User login
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
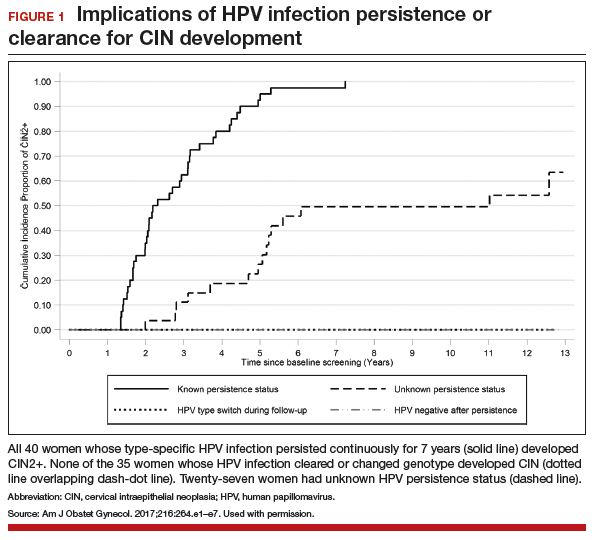
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.
Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).
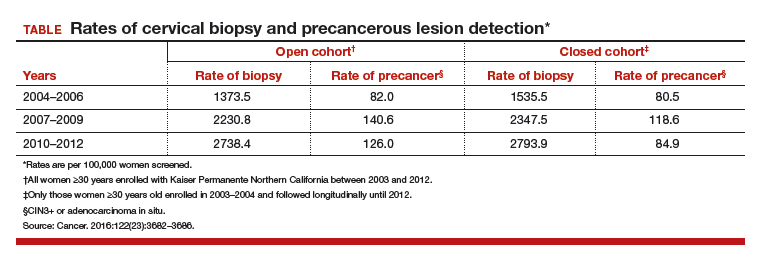
In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
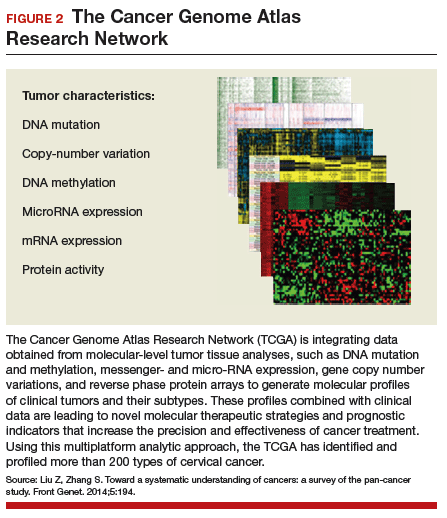
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.

Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).

In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Vaccination against human papillomavirus (HPV) infection and periodic cervical screening have significantly decreased the incidence of invasive cervical cancer. But cancers still exist despite the availability of these useful clinical tools, especially in women of reproductive age in developing regions of the world. In the 2016 update on cervical disease, I reviewed studies on 2 promising and novel immunotherapies for cervical cancer: HPV therapeutic vaccine and adoptive T-cell therapy. This year the focus is on remarkable advances in the field of genomics and related studies that are rapidly expanding our understanding of the molecular characteristics of cervical cancer. Rewards of this research already being explored include novel immunotherapeutic agents as well as the repurposed use of existing drugs.
But first, with regard to cervical screening and follow-up, 2 recent large studies have yielded findings that have important implications for patient management. One pertains to the monitoring of women who have persistent infection with high-risk HPV but cytology results that are negative. Its conclusion was unequivocal and very useful in the management of our patients. The other study tracked HPV screening performed every 3 years and reported on the diagnostic efficiency of this shorter interval screening strategy.
Read about persistent HPV infection and CIN
Persistent HPV infection has a higher risk than most clinicians might think
Elfgren K, Elfström KM, Naucler P, Arnheim-Dahlström L, Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):264.e1-e7.
