User login
Establishing the Diagnosis of Rosacea in Skin of Color Patients
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
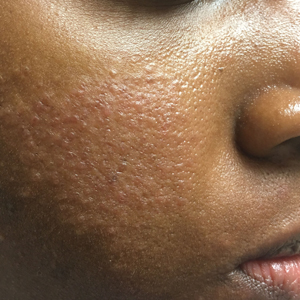
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.

Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.

Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Practice Points
- The clinical signs of rosacea may be similar in all skin types; however, dermatologists must have a high clinical index of suspicion for rosacea in patients with skin of color (SOC).
- Dermatologists should consider a wide differential diagnosis when presented with an SOC patient with facial erythema and/or papules and pustules.
Changing Public Perception of Vitiligo
Is Vitiligo in Vogue? The Changing Face of Vitiligo
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
Approach to Treatment of Medical and Cosmetic Facial Concerns in Skin of Color Patients
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
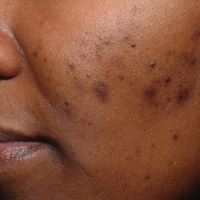
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16
Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26
Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
The approach to the treatment of common skin disorders and cosmetic concerns in patients with skin of color (SOC) requires the clinician to understand the biological differences, nuances, and special considerations that are unique to patients with darker skin types.1-3 This article addresses 4 common facial concerns in SOC patients—acne, rosacea, facial hyperpigmentation, and cosmetic enhancement—and provides treatment recommendations and management pearls to assist the clinician with optimal outcomes for SOC patients.
Acne in SOC Patients
Acne vulgaris is one of the most common conditions that dermatologists treat and is estimated to affect 40 to 50 million individuals in the United States.1 Many of these acne patients are individuals with SOC.2-4 A study of 2835 females (aged 10–70 years) conducted in 4 different cities—Los Angeles, California; London, United Kingdom; Akita, Japan; and Rome, Italy—demonstrated acne prevalence of 37% in blacks, 32% in Hispanics, 30% in Asians, 24% in whites, and 23% in Continental Indians.5 Blacks, Hispanics, and Continental Indians demonstrated equal prevalence with comedonal and inflammatory acne. Asians displayed more inflammatory acne lesions than comedones. In contrast, whites demonstrated more comedones than inflammatory acne. Dyspigmentation, postinflammatory hyperpigmentation (PIH), and atrophic scars were more common in black and Hispanic females than other ethnicities.5 This study illustrated that acne-induced PIH is a common sequela in SOC patients and is the main reason they seek treatment.6,7
The pathogenesis of acne is the same in all racial and ethnic groups: (1) follicular hyperkeratinization and the formation of a microcomedone caused by abnormal desquamation of the keratinocytes within the sebaceous follicle, (2) production of sebum by circulating androgens, (3) proliferation of Propionibacterium acnes, and (4) inflammation. Subclinical inflammation is present throughout all stages of acne, including normal-appearing skin, inflammatory lesions, comedones, and scarring, and may contribute to PIH in acne patients with SOC (Figure 1).8 A thorough history should be obtained from acne patients, including answers to the following questions7:
- What skin and hair care products do you use?
- Do you use sunscreen daily?
- What cosmetic products or makeup do you use?
- Do you use any ethnic skin care products, including skin lightening creams?
- Do you have a history of keloids?

It is important to ask these questions to assess if the SOC patient has developed pomade acne,9 acne cosmetica,10 or a potential risk of skin irritation from the use of skin care practices. It is best to take total control of the patient’s skin care regimen and discontinue use of toners, astringents, witch hazel, exfoliants, and rubbing alcohol, which may lead to skin dryness and irritation, particularly when combined with topical acne medications.
Treatment
Treatment of acne in SOC patients is similar to generally recommended treatments, with special considerations. Consider the following key points when treating acne in SOC patients:
- Treat acne early and aggressively to prevent or minimize subsequent PIH and acne scarring.
- Balance aggressive treatment with nonirritating topical skin care.
- Most importantly, target PIH in addition to acne and choose a regimen that limits skin irritation that might exacerbate existing PIH.7
Develop a maintenance program to control future breakouts. Topical agents can be used as monotherapy or in fixed combinations and may include benzoyl peroxide, antibiotics, dapsone, azelaic acid (AZA), and retinoids. Similar to white patients, topical retinoids remain a first-line treatment for acne in patients with SOC.11,12
Tolerability must be managed in SOC acne patients. Therapeutic maneuvers that can be instituted should include a discussion on using gentle skin care, initiating therapy with a retinoid applied every other night starting with a low concentration and gradually titrating up, and applying a moisturizer before or after applying acne medication. Oral therapies consist of antibiotics (doxycycline, minocycline), retinoids (isotretinoin), and hormonal modulators (oral contraceptives, spironolactone). Isotretinoin, recommended for patients with nodulocystic acne, may play a possible role in treating acne-induced PIH.13
Two common procedural therapies for acne include comedone extraction and intralesional corticosteroid injection. A 6- to 8-week course of a topical retinoid prior to comedonal extraction may facilitate the procedure and is recommended in SOC patients to help reduce cutaneous trauma and PIH.11 Inflammatory acne lesions can be treated with intralesional injection of triamcinolone acetonide 2.5 or 5.0 mg/mL, which usually reduces inflammation within 2 to 5 days.11
Treatment of acne-induced PIH includes sun protection, topical and oral medications, chemical peels, lasers, and energy devices. Treatment of hypertrophic scarring and keloids involves intralesional injection of triamcinolone acetonide 20, 30, or 40 mg/mL every 4 weeks until the lesion is flat.11
Superficial chemical peels can be used to treat acne and PIH in SOC patients,14 such as salicylic acid (20%–30%), glycolic acid (20%–70%), trichloroacetic acid (15%–30%), and Jessner peels.
Acne Scarring
Surgical approaches to acne scarring in patients with SOC include elliptical excision, punch excision, punch elevation, punch autografting, dermal grafting, dermal planning, subcutaneous incision (subcision), dermabrasion, microneedling, fillers, and laser skin resurfacing. The treatment of choice depends on the size, type, and depth of the scar and the clinician’s preference.
