User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AGA guideline defines role of biomarkers in ulcerative colitis
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
FROM GASTROENTEROLOGY
Meningococcal vaccine shows benefit in STI prevention
The latest study to show high efficacy of doxycycline post-exposure prophylaxis (Doxy PEP) in preventing sexually transmitted infections among men who have sex with men (MSM) adds a new twist, showing – for the first time – reductions in gonorrhea among those receiving the meningococcal B vaccine.
said first author Jean-Michel Molina, MD, PhD, in presenting the findings at the Conference on Retroviruses and Opportunistic Infections.
In addition, “two doses of the meningococcal B vaccine reduced the incidence of a first episode of gonorrhea by roughly 50% among men who have sex with men,” said Dr. Molina, a professor of infectious diseases at the University of Paris, and head of the Infectious Diseases Department at the Saint-Louis and Lariboisière Hospitals, Paris.
Whereas the advent of PrEP has been associated with significant reductions in HIV transmission, rates of STIs have conversely been on the rise among MSM, specifically among those receiving PrEP.
Post-exposure prophylaxis with Doxy PEP has been shown to reduce the incidence of chlamydia and syphilis by approximately 70%; however, effects on prevention of gonorrhea have been less clear.
Meningococcal B vaccination has, meanwhile, shown intriguing reductions of gonorrhea incidence of as much as 26%-46% in some observational studies.
Therefore, Dr. Molina and colleagues decided to further investigate Doxy PEP as well as the meningococcal B vaccine in prevention of STIs.
For the ANRS 174 DOXYVAC trial, they enrolled 546 MSM in the open-label, multicenter study between January 2021 and July 2022.
The men were randomly assigned to one of 4 groups: doxycycline postexposure prophylaxis (Doxy PEP: 200 mg; n = 332), no Doxy PEP (n = 170), two shots of meningococcal B vaccine (4CMenB vaccine; n = 257), or no 4CMenB vaccine (n = 245).
All participants were assigned to their groups within 72 hours of condomless sex.
The men, who had a median age of 39, had a median time of PrEP use of 42 months, a history of an STI in the past year, and their median number of sexual partners in the past 3 months was 10.
Their characteristics were well-balanced across the treatment groups. After discontinuations of 54 patients across the groups, the final analysis included 502 participants.
With a median follow-up of 9 months, the intent-to-treat analysis showed 13 subjects had a first episode of chlamydia or syphilis in the Doxy PEP group, versus 49 subjects infected in the no Doxy PEP arm, for an incidence of 5.6 versus 35.4 per 100 person-years, respectively (adjusted hazard ratio, 0.16; P < .0001).
Infection specifically with chlamydia occurred among 21 men with no Doxy PEP versus 5 receiving Dox PEP (19.3 vs. 2.1 per 100 person-years, respectively; HR, 0.11; P < .0001).
And infection with syphilis occurred in 18 men receiving no Doxy PEP versus 8 receiving the treatment (16.3 vs. 3.4 per 100 person-years, respectively; HR, 0.21; P < .001).
The corresponding rates for gonorrhea infection were an incidence 41.3 versus 20.5 per 100 person-years, in the no Doxy PEP versus Doxy PEP arms, respectively (adjusted HR, 0.49; P = .001), and 29.4 versus 16.8 per 100 person-years for Mycoplasma genitalium infection (aHR, 0.55; P = .015).
Throughout the study, about 80% of patients in the Doxy PEP group reported using the prophylaxis treatment after their most recent sexual intercourse, with subjects reporting taking a median of seven pills per month.
In the vaccine/no vaccine comparisons, 32 subjects in the no meningococcal vaccine group were infected with a first gonorrhea infection, compared with 17 in the vaccine group, representing an incidence of 19.7 versus 9.8 per 100 person-years, respectively (adjusted HR, 0.49; P = .016), which Dr. Molina called “highly significant.”
An analysis of the cumulative incidence of gonorrhea infection with the meningococcal vaccine showed rates in the no vaccine versus vaccine groups of 30.4 versus 20.1 per 100 person-years, respectively; however, statistical significance was not reached (aHR, 0.66; P = .052).
Importantly, there were no significant interactions in the results between those receiving Doxy PEP or the 4CMenB vaccine group, and there were no significant differences in drug-related serious adverse events between the groups.
Dr. Molina noted that the meningococcal B vaccine is known to contain key antigens that are shared between meningitis and gonorrhea, which could explain the benefits.
Although chlamydia and syphilis thus far appear to remain susceptible to Doxy PEP, resistances with gonorrhea remain a concern, hence the ability of the vaccine to provide some protection could be an added bonus.
“We know that [gonorrhea] is able to very quickly develop resistances to any antibiotics, so that was why we wanted to look beyond the antibiotic prophylaxis,” said Dr. Molina.
Among questions to explore looking ahead is the potential longevity of protection with the vaccine.
“We don’t know at this point how long the protection with the vaccine could last, or if [people] may need booster injections, for instance, but the literature suggests benefits for at least a year,” Dr. Molina said. “We are still monitoring the patients in the study to see what happens.”
He added that combination of the interventions may be of benefit.
“In the future, we think we may need to combine these approaches if we want to meet the WHO/UNAIDS targets to reduce the incidence of HIV and STIs by 90% by 2030.”
Commenting on the study, CROI vice-chair Landon Myer, MD, PhD, noted that “gonorrhea develops resistance quickly and can be hard to treat or prophylaxis, so the vaccine finding, which was hinted at by previous observational data, is really important.”
He agrees that “the duration of protective efficacy – a big thing in vaccines – is unknown.”
“Still, this is really significant,” Dr. Myer stressed. “An efficacious vaccine against a stubborn sexually transmitted infection.”
A version of this article first appeared on Medscape.com.
The latest study to show high efficacy of doxycycline post-exposure prophylaxis (Doxy PEP) in preventing sexually transmitted infections among men who have sex with men (MSM) adds a new twist, showing – for the first time – reductions in gonorrhea among those receiving the meningococcal B vaccine.
said first author Jean-Michel Molina, MD, PhD, in presenting the findings at the Conference on Retroviruses and Opportunistic Infections.
In addition, “two doses of the meningococcal B vaccine reduced the incidence of a first episode of gonorrhea by roughly 50% among men who have sex with men,” said Dr. Molina, a professor of infectious diseases at the University of Paris, and head of the Infectious Diseases Department at the Saint-Louis and Lariboisière Hospitals, Paris.
Whereas the advent of PrEP has been associated with significant reductions in HIV transmission, rates of STIs have conversely been on the rise among MSM, specifically among those receiving PrEP.
Post-exposure prophylaxis with Doxy PEP has been shown to reduce the incidence of chlamydia and syphilis by approximately 70%; however, effects on prevention of gonorrhea have been less clear.
Meningococcal B vaccination has, meanwhile, shown intriguing reductions of gonorrhea incidence of as much as 26%-46% in some observational studies.
Therefore, Dr. Molina and colleagues decided to further investigate Doxy PEP as well as the meningococcal B vaccine in prevention of STIs.
For the ANRS 174 DOXYVAC trial, they enrolled 546 MSM in the open-label, multicenter study between January 2021 and July 2022.
The men were randomly assigned to one of 4 groups: doxycycline postexposure prophylaxis (Doxy PEP: 200 mg; n = 332), no Doxy PEP (n = 170), two shots of meningococcal B vaccine (4CMenB vaccine; n = 257), or no 4CMenB vaccine (n = 245).
All participants were assigned to their groups within 72 hours of condomless sex.
The men, who had a median age of 39, had a median time of PrEP use of 42 months, a history of an STI in the past year, and their median number of sexual partners in the past 3 months was 10.
Their characteristics were well-balanced across the treatment groups. After discontinuations of 54 patients across the groups, the final analysis included 502 participants.
With a median follow-up of 9 months, the intent-to-treat analysis showed 13 subjects had a first episode of chlamydia or syphilis in the Doxy PEP group, versus 49 subjects infected in the no Doxy PEP arm, for an incidence of 5.6 versus 35.4 per 100 person-years, respectively (adjusted hazard ratio, 0.16; P < .0001).
Infection specifically with chlamydia occurred among 21 men with no Doxy PEP versus 5 receiving Dox PEP (19.3 vs. 2.1 per 100 person-years, respectively; HR, 0.11; P < .0001).
And infection with syphilis occurred in 18 men receiving no Doxy PEP versus 8 receiving the treatment (16.3 vs. 3.4 per 100 person-years, respectively; HR, 0.21; P < .001).
The corresponding rates for gonorrhea infection were an incidence 41.3 versus 20.5 per 100 person-years, in the no Doxy PEP versus Doxy PEP arms, respectively (adjusted HR, 0.49; P = .001), and 29.4 versus 16.8 per 100 person-years for Mycoplasma genitalium infection (aHR, 0.55; P = .015).
Throughout the study, about 80% of patients in the Doxy PEP group reported using the prophylaxis treatment after their most recent sexual intercourse, with subjects reporting taking a median of seven pills per month.
In the vaccine/no vaccine comparisons, 32 subjects in the no meningococcal vaccine group were infected with a first gonorrhea infection, compared with 17 in the vaccine group, representing an incidence of 19.7 versus 9.8 per 100 person-years, respectively (adjusted HR, 0.49; P = .016), which Dr. Molina called “highly significant.”
An analysis of the cumulative incidence of gonorrhea infection with the meningococcal vaccine showed rates in the no vaccine versus vaccine groups of 30.4 versus 20.1 per 100 person-years, respectively; however, statistical significance was not reached (aHR, 0.66; P = .052).