It is well known that most cases of cervical cancer arise from persistent HPV infection, with the highest percentage of cancers caused by high-risk types 16 or 18. What has been uncertain, however, is the actual degree of risk that persistent infection confers over time for the development of cervical intraepithelial neoplasia (CIN) or worse when a woman's repeated cytology reports are negative. In an analysis of a long-term double-blind, randomized, controlled screening study, Elfgren and colleagues showed that all women whose HPV infection persisted up to 7 years developed CIN grade 2 (CIN2+), while those whose infection cleared in that period, or changed genotype, had no precancerous lesions out to 13 years of follow-up.
Related Article:
It is time for HPV vaccination to be considered part of routine preventive health care
Details of the study
Between 1997 and 2000, 12,527 Swedish women between the ages of 32 and 38 years who were undergoing organized cervical cancer screening agreed to participate in a 1:1-randomized prospective trial to determine the benefit of screening with HPV and cytology (intervention group) compared with cytology screening alone (control group). However, brush sampling for HPV was performed even on women in the control group, with the samples frozen for later testing. All participants were identified in the Swedish National Cervical Screening Registry.
Women in the intervention group who initially tested positive for HPV but whose cytology test results were negative (n = 341) were invited to return a year later for repeat HPV testing; 270 women returned and 119 had type-specific HPV persistence. Of those with persistent infection, 100 agreed to undergo colposcopy; 111 women from the control group were randomly selected to undergo sham HPV testing and colposcopy, and 95 attended. Women with evident cytologic abnormalities received treatment per protocol. Those with negative cytology results were offered annual HPV testing thereafter, and each follow-up with documented type-specific HPV persistence led to repeat colposcopy. A comparable number of women from the control group had repeat colposcopies.
Although some women were lost to clinical follow-up throughout the trial, all 195 who attended the first colposcopy were followed for at least 5 years in the Swedish registry, and 191 were followed in the registry for 13 years. Of 102 women with known HPV persistence at baseline (100 in the treatment group; 2 in the randomly selected control group), 31 became HPV negative, 4 evidenced a switch in HPV type but cleared the initial infection, 27 had unknown persistence status due to missed HPV tests, and 40 had continuously type-specific persistence. Of note, persistent HPV16 infection seemed to impart a higher risk of CIN development than did persistent HPV18 infection.
All 40 participants with clinically verified continuously persistent HPV infection developed CIN2+ within 7 years of baseline documentation of persistence (FIGURE 1). Among the 27 women with unknown persistence status, risk of CIN2+ occurrence within 7 years was 50%. None of the 35 women who cleared their infection or switched HPV type developed CIN2+.

Read about HPV-cytology cotesting
HPV−cytology cotesting every 3 years lowers population rates of cervical precancer and cancer
Silver MI, Schiffman M, Fetterman B, et al. The population impact of human papillomavirus/cytology cervical cotesting at 3-year intervals: reduced cervical cancer risk and decreased yield of precancer per screen. Cancer. 2016;122(23):3682−3686.
Current guidelines on screening for cervical cancer in women 30 to 65 years of age advise the preferred strategy of using cytology alone every 3 years or combining HPV testing and cytology every 5 years.1 These guidelines, based on data available at the time they were written, were meant to offer a reasonable balance between timely detection of abnormalities and avoidance of potential harms from screening too frequently. However, many patients are reluctant to postpone repeat testing to the extent recommended. Several authorities have in fact asked that screening intervals be revisited, perhaps allowing for a range of strategies, contending that the level of protection once provided by annual screening should be the benchmark by which evolving strategies are judged.2 Today, they point out, the risk of cancer doubles in the 3 years following an initial negative cytology result, and it also increases by lengthening the cotesting interval from 3 to 5 years. They additionally question the validity of using frequency of colposcopies as a surrogate to measure harms of screening, and suggest that many women would willingly accept the procedure's minimal discomfort and inconvenience to gain peace of mind.
The study by Silver and colleagues gives credence to considering a shorter cotesting interval. Since 2003, Kaiser Permanente Northern California (KPNC) has implemented 3-year cotesting. To determine actual clinical outcomes of cotesting at this interval, KPNC analyzed data on more than 1 million women in its care between 2003 and 2012. Although investigators expected that they might see decreasing efficiency in cotesting over time, they instead found an increased detection rate of precancerous lesions per woman screened in the larger of 2 study cohorts.