Lasers
Fractional photothermolysis has emerged as a treatment option for acne scars in SOC patients. This procedure produces microscopic columns of thermal injury in the epidermis and dermis, sparing the surrounding tissue and minimizing downtime and adverse events. Because fractional photothermolysis does not target melanin and produces limited epidermal injury, darker Fitzpatrick skin types (IV–VI) can be safely and effectively treated with this procedure.15
Rosacea in SOC Patients
Rosacea is a chronic inflammatory disorder that affects the vasculature and pilosebaceous units of the face. It commonly is seen in Fitzpatrick skin types I and II; however, rosacea can occur in all skin types (Figure 2). Triggers include emotional stress, extreme environmental temperatures, hot and spicy foods, red wine or alcohol, and topical irritants or allergens found in common cosmetic products.16

Data suggest that 4% of rosacea patients in the United States are of African, Latino, or Asian descent.11 National Ambulatory Medical Care Survey data revealed that of 31.5 million rosacea visits, 2% of patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino. In a 5-year longitudinal study of 2587 rosacea patients enrolled in Medicaid in North Carolina who were prescribed at least 1 topical treatment for rosacea, 16.27% were black and 10% were of a race other than white.17
Although the pathogenesis of rosacea is unclear, hypotheses include immune system abnormalities, neurogenic dysregulation, presence of microorganisms (eg, Demodex folliculorum), UV damage, and skin barrier dysfunction.18
The 4 major subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea.16 Interestingly, rosacea in SOC patients may present with hypopigmentation surrounding the borders of the facial erythema. For phymatous rosacea, isotretinoin may reduce incipient rhinophyma but must be carefully monitored and pregnancy must be excluded. Surgical or laser therapy may be indicated to recontour the nose if severe.
There are several skin conditions that can present with facial erythema in patients with SOC, including seborrheic dermatitis, systemic lupus erythematosus, and contact dermatitis. It is important to note that the detection of facial erythema in darker skin types may be difficult; therefore, laboratory evaluation (antinuclear antibodies), patch testing, and skin biopsy should be considered if the clinical diagnosis is unclear.
Treatment
Treatment of rosacea in SOC patients does not differ from other racial groups. Common strategies include gentle skin care, sun protection (sun protection factor 30+), and barrier repair creams. Topical agents include metronidazole, AZA, sodium sulfacetamide/sulfur, ivermectin, and retinoids.16 Oral treatments include antibiotics in the tetracycline family (eg, subantimicrobial dose doxycycline) and isotretinoin.16 Persistent erythema associated with rosacea can be treated with brimonidine19 and oxymetazoline.20 Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea21; however, the latter is not recommended in Fitzpatrick skin types IV through VI.
Facial Hyperpigmentation in SOC Patients
Hyperpigmentation disorders can be divided into conditions that affect Fitzpatrick skin types I through III and IV though VI. Mottled hyperpigmentation (photodamage) and solar lentigines occur in patients with lighter skin types as compared to melasma, PIH, and age-related (UV-induced) hyperpigmentation, which occur more commonly in patients with darker skin types. Facial hyperpigmentation is a common concern in SOC patients. In a survey of cosmetic concerns of 100 women with SOC, hyperpigmentation or dark spots (86%) and blotchy uneven skin (80%) were the top concerns.22 In addition, facial hyperpigmentation has been shown to negatively impact quality of life.23
Postinflammatory hyperpigmentation occurs from a pathophysiological response to inflammation, cutaneous irritation or injury, and subsequent melanocyte lability. Postinflammatory hyperpigmentation is a common presenting concern in patients with SOC and is seen as a result of many inflammatory skin disorders (eg, acne, eczema) and dermatologic procedures (eg, adverse reaction to electrodesiccation, microdermabrasion, chemical peels, laser surgery).24
Melasma is an acquired idiopathic disorder of hyperpigmentation and often referred to as the mask of pregnancy (Figure 3). It occurs on sun-exposed areas of skin, mainly in women with Fitzpatrick skin types III through V. Associated factors or triggers include pregnancy, hormonal treatments, exposure to UV radiation, and medications.25 Hereditary factors play a role in more than 40% of cases.26

Other not-so-common facial dyschromias include contact dermatitis, acanthosis nigricans, exogenous ochronosis, lichen planus pigmentosus (associated with frontal fibrosing alopecia),27 drug-induced hyperpigmentation (associated with minocycline or diltiazem),28,29 and UV-induced (age-related) hyperpigmentation.
Treatment
The treatment of hyperpigmentation should provide the following: (1) protection from sun exposure; (2) inhibition of tyrosinase, the enzyme responsible for the conversion of tyrosine to melanin; (3) inhibition of melanosome transfer from the melanocyte to the keratinocyte; (4) removal of melanin from the epidermis through exfoliation; and (5) destruction or disruption of melanin in the dermis.30 Therapies for facial hyperpigmentation are listed in Table 1.

Topical therapies include prescription medications and nonprescription cosmeceuticals. Prescription medications include hydroquinone (HQ), topical retinoids, and AZA. Hydroquinone, a tyrosinase inhibitor, is the gold standard for skin lightening and often is used as a first-line therapy. It is used as a monotherapy (HQ 4%) or as a fixed combination with tretinoin 0.05% and fluocinolone 0.01%.31 Use caution with HQ in high concentrations (6% and higher) and low concentrations (2% [over-the-counter strength]) used long-term due to the potential risk of exogenous ochronosis.
Topical retinoids have been shown to be effective therapeutic agents for melasma and PIH. Tretinoin,32 tazarotene,33 and adapalene34 all have demonstrated efficacy for acne and acne-induced PIH in SOC patients. Patients must be monitored for the development of retinoid dermatitis and worsening of hyperpigmentation.
Azelaic acid is a naturally occurring dicarboxylic acid obtained from cultures of Malassezia furfur. Azelaic acid inhibits tyrosinase activity, DNA synthesis, and mitochondrial enzymes, thus blocking direct cytotoxic effects toward melanocytes. Azelaic acid is approved by the US Food and Drug Administration for acne in a 20% cream formulation and rosacea in 15% gel and foam formulations, and it is used off label for melasma and PIH.35
Oral tranexamic acid is currently used as a hemostatic agent due to its ability to inhibit the plasminogen-plasmin pathway. In melasma, it blocks the interaction between melanocytes and keratinocytes in the epidermis and modulates the vascular component of melasma in the dermis. In an open-label study, 561 Asian melasma patients were treated with oral tranexamic acid 250 mg twice daily for 4 months. Results demonstrated improvement in 90% of patients, and 7.1% reported adverse effects (eg, abdominal bloating and pain, nausea, vomiting, headache, tinnitus, numbness, menstrual irregularities).36 Coagulation screening should be monitored monthly, and any patient with a history of clotting abnormalities should be excluded from off-label treatment with oral tranexamic acid.
Nonprescription cosmeceuticals are available over-the-counter or are office dispensed.37 For optimal results, cosmeceutical agents for skin lightening are used in combination. Most of these combinations are HQ free and have additive benefits such as a multimodal skin lightening agent containing key ingredients that correct and prevent skin pigmentation via several pathways affecting melanogenesis.38 It is an excellent alternative to HQ for mottled and diffuse UV-induced hyperpigmentation and can be used for maintenance therapy in patients with melasma.
