User login
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
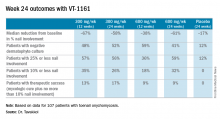
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
VIENNA – A once-weekly oral antifungal drug known as VT-1161 will move into phase III clinical testing in 2017 based on its impressive performance in an interim analysis of a phase IIb study, Amir Tavakkol, PhD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“We saw robust evidence of clinical and mycologic efficacy in patients with moderate to severe onychomycosis. This was true even in patients with nails considered very difficult to treat because of dermatophytomas and spikes, which are usually poor prognostic elements,” said Dr. Tavakkol, chief development officer at Viamet Pharmaceuticals in Durham, N.C., which is developing VT-1161.
He reported on 107 patients with toenail onychomycosis who enrolled in the phase IIb, double-blind, placebo-controlled RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study. At enrollment they averaged 47% disease involvement of the big toenail, the target nail for the trial. Participants in the five study arms had an average of 4.2-5.0 affected toenails. Both the percentage of nail involvement and the number of diseased toenails were roughly twice as great as is typical in studies of topical antifungals, underscoring that participants in the VT-1161 trial had fairly severe onychomycosis.
Patients were assigned to one of five study arms: 300 mg of VT-1161 once weekly for 12 weeks, then 12 weeks of placebo; 600 mg of VT-1161 once weekly for 12 weeks, followed by 12 weeks of placebo; either 300 or 600 mg of the antifungal agent once weekly for the full 24 weeks; or 24 weeks of once-per-week placebo. Immediately prior to the formal start of the study, however, everyone received 14 days of a once-daily loading dose of VT-1161 or placebo at the dose they would subsequently take once weekly during the trial.
The primary outcome in the ongoing study is the percentage of patients with a complete cure, both mycologic and clinical, at 48 weeks. Those data aren’t in yet, but Dr. Tavakkol presented the results of the prespecified interim analysis at 24 weeks.
“Please keep in mind that this is only at most 24 weeks of therapy. Given the rate of nail growth at 1 mm per month when it is healthy, these are remarkable data. Ten percent or less nail involvement is basically 1-2 mm at the distal end. I believe a substantial percentage of these patients will reach clinical cure by 48 weeks,” he said.
VT-1161, a molecule with high selectivity for fungal CYP51, blocks the production of ergosterol, a key component of the fungal cell membrane, according to the company. It has no known drug interactions. At all doses studied in the trial, it was safe and well tolerated, with no abnormal liver function tests, no effect on bilirubin, and no change in QTc interval. Only 8 of the 107 patients reported adverse events deemed possibly related to the study drug by blinded investigators. No one dropped out of the study.
“VT-1161 is also being developed for recurrent vulvovaginal candidiasis. The results there are outstanding, too,” Dr. Tavakkol said.
The trial was funded by Viamet, where he is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Among onychomycosis patients with an average 47% target toenail involvement at baseline, 35% had improved to no more than 10% nail involvement at 24 weeks after 12 weeks of once-weekly VT-1161 followed by 12 weeks of placebo.
Data source: A double-blind, randomized, phase IIb clinical trial involving 107 patients with toenail onychomycosis.
Disclosures: The trial was funded by Viamet Pharmaceuticals, where the study presenter is employed.