Importantly, there were no significant interactions in the results between those receiving Doxy PEP or the 4CMenB vaccine group, and there were no significant differences in drug-related serious adverse events between the groups.
Dr. Molina noted that the meningococcal B vaccine is known to contain key antigens that are shared between meningitis and gonorrhea, which could explain the benefits.
Although chlamydia and syphilis thus far appear to remain susceptible to Doxy PEP, resistances with gonorrhea remain a concern, hence the ability of the vaccine to provide some protection could be an added bonus.
“We know that [gonorrhea] is able to very quickly develop resistances to any antibiotics, so that was why we wanted to look beyond the antibiotic prophylaxis,” said Dr. Molina.
Among questions to explore looking ahead is the potential longevity of protection with the vaccine.
“We don’t know at this point how long the protection with the vaccine could last, or if [people] may need booster injections, for instance, but the literature suggests benefits for at least a year,” Dr. Molina said. “We are still monitoring the patients in the study to see what happens.”
He added that combination of the interventions may be of benefit.
“In the future, we think we may need to combine these approaches if we want to meet the WHO/UNAIDS targets to reduce the incidence of HIV and STIs by 90% by 2030.”
Commenting on the study, CROI vice-chair Landon Myer, MD, PhD, noted that “gonorrhea develops resistance quickly and can be hard to treat or prophylaxis, so the vaccine finding, which was hinted at by previous observational data, is really important.”
He agrees that “the duration of protective efficacy – a big thing in vaccines – is unknown.”
“Still, this is really significant,” Dr. Myer stressed. “An efficacious vaccine against a stubborn sexually transmitted infection.”
A version of this article first appeared on Medscape.com.
The latest study to show high efficacy of doxycycline post-exposure prophylaxis (Doxy PEP) in preventing sexually transmitted infections among men who have sex with men (MSM) adds a new twist, showing – for the first time – reductions in gonorrhea among those receiving the meningococcal B vaccine.
said first author Jean-Michel Molina, MD, PhD, in presenting the findings at the Conference on Retroviruses and Opportunistic Infections.
In addition, “two doses of the meningococcal B vaccine reduced the incidence of a first episode of gonorrhea by roughly 50% among men who have sex with men,” said Dr. Molina, a professor of infectious diseases at the University of Paris, and head of the Infectious Diseases Department at the Saint-Louis and Lariboisière Hospitals, Paris.
Whereas the advent of PrEP has been associated with significant reductions in HIV transmission, rates of STIs have conversely been on the rise among MSM, specifically among those receiving PrEP.
Post-exposure prophylaxis with Doxy PEP has been shown to reduce the incidence of chlamydia and syphilis by approximately 70%; however, effects on prevention of gonorrhea have been less clear.
Meningococcal B vaccination has, meanwhile, shown intriguing reductions of gonorrhea incidence of as much as 26%-46% in some observational studies.
Therefore, Dr. Molina and colleagues decided to further investigate Doxy PEP as well as the meningococcal B vaccine in prevention of STIs.
For the ANRS 174 DOXYVAC trial, they enrolled 546 MSM in the open-label, multicenter study between January 2021 and July 2022.
The men were randomly assigned to one of 4 groups: doxycycline postexposure prophylaxis (Doxy PEP: 200 mg; n = 332), no Doxy PEP (n = 170), two shots of meningococcal B vaccine (4CMenB vaccine; n = 257), or no 4CMenB vaccine (n = 245).
All participants were assigned to their groups within 72 hours of condomless sex.
The men, who had a median age of 39, had a median time of PrEP use of 42 months, a history of an STI in the past year, and their median number of sexual partners in the past 3 months was 10.
Their characteristics were well-balanced across the treatment groups. After discontinuations of 54 patients across the groups, the final analysis included 502 participants.
With a median follow-up of 9 months, the intent-to-treat analysis showed 13 subjects had a first episode of chlamydia or syphilis in the Doxy PEP group, versus 49 subjects infected in the no Doxy PEP arm, for an incidence of 5.6 versus 35.4 per 100 person-years, respectively (adjusted hazard ratio, 0.16; P < .0001).
Infection specifically with chlamydia occurred among 21 men with no Doxy PEP versus 5 receiving Dox PEP (19.3 vs. 2.1 per 100 person-years, respectively; HR, 0.11; P < .0001).
And infection with syphilis occurred in 18 men receiving no Doxy PEP versus 8 receiving the treatment (16.3 vs. 3.4 per 100 person-years, respectively; HR, 0.21; P < .001).
The corresponding rates for gonorrhea infection were an incidence 41.3 versus 20.5 per 100 person-years, in the no Doxy PEP versus Doxy PEP arms, respectively (adjusted HR, 0.49; P = .001), and 29.4 versus 16.8 per 100 person-years for Mycoplasma genitalium infection (aHR, 0.55; P = .015).
Throughout the study, about 80% of patients in the Doxy PEP group reported using the prophylaxis treatment after their most recent sexual intercourse, with subjects reporting taking a median of seven pills per month.
In the vaccine/no vaccine comparisons, 32 subjects in the no meningococcal vaccine group were infected with a first gonorrhea infection, compared with 17 in the vaccine group, representing an incidence of 19.7 versus 9.8 per 100 person-years, respectively (adjusted HR, 0.49; P = .016), which Dr. Molina called “highly significant.”
An analysis of the cumulative incidence of gonorrhea infection with the meningococcal vaccine showed rates in the no vaccine versus vaccine groups of 30.4 versus 20.1 per 100 person-years, respectively; however, statistical significance was not reached (aHR, 0.66; P = .052).
Importantly, there were no significant interactions in the results between those receiving Doxy PEP or the 4CMenB vaccine group, and there were no significant differences in drug-related serious adverse events between the groups.
Dr. Molina noted that the meningococcal B vaccine is known to contain key antigens that are shared between meningitis and gonorrhea, which could explain the benefits.
Although chlamydia and syphilis thus far appear to remain susceptible to Doxy PEP, resistances with gonorrhea remain a concern, hence the ability of the vaccine to provide some protection could be an added bonus.
“We know that [gonorrhea] is able to very quickly develop resistances to any antibiotics, so that was why we wanted to look beyond the antibiotic prophylaxis,” said Dr. Molina.
Among questions to explore looking ahead is the potential longevity of protection with the vaccine.
“We don’t know at this point how long the protection with the vaccine could last, or if [people] may need booster injections, for instance, but the literature suggests benefits for at least a year,” Dr. Molina said. “We are still monitoring the patients in the study to see what happens.”
He added that combination of the interventions may be of benefit.
“In the future, we think we may need to combine these approaches if we want to meet the WHO/UNAIDS targets to reduce the incidence of HIV and STIs by 90% by 2030.”
Commenting on the study, CROI vice-chair Landon Myer, MD, PhD, noted that “gonorrhea develops resistance quickly and can be hard to treat or prophylaxis, so the vaccine finding, which was hinted at by previous observational data, is really important.”
He agrees that “the duration of protective efficacy – a big thing in vaccines – is unknown.”
“Still, this is really significant,” Dr. Myer stressed. “An efficacious vaccine against a stubborn sexually transmitted infection.”
A version of this article first appeared on Medscape.com.
FROM CROI 2023
COVID vs. flu: Which is deadlier?
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
a new study shows.
People who were hospitalized with Omicron COVID-19 infections were 54% more likely to die, compared with people who were hospitalized with the flu, Swiss researchers found.
The results of the study continue to debunk an earlier belief from the start of the pandemic that the flu was the more dangerous of the two respiratory viruses. The researchers noted that the deadliness of COVID-19, compared with flu, persisted “despite virus evolution and improved management strategies.”
The study was published in JAMA Network Open and included 5,212 patients in Switzerland hospitalized with COVID-19 or the flu. All the COVID patients were infected with the Omicron variant and hospitalized between Jan. 15, 2022, and March 15, 2022. Flu data included cases from January 2018 to March 15, 2022.
Overall, 7% of COVID-19 patients died, compared with 4.4% of flu patients. Researchers noted that the death rate for hospitalized COVID patients had declined since their previous study, which was conducted during the first COVID wave in the first half of 2020. At that time, the death rate of hospitalized COVID patients was 12.8%.
Since then, 98% of the Swiss population has been vaccinated. “Vaccination still plays a significant role regarding the main outcome,” the authors concluded, since a secondary analysis in this most recent study showed that unvaccinated COVID patients were twice as likely to die, compared with flu patients.
“Our results demonstrate that COVID-19 still cannot simply be compared with influenza,” they wrote.
While the death rate among COVID patients was significantly higher, there was no difference in the rate that COVID or flu patients were admitted to the ICU, which was around 8%.
A limitation of the study was that all the COVID cases did not have laboratory testing to confirm the Omicron variant. However, the study authors noted that Omicron accounted for at least 95% of cases during the time the patients were hospitalized. The authors were confident that their results were not biased by the potential for other variants being included in the data.
Four coauthors reported receiving grants and personal fees from various sources.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Dietitian-led weight loss program improves difficult-to-treat asthma in obese patients
In a proof-of-concept feasibility study among adults with difficult-to-treat asthma and body mass index ≥ 30kg/m2, an evidence-based, dietitian-led program resulted in clinically important improvements in asthma control and quality of life over 16 weeks compared to usual care.
The Counterweight-Plus weight management program (CWP) used in the study includes 12 weeks of total diet replacement (TDR), stepwise food reintroduction in weeks 13-18, and weight loss maintenance up to 1 year, according to a report by Varun Sharma, MBChB, and fellow researchers at University of Glasgow.
