patients with measurable disease (55% vs. 39%), and in the clinical benefit rate (81% vs. 58%).
The median overall survival increased from 33.3 months among patients in the letrozole-only arm to 37.5 months among patients in the letrozole-plus-palbociclib arm. That translated into a hazard ratio of 0.813, "which at this time is not statistically significant," Dr. Finn noted.
The most common adverse events were neutropenia, leukopenia, and fatigue. "This neutropenia is not the type we see with chemotherapy," he said. "It is self limited and recovers quickly. We did not see neutropenic fever."
A randomized phase III study is under way to confirm these findings in a similar patient population.
In an interview, Dr. Patricia M. LoRusso, director of the Center for Translational Therapeutics at Wayne State University’s Karmanos Cancer Institute, Detroit, characterized the findings as impressive. "This is just as impressive as the everolimus data that led to a subsequent phase III [trial] that led to subsequent approval of that drug," she said.
The study was supported by Pfizer. Dr. Finn disclosed that he has received research support from the company. Dr. LoRusso disclosed that she has received research funding from Agios Pharmaceuticals.
SAN DIEGO – Palbociclib, a first-in-class cyclin-dependent kinase 4/6 inhibitor, in combination with letrozole, significantly improved median progression-free survival in patients with advanced estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer in the first-line setting.
In preclinical studies, palbociclib, previously known as PD 0332991, "has been shown to block the transition from G1 to S in the cell cycle," Dr. Richard S. Finn said during a press briefing at the annual meeting of the American Association for Cancer Research. "That’s a place where CDK 4 and 6 play a critical role in regulating cell metabolism. What is important to note is that palbociclib, unlike prior CDK inhibitors, is very specific for CDK 4 and 6. Earlier [nonspecific] CDK inhibitors had trouble in clinical development."
In a study supported by Pfizer Inc., which is developing palbociclib, Dr. Finn of the University of California, Los Angeles, and his associates conducted an open-label, randomized, phase II study of palbociclib in combination with letrozole vs. letrozole alone for first-line treatment of ER-positive, HER2-negative advanced breast cancer.
The study, known as PALOMA-1, included 165 patients and was conducted in two parts. Part one included 66 postmenopausal women with ER-positive/HER2-negative breast cancer, while part two included 99 postmenopausal women with the same cancer subtype who were additionally screened for CCND1 amplification and/or loss of p16. Women in both study arms were randomized 1:1 open label to letrozole vs. palbociclib 125 mg on a 3-week-on, 3-week-off course and were followed for tumor assessments every 2 months. The primary endpoint was investigator-assessed progression-free survival defined as the time from randomization to objective progression or death.
The median age of the patients was 64 years, and about one-third had received prior hormone replacement therapy in the adjuvant setting. The median progression-free survival was 10.2 months among patients who received letrozole alone, compared with 20.2 months among patients who received letrozole plus palbociclib. This difference reached statistical significance with a hazard ratio of 0.488, or a 51% decrease in the risk of progression with the addition of palbociclib (P = .0004), Dr. Finn reported.
In secondary measures, the researchers observed a benefit of letrozole plus palbociclib, compared with letrozole only, in terms of the objective response rate among all randomized patients (43% vs. 33%, respectively), the objective response rate among patients with measurable disease (55% vs. 39%), and in the clinical benefit rate (81% vs. 58%).
The median overall survival increased from 33.3 months among patients in the letrozole-only arm to 37.5 months among patients in the letrozole-plus-palbociclib arm. That translated into a hazard ratio of 0.813, "which at this time is not statistically significant," Dr. Finn noted.
The most common adverse events were neutropenia, leukopenia, and fatigue. "This neutropenia is not the type we see with chemotherapy," he said. "It is self limited and recovers quickly. We did not see neutropenic fever."
A randomized phase III study is under way to confirm these findings in a similar patient population.
In an interview, Dr. Patricia M. LoRusso, director of the Center for Translational Therapeutics at Wayne State University’s Karmanos Cancer Institute, Detroit, characterized the findings as impressive. "This is just as impressive as the everolimus data that led to a subsequent phase III [trial] that led to subsequent approval of that drug," she said.