Related Article:
Women’s Preventive Services Initiative Guidelines provide consensus for practicing ObGyns
Details of the study
Included were all women 30 years of age or older enrolled in this study at KPNC between 2003 and 2012 who underwent HPV−cytology cotesting every 3 years. The population in its entirety (1,065,273 women) was deemed the "open cohort" and represented KPNC's total annual experience. A subset of this population, the "closed cohort," was designed to gauge the effect of repeated screening on a fixed population and comprised only those women enrolled and initially screened between 2003 and 2004 and then followed longitudinally until 2012.
For each cohort, investigators calculated the ratios of precancer and cancer diagnoses to the total number of cotests performed on the cohort's population. The 3-year testing periods were 2004−2006, 2007−2009, and 2010−2012. Also calculated in these periods were the ratios of colposcopic biopsies to cotests and the rates of precancer diagnoses (TABLE).

In the open cohort, the biopsy rate nearly doubled over the course of the study. Precancer diagnoses per number of cotests rose by 71.5% between the first and second testing periods (P = .001) and then eased off by 10% in the third period (P<.001). These corresponding increases throughout the study yielded a stable number of biopsies (16 to 22) needed to detect precancer.
In the closed long-term cohort, the biopsy rate rose, but not as much as in the open cohort. Precancer diagnoses per number of cotests rose by 47% between the first and second periods (P≤.001), but in the third period fell back by 28% (P<.001) to a level just above the first period results. The number of biopsies needed to detect a precancerous lesion in the closed cohort rose from 19 to 33 over the course of the study, suggesting there may have been some loss of screening efficiency in the fixed group.
Read about molecular profiling of cervical cancer
Molecular profiling of cervical cancer is revolutionizing treatment
The Cancer Genome Atlas Research Network. Integratedgenomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378−384.
Effective treatments for cervical cancer could be close at hand, thanks to a recent explosion of knowledge at the molecular level about how specific cancers arise and what drives them other than HPV. The Cancer Genome Atlas Research Network (TCGA) recently published the results of its genomic and proteomic analyses, which yielded distinct profiles for 178 cervical cancers with important patterns common to other cancers, such as uterine and breast cancer. These recently published findings on cervical cancer highlight areas of gene and protein dysfunction it shares with these other cancers, which could open the doors for new targets for treatments already developed or in the pipeline.
Related Article:
2016 Update on cervical disease
How molecular profiling is paying off for cervical cancer
Cancers develop in any given tissue through the altered function of different genes and signaling pathways in the tissue's cells. The latest extensive investigation conducted by the TCGA network has identified significant mutations in 5 genes previously unrecognized in association with cervical cancer, bringing the total now to 14.
Several highlights are featured in the TCGA's recently published work. One discovery is the amplification of genes CD274 and PDCD1LG2, which are involved with the expression of 2 cytolytic effector genes and are therefore likely targets for immunotherapeutic strategies. Another line of exploration, whole-genome sequencing, has detected an aberration in some cervical cancer tissue with the potential for immediate application. Duplication and copy number gain of BCAR4, a noncoding RNA, facilitates cell proliferation through the HER2/HER3 pathway, a target of the tyrosine-kinase inhibitor, lapatinib, which is currently used to treat breast cancer.
The integration of data from multiple layers of analysis (FIGURE 2) is helping investigators identify variations in cancers. DNA methylation, for instance, is a means by which cells control gene expression. An analysis of this process in cervical tumor tissue has revealed additional cancer subgroups in which messenger RNA increases the transition of epithelial cells to invasive mesenchymal cells. Targeting that process in these subgroups would likely enhance the effectiveness of novel small-molecule inhibitors and some standard cytotoxic chemotherapy.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542.
- Kinney W, Wright TC, Dinkelspiel HE, DeFrancesco M, Thomas Cox J, Huh W. Increased cervical cancer risk associated with screening at longer intervals. Obstet Gynecol. 2015;125(2):311–315.