Photoprotection is an essential component of therapy for melasma and PIH, but there is a paucity of data on the benefits for SOC patients. Halder et al39 performed a randomized prospective study of 89 black and Hispanic patients who applied sunscreen with a sun protection factor of 30 or 60 daily for 8 weeks. Clinical grading, triplicate L*A*B chromameter, and clinical photography were taken at baseline and weeks 4 and 8. The results demonstrated skin lightening in both black and Hispanic patients and support the use of sunscreen in the prevention and management of dyschromia in SOC patients.39 Visible light also may play a role in melasma development, and thus use of sunscreens or makeup containing iron oxides are recommended.40
Procedural treatments for facial hyperpigmentation include microdermabrasion, chemical peels, lasers, energy-based devices, and microneedling. There are many types and formulations of chemical peeling agents available; however, superficial and medium-depth chemical peels are recommended for SOC patients (Table 2). Deep chemical peels are not recommended for SOC patients due to the potential increased risk for PIH and scarring.

Cosmetic Enhancement in SOC Patients
Cosmetic procedures are gaining popularity in the SOC population and account for more than 20% of cosmetic procedures in the United States.41 Facial cosmetic concerns in SOC include dyschromia, benign growths (dermatosis papulosa nigra), hyperkinetic facial lines, volume loss, and skin laxity.42 Key principles to consider when treating SOC patients are the impact of ethnicity on aging and facial structure, the patient’s desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient’s expectations for recommended therapies.
Aging in SOC Patients
Skin aging can be classified as intrinsic aging or extrinsic aging. Intrinsic aging is genetic and involves subsurface changes such as volume loss, muscle atrophy, and resorption of bony structure. Extrinsic aging (or photoaging) involves surface changes of the epidermis/dermis and manifests as mottled pigmentation, textural changes, and fine wrinkling. Due to the photoprotection of melanin (black skin=SPF 13.4), skin aging in SOC patients is delayed by 10 to 20 years.43 In addition, SOC patients have more reactive collagen and can benefit from noninvasive cosmetic procedures such as fillers and skin-tightening procedures.42
Cosmetic Treatments and Procedures
Dermatosis papulosa nigra (benign growths of skin that have a genetic predisposition)44 occur mainly on the face but can involve the entire body. Treatment modalities include electrodesiccation, cryotherapy, scissor excision, and laser surgery.45
Treatment of hyperkinetic facial lines with botulinum toxin type A is a safe and effective procedure in patients with SOC. Grimes and Shabazz46 performed a 4-month, randomized, double-blind study that evaluated the treatment of glabellar lines in women with Fitzpatrick skin types V and VI. The results demonstrated that the duration of effects was the same in the patients who received either 20 or 30 U of botulinum toxin type A.46 Dynamic rhytides (furrows and frown/scowl lines arising from laughing, frowning, or smiling) can be treated safely in patients with SOC using botulinum toxin type A off label for relaxation of the upper and lower hyperkinetic muscles that result in these unwanted signs of aging. Botulinum toxin type A often is used for etched-in crow’s-feet, which rarely are evident in SOC patients.47 Facial shaping also can be accomplished by injecting botulinum toxin type A in combination with soft-tissue dermal fillers.47
Although black individuals do not experience perioral rhytides at the frequency of white individuals, they experience a variety of other cosmetic issues related to skin sagging and sinking. Currently available hyaluronic acid (HA) fillers have been shown to be safe in patients with Fitzpatrick skin types IV through VI.48 Two studies evaluated fillers in patients with SOC, specifically HA49 and calcium hydroxylapatite,50 focused on treatment of the nasolabial folds and the potential risk for dyspigmentation and keloidal scarring. Taylor et al49 noted that the risk of hyperpigmentation was 6% to 9% for large- and small-particle HA, respectively, and was associated with the serial or multiple puncture injection technique. No hypertrophic or keloidal scarring occurred in both studies.49,50
Facial contouring applications with fillers include glabellar lines, temples, nasal bridge, tear troughs, malar and submalar areas, nasolabial folds, radial lines, lips, marionette lines, mental crease, and chin. Hyaluronic acid fillers also can be used for lip enhancement.47 Although white women are looking to increase the size of their lips, black women are seeking augmentation to restore their lip size to that of their youth. Black individuals do not experience the same frequency of perioral rhytides as white patients, but they experience a variety of other issues related to skin sagging and sinking. Unlike white women, enhancement of the vermilion border rarely is performed in black women due to development of rhytides, predominantly in the body of the lip below the vermilion border in response to volume loss in the upper lip while the lower lip usually maintains its same appearance.47
Facial enhancement utilizing poly-L-lactic acid can be used safely in SOC patients.51 Poly-L-lactic acid microparticles induce collagen formation, leading to dermal thickening over 3 to 6 months; however, multiple sessions are required to achieve optimal aesthetic results.
Patients with more reactive collagen can benefit from noninvasive cosmetic procedures such as skin-tightening procedures.52 Radiofrequency and microfocused ultrasound are cosmetic procedures used to provide skin tightening and facial lifting. They are safe and effective treatments for patients with Fitzpatrick skin types IV to VI.53 Histologically, there is less thinning of collagen bundles and elastic tissue in ethnic skin. Due to stimulation of collagen by these procedures, most SOC patients will experience a more enhanced response, requiring fewer treatment sessions than white individuals.
Conclusion
Medical and aesthetic facial concerns in SOC patients vary and can be a source of emotional and psychological distress that can negatively impact quality of life. The approach to the treatment of SOC patients should be a balance between tolerability and efficacy, considering the potential risk for PIH.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39(2 pt 3):S34-S37.
- Halder RM, Grimes PE, McLaurin CL, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasians, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl):S98-S106.
- Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
- Halder RM, Holmes YC, Bridgeman-Shah S, et al. A clinicohistologic study of acne vulgaris in black females (abstract). J Invest Dermatol. 1996;106:888.
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101:580-584.
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106:893-897.
- Halder RM, Brooks HL, Callender VD. Acne in ethnic skin. Dermatol Clin. 2003;21:609-615.
- Callender VD. Acne in ethnic skin: special considerations for therapy. Dermatol Ther. 2004;17:184-195.
- Winhoven SM. Postinflammatory hyperpigmentation in an Asian patient. a dramatic response to oral isotretinoin (13-cis-retinoic acid). Br J Med. 2005;152:368-403.
- Sarkar R, Bansal S, Garg VK. Chemical peels for melasma in dark-skinned patients. J Cutan Aesthet Surg. 2012;5:247-253.
- Alexis AF, Coley MK, Nijhawan RI, et al. Nonablative fractional laser resurfacing for acne scarring in patients with Fitzpatrick skin phototypes IV-VI. Dermatol Surg. 2016;42:392-402.
- Culp B, Scheinfeld N. Rosacea: a review. P T. 2009;34:38-45.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014:20. pii:13030/qt1mv9r0ss.
- Del Rosso JQ. Advances in understanding and managing rosacea: part 1: connecting the dots between pathophysiological mechanisms and common clinical features of rosacea with emphasis on vascular changes and facial erythema. J Clin Aesthet Dermatol. 2012;5:16-25.
