User login
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
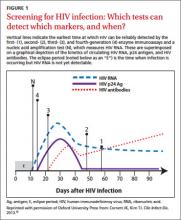
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
› Screen all adolescents and adults ages 15 to 65 years for human immunodeficiency virus (HIV) infection. A
› Screen younger adolescents and older adults who are at increased risk for HIV infection on an annual basis. A
› Screen all pregnant women for HIV infection, including those who are in labor and who are untested or whose HIV status is unknown. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
For the first 15 years of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDs) was uniformly fatal. Between 1981 and 1996, approximately 362,000 people in the United States succumbed to the disease.1 That began to change in the mid 1990s, though, when highly active antiretroviral therapy (HAART) came into routine use. From that point forward, HIV became a chronic, manageable disease for most patients; an estimated 1.2 million people in the United States are now living with HIV infection.2
Unfortunately, the number of new infections continues to grow. There are more than 50,000 new infections in the United States each year,2 and an estimated approximately 200,000 people have it but are undiagnosed, leading to further spread of the disease.3 The Office of National AIDS Policy has issued a National HIV/AIDS Strategy that seeks to reduce new infections by 25% in 2015, in part by identifying people with the disease who do not know their HIV status.4
But screening still has not gotten the uptake by clinicians that health officials would like.
Lack of awareness by physicians? Or an unwillingness of patients?
In 2006, the Centers for Disease Control and Prevention (CDC) began recommending routine HIV screening for individuals between the ages of 13 and 64, with patients given the ability to opt out of such testing.5 That same year, the CDC also removed some prior barriers to testing, such as requiring written consent and pretest counseling. But as of 2009, fewer than 50% of US adults had ever been tested for HIV6—possibly the result of physicians being unaware of the guidelines, patients being unwilling to be tested, and/or reimbursement issues.
Conflicting recommendations may have played a role. When the CDC released its 2006 recommendations, the United States Preventive Services Task Force (USPSTF) felt there was insufficient evidence to support routine HIV screening and issued a grade C recommendation. At that time, the USPSTF recommended that only high-risk individuals and pregnant women be tested (A recommendation, meaning there was high certainty that the net benefit was substantial).
However, in April 2013, based on new evidence regarding the clinical and public health benefits of early identification of HIV infection and subsequent treatment, the USPSTF updated its recommendations. The USPSTF now encourages clinicians to screen all adolescents and adults age 15 to 65 years for HIV (A recommendation).7 Shortly thereafter, the American Academy of Family Physicians (AAFP) also endorsed routine HIV screening, although the AAFP calls for such screening to begin at age 18.8
Insurance now covers it… A USPSTF A recommendation carries significant health policy implications because the Affordable Care Act requires private and public health insurance plans to cover preventive services recommended by USPSTF.9
Integrating screening into your practice
Serologic tests have come a long way. The first HIV antibody test was an enzyme immunoassay (EIA) that was introduced in 1985 and used mainly to screen the blood supply. This first-generation EIA identified only immunoglobulin G (IgG) antibodies to HIV type 1 (HIV-1). More sensitive and specific second- and third-generation EIAs have since been developed to detect both IgG and IgM antibodies, as well as antibodies to HIV-2. The third-generation assays also can detect antibodies as soon as 3 weeks after infection.
The fourth-generation EIAs were approved by the US Food and Drug Administration (FDA) in 201010 and are the first step in the CDC’s current HIV diagnostic testing algorithm. These tests can detect HIV-1/HIV-2 IgG and IgM antibodies and also p24 antigen, which is present within 7 days of the appearance of HIV RNA.11 The fourth-generation assay allows for reliable detection within about 2 weeks of infection (FIGURE 1).10
Rapid HIV tests are also an option.12 These tests can detect IgG and IgM antibodies in samples of saliva, whole blood, serum, and plasma. Results of rapid tests usually are available in 20 to 30 minutes and allow physicians to give patients the results while they are still in the office. In 2013 the FDA approved a combination p24 antigen/antibody rapid HIV assay that according to the manufacturer can detect infection earlier than other currently available rapid tests.13
When rapid tests are most useful. Rapid tests can be particularly useful for testing women presenting in labor who have not been screened for HIV as part of prenatal care. They also can be used to determine the need for postexposure prophylaxis in the event of a needlestick injury. According to manufacturer’s data, the sensitivity of rapid tests ranges from 99.3% to 100% and specificity from 99.7% to 99.9%.12 However, in real-world experience these numbers have been slightly lower.12 By comparison, the sensitivity and specificity of the fourth-generation EIAs are 99.4% and 99.5%, respectively.14
The downside... A disadvantage of rapid HIV testing is that under current FDA-approval status and CDC guidance, tests performed on oral fluid must have serologic confirmation. In addition, patients tested during the “window period” of seroconversion (after infection occurs but before antibodies are detectable) will test negative with rapid HIV tests and must be reminded that repeat testing should be done within 4 to 6 weeks of their last potential exposure to the virus. In high-prevalence settings such as urban emergency departments (EDs), rapid HIV tests have detected a significant number of new infections.15 However, ED physicians and urgent care providers have been reluctant to perform HIV tests due to the lack of follow-up for most patients treated in these settings.