Difficult-to-treat asthma, found among about 17% of asthma-affected patients, may be attributed to factors such as poor inhaler technique, treatment nonadherence, and comorbidities such as obesity. Obesity is frequently associated with difficult-to-treat, uncontrolled asthma and increased morbidity and mortality. Among multifactorial effects of obesity on asthma are direct ones on thoracic wall mechanics, increased airway closure, airway hyper-responsiveness and airway inflammation. Prior research showing that weight loss may improve asthma outcomes has been conducted among heterogeneous asthma populations, with no clear consensus regarding optimal methods of weight management, according to the authors.
They tested whether use of the CWP compared to usual care (1:1) would improve asthma control and quality of life in this population of patients with obesity. The TDR phase comprised a low-energy liquid diet consisting of 825-853 kcal/day (approximately 59% carbohydrate, 13% fat, 26% protein, 2% fiber), with meals supplied dried in sachets by the dietitian team and reconstituted with water by the participants. A review by the dietitian team at 1 week was followed by reviews every other week.
The primary outcome was difference in change in Asthma Control Questionnaire (ACQ6) from baseline (visit 1) to 16 weeks (visit 2), between CWP and usual care.
The single-center trial included 33 evaluable adult patients (75 years or younger; mean age 53 years; 63% women) with asthma (as per Global Initiative for Asthma guidelines) that was difficult to treat (as per Scottish Intercollegiate Guidelines Network/British Thoracic Society guidelines). The study population consisted of patients with frequent exacerbations with uncontrolled disease as reflected by the median interquartile range (IQR) for oral corticosteroid courses in the previous 12 months of 3 (2 to 5) and mean ACQ6 of 2.8 (2.4 to 3.1). Mean overall Asthma Quality of Life Questionnaire (AQLQ) was 3.8 (3.4 to 4.2). Median weight was 101.7 (91.4 to 118.7) kg, with a median BMI of 37.5 (35.0 to 42.3) kg/m2. Recruitment was discontinued before the target of 40 patients because the CWP dropout rate (n = 2) was lower than expected.
The researchers reported that the mean change in ACQ6 over 16 weeks was –0.45 for CWP and 0.23 for usual care with a mean difference of –0.69 (P = .048) between groups. The secondary outcome of mean change in overall AQLQ was 0.81 for CWP and 0.08 for usual care with a mean difference of 0.76 (P = .013) between groups.
No unexpected serious adverse events or intervention-related adverse events were observed during the trial.
“In this pragmatic open label, randomized, controlled trial we showed that delivery of a supported low-calorie total diet replacement program (Counterweight-Plus) to patients with difficult-to treat asthma and obesity, was safe and led to significant improvements in asthma control and quality of life compared to usual care over 16 weeks,” the authors wrote.
“Findings from the study are a welcome addition to this field of study,” Diego J. Maselli, MD, associate professor of medicine and interim chief, division of pulmonary diseases and critical care, UT Health at San Antonio, said in an interview. “ Obesity is an important comorbid condition because, although by itself it may have an effect on asthma patients, it is also associated with other comorbidities such as gastroesophageal reflux disease, obstructive sleep apnea, anxiety, depression, and others that in turn can affect asthmatics. Also, obesity may influence pulmonary physiology and it’s considered a proinflammatory state by many, and this can favor uncontrolled disease.”
While underscoring the clinically relevant weight loss and improvements in ACQ6 and AQLQ, Dr. Maselli said that the study did not follow the patients long enough to determine if weight loss was associated with a reduction in exacerbations and other long-term outcomes in asthma such as resource utilization and changes in maintenance medications, which may be explored in future studies.
“It remains to be seen if the weight loss of these types of programs can be sustained over longer periods of time, given the considerable caloric restriction in the initial stages of the weight reduction program. Interestingly, the majority of the patients in the study did not exhibit features of type 2 inflammation and had low-T2 endotype with low eosinophil count and low FeNO [fractional exhaled nitric oxide],” Dr. Maselli added. “Although obesity has been linked to this phenotype, the vast majority of [people with asthma], about 80%, have high T2 phenotype. Future studies are still need with larger and more representative samples and with longer follow-up times to determine the effects of weight loss on asthma outcomes, especially in severe asthma,” he concluded.
The trial was funded by an NHS Greater Glasgow and Clyde Endowment Fund grant. Several of the authors reported having received travel awards to attend conferences and funding from Cambridge Weight Plan and one author is an employee of and another a medical adviser for Counterweight Ltd., the developer of the program used. Other authors reported receiving funding from a variety of pharmaceutical companies. Dr. Maselli reported no relevant conflicts.
In a proof-of-concept feasibility study among adults with difficult-to-treat asthma and body mass index ≥ 30kg/m2, an evidence-based, dietitian-led program resulted in clinically important improvements in asthma control and quality of life over 16 weeks compared to usual care.
The Counterweight-Plus weight management program (CWP) used in the study includes 12 weeks of total diet replacement (TDR), stepwise food reintroduction in weeks 13-18, and weight loss maintenance up to 1 year, according to a report by Varun Sharma, MBChB, and fellow researchers at University of Glasgow.
Difficult-to-treat asthma, found among about 17% of asthma-affected patients, may be attributed to factors such as poor inhaler technique, treatment nonadherence, and comorbidities such as obesity. Obesity is frequently associated with difficult-to-treat, uncontrolled asthma and increased morbidity and mortality. Among multifactorial effects of obesity on asthma are direct ones on thoracic wall mechanics, increased airway closure, airway hyper-responsiveness and airway inflammation. Prior research showing that weight loss may improve asthma outcomes has been conducted among heterogeneous asthma populations, with no clear consensus regarding optimal methods of weight management, according to the authors.
They tested whether use of the CWP compared to usual care (1:1) would improve asthma control and quality of life in this population of patients with obesity. The TDR phase comprised a low-energy liquid diet consisting of 825-853 kcal/day (approximately 59% carbohydrate, 13% fat, 26% protein, 2% fiber), with meals supplied dried in sachets by the dietitian team and reconstituted with water by the participants. A review by the dietitian team at 1 week was followed by reviews every other week.
The primary outcome was difference in change in Asthma Control Questionnaire (ACQ6) from baseline (visit 1) to 16 weeks (visit 2), between CWP and usual care.
The single-center trial included 33 evaluable adult patients (75 years or younger; mean age 53 years; 63% women) with asthma (as per Global Initiative for Asthma guidelines) that was difficult to treat (as per Scottish Intercollegiate Guidelines Network/British Thoracic Society guidelines). The study population consisted of patients with frequent exacerbations with uncontrolled disease as reflected by the median interquartile range (IQR) for oral corticosteroid courses in the previous 12 months of 3 (2 to 5) and mean ACQ6 of 2.8 (2.4 to 3.1). Mean overall Asthma Quality of Life Questionnaire (AQLQ) was 3.8 (3.4 to 4.2). Median weight was 101.7 (91.4 to 118.7) kg, with a median BMI of 37.5 (35.0 to 42.3) kg/m2. Recruitment was discontinued before the target of 40 patients because the CWP dropout rate (n = 2) was lower than expected.
The researchers reported that the mean change in ACQ6 over 16 weeks was –0.45 for CWP and 0.23 for usual care with a mean difference of –0.69 (P = .048) between groups. The secondary outcome of mean change in overall AQLQ was 0.81 for CWP and 0.08 for usual care with a mean difference of 0.76 (P = .013) between groups.
No unexpected serious adverse events or intervention-related adverse events were observed during the trial.
“In this pragmatic open label, randomized, controlled trial we showed that delivery of a supported low-calorie total diet replacement program (Counterweight-Plus) to patients with difficult-to treat asthma and obesity, was safe and led to significant improvements in asthma control and quality of life compared to usual care over 16 weeks,” the authors wrote.
“Findings from the study are a welcome addition to this field of study,” Diego J. Maselli, MD, associate professor of medicine and interim chief, division of pulmonary diseases and critical care, UT Health at San Antonio, said in an interview. “ Obesity is an important comorbid condition because, although by itself it may have an effect on asthma patients, it is also associated with other comorbidities such as gastroesophageal reflux disease, obstructive sleep apnea, anxiety, depression, and others that in turn can affect asthmatics. Also, obesity may influence pulmonary physiology and it’s considered a proinflammatory state by many, and this can favor uncontrolled disease.”
While underscoring the clinically relevant weight loss and improvements in ACQ6 and AQLQ, Dr. Maselli said that the study did not follow the patients long enough to determine if weight loss was associated with a reduction in exacerbations and other long-term outcomes in asthma such as resource utilization and changes in maintenance medications, which may be explored in future studies.
“It remains to be seen if the weight loss of these types of programs can be sustained over longer periods of time, given the considerable caloric restriction in the initial stages of the weight reduction program. Interestingly, the majority of the patients in the study did not exhibit features of type 2 inflammation and had low-T2 endotype with low eosinophil count and low FeNO [fractional exhaled nitric oxide],” Dr. Maselli added. “Although obesity has been linked to this phenotype, the vast majority of [people with asthma], about 80%, have high T2 phenotype. Future studies are still need with larger and more representative samples and with longer follow-up times to determine the effects of weight loss on asthma outcomes, especially in severe asthma,” he concluded.
The trial was funded by an NHS Greater Glasgow and Clyde Endowment Fund grant. Several of the authors reported having received travel awards to attend conferences and funding from Cambridge Weight Plan and one author is an employee of and another a medical adviser for Counterweight Ltd., the developer of the program used. Other authors reported receiving funding from a variety of pharmaceutical companies. Dr. Maselli reported no relevant conflicts.
In a proof-of-concept feasibility study among adults with difficult-to-treat asthma and body mass index ≥ 30kg/m2, an evidence-based, dietitian-led program resulted in clinically important improvements in asthma control and quality of life over 16 weeks compared to usual care.