- Jackson JM, Knuckles M, Minni JP, et al. The role of brimonidine tartrate gel in the treatment of rosacea. Clin Cosmet Investig Dermatol. 2015;23:529-538.
- Patel NU, Shukla S, Zaki J, et al. Oxymetazoline hydrochloride cream for facial erythema associated with rosacea. Expert Rev Clin Pharmacol. 2017;10:104954.
- Weinkle AP, Doktor V, Emer J. Update on the management of rosacea. Clin Cosmet Investig Dermatol. 2015;8:159-177.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;19:659-665.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7:164-168.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation: a review of the epidemiology, clinical features, and treatment options in skin of color. J Clin Aesthet Dermatol. 2010;3:20-31.
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Callender VD, Reid SD, Obayan O, et al. Diagnostic clues to frontal fibrosing alopecia in patients of African descent. J Clin Aesthet Dermatol. 2016;9:45-51.
- Narang T, Sawatkar GU, Kumaran MS, et al. Minocycline for recurrent and/or chronic erythema nodosum leprosum. JAMA Dermatol. 2015;151:1026-1028.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10.
- Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91-98.
- Taylor SC, Torok H, Jones T, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003;72:67-72.
- Bulengo-Ransby SM. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438-1443.
- Grimes P, Callender V. Tazarotene cream for postinflammatory hyperpigmentation and acne vulgaris in darker skin: a double-blind, randomized, vehicle-controlled study. Cutis. 2006;77:45-50.
- Jacyk WK. Adapalene in the treatment of African patients. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):37-42.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of postinflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10:586-590.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma. J Am Acad Dermatol. 2016;75:385-392.
- Kindred C, Okereke U, Callender VD. Skin-lightening agents: an overview of prescription, office-dispensed, and over-the-counter products. Cosmet Dermatol. 2013;26:18-26.
- Makino ET, Kadoya K, Sigler ML, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol. 2016;15:1562-1570.
- Halder R, Rodney I, Munhutu M, et al. Evaluation and effectiveness of a photoprotection composition (sunscreen) on subjects of skin of color. J Am Acad Dermatol. 2015;72(suppl 1):AB215.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- American Society for Aesthetic Plastic Surgery. 2016 Cosmetic Surgery National Data Bank Statistics. https://www.surgery.org/sites/default/files/ASAPS-Stats2016.pdf. Accessed November 15, 2017.
- Burgess CM. Soft tissue augmentation in skin of color: market growth, available fillers and successful techniques. J Drugs Dermatol. 2007;6:51-55.
- Davis EC, Callender VD. Aesthetic dermatology for aging ethnic skin. Dermatol Surg. 2011;37:901-917.
- Grimes PE, Arora S, Minus HR, et al. Dermatosis papulosa nigra. Cutis. 1983;32:385-386.
- Lupo M. Dermatosis papulosa nigra: treatment options. J Drugs Dermatol. 2007;6:29-30.
- Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg. 2009;35:429-435.
- Burgess CM, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg. 2015;136(5 suppl):28S-31S.
- Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill Education; 2016.
- Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(suppl 2):1653-1660.
- Marmur ES, Taylor SC, Grimes PE, et al. Six-month safety results of calcium hydroxylapatite for treatment of nasolabial folds in Fitzpatrick skin types IV to VI. Dermatol Surg. 2009;35(suppl 2):1641-1645.
- Hamilton TK, Burgess CM. Consideration for the use of injectable poly-L-lactic acid in people of color. J Drugs Dermatol. 2010;9:451-456.
- Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening of the face and neck. Dermatol Surg. 2014;40:569-575.
- Harris MO, Sundaram HA. Safety of microfocused ultrasound with visualization in patients with Fitzpatrick skin phototypes III to VI. JAMA Facial Plast Surg. 2015;17:355-357.
Practice Points
- Treat acne in skin of color (SOC) patients early and aggressively to prevent or minimize subsequent postinflammatory hyperpigmentation (PIH) and acne scarring.
- Vascular lasers and intense pulsed light may be used to address the vascular components of rosacea; however, the latter is not recommended in Fitzpatrick skin types IV to VI.
- Hydroquinone is the gold standard for skin lightening and is often used as a first-line therapy for melasma and PIH.
- Photoprotection is an essential component of therapy for hyperpigmented skin disorders.
- Cosmetic procedures are gaining popularity in the SOC population. When treating SOC patients, consider the impact of ethnicity on aging and facial structure, the patient's desired cosmetic outcome, tissue reaction to anticipated treatments, and the patient's expectations for recommended therapies.
Assessing the Effectiveness of Knowledge-Based Interventions in Increasing Skin Cancer Awareness, Knowledge, and Protective Behaviors in Skin of Color Populations
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).
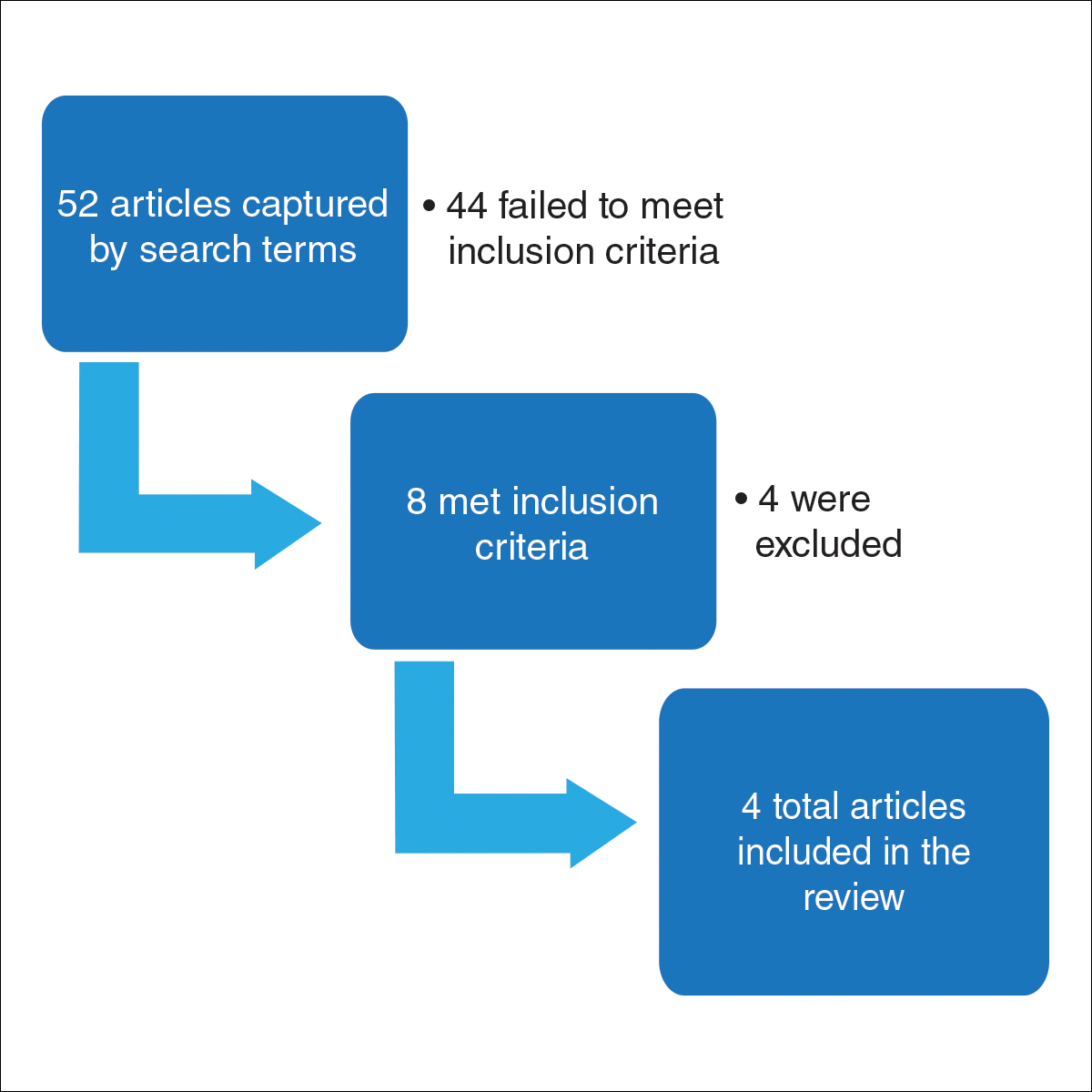
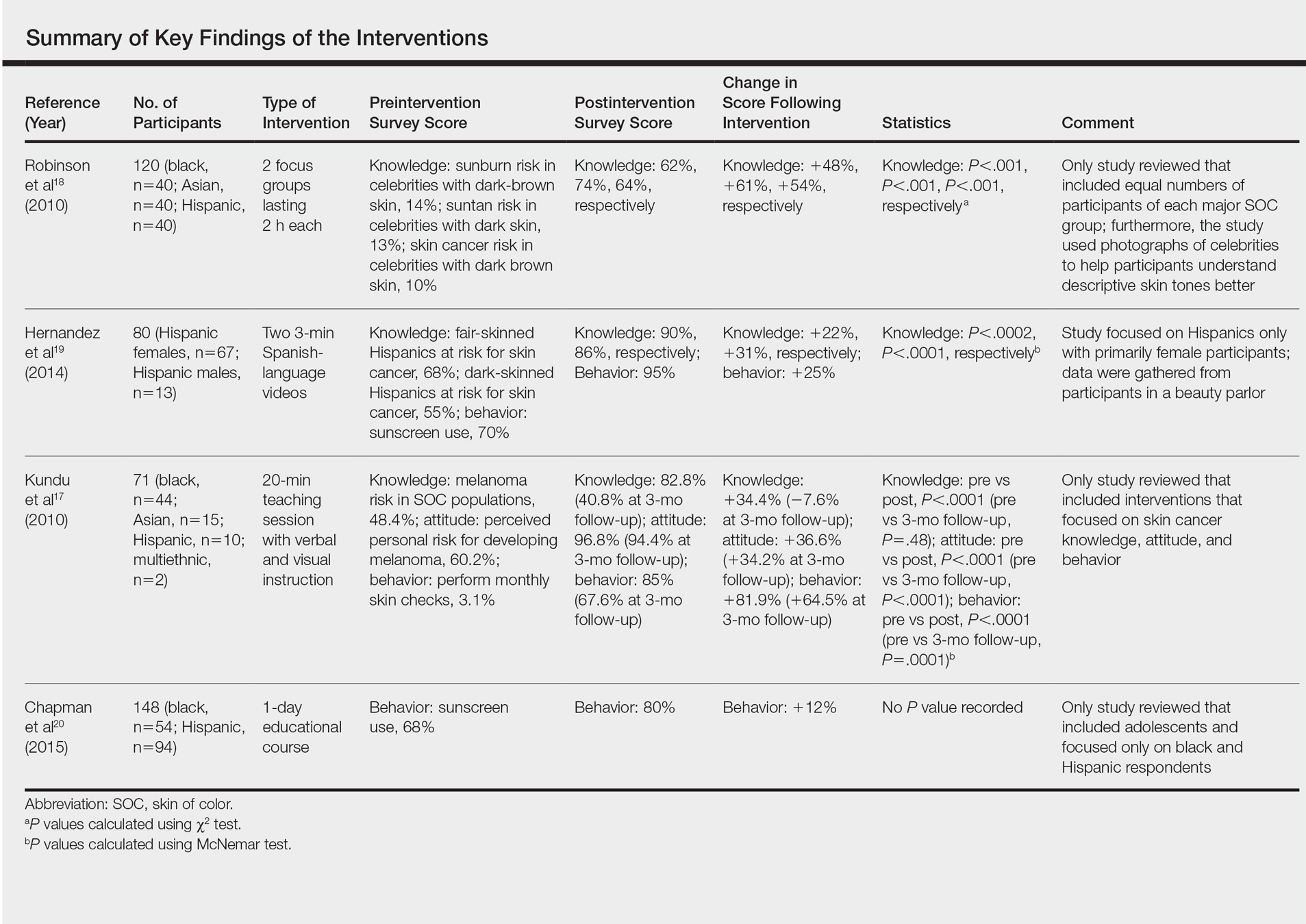
Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Practice Points
- Patients of color should be informed that they are at risk for skin cancer including melanoma.
- Patients of color should be taught to identify suspicious skin lesions including the ABCDEs of melanoma.
- Patients of color should be instructed to perform self-body skin examinations, especially of the palms and soles, for any evolving skin lesions. Patients should be instructed on the importance of visiting a physician for an evolving or suspicious mole or lesion.
Hair and Scalp Disorders in Patients With Skin of Color
Hair and Scalp Disorders in Adult and Pediatric Patients With Skin of Color
One of the most common concerns among black patients is hair- and scalp-related disease. As increasing numbers of black patients opt to see dermatologists, it is imperative that all dermatologists be adequately trained to address the concerns of this patient population. When patients ask for help with common skin diseases of the hair and scalp, there are details that must be included in diagnosis, treatment, and hair care recommendations to reach goals for excellence in patient care. Herein, we provide must-know information to effectively approach this patient population.
Seborrheic Dermatitis
A study utilizing data from the National Ambulatory Medical Care Survey from 1993 to 2009 revealed seborrheic dermatitis (SD) as the second most common diagnosis for black patients who visit a dermatologist.1 Prevalence data from a population of 1408 white, black, and Chinese patients from the United States and China revealed scalp flaking in 81% to 95% of black patients, 66% to 82% in white patients, and 30% to 42% in Chinese patients.2 Seborrheic dermatitis has a notable prevalence in black women and often is considered normal by patients. It can be exacerbated by infrequent shampooing (ranging from once per month or longer in between shampoos) and the inappropriate use of hair oils and pomades; it also has been associated with hair breakage, lichen simplex chronicus, and folliculitis. Seborrheic dermatitis must be distinguished from other disorders including sarcoidosis, psoriasis, discoid lupus erythematosus, tinea capitis, and lichen simplex chronicus.
