User login
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
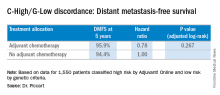
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
A large multinational European trial shows that genetic analysis of breast tumors can spare women unnecessary adjuvant chemotherapy, even when clinical factors indicate a high risk of recurrence.
Using MammaPrint, a 70-gene signature test, led to a 14% absolute reduction in the use of chemotherapy, compared with a clinical strategy, according to the highly anticipated results of the Phase III Microarray in Node-negative and 1 to 3 positive lymph node Disease may Avoid Chemo Therapy (MINDACT) trial, presented at the annual meeting of the American Association for Cancer Research.
While three decades of clinical trials have shown survival benefits of adjuvant chemotherapy, it carries later risks. “And it will happen when the survival gain associated with adjuvant chemotherapy is small, in the range of 2%-3% – it happens in good prognosis patients – and is counterbalanced by the long-term severe risks of adjuvant chemotherapy,” said Dr. Martine Piccart, director of medicine at the Jules Bordet Institute in Brussels, and principal investigator for the trial.
Risks include secondary cancers, cardiotoxicity, early menopause, and a decline in cognitive function, as well as negative socioeconomic effects. So avoiding chemotherapy when outcomes would be nearly the same without it is a reasonable goal.
To investigate the best method to determine which breast cancer patients could do just as well without adjuvant chemotherapy, European investigators at 111 centers in 9 countries screened 11,288 patients with early-stage disease and enrolled 6,693 in the MINDACT trial. Speaking at a news conference during the annual meeting, Dr. Piccart said MINDACT compared tumor genetic testing with clinical and pathology parameters to gauge the risk of disease recurrence and is the only trial to date to compare the utility of the two strategies.
MammaPrint is a genetic test (G) of early stage breast cancer tumors that produces a 70-gene signature indicating a high or low 10-year risk of metastatic recurrence. The Adjuvant! Online tool takes into account clinical factors and pathology markers, including a patient’s age and comorbidities and a tumor’s estrogen receptor status, grade, size, and the number of positive lymph nodes, to calculate a ten-year “clinical risk” (C) of negative outcomes with or without adjuvant chemotherapy or hormonal therapy.
After surgery, tissue samples underwent local pathology examination to determine tumor stage and nodal, hormone receptor, and HER2 status. Samples were also sent to a central facility for MammaPrint testing. For women with low-risk tumors by both tests, no chemotherapy was indicated. For high-risk tumors by both tests, chemotherapy was prescribed. But those with discordant results were randomized equally to receive chemotherapy or not.
MammaPrint better predictor than clinical factors
The median age of patients at enrollment was 55 years, 80% of tumors were node-negative, 58% were T1, 88% hormone receptor–positive, and 10% HER2-positive. At a median follow-up of 5 years, 362 (5.4%) women had distant metastases or had died.
Among 3,356 patients classified as high risk based on the Adjuvant! Online test, there were 1,550 classified by genetic criteria as low risk (C-High/G-Low), including 48% with positive nodes. For this C-High/G-Low group, the 5-year distant metastasis-free survival was greater than 94% regardless of adjuvant chemotherapy and was not statistically different for the two groups. These are patients who would normally be prescribed adjuvant chemotherapy if based solely on the C criteria.
Going by MammaPrint test results would thereby reduce chemotherapy prescriptions by 46% (1,550/3,356) for the study cohort. Considering the entire initial 11,288 patients screened, the genomic strategy would lead to a 14% absolute reduction in chemotherapy, compared with the clinical strategy, according to Dr. Piccart. “MINDACT results provide level 1A evidence of the clinical utility of MammaPrint for assessing the lack of a clinically relevant chemotherapy benefit in the clinically high-risk population,” she said.
When asked about the use of MammaPrint in the United States, she said it is more common in Europe and other places in the world, whereas the use of Oncotype DX genetic testing seems to be more prevalent in the U.S.
News conference moderator Dr. Nancy Davidson, director of the University of Pittsburgh Cancer Institute, said that both tests are used at her institution. “I would say Oncotype is probably the dominant test that’s ordered,” she said. “But there are folks who are big fans of MammaPrint, and they’re going to be very excited to see these major results come out from this trial to help us to discuss this further and to think about how to guide our thinking and our patients.”
Entities and funding sources involved in the study included Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.
FROM THE AACR ANNUAL MEETING
Key clinical point: MammaPrint bests Adjuvant! Online for sparing low-risk patients from chemotherapy.
Major finding: Genotyping results in a 14% reduction in the use of chemotherapy, compared with a clinical strategy.
Data source: Phase III randomized controlled study of 6,693 women with early stage breast cancer.
Disclosures: Entities and funding sources involved in the study include Adjuvant! Online, Agendia, Novartis, Roche, Sanofi-Aventis, Veridex, and Eli Lilly. Dr. Piccart disclosed that she is a board member of Radius, and a consultant for AstraZeneca, Eli Lilly, Invivus, Merck Sharp & Dohme, Novartis, Pfizer, Roche-Genentech, Synthon, Debiopharm, and PharmaMar. The Jules Bordet Institute receives grant/research funds from most companies in the field.