The Counterweight-Plus weight management program (CWP) used in the study includes 12 weeks of total diet replacement (TDR), stepwise food reintroduction in weeks 13-18, and weight loss maintenance up to 1 year, according to a report by Varun Sharma, MBChB, and fellow researchers at University of Glasgow.
Difficult-to-treat asthma, found among about 17% of asthma-affected patients, may be attributed to factors such as poor inhaler technique, treatment nonadherence, and comorbidities such as obesity. Obesity is frequently associated with difficult-to-treat, uncontrolled asthma and increased morbidity and mortality. Among multifactorial effects of obesity on asthma are direct ones on thoracic wall mechanics, increased airway closure, airway hyper-responsiveness and airway inflammation. Prior research showing that weight loss may improve asthma outcomes has been conducted among heterogeneous asthma populations, with no clear consensus regarding optimal methods of weight management, according to the authors.
They tested whether use of the CWP compared to usual care (1:1) would improve asthma control and quality of life in this population of patients with obesity. The TDR phase comprised a low-energy liquid diet consisting of 825-853 kcal/day (approximately 59% carbohydrate, 13% fat, 26% protein, 2% fiber), with meals supplied dried in sachets by the dietitian team and reconstituted with water by the participants. A review by the dietitian team at 1 week was followed by reviews every other week.
The primary outcome was difference in change in Asthma Control Questionnaire (ACQ6) from baseline (visit 1) to 16 weeks (visit 2), between CWP and usual care.
The single-center trial included 33 evaluable adult patients (75 years or younger; mean age 53 years; 63% women) with asthma (as per Global Initiative for Asthma guidelines) that was difficult to treat (as per Scottish Intercollegiate Guidelines Network/British Thoracic Society guidelines). The study population consisted of patients with frequent exacerbations with uncontrolled disease as reflected by the median interquartile range (IQR) for oral corticosteroid courses in the previous 12 months of 3 (2 to 5) and mean ACQ6 of 2.8 (2.4 to 3.1). Mean overall Asthma Quality of Life Questionnaire (AQLQ) was 3.8 (3.4 to 4.2). Median weight was 101.7 (91.4 to 118.7) kg, with a median BMI of 37.5 (35.0 to 42.3) kg/m2. Recruitment was discontinued before the target of 40 patients because the CWP dropout rate (n = 2) was lower than expected.
The researchers reported that the mean change in ACQ6 over 16 weeks was –0.45 for CWP and 0.23 for usual care with a mean difference of –0.69 (P = .048) between groups. The secondary outcome of mean change in overall AQLQ was 0.81 for CWP and 0.08 for usual care with a mean difference of 0.76 (P = .013) between groups.
No unexpected serious adverse events or intervention-related adverse events were observed during the trial.
“In this pragmatic open label, randomized, controlled trial we showed that delivery of a supported low-calorie total diet replacement program (Counterweight-Plus) to patients with difficult-to treat asthma and obesity, was safe and led to significant improvements in asthma control and quality of life compared to usual care over 16 weeks,” the authors wrote.
“Findings from the study are a welcome addition to this field of study,” Diego J. Maselli, MD, associate professor of medicine and interim chief, division of pulmonary diseases and critical care, UT Health at San Antonio, said in an interview. “ Obesity is an important comorbid condition because, although by itself it may have an effect on asthma patients, it is also associated with other comorbidities such as gastroesophageal reflux disease, obstructive sleep apnea, anxiety, depression, and others that in turn can affect asthmatics. Also, obesity may influence pulmonary physiology and it’s considered a proinflammatory state by many, and this can favor uncontrolled disease.”
While underscoring the clinically relevant weight loss and improvements in ACQ6 and AQLQ, Dr. Maselli said that the study did not follow the patients long enough to determine if weight loss was associated with a reduction in exacerbations and other long-term outcomes in asthma such as resource utilization and changes in maintenance medications, which may be explored in future studies.
“It remains to be seen if the weight loss of these types of programs can be sustained over longer periods of time, given the considerable caloric restriction in the initial stages of the weight reduction program. Interestingly, the majority of the patients in the study did not exhibit features of type 2 inflammation and had low-T2 endotype with low eosinophil count and low FeNO [fractional exhaled nitric oxide],” Dr. Maselli added. “Although obesity has been linked to this phenotype, the vast majority of [people with asthma], about 80%, have high T2 phenotype. Future studies are still need with larger and more representative samples and with longer follow-up times to determine the effects of weight loss on asthma outcomes, especially in severe asthma,” he concluded.
The trial was funded by an NHS Greater Glasgow and Clyde Endowment Fund grant. Several of the authors reported having received travel awards to attend conferences and funding from Cambridge Weight Plan and one author is an employee of and another a medical adviser for Counterweight Ltd., the developer of the program used. Other authors reported receiving funding from a variety of pharmaceutical companies. Dr. Maselli reported no relevant conflicts.
FROM CHEST
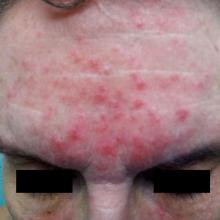
Treating rosacea: Combination therapy, benzoyl peroxide, and the ‘STOP’ mnemonic
HONOLULU – More often than not, patients with rosacea require a combination of treatments to optimize the management of the disease, according to Julie C. Harper, MD.
“We’ve been more comfortable with the idea of combination therapy for acne than we have been for rosacea,” Dr. Harper, who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “If patients are doing great on one treatment, then don’t change it. But if there’s room for improvement, think about combinations.”
Treatment options for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and microencapsulated benzoyl peroxide, 5%. Options for persistent background erythema include brimonidine and oxymetazoline, as well as device-based treatments, which include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Dr. Harper said that she has been especially surprised by the effectiveness of one of these options, microencapsulated benzoyl peroxide cream, 5% (Epsolay), which is approved by the Food and Drug Administration for treating inflammatory lesions of rosacea in adults. In two identical, phase 3 randomized clinical trials of patients with inflammatory rosacea lesions, those treated with microencapsulated benzoyl peroxide achieved a 68.8% reduction in inflammatory lesions at 12 weeks (including 42.5% at week 2), compared with 38%-46% of those on the vehicle, according to the April 2022 announcement of the approval from the manufacturers, Sol-Gel Technologies and Galderma.
“A common drug is playing a key role,” Dr. Harper said. “What’s the mechanism of action? I have no idea. I wonder if there may be a bacterial pathogen after all,” possibly Staphylococcus epidermidis, she added. However, she noted, “it does appear that benzoyl peroxide has an impact on Demodex, so maybe that’s the primary way it’s working.”
In her opinion, a key standout from the clinical trial data is the drug’s rapid onset of action, with a 42.5% reduction of lesions at week 2. “What makes this different is that the 5% microencapsulated benzoyl peroxide cream is wrapped up in a silica shell,” said Dr. Harper, a past president of the American Acne and Rosacea Society. “The silica shell kind of acts like a speed bump that slows the release of drug onto the skin. We think that’s what may be giving us this better tolerability.”
In an interview at the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that prior to the approval of Epsolay, benzoyl peroxide was never considered a first-line treatment for rosacea. “The problem is, the conventional formulation is irritating to the skin,” said Dr. Stein Gold, who was involved in clinical trials of Epsolay.
“The benzoyl peroxide encapsulated in the silica shell allows for a slow and steady delivery of medication to the skin in a very controlled manner. It is exceptionally good at getting rosacea under control. In the clinical trials, when we looked at the baseline irritation of the skin and followed those patients when they used the benzoyl 5% microencapsulated benzoyl peroxide cream, the irritation improved.”
‘STOP’ mnemonic
When treating her patients with rosacea, Dr. Harper incorporates the mnemonic “STOP” to these patient visits:
S: Identify signs and symptoms of rosacea.
T: Discuss triggers. “We cannot make this disease triggerless, so when you’re talking to your patients, you need to find out what’s triggering their rosacea,” she said.
O: Agree on a treatment outcome. “What is it that’s important to the patient?” she said. “They may tell you, ‘I want to be able to not be so red,’ or ‘I want to get rid of the bumps,’ or ‘I want my eyes to not feel so dry.’ ”
P: Develop a plan that helps achieve that desired outcome with patients.
Dr. Harper disclosed ties with Almirall, Cassiopeia, Cutera, Galderma, EPI, L’Oréal, Ortho Dermatologics, Sol Gel, Sun Pharmaceutical Industries, and Vyne.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries.
Medscape and this news organization are owned by the same parent company.
HONOLULU – More often than not, patients with rosacea require a combination of treatments to optimize the management of the disease, according to Julie C. Harper, MD.
“We’ve been more comfortable with the idea of combination therapy for acne than we have been for rosacea,” Dr. Harper, who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “If patients are doing great on one treatment, then don’t change it. But if there’s room for improvement, think about combinations.”
Treatment options for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and microencapsulated benzoyl peroxide, 5%. Options for persistent background erythema include brimonidine and oxymetazoline, as well as device-based treatments, which include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Dr. Harper said that she has been especially surprised by the effectiveness of one of these options, microencapsulated benzoyl peroxide cream, 5% (Epsolay), which is approved by the Food and Drug Administration for treating inflammatory lesions of rosacea in adults. In two identical, phase 3 randomized clinical trials of patients with inflammatory rosacea lesions, those treated with microencapsulated benzoyl peroxide achieved a 68.8% reduction in inflammatory lesions at 12 weeks (including 42.5% at week 2), compared with 38%-46% of those on the vehicle, according to the April 2022 announcement of the approval from the manufacturers, Sol-Gel Technologies and Galderma.