Although there is a paucity of literature on the treatment of SD in black patients, components of treatment are similar to those recommended for other populations. Black women are advised to carefully utilize antidandruff shampoos containing zinc pyrithione, selenium sulfide, or tar to avoid hair shaft damage and dryness. Ketoconazole shampoo rarely is recommended and may be more appropriately used in men and boys, as hair fragility is less of a concern for them. The shampoo should be applied directly to the scalp rather than the hair shafts to minimize dryness, with no particular elongated contact time needed for these medicated shampoos to be effective. Because conditioners can wash off the active ingredients in therapeutic shampoos, antidandruff conditioners are recommended. Potent or ultrapotent topical corticosteroids applied to the scalp 3 to 4 times weekly initially will control the symptoms of itching as well as scaling, and mid-potency topical corticosteroid oil may be used at weekly intervals.
Hairline and facial involvement of SD often co-occurs, and low-potency topical steroids may be applied to the affected areas twice daily for 3 to 4 weeks, which may be repeated for flares. Topical calcineurin inhibitors or antifungal creams such as ketoconazole or econazole may then provide effective control. Encouraging patients to increase shampooing to once weekly or every 2 weeks and discontinue use of scalp pomades and oils also is recommended. Patients must know that an itchy scaly scalp represents a treatable disorder.
Acquired Trichorrhexis Nodosa
Hair fragility and breakage is common and multifactorial in black patients. Hair shaft breakage can occur on the vertex scalp in central centrifugal cicatricial alopecia (CCCA), with random localized breakage due to scratching in SD. Heat, hair colorants, and chemical relaxers may result in diffuse damage and breakage.3 Sodium-, potassium-, and guanine hydroxide–containing chemical relaxers change the physical properties of the hair by rearranging disulfide bonds. They remove the monomolecular layer of fatty acids covalently bound to the cuticle that help prevent penetration of water into the hair shaft. Additionally, chemical relaxers weaken the hair shaft and decrease tensile strength.
Unlike hair relaxers, colorants are less likely to lead to catastrophic hair breakage after a single use and require frequent use, which leads to cumulative damage. Thermal straightening is another cause of hair-shaft weakening in black patients.4,5 Flat irons and curling irons can cause substantially more damage than blow-dryers due to the amount of heat generated. Flat irons may reach a high temperature of 230ºC (450ºF) as compared to 100°C (210°F) for a blow-dryer. Even the simple act of combing the hair can cause hair breakage, as demonstrated in African volunteers whose hair remained short in contrast to white and Asian volunteers, despite the fact that they had not cut their hair for 1 or more years.6,7 These volunteers had many hair strand knots that led to breakage during combing and hair grooming.6
There is no known prevalence data for acquired trichorrhexis nodosa, though a study of 30 white and black women demonstrated that broken hairs were significantly increased in black women (P=.0001).8 Another study by Hall et al9 of 103 black women showed that 55% of the women reported breakage of hair shafts with normal styling. Khumalo et al6 investigated hair shaft fragility and reported no trichothiodystrophy; the authors concluded that the cause of the hair fragility likely was physical trauma or an undiscovered structural abnormality. Franbourg et al10 examined the structure of hair fibers in white, Asian, and black patients and found no differences, but microfractures were only present in black patients and were determined to be the cause of hair breakage. These studies underscore the need for specific questioning of the patient on hair care including combing, washing, drying, and using products and chemicals.
The approach to the treatment of hair breakage involves correcting underlying abnormalities (eg, iron deficiency, hypothyroidism, nutritional deficiencies). Patients should “give their hair a rest” by discontinuing use of heat, colorants, and chemical relaxers. For patients who are unable to comply, advising them to stop these processes for 6 to 12 months will allow for repair of the hair shaft. To minimize damage from colorants, recommend semipermanent, demipermanent, or temporary dyes. Patients should be counseled to stop bleaching their hair or using permanent colorants. The use of heat protectant products on the hair before styling as well as layering moisturizing regimens starting with a moisturizing shampoo followed by a leave-in, dimethicone-containing conditioner marketed for dry damaged hair is suggested. Dimethicone thinly coats the hair shaft to restore hydrophobicity, smoothes cuticular scales, decreases frizz, and protects the hair from damage. Use of a 2-in-1 shampoo and conditioner containing anionic surfactants and wide-toothed, smooth (no jagged edges in the grooves) combs along with rare brushing are recommended. The hair may be worn in its natural state, but straightening with heat should be avoided. Air drying the hair can minimize breakage, but if thermal styling is necessary, patients should turn the temperature setting of the flat or curling iron down. Protective hair care practices may include placing a loosely sewn-in hair weave that will allow for good hair care, wearing loose braids, or using a wig. Serial trimming of the hair every 6 to 8 weeks is recommended. Improvement may take time, and patients should be advised of this timeline to prevent frustration.
Acne Keloidalis Nuchae
Acne keloidalis nuchae (AKN) is characterized by papules and pustules located on the occipital scalp and/or the nape of the neck, which may result in keloidal papules and plaques. The etiology is unknown, but ingrown hairs, genetics, trauma, infection, inflammation, and androgen hormones have been proposed to play a role.11 Although AKN may occur in black women, it is primarily a disorder in black men. The diagnosis is made based primarily on clinical findings, and a history of short haircuts may support the diagnosis. Treatment is tailored to the severity of the disease (Table 1). Avoidance of short haircuts and irritation from shirt collars may be helpful. Patients should be advised that the condition is controllable but not curable.