The study was supported by Pfizer. Dr. Finn disclosed that he has received research support from the company. Dr. LoRusso disclosed that she has received research funding from Agios Pharmaceuticals.
SAN DIEGO – Palbociclib, a first-in-class cyclin-dependent kinase 4/6 inhibitor, in combination with letrozole, significantly improved median progression-free survival in patients with advanced estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast cancer in the first-line setting.
In preclinical studies, palbociclib, previously known as PD 0332991, "has been shown to block the transition from G1 to S in the cell cycle," Dr. Richard S. Finn said during a press briefing at the annual meeting of the American Association for Cancer Research. "That’s a place where CDK 4 and 6 play a critical role in regulating cell metabolism. What is important to note is that palbociclib, unlike prior CDK inhibitors, is very specific for CDK 4 and 6. Earlier [nonspecific] CDK inhibitors had trouble in clinical development."
In a study supported by Pfizer Inc., which is developing palbociclib, Dr. Finn of the University of California, Los Angeles, and his associates conducted an open-label, randomized, phase II study of palbociclib in combination with letrozole vs. letrozole alone for first-line treatment of ER-positive, HER2-negative advanced breast cancer.
The study, known as PALOMA-1, included 165 patients and was conducted in two parts. Part one included 66 postmenopausal women with ER-positive/HER2-negative breast cancer, while part two included 99 postmenopausal women with the same cancer subtype who were additionally screened for CCND1 amplification and/or loss of p16. Women in both study arms were randomized 1:1 open label to letrozole vs. palbociclib 125 mg on a 3-week-on, 3-week-off course and were followed for tumor assessments every 2 months. The primary endpoint was investigator-assessed progression-free survival defined as the time from randomization to objective progression or death.
The median age of the patients was 64 years, and about one-third had received prior hormone replacement therapy in the adjuvant setting. The median progression-free survival was 10.2 months among patients who received letrozole alone, compared with 20.2 months among patients who received letrozole plus palbociclib. This difference reached statistical significance with a hazard ratio of 0.488, or a 51% decrease in the risk of progression with the addition of palbociclib (P = .0004), Dr. Finn reported.
In secondary measures, the researchers observed a benefit of letrozole plus palbociclib, compared with letrozole only, in terms of the objective response rate among all randomized patients (43% vs. 33%, respectively), the objective response rate among patients with measurable disease (55% vs. 39%), and in the clinical benefit rate (81% vs. 58%).
The median overall survival increased from 33.3 months among patients in the letrozole-only arm to 37.5 months among patients in the letrozole-plus-palbociclib arm. That translated into a hazard ratio of 0.813, "which at this time is not statistically significant," Dr. Finn noted.
The most common adverse events were neutropenia, leukopenia, and fatigue. "This neutropenia is not the type we see with chemotherapy," he said. "It is self limited and recovers quickly. We did not see neutropenic fever."
A randomized phase III study is under way to confirm these findings in a similar patient population.
In an interview, Dr. Patricia M. LoRusso, director of the Center for Translational Therapeutics at Wayne State University’s Karmanos Cancer Institute, Detroit, characterized the findings as impressive. "This is just as impressive as the everolimus data that led to a subsequent phase III [trial] that led to subsequent approval of that drug," she said.
The study was supported by Pfizer. Dr. Finn disclosed that he has received research support from the company. Dr. LoRusso disclosed that she has received research funding from Agios Pharmaceuticals.
AT THE AACR ANNUAL MEETING
Major Finding: Median progression-free survival was 10.2 months among patients who received letrozole alone, compared with 20.2 months in patients who received letrozole plus palbociclib (hazard ratio, 0.488).
Data Source: Final results from a phase II study in which 165 postmenopausal women with hormone receptor–positive metastatic breast cancer were randomized 1:1 open label to letrozole vs. palbociclib 125 mg on a 3-week-on, 3-week-off course and were followed for tumor assessments every 2 months.
Disclosures: Pfizer funded the study. Dr. Finn disclosed that he has received research support from the company.