“A common drug is playing a key role,” Dr. Harper said. “What’s the mechanism of action? I have no idea. I wonder if there may be a bacterial pathogen after all,” possibly Staphylococcus epidermidis, she added. However, she noted, “it does appear that benzoyl peroxide has an impact on Demodex, so maybe that’s the primary way it’s working.”
In her opinion, a key standout from the clinical trial data is the drug’s rapid onset of action, with a 42.5% reduction of lesions at week 2. “What makes this different is that the 5% microencapsulated benzoyl peroxide cream is wrapped up in a silica shell,” said Dr. Harper, a past president of the American Acne and Rosacea Society. “The silica shell kind of acts like a speed bump that slows the release of drug onto the skin. We think that’s what may be giving us this better tolerability.”
In an interview at the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that prior to the approval of Epsolay, benzoyl peroxide was never considered a first-line treatment for rosacea. “The problem is, the conventional formulation is irritating to the skin,” said Dr. Stein Gold, who was involved in clinical trials of Epsolay.
“The benzoyl peroxide encapsulated in the silica shell allows for a slow and steady delivery of medication to the skin in a very controlled manner. It is exceptionally good at getting rosacea under control. In the clinical trials, when we looked at the baseline irritation of the skin and followed those patients when they used the benzoyl 5% microencapsulated benzoyl peroxide cream, the irritation improved.”
‘STOP’ mnemonic
When treating her patients with rosacea, Dr. Harper incorporates the mnemonic “STOP” to these patient visits:
S: Identify signs and symptoms of rosacea.
T: Discuss triggers. “We cannot make this disease triggerless, so when you’re talking to your patients, you need to find out what’s triggering their rosacea,” she said.
O: Agree on a treatment outcome. “What is it that’s important to the patient?” she said. “They may tell you, ‘I want to be able to not be so red,’ or ‘I want to get rid of the bumps,’ or ‘I want my eyes to not feel so dry.’ ”
P: Develop a plan that helps achieve that desired outcome with patients.
Dr. Harper disclosed ties with Almirall, Cassiopeia, Cutera, Galderma, EPI, L’Oréal, Ortho Dermatologics, Sol Gel, Sun Pharmaceutical Industries, and Vyne.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries.
Medscape and this news organization are owned by the same parent company.
HONOLULU – More often than not, patients with rosacea require a combination of treatments to optimize the management of the disease, according to Julie C. Harper, MD.
“We’ve been more comfortable with the idea of combination therapy for acne than we have been for rosacea,” Dr. Harper, who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “If patients are doing great on one treatment, then don’t change it. But if there’s room for improvement, think about combinations.”
Treatment options for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and microencapsulated benzoyl peroxide, 5%. Options for persistent background erythema include brimonidine and oxymetazoline, as well as device-based treatments, which include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Dr. Harper said that she has been especially surprised by the effectiveness of one of these options, microencapsulated benzoyl peroxide cream, 5% (Epsolay), which is approved by the Food and Drug Administration for treating inflammatory lesions of rosacea in adults. In two identical, phase 3 randomized clinical trials of patients with inflammatory rosacea lesions, those treated with microencapsulated benzoyl peroxide achieved a 68.8% reduction in inflammatory lesions at 12 weeks (including 42.5% at week 2), compared with 38%-46% of those on the vehicle, according to the April 2022 announcement of the approval from the manufacturers, Sol-Gel Technologies and Galderma.
“A common drug is playing a key role,” Dr. Harper said. “What’s the mechanism of action? I have no idea. I wonder if there may be a bacterial pathogen after all,” possibly Staphylococcus epidermidis, she added. However, she noted, “it does appear that benzoyl peroxide has an impact on Demodex, so maybe that’s the primary way it’s working.”
In her opinion, a key standout from the clinical trial data is the drug’s rapid onset of action, with a 42.5% reduction of lesions at week 2. “What makes this different is that the 5% microencapsulated benzoyl peroxide cream is wrapped up in a silica shell,” said Dr. Harper, a past president of the American Acne and Rosacea Society. “The silica shell kind of acts like a speed bump that slows the release of drug onto the skin. We think that’s what may be giving us this better tolerability.”
In an interview at the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that prior to the approval of Epsolay, benzoyl peroxide was never considered a first-line treatment for rosacea. “The problem is, the conventional formulation is irritating to the skin,” said Dr. Stein Gold, who was involved in clinical trials of Epsolay.
“The benzoyl peroxide encapsulated in the silica shell allows for a slow and steady delivery of medication to the skin in a very controlled manner. It is exceptionally good at getting rosacea under control. In the clinical trials, when we looked at the baseline irritation of the skin and followed those patients when they used the benzoyl 5% microencapsulated benzoyl peroxide cream, the irritation improved.”
‘STOP’ mnemonic
When treating her patients with rosacea, Dr. Harper incorporates the mnemonic “STOP” to these patient visits:
S: Identify signs and symptoms of rosacea.
T: Discuss triggers. “We cannot make this disease triggerless, so when you’re talking to your patients, you need to find out what’s triggering their rosacea,” she said.
O: Agree on a treatment outcome. “What is it that’s important to the patient?” she said. “They may tell you, ‘I want to be able to not be so red,’ or ‘I want to get rid of the bumps,’ or ‘I want my eyes to not feel so dry.’ ”
P: Develop a plan that helps achieve that desired outcome with patients.
Dr. Harper disclosed ties with Almirall, Cassiopeia, Cutera, Galderma, EPI, L’Oréal, Ortho Dermatologics, Sol Gel, Sun Pharmaceutical Industries, and Vyne.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR
Metformin linked to reductions in COVID-19 viral load
These findings add to a multitude of benefits the drug has been shown to have in COVID infection.
COVID-OUT did not meet its primary endpoint, but it did show important secondary outcomes including a 42% reduction in ED visits and in hospitalizations and/or deaths by day 14, and a 58% reduction in hospitalizations/death by day 28. A further subanalysis has shown a 42% reduction in long COVID, compared with placebo.
“In this phase 3 randomized controlled trial, metformin showed prevention of severe COVID, prevention of long COVID, and an antiviral effect, and this is consistent with other data,” said coauthor Carolyn Bramante, MD, University of Minnesota, Minneapolis, in presenting the findings at the Conference on Retroviruses & Opportunistic Infections.
Study details
For the new subanalysis, the authors further evaluated the effects of metformin treatment on SARS-CoV-2 viral load.
A total of 1,323 patients in the study, enrolled at six centers, were randomized to treatment either with metformin 1,000 mg per day on days 2-5 and 1,500 mg per day on days 6 to 14 (n = 187), or to ivermectin 390-470 mcg/kg per day for 3 days (n = 187), fluvoxamine 50 mg twice daily for 14 days, and/or an exact-matching placebo in a 2 x 3 factorial trial design.
The subanalysis on viral load included 483 patients from the trial who were treated with metformin versus 462 who received placebo, who were all enrolled within 3 days of a documented SARS-CoV-2 infection and less than 7 days after symptom onset.
The patients had a median age of 46 years, and all had either overweight or obesity. Only about 2% had diabetes, and only patients considered low-risk were excluded from the trial, including those under age 30 and those with a body mass index under 25.
About half of patients had received a primary vaccine and about 5% had received a vaccine booster. SARS-CoV-2 variants that were prominent during the study included Alpha, Delta, and Omicron.
The viral samples available on days 1, 5, and 10 showed a mean change in viral load from baseline to follow-up; the viral load was significantly lower with metformin versus placebo (–0.64 log10 copies/mL), representing a 4.4-fold greater decrease in viral load with metformin.
The mean rate of undetectable SARS-CoV-2 viral load at day 5 was 49.9% in the metformin group versus 54.6% in the placebo group (odds ratio, 1.235), and the undetectable rate at day 10 was 14.3% in the metformin group and 22.6% in the placebo group (OR, 1.663; P = .003).
An increased antiviral effect corresponded with increases in metformin dosing on days 6 through 14. Furthermore, the antiviral effect became stronger when metformin was started earlier in the course of infection.
Of note, the antiviral effect was more pronounced among those who were not vaccinated (mean, –0.95 log copies/mL), compared with the vaccinated (mean, –0.39 log copies/mL).
The antiviral effect with metformin was similar to that seen with nirmatrelvir at day 5 and was greater than nirmatrelvir at day 10.
No similar relationships in SARS-CoV-2 viral load were observed between ivermectin or fluvoxamine and placebo.
The findings are consistent with results of other recent observational studies, including research showing metformin to be associated with reductions in COVID-19 severity in patients with prediabetes, Dr. Bramante noted.
The authors’ previous analysis looking at long COVID in the COVID-OUT study showed that metformin treatment during acute COVID significantly reduced the risk for a diagnosis of long COVID versus placebo at 300 days following randomization, with a hazard ratio of 0.59 after adjustment for the study drug and vaccination at baseline.
Dr. Bramante noted that metformin’s potential antiviral properties have long been speculated, with some of the earliest research on the drug suggesting less severe outcomes in influenza, and more recently, RNA assays suggesting effects against other RNA viruses, including the Zika virus.
In terms of COVID, Dr. Bramante noted that the drug has plenty of potentially favorable benefits.
“Metformin is very safe and is known to have very few contraindications, so the next steps could be to consider looking at this in terms of a combination therapy,” she said.
‘Data from other studies are conflicting’
Commenting on the study, Diane V. Havlir, MD, cautioned that “metformin is currently not recommended in treatment guidelines, [and] data from other studies are conflicting; side effects can be an issue, and the study presented here was in a select population,” she said in an interview.