Over-the-counter (OTC) tests. Approved by the FDA in 2012, the OraQuick In-Home HIV Test is the only available OTC test for use at home. Patients can go to the company’s Web site at www.oraquick.com to learn more about HIV and testing, and the company offers 24-hour phone support. It’s not clear how many patients are taking advantage of this home testing option. The test costs approximately $40 and several studies suggest that this price may deter patients from using it.16 In addition, it is not clear how patients who test positive using an OTC test will access medical care or get appropriate medical follow-up.
New testing algorithm eliminates Western blot
Historically, a patient with a reactive (positive) EIA result would undergo the Western blot assay as a confirmatory test. Although the Western blot for HIV is highly specific (99.7%), it tests only for the IgG antibody. This could lead to a false negative test in a patient in whom IgG seroconversion has not yet occurred. Additionally, the time for HIV confirmation with the Western blot often is one week or longer.
Recently, the CDC has made available for public comment a diagnostic algorithm that removes the Western blot as a recommended test (FIGURE 2).17 This algorithm replaces the Western blot with an assay to differentiate HIV-1 and HIV-2 antibodies. Patients for whom this test is negative should undergo additional testing for HIV RNA to determine if HIV-1 is present. Positive HIV RNA would indicate acute or more recent infection. Studies suggest that this new algorithm is better than the existing algorithm at detecting HIV infections, and many reference labs have already adapted it.17,18
Choosing your words carefully when giving patients their results
Patients can be given the results of a rapid HIV test during their visit, but a positive result on a rapid test should be confirmed by serologic testing. When speaking with a patient who tests positive on a rapid test, consider using the phrase “preliminary positive” results. This allows the patient to more easily process the results, knowing that a confirmatory blood draw will be done. State laws vary regarding how patients can receive HIV test results. Most states allow negative serologic test results to be given over the telephone (or electronically). For positive tests, it is preferable to give these results at a face-to-face consultation so that you can ensure the patient will have access to medical care. For more on HIV testing and lab reporting laws by state, see http://www.cdc.gov/hiv/policies/law/states/index.html.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Family and Community Medicine, Lancaster General Hospital, 555 N. Duke Street #3555, Lancaster, PA 17602; jtkirchn@lghealth.org
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.
1. amFAR. Thirty years of HIV/AIDS: Snapshots of an epidemic. amfAR, The Foundation for AIDS Research Web site. Available at: http://www.amfar.org/thirty-years-of-hiv/aids-snapshots-of-anepidemic. Accessed May 9, 2014.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. Published February 2013. Accessed October 19, 2013.
3. Centers for Disease Control and Prevention. HIV in the United States: At a glance. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed May 9, 2014.
4. The White House Office of National AIDS Policy. National HIV/ AIDS Strategy for the United States. AIDS.gov Web site. Available at: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf. Accessed October 25, 2013.
5. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17;quiz CE1-CE4.
6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV testing and diagnosis among adults-- United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550-1555.
7. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
8. Brown M. AAFP, USPSTF recommend routine HIV screening but differ on age to begin. Available at: http://www.aafp.org/news/health-of-the-public/20130429hivscreenrecs.html. Accessed August 17, 2013.
9. Bayer R, Oppenheimer GM. Routine HIV testing, public health, and the USPSTF--an end to the debate. N Engl J Med. 2013;368:881-884
10. Cornett JK, Kirn TJ. Laboratory diagnosis of HIV in adults: a review of current methods. Clin Infect Dis. 2013;57:712-718.
11. Baron EJ, Miller MJ, Weinstein MP, et al. A guide to the utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin Infect Dis. 2013;57:e22-e121.
12. Delaney KP, Branson BM, Uniyal A, et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin Infect Dis. 2011;52:257-263.
13. FDA. FDA approves first rapid diagnostic test to detect both HIV-1 antigen and HIV-1/2 antibodies [press release]. US Food and Drug Administration Web site. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm364480.htm. Silver Spring, MD: US Food and Drug Administration; August 8, 2013. Accessed October 26, 2013.
14. Chavez P, Wesolowski L, Patel P, et al. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab Combo Assay. J Clin Virol. 2011;52:S51-S55.
15. Centers for Disease Control and Prevention (CDC). Rapid HIV testing in emergency departments—three U.S. sites, January 2005-March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597-601.
16. Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57:126-138.
17. Centers for Disease Control and Prevention (CDC). Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62:489-494.
18. Nasrullah M, Wesolowski LG, Meyer WA 3rd, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. AIDS. 2013;27:731-737.