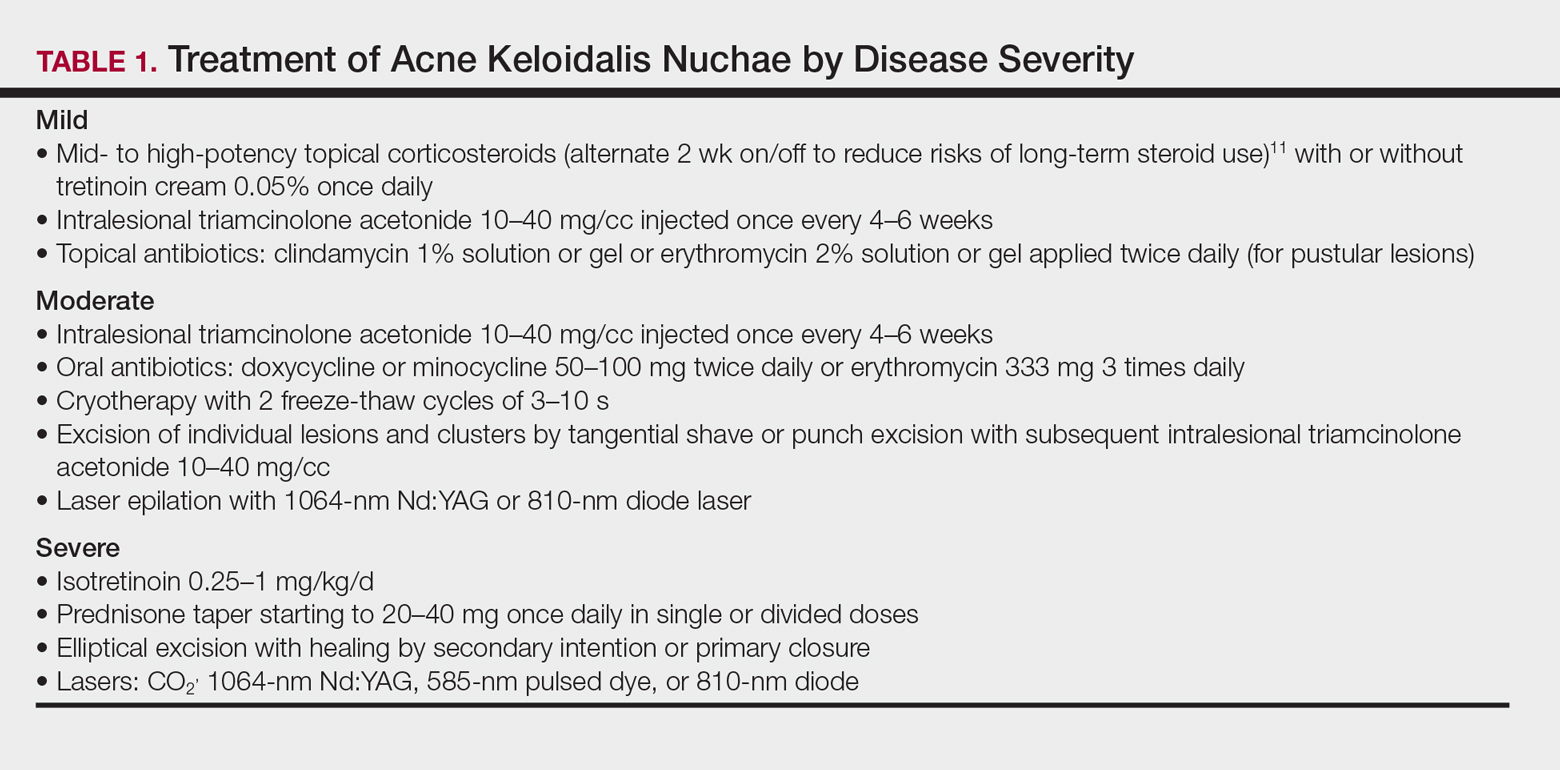
Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB) is characterized by papules and pustules in the beard region that may result in postinflammatory hyperpigmentation, keloidal scar formation, and/or linear scarring. The coarse curled hairs characteristic of black men penetrate the follicle before exiting the skin and penetrate the skin after exiting the follicle, resulting in inflammation. Shaving methods and genetics also may contribute to the development of PFB. As with AKN, diagnosis is made clinically and does not require a skin biopsy. Important components of the patient’s history that should be obtained are hair removal practices and the use of over-the-counter products (eg, shave [pre and post] moisturizers, exfoliants, shaving creams or gels, keratin-softening agents containing α- or β-hydroxy acids). A bacterial culture may be appropriate if a notable pustular component is present. The patient should be advised to discontinue shaving if possible, which may require a physician’s letter explaining the necessity to the patient’s employer. Pseudofolliculitis barbae often can be prevented or lessened with the right hair removal strategy. Because there is not one optimal hair removal strategy that suits every patient, encourage the patient to experiment with different hair removal techniques, from depilatories to electric shavers, foil-guard razors, and multiple-blade razors. Preshave hydration and postshave moisturiza-tion also should be encouraged.12 Benzoyl peroxide–containing shave gels and cleansers, as well as moisturizers containing glycolic, salicylic, and phytic acids, may minimize ingrown hairs, papules, and inflammation.
Other useful topical agents include eflornithine hydrochloride to decrease hair growth, retinoids to soften hair fibers, mild topical steroids to reduce inflammation, and/or topical erythromycin or clindamycin if pustules are present.13 Oral antibiotics such as doxycycline, minocycline, or erythromycin can be added for more severe cases of inflammation or infection. Procedural interventions include laser hair removal to prevent PFB and intralesional triamcinolone 10 to 40 mg/cc every 4 to 6 weeks, with the total volume depending on the size and number of lesions.
Alopecia
Alopecia is the sixth most common diagnosis seen in black patients visiting a dermatologist.14 The physician’s response to the patient’s chief concern of hair loss is key to building a relationship of confidence and trust. Trivializing the concern or dismissing it will undermine the physician-patient relationship. A survey by Gathers and Mahan15 revealed that 68% of patients thought that physicians did not understand their hair.
Hair loss negatively impacts quality of life, and a study of 50 black South African women with alopecia demonstrated a notable disease burden. Factors with the highest impact were those related to self-image, relationships, and interactions with others.16
It is not unusual for black women to have multiple types of alopecia identified in one biopsy specimen. Wohltmann and Sperling17 demonstrated 2 or more different types of alopecia in more than 10% of biopsy specimens of alopecia, including CCCA, androgenetic alopecia, end-stage traction alopecia, telogen effluvium, and tinea capitis. A complete history, physical examination, and appropriate procedures (eg, hair pull test, dermatoscopic examination and scalp biopsy) likely will yield an accurate diagnosis. Table 2 highlights important questions that should be asked about the patient’s history.