However, “what is both new and interesting in this presentation is the reduction of viral load, which [was observed] in the samples collected not only on days 1-5, but also days 6-14,” said Dr. Havlir, who is professor and associate chair of clinical research, department of medicine, and chief of the division of HIV, infectious diseases and global medicine and director of the AIDS Research Institute at the University of California, San Francisco.
Key questions the findings raise include whether the results correlate with clinical outcomes or transmission, and whether the findings are generalizable to other populations and settings, Dr. Havlir said.
Ultimately, “we need to continue to pursue all aspects of outpatient treatments for COVID to address questions like these for new and existing agents,” she added.
The trial received funding from the Parsemus Foundation, the Rainwater Charitable Foundation, Fast Grants, and the United Health Group. The authors and Dr. Havlir disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
These findings add to a multitude of benefits the drug has been shown to have in COVID infection.
COVID-OUT did not meet its primary endpoint, but it did show important secondary outcomes including a 42% reduction in ED visits and in hospitalizations and/or deaths by day 14, and a 58% reduction in hospitalizations/death by day 28. A further subanalysis has shown a 42% reduction in long COVID, compared with placebo.
“In this phase 3 randomized controlled trial, metformin showed prevention of severe COVID, prevention of long COVID, and an antiviral effect, and this is consistent with other data,” said coauthor Carolyn Bramante, MD, University of Minnesota, Minneapolis, in presenting the findings at the Conference on Retroviruses & Opportunistic Infections.
Study details
For the new subanalysis, the authors further evaluated the effects of metformin treatment on SARS-CoV-2 viral load.
A total of 1,323 patients in the study, enrolled at six centers, were randomized to treatment either with metformin 1,000 mg per day on days 2-5 and 1,500 mg per day on days 6 to 14 (n = 187), or to ivermectin 390-470 mcg/kg per day for 3 days (n = 187), fluvoxamine 50 mg twice daily for 14 days, and/or an exact-matching placebo in a 2 x 3 factorial trial design.
The subanalysis on viral load included 483 patients from the trial who were treated with metformin versus 462 who received placebo, who were all enrolled within 3 days of a documented SARS-CoV-2 infection and less than 7 days after symptom onset.
The patients had a median age of 46 years, and all had either overweight or obesity. Only about 2% had diabetes, and only patients considered low-risk were excluded from the trial, including those under age 30 and those with a body mass index under 25.
About half of patients had received a primary vaccine and about 5% had received a vaccine booster. SARS-CoV-2 variants that were prominent during the study included Alpha, Delta, and Omicron.
The viral samples available on days 1, 5, and 10 showed a mean change in viral load from baseline to follow-up; the viral load was significantly lower with metformin versus placebo (–0.64 log10 copies/mL), representing a 4.4-fold greater decrease in viral load with metformin.
The mean rate of undetectable SARS-CoV-2 viral load at day 5 was 49.9% in the metformin group versus 54.6% in the placebo group (odds ratio, 1.235), and the undetectable rate at day 10 was 14.3% in the metformin group and 22.6% in the placebo group (OR, 1.663; P = .003).
An increased antiviral effect corresponded with increases in metformin dosing on days 6 through 14. Furthermore, the antiviral effect became stronger when metformin was started earlier in the course of infection.
Of note, the antiviral effect was more pronounced among those who were not vaccinated (mean, –0.95 log copies/mL), compared with the vaccinated (mean, –0.39 log copies/mL).
The antiviral effect with metformin was similar to that seen with nirmatrelvir at day 5 and was greater than nirmatrelvir at day 10.
No similar relationships in SARS-CoV-2 viral load were observed between ivermectin or fluvoxamine and placebo.
The findings are consistent with results of other recent observational studies, including research showing metformin to be associated with reductions in COVID-19 severity in patients with prediabetes, Dr. Bramante noted.
The authors’ previous analysis looking at long COVID in the COVID-OUT study showed that metformin treatment during acute COVID significantly reduced the risk for a diagnosis of long COVID versus placebo at 300 days following randomization, with a hazard ratio of 0.59 after adjustment for the study drug and vaccination at baseline.
Dr. Bramante noted that metformin’s potential antiviral properties have long been speculated, with some of the earliest research on the drug suggesting less severe outcomes in influenza, and more recently, RNA assays suggesting effects against other RNA viruses, including the Zika virus.
In terms of COVID, Dr. Bramante noted that the drug has plenty of potentially favorable benefits.
“Metformin is very safe and is known to have very few contraindications, so the next steps could be to consider looking at this in terms of a combination therapy,” she said.
‘Data from other studies are conflicting’
Commenting on the study, Diane V. Havlir, MD, cautioned that “metformin is currently not recommended in treatment guidelines, [and] data from other studies are conflicting; side effects can be an issue, and the study presented here was in a select population,” she said in an interview.
However, “what is both new and interesting in this presentation is the reduction of viral load, which [was observed] in the samples collected not only on days 1-5, but also days 6-14,” said Dr. Havlir, who is professor and associate chair of clinical research, department of medicine, and chief of the division of HIV, infectious diseases and global medicine and director of the AIDS Research Institute at the University of California, San Francisco.
Key questions the findings raise include whether the results correlate with clinical outcomes or transmission, and whether the findings are generalizable to other populations and settings, Dr. Havlir said.
Ultimately, “we need to continue to pursue all aspects of outpatient treatments for COVID to address questions like these for new and existing agents,” she added.
The trial received funding from the Parsemus Foundation, the Rainwater Charitable Foundation, Fast Grants, and the United Health Group. The authors and Dr. Havlir disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
These findings add to a multitude of benefits the drug has been shown to have in COVID infection.
COVID-OUT did not meet its primary endpoint, but it did show important secondary outcomes including a 42% reduction in ED visits and in hospitalizations and/or deaths by day 14, and a 58% reduction in hospitalizations/death by day 28. A further subanalysis has shown a 42% reduction in long COVID, compared with placebo.
“In this phase 3 randomized controlled trial, metformin showed prevention of severe COVID, prevention of long COVID, and an antiviral effect, and this is consistent with other data,” said coauthor Carolyn Bramante, MD, University of Minnesota, Minneapolis, in presenting the findings at the Conference on Retroviruses & Opportunistic Infections.
Study details
For the new subanalysis, the authors further evaluated the effects of metformin treatment on SARS-CoV-2 viral load.
A total of 1,323 patients in the study, enrolled at six centers, were randomized to treatment either with metformin 1,000 mg per day on days 2-5 and 1,500 mg per day on days 6 to 14 (n = 187), or to ivermectin 390-470 mcg/kg per day for 3 days (n = 187), fluvoxamine 50 mg twice daily for 14 days, and/or an exact-matching placebo in a 2 x 3 factorial trial design.
The subanalysis on viral load included 483 patients from the trial who were treated with metformin versus 462 who received placebo, who were all enrolled within 3 days of a documented SARS-CoV-2 infection and less than 7 days after symptom onset.
The patients had a median age of 46 years, and all had either overweight or obesity. Only about 2% had diabetes, and only patients considered low-risk were excluded from the trial, including those under age 30 and those with a body mass index under 25.
About half of patients had received a primary vaccine and about 5% had received a vaccine booster. SARS-CoV-2 variants that were prominent during the study included Alpha, Delta, and Omicron.
The viral samples available on days 1, 5, and 10 showed a mean change in viral load from baseline to follow-up; the viral load was significantly lower with metformin versus placebo (–0.64 log10 copies/mL), representing a 4.4-fold greater decrease in viral load with metformin.
The mean rate of undetectable SARS-CoV-2 viral load at day 5 was 49.9% in the metformin group versus 54.6% in the placebo group (odds ratio, 1.235), and the undetectable rate at day 10 was 14.3% in the metformin group and 22.6% in the placebo group (OR, 1.663; P = .003).
An increased antiviral effect corresponded with increases in metformin dosing on days 6 through 14. Furthermore, the antiviral effect became stronger when metformin was started earlier in the course of infection.
Of note, the antiviral effect was more pronounced among those who were not vaccinated (mean, –0.95 log copies/mL), compared with the vaccinated (mean, –0.39 log copies/mL).
The antiviral effect with metformin was similar to that seen with nirmatrelvir at day 5 and was greater than nirmatrelvir at day 10.
No similar relationships in SARS-CoV-2 viral load were observed between ivermectin or fluvoxamine and placebo.
The findings are consistent with results of other recent observational studies, including research showing metformin to be associated with reductions in COVID-19 severity in patients with prediabetes, Dr. Bramante noted.
The authors’ previous analysis looking at long COVID in the COVID-OUT study showed that metformin treatment during acute COVID significantly reduced the risk for a diagnosis of long COVID versus placebo at 300 days following randomization, with a hazard ratio of 0.59 after adjustment for the study drug and vaccination at baseline.
Dr. Bramante noted that metformin’s potential antiviral properties have long been speculated, with some of the earliest research on the drug suggesting less severe outcomes in influenza, and more recently, RNA assays suggesting effects against other RNA viruses, including the Zika virus.
In terms of COVID, Dr. Bramante noted that the drug has plenty of potentially favorable benefits.
“Metformin is very safe and is known to have very few contraindications, so the next steps could be to consider looking at this in terms of a combination therapy,” she said.
‘Data from other studies are conflicting’
Commenting on the study, Diane V. Havlir, MD, cautioned that “metformin is currently not recommended in treatment guidelines, [and] data from other studies are conflicting; side effects can be an issue, and the study presented here was in a select population,” she said in an interview.
