User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Cardiac issues after COVID infection and vaccination: New data
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
Fourth Pfizer dose better for severe than symptomatic COVID: Study
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Monoclonal antibodies for COVID – Give IV infusion or an injection?
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Woman who faked medical degree practiced for 3 years
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
COVID-19 cardiovascular complications in children: AHA statement
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Nontuberculous mycobacterial lung disease can be challenging to treat
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Long-term smell loss in COVID-19 tied to damage in the brain’s olfactory bulb
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Meningococcal vaccine shows moderate protective effect against gonorrhea
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM THE LANCET INFECTIOUS DISEASES
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.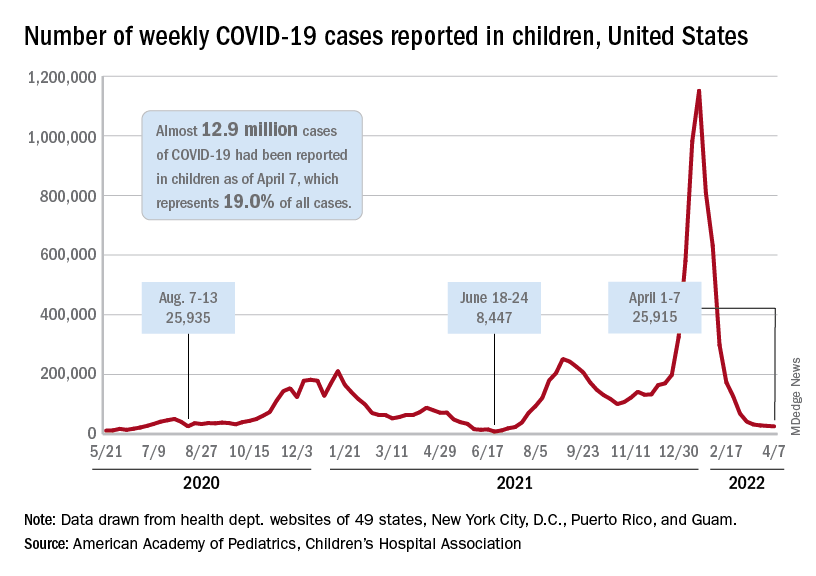
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
Counterfeit HIV drugs: Justice Department opens investigation
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.