User login
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
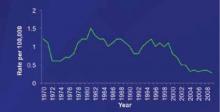
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
The Advisory Committee on Immunization Practices (ACIP) is evaluating whether to recommend the use of meningococcal vaccines for infants and children <2 years.1 The decision may be made within the next 4 to 8 months. In its deliberation, ACIP must consider several issues, which I review here.
Current and impending vaccine options. Two quadrivalent meningococcal conjugate vaccines (MCV4) are licensed by the US Food and Drug Administration (FDA) for use in the United States: Men ACWY-CRM (Menveo, Novartis) and Men ACWY-D (Menactra, Sanofi Pasteur).2 Both vaccines protect against 4 meningococcal serogroups (A, C, Y, and W-135) and are approved for use among those ages 2 to 55 years. In addition, Menactra was recently licensed as a 2-dose series for children ages 9 to 23 months. ACIP recommends routine use of MCV4 for adolescents ages 11 to 18 years, with a preference for the first dose at ages 11 to 12 years; and for all individuals between the ages of 2 and 55 years who are at increased risk for meningococcal disease ( TABLE ).
Complicating matters is the pending availability of more formulations. In addition to the 2-dose Menactra option for children 9 to 23 months, Novartis has an application before the FDA for a 4-dose schedule with Menveo, given at ages 2, 4, 6, and 12 months. GlaxoSmithKline has just received approval from the FDA for MenHibrix, a combination vaccine that contains antigens against Haemophilus influenzae type b (Hib) and 2 meningococcal serogroups, C and Y, licensed as a 4-dose series given at ages 2, 4, 6, and 12 months.
These vaccines have proven to be immunogenic in infants without diminishing the effectiveness of other, co-administered vaccines in normal infant populations. They also appear to be safe, although the studies to date have not been sufficiently large to detect uncommon adverse events.3-7
Table
Patients at high risk for meningococcal disease
|
| Source: CDC. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76. |
Meningococcal disease incidence and prevalence are declining
One major consideration for ACIP is the changing epidemiology of meningococcal disease and the low prevalence of disease in all age groups, including infants. The incidence of meningococcal disease has declined in the United States since 1980, with a marked and sustained decline since 2000 ( FIGURE 1 ).8 This decline has occurred in all age groups including infants, who have the highest rate of infection ( FIGURE 2 ).8 This decline in incidence occurred for all serogroups, including serogroup B.8
Serogroup B. Among children <5 years, including infants, half of meningococcal disease is caused by serogroup B,8 and these infections would not be prevented by any of the currently licensed vaccines or by those under review. Furthermore, half of all infections occur before age 9 months8 —an age range for which Menactra in not approved.
Serogroup C and Y. One-third of infections with serogroups C and Y occur before the age of 6 months8 and would not be prevented by any of the new products. Also of note: From 2007 to 2009, the mean number of cases of serotype A or C infection occurring each year in children <5 years was 77.8
The impact on children vs adults. Meningococcal disease in children is generally less severe than that occurring in older age groups. Overall case fatality in children is 6%; 10% in those with serogroup B and 1% in those with serogroup Y.8 The disease in children does result in significant sequelae, however, with 10% suffering hearing loss and 1% to 2% requiring amputation. From 2007 to 2009, there were 4 to 8 deaths per year among children under age 5, and 8 to 12 children per year experienced serious sequelae.8
FIGURE 1
The incidence of meningococcal disease has declined steadily since 20008
1970-1996 National Notifiable Diseases Surveillance System data. 1997-2009 Active Bacterial Core surveillance data estimated to the US population.
FIGURE 2
Meningococcal disease has declined among infants and other age groups8
Active Bacterial Core surveillance cases from 1993-2009 estimated to the US population with 18% correction for underreporting.
ACIP’s dilemma
The low morbidity and mortality associated with meningococcal disease is one issue to consider when deciding whether to recommend new vaccines as part of the routine infant and child immunization schedule. The vaccine schedule is already crowded and complex, and parents increasingly are questioning the need for additional antigens.
In addition, the cost of vaccines for children has escalated over the past decade due mainly to the new, more expensive formulations.
The reason for a declining incidence of meningococcal disease is not fully known. It may be partly explained by increasing rates of vaccination among adolescents. However, the overall low rate of disease in the population makes assessing herd immunity difficult.
If ACIP decides to recommend vaccinating infants against meningococcal disease, it is unclear how long immunity will last, potentially necessitating a booster dose before the currently recommended adolescent dose.
Finally, in children at high risk, it is not fully known how meningococcal vaccines will affect the immune response to pneumococcal conjugate vaccine. This is an important consideration because the incidence of pneumococcal disease among these children is much higher than that of meningococcal disease.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.
1. CDC. Report from the Advisory Committee on Immunization Practices (ACIP): decision not to recommend routine vaccination of all children aged 2-10 years with quadrivalent meningococcal conjugate vaccine (MCV4). MMWR Morb Mortal Wkly Rep. 2008;57:462-465.
2. CDC. Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018-1019.
3. Marshall GS, Marchant CD, Blatter M, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type B and Neisseria meningitides serogroups C and T-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469-471.
4. Nolan T, Richmond P, Marshall H, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190-196.
5. Bryant KA, Marshall GS, Marchant CD, et al. Immunogenicity and safety of H influenzae type b-N meningitides C/Y conjugate vaccine in infants. Pediatrics. 2011;127:e1375-e1385.
6. Marshall GS, Marchant CD, Blatter M, et al. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitides serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Hum Vaccin. 2011;7:258-264.
7. Perrett KP, Snape MD, Ford KJ, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186-193.
8. MacNeil J. Epidemiology of meningococcal diseases in infants and young children. Paper presented at: Meeting of the Advisory Committee on Immunization Practices; October 25, 2011; Atlanta, GA.