However, “what is both new and interesting in this presentation is the reduction of viral load, which [was observed] in the samples collected not only on days 1-5, but also days 6-14,” said Dr. Havlir, who is professor and associate chair of clinical research, department of medicine, and chief of the division of HIV, infectious diseases and global medicine and director of the AIDS Research Institute at the University of California, San Francisco.
Key questions the findings raise include whether the results correlate with clinical outcomes or transmission, and whether the findings are generalizable to other populations and settings, Dr. Havlir said.
Ultimately, “we need to continue to pursue all aspects of outpatient treatments for COVID to address questions like these for new and existing agents,” she added.
The trial received funding from the Parsemus Foundation, the Rainwater Charitable Foundation, Fast Grants, and the United Health Group. The authors and Dr. Havlir disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CROI 2023
Zika virus still calls for preparedness and vaccine development
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Warming U.S. temperatures, the resumption of travel, and new knowledge about Zika’s long-term effects on children signal that Zika prevention and vaccine development should be on public health officials’, doctors’, and communities’ radar, even when community infection is not occurring.
“Although we haven’t seen confirmed Zika virus circulation in the continental United States or its territories for several years, it’s still something that we are closely monitoring, particularly as we move into the summer months,” Erin Staples, MD, PhD, medical epidemiologist at the Arboviral Diseases Branch of the Centers for Disease Control and Prevention in Fort Collins, Colo., told this news organization.
“This is because cases are still being reported in other countries, particularly in South America. Travel to these places is increasing following the pandemic, leaving more potential for individuals who might have acquired the infection to come back and restart community transmission.”
How Zika might reemerge
The Aedes aegypti mosquito is the vector by which Zika spreads, and “during the COVID pandemic, these mosquitoes moved further north in the United States, into southern California, and were identified as far north as Washington, D.C.,” said Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at UCLA Medical Center in Los Angeles.
“On a population level, Americans have essentially no immunity to Zika from prior infection, and there is no vaccine yet approved. If individuals infected with Zika came into a U.S. region where the Aedes aegypti mosquito was present, that population could be very susceptible to infection spread and even another outbreak. This would be a confluence of bad circumstances, but that’s exactly what infectious disease specialists continue to be watchful about, especially because Zika is so dangerous for fetuses,” said Dr. Silverman.
How the public can prepare
The CDC recommends that pregnant women or women who plan to become pregnant avoid traveling to regions where there are currently outbreaks of Zika, but this is not the only way that individuals can protect themselves.
“The message we want to deliver to people is that in the United States, people are at risk for several mosquito-borne diseases every summer beyond just Zika,” Dr. Staples said. “It’s really important that people are instructed to make a habit of wearing EPA [U.S. Environmental Protection Agency)–registered insect repellents when they go outside. Right now, that is the single best tool that we have to prevent mosquito-borne diseases in the U.S.
“From a community standpoint, there are several emerging mosquito control methods that are being evaluated right now, such as genetic modification and irradiation of mosquitoes. These methods are aimed at producing sterile mosquitoes that are released into the wild to mate with the local mosquito population, which will render them infertile. This leads, over time, to suppression of the overall Aedes aegypti mosquito population – the main vector of Zika transmission,” said Dr. Staples.
Monica Gandhi, MD, MPH, professor of medicine and associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, encourages her patients to wear mosquito repellent but cautioned that “there’s no antiviral that you can take for Zika. Until we have a vaccine, the key to controlling/preventing Zika is controlling the mosquitoes that spread the virus.”
Vaccines
The National Institute of Allergy and Infectious Diseases (NIAID) is currently investigating a variety of Zika vaccines, including a DNA-based vaccine, (phase 2), a purified inactivated virus vaccine (phase 1), live attenuated vaccines (phase 2), and mRNA vaccines (phase 2).
“I’m most excited about mRNA vaccines because they help patients produce a lot of proteins. The protein from a typical protein-adjuvant vaccine will break down, and patients can only raise an immune response to whatever proteins are left. On the other hand, mRNA vaccines provide the body [with] a recipe to make the protein from the pathogen in high amounts, so that a strong immune response can be raised for protection,” noted Dr. Gandhi.
Moderna’s mRNA-1893 vaccine was recently studied in a randomized, observer-blind, controlled, phase 1 trial among 120 adults in the United States and Puerto Rico, the results of which were published online in The Lancet. “The vaccine was found to be generally well tolerated with no serious adverse events considered related to vaccine. Furthermore, the vaccine was able to generate a potent immune response that was capable of neutralizing the virus in vitro,” said Brett Leav, MD, executive director of clinical development for public health vaccines at Moderna.
“Our mRNA platform technology ... can be very helpful against emerging pandemic threats, as we saw in response to COVID-19. What is unique in our approach is that if the genetic sequence of the virus is known, we can quickly generate vaccines to test for their capability to generate a functional immune response. In the case of the mRNA-1893 trial, the vaccine was developed with antigens that were present in the strain of virus circulating in 2016, but we could easily match whatever strain reemerges,” said Dr. Leav.
A phase 2 trial to confirm the dose of mRNA-1893 in a larger study population is underway.
Although it’s been demonstrated that Moderna’s mRNA vaccine is safe and effective, moving from a phase 2 to a phase 3 study presents a challenge, given the fact that currently, the disease burden from Zika is low. If an outbreak were to occur in the future, these mRNA vaccines could potentially be given emergency approval, as occurred during the COVID pandemic, according to Dr. Silverman.
If approved, provisionally or through a traditional route, the vaccine would “accelerate the ability to tamp down any further outbreaks, because vaccine-based immunity could be made available to a large portion of the population who were pregnant or planning a pregnancy, not just in the U.S. but also in these endemic areas,” said Dr. Silverman.
Takeaways from the last Zika outbreak
Practical steps such as mosquito eradication and development of vaccines are not the only takeaway from the recent Zika epidemics inside and outside the United States. A clearer picture of the short- and long-term stakes of the disease has emerged.
According to the CDC, most people who become infected with Zika experience only mild symptoms, such as fever, rash, headache, and muscle pain, but babies conceived by mothers infected with Zika are at risk for stillbirth, miscarriage, and microcephaly and other brain defects.
Although a pregnant woman who tests positive for Zika is in a very high-risk situation, “data show that only about 30% of mothers with Zika have a baby with birth defects. If a pregnant woman contracted Zika, what would happen is we would just do very close screening by ultrasound of the fetus. If microcephaly in utero or fetal brain defects were observed, then a mother would be counseled on her options,” said Dr. Gandhi.
Dr. Silverman noted that “new data on children who were exposed in utero and had normal exams, including head measurements when they were born, have raised concerns. In recently published long-term follow-up studies, even when children born to mothers infected with Zika during pregnancy had normal head growth at least 3 years after birth, they were still at risk for neurodevelopmental delay and behavioral disorders, including impact on coordination and executive function.
“This is another good reason to keep the potential risks of Zika active in the public’s consciousness and in public health planning.”
Dr. Silverman, Dr. Gandhi, and Dr. Staples have disclosed no relevant financial relationships. Dr. Leav is an employee of Moderna and owns stock in the company.
A version of this article originally appeared on Medscape.com.
Pfizer COVID vaccine effective in young children, study shows
A new study shows the Pfizer vaccine is safe and highly effective against COVID-19 in children as young as 6 months old.
A three-dose series of the Pfizer COVID-19 vaccine was 73% effective at preventing symptomatic COVID-19 in children aged 6 months to 4 years, the researchers found. They also found that an examination of reactions and safety results “did not suggest any concerns.”
The study, published in the New England Journal of Medicine, included 1,776 children aged 6 months to 2 years old, and 2,750 children aged 2-4 years. Children were randomly assigned to receive either the three-shot series of the Pfizer vaccine or placebo shots. Participants received the first dose of the vaccine by March 31, 2022, and lived in Brazil, Finland, Poland, Spain, or the United States.
The authors wrote that having safe and effective COVID vaccines for young children is important to protect them from hospitalization or death and because young children play a role in spreading highly transmissible variants of the virus. COVID hospitalizations for children under 5 years old peaked at a rate of 14.5 per 100,000 in January 2022, the authors wrote, noting that the Omicron virus variant appeared to affect young children more severely than the previous variant, Delta.
When the researchers evaluated vaccine effectiveness by age group, they found that it prevented symptomatic COVID in 75.8% of children aged 6 months to 2 years, and in 71.8% of children aged 2-4 years.
Less than 0.5% of participants reported severe reactions to the vaccine. The most common reactions reported were tenderness or pain. Reactions typically appeared within the first couple days following vaccine administration and resolved within 2 days. No cases of inflammation of the heart muscle or its lining were reported among participants.
Uptake of COVID vaccines for young children has been lower than other age groups in the United States. The Centers for Disease Control and Prevention says 10% of children younger than 5 have received at least one dose of a COVID-19 vaccine, and 5% have completed a primary vaccine series.
A version of this article originally appeared on WebMD.com.
A new study shows the Pfizer vaccine is safe and highly effective against COVID-19 in children as young as 6 months old.
A three-dose series of the Pfizer COVID-19 vaccine was 73% effective at preventing symptomatic COVID-19 in children aged 6 months to 4 years, the researchers found. They also found that an examination of reactions and safety results “did not suggest any concerns.”
The study, published in the New England Journal of Medicine, included 1,776 children aged 6 months to 2 years old, and 2,750 children aged 2-4 years. Children were randomly assigned to receive either the three-shot series of the Pfizer vaccine or placebo shots. Participants received the first dose of the vaccine by March 31, 2022, and lived in Brazil, Finland, Poland, Spain, or the United States.
The authors wrote that having safe and effective COVID vaccines for young children is important to protect them from hospitalization or death and because young children play a role in spreading highly transmissible variants of the virus. COVID hospitalizations for children under 5 years old peaked at a rate of 14.5 per 100,000 in January 2022, the authors wrote, noting that the Omicron virus variant appeared to affect young children more severely than the previous variant, Delta.
When the researchers evaluated vaccine effectiveness by age group, they found that it prevented symptomatic COVID in 75.8% of children aged 6 months to 2 years, and in 71.8% of children aged 2-4 years.
Less than 0.5% of participants reported severe reactions to the vaccine. The most common reactions reported were tenderness or pain. Reactions typically appeared within the first couple days following vaccine administration and resolved within 2 days. No cases of inflammation of the heart muscle or its lining were reported among participants.
Uptake of COVID vaccines for young children has been lower than other age groups in the United States. The Centers for Disease Control and Prevention says 10% of children younger than 5 have received at least one dose of a COVID-19 vaccine, and 5% have completed a primary vaccine series.
A version of this article originally appeared on WebMD.com.
A new study shows the Pfizer vaccine is safe and highly effective against COVID-19 in children as young as 6 months old.
A three-dose series of the Pfizer COVID-19 vaccine was 73% effective at preventing symptomatic COVID-19 in children aged 6 months to 4 years, the researchers found. They also found that an examination of reactions and safety results “did not suggest any concerns.”
The study, published in the New England Journal of Medicine, included 1,776 children aged 6 months to 2 years old, and 2,750 children aged 2-4 years. Children were randomly assigned to receive either the three-shot series of the Pfizer vaccine or placebo shots. Participants received the first dose of the vaccine by March 31, 2022, and lived in Brazil, Finland, Poland, Spain, or the United States.
The authors wrote that having safe and effective COVID vaccines for young children is important to protect them from hospitalization or death and because young children play a role in spreading highly transmissible variants of the virus. COVID hospitalizations for children under 5 years old peaked at a rate of 14.5 per 100,000 in January 2022, the authors wrote, noting that the Omicron virus variant appeared to affect young children more severely than the previous variant, Delta.
When the researchers evaluated vaccine effectiveness by age group, they found that it prevented symptomatic COVID in 75.8% of children aged 6 months to 2 years, and in 71.8% of children aged 2-4 years.
Less than 0.5% of participants reported severe reactions to the vaccine. The most common reactions reported were tenderness or pain. Reactions typically appeared within the first couple days following vaccine administration and resolved within 2 days. No cases of inflammation of the heart muscle or its lining were reported among participants.
Uptake of COVID vaccines for young children has been lower than other age groups in the United States. The Centers for Disease Control and Prevention says 10% of children younger than 5 have received at least one dose of a COVID-19 vaccine, and 5% have completed a primary vaccine series.
A version of this article originally appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
What does the future of psoriasis treatment look like?
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR
How to get started with prescribing and advising on CGM
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.
Continuous glucose monitoring (CGM) is gaining ground with both patients and providers because of an array of driving forces, including broadening eligibility, insulin price caps, public awareness, and an increasing number of educational initiatives for doctors.
While professional organizations aim to familiarize doctors with this relatively new technology, more patients are learning independently that finger sticks may be optional, leading them to request CGM from their provider, according to Neil Skolnik, MD.
“We in primary care are being shepherded into this space by our patients who have seen an advertisement or talked to a friend about the benefits of CGM, and then asked us to prescribe it,” said Dr. Skolnik, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health.
Systemic factors are also accelerating CGM uptake, he added, highlighting recent Medicare rule changes to expand eligibility, with insurance companies beginning to follow suit.
Warren A. Jones, MD, FAAFP, professor emeritus at the University of Mississippi, Jackson, and past president of the AAFP, said that insulin price regulations have also opened doors to CGM.
“When you had patients trying to determine whether they were going to buy food or pay for high-priced insulin, that was a big challenge,” Dr. Jones said in an interview. “But that barrier has recently been removed, so we’re at the dawn of a new era.”
Like any paradigm shift, however,
Overview of online resources and navigating coverage
The latest learning resource on CGM for physicians comes from the American Academy of Family Physicians in the form of a new online educational hub with a 2-credit, ACCME-accredited course. It offers comprehensive guidance for employing CGM in daily practice. Topics include both medical and practical considerations, from interpretation of curves and glucose goal-setting to choosing a device and navigating coverage.
The AAFP’s new offering joins a growing number of similar educational efforts launched over the past few years by the Association of Diabetes Care & Education Specialists, the American Pharmacists Association, the American Diabetes Association, and the American Association of Clinical Endocrinologists.
Checking for coverage is a key first step when considering CGM for a particular patient, Dr. Jones said, noting that CGM, like any new form of care, presents unique challenges with coding and claims that must be overcome to get reimbursed.
“No margin, no mission,” Dr. Jones said. “If you are not able to pay your bills, you can’t be available for your patients. Our goal at the AAFP is to make sure that physicians get this knowledge [about reimbursement].”
To this end, the AAFP’s new online educational hub and the guide provided by APhA present CGM eligibility criteria for various patient groups, including those with Medicare, Medicaid, private insurance, and without coverage.
Medicare criteria include a diagnosis of diabetes, treatment with three or more daily administrations of insulin or continuous infusion via a pump, frequent adjustment to insulin treatment based on glucose readings, and presentation for diabetes in the past 6 months.
Once these requirements are clearly documented in the patient’s record, providers need to write the script, complete a certificate of medical necessity, and choose a supplier. Medicare covers CGM as a durable medical equipment benefit instead of a pharmacy benefit, according to the AAFP and APhA.
Exact coverage criteria and reimbursement processes for non-Medicare patients follow similar paths, although details vary by state and insurer, so personalized investigation is required.
When exploring coverage, the AAFP recommends paying attention to information needed for prior authorization, the patient’s diabetes type and age, and other medical requirements, such as minimum number of daily finger sticks or insulin doses per day.
Looking ahead, Dr. Jones predicted that authorization obstacles stemming from short-term cost concerns are going to fade as long-term savings are uncovered.
“I think pharmacy benefit managers and payers are going to recognize that we have better patient compliance, and that continuous glucose monitoring is going to bring the cost of care down and decrease the rate of hospitalizations,” Dr. Jones said. “So I think they’re going to be willing to pay clinicians to engage in this more readily over time.”
Patients who fail to qualify for personal CGM can still benefit from professional CGM, in which they borrow necessary equipment on a short-term basis. This avenue typically requires minimal or no insurance authorization. In addition, providers have the “opportunity to cover/exceed expenses by enhancing revenue with separately billable procedures, which can be billed in addition to [evaluation and management] if done on same day,” according to the AAFP guide, which goes on to provide appropriate codes.
Learning CGM through first-hand experience
Getting started with CGM can be intimidating for providers, Dr. Skolnik said, although he offered some reassurance, suggesting that the learning process may be more forgiving than prescribing a new drug for the first time.
“I think the best way to figure out CGM is to prescribe it to a couple of patients and learn with them,” Dr. Skolnik said. “You can’t do that with medicines. With medicines, you need to know what you’re doing before you choose who to give a medicine to.”
Instead of “reading everything under the sun” about CGM, he recommends starting with several of the ADA’s resources focusing on time in range, including an article, webinar, and podcast.
After that, physicians can learn on the job. A beginner’s mindset to CGM is well received by patients, he said, especially if you share your natural curiosity with them.
“Share your patients’ wonder at what they see,” Dr. Skolnik said. “They’ll open the app and you’ll look at their time and range and together you’ll go, ‘Wow, isn’t that something? I wonder why?’ ”
With this approach, providers and patients can join forces to explore trends and troubleshoot anomalous readings.
“Together you’ll go: ‘Hmm, I wonder why on Thursday, that graph is looking so far off from the other days? Wow. And then the patient remembers: they ate out on Thursday. They had a big pasta meal, perhaps. Everyone’s different in how they respond to different carbs. And you’ll both have this epiphany together about: ‘Wow, what I do matters.’ And I think that’s actually the best way to jump in.”
According to the AAFP, ADCES, and APhA resources, providers should first address time below range, as hypoglycemia can be imminently dangerous.
Next, providers should consider time in range, average glucose, and glucose management indicator, the latter of which acts as a surrogate for HbA1c. The first couple weeks of monitoring should be viewed as an information gathering phase, after which specific targets can be addressed through behavioral modifications and insulin adjustments, the AAFP advises.
The ADA guide highlights CGM usage, glucose variability, time in range, time above range, and average glucose as key metrics to monitor and offers corresponding actions when targets are unmet.
Encouraging patients to start CGM
Like providers, patients may also be intimidated by CGM, Dr. Jones said, typically because they don’t know how it works, or it seems complicated. Fortunately, he said, these fears are easily overcome when patients learn that they don’t need to stick themselves, record any of their readings, or really do anything at all for the first few weeks.
“You don’t even worry about it,” Dr. Jones tells his patients, who typically feel “more in control and engaged in their own care” after experiencing CGM for themselves.
Dr. Jones speaks from both professional and personal experience. A member of his family recently started CGM after being discharged from the hospital, and the benefits have been significant for everyone involved.
“I see how effectively we can control [my family member’s] blood pressure and insulin requirements, as opposed to several months ago when we didn’t have it,” Dr. Jones said. “So I’m giving it to you from two perspectives: one, of the clinician who knows, intellectually, what should go on, and two, experientially, from a family trying to take care of someone they love.”
Dr. Skolnik disclosed relationships with AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, GSK, Bayer, Genentech, Abbott, Idorsia, Merck, Novartis, Heartland, and Novo Nordisk. Dr Jones disclosed no relevant conflicts of interest.