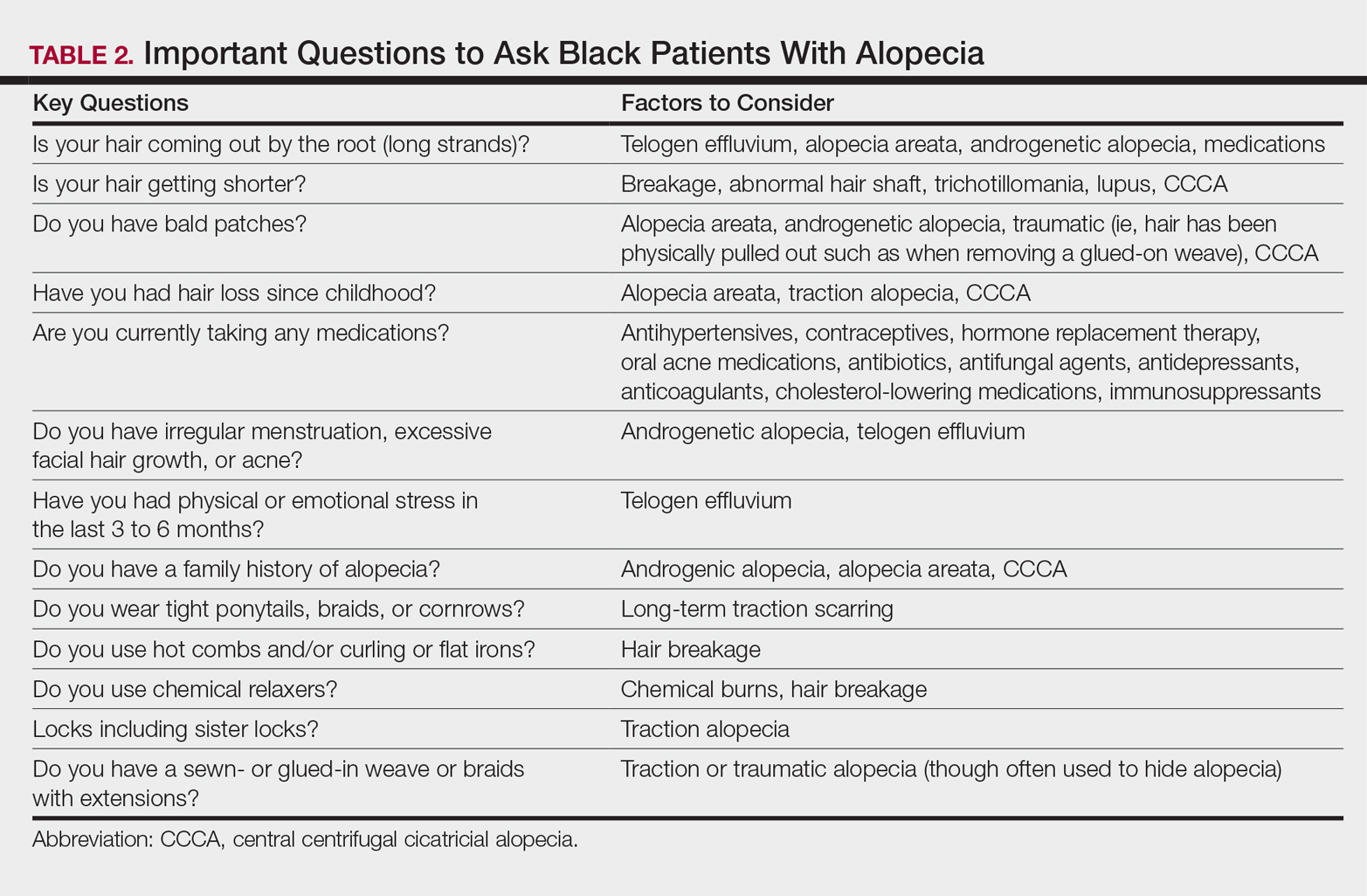
Physical examination of the scalp including dermatoscopic examination and a hair pull test as well as an evaluation of other hair-bearing areas may suggest a diagnosis that can be confirmed with a scalp biopsy.18,19 Selection of a biopsy site at the periphery of the alopecic area that includes hair and consultation with a dermatopathologist familiar with features of CCCA, traction, and traumatic alopecia are important for making an accurate diagnosis.
Tinea Capitis in Black Pediatric Patients
Tinea capitis, a fungal infection of the scalp and hair, is one of the most common issues in children with skin of color. Clinical presentation may include widely distributed scaling, annular scaly plaques, annular patches of alopecia studded with black dots (broken hairs), and/or annular inflammatory plaques. Although scalp hyperkeratosis often is a hallmark of pediatric tinea capitis, it is not diagnostic. The differential diagnosis of pediatric scalp hyperkeratosis/scaling includes tinea capitis, SD, atopic dermatitis, psoriasis, and sebopsoriasis.20,21 Clues to accurate diagnosis of tinea capitis may be found by examination of the adult who combs the child’s hair, as erythematous annular scaly plaques representing tinea corporis may be observed on the forearms or thighs. Although the thighs are a seemingly unusual location, the frequent practice of the child sitting on the floor between the legs of the adult during hairstyling provides a point of contact for the transmission of tinea from the child’s scalp to the thighs or forearms of the adult. Once tinea capitis is clinically suspected, the diagnosis is confirmed by a fungal culture. Adequate sampling is obtained by clipping hairs in an area of scaling for submission and vigorously rubbing the area of black dots or hyperkeratosis with a cotton swab.
Hubbard22 shed light on the decision to treat tinea capitis empirically or await the culture results. One hundred consecutive children (98 were black) presented with the constellation of scalp alopecia, scaling, pruritus, and occipital lymphadenopathy. Sixty-eight of those children had positive fungal cultures, and of them, 60 had both occipital lymphadenopathy and scaling and 55 had both occipital lymphadenopathy and alopecia.22 Thus, occipital lymphadenopathy in conjunction with alopecia and/or scaling is predictive of tinea capitis in this population and suggests that the initiation of treatment prior to confirmative culture results is appropriate.
The mainstay of treatment for tinea capitis is griseofulvin, but it is often underdosed and not continued for an adequate period of time to ensure clearance of the infection. Griseofulvin microsize (125 mg/5 mL) at the dosage of 20 to 25 mg/kg once daily for 8 to 12 weeks is recommended instead of a lower-dosed 4- to 6-week course.23,24
Options for treating a child with residual disease include increasing and/or extending the griseofulvin dosage, encouraging ingestion of fatty foods to enhance absorption, dividing the dosage of griseofulvin from once daily to twice daily, changing therapy to oral terbinafine due to resistance to griseofulvin, examining siblings as a source of reinfection, and reviewing the positive fungal culture report to distinguish Trichophyton tonsurans versus Microsporum canis as the causative agent and adjust treatment accordingly. Although griseofulvin is the first-line treatment for M canis, terbinafine, which is approved for children 4 years and older for tineacapitis, is most efficacious for T tonsurans.25 Treatment with terbinafine is weight based and should extend for 2 to 4 weeksfor T tonsurans and 8 to 12 weeks for M canis.
Antifungal shampoos may help reduce household spread of tinea and decrease transmissible fungal spores, but they may cause hair dryness and breakage.26,27 Antifungal shampoos can be applied directly onto the scalp for a 5- to 10-minute contact time and rinsed, and then the hair should be shampooed with a moisturizing shampoo followed by a moisturizing conditioner. Hair conditioners may decrease household spread of tinea capitis and should be used by the patient and other members of the household.28 Infection control may be enhanced by advising parents to dispose of hair pomades and washing hair accessories, combs, and brushes in hot soapy water, preferably in the dishwasher.
Hair Growth
The inability of the hair of black children to grow long is a common concern for parents of toddlers and preschool-aged children. Although the hair does grow, it grows more slowly than hair in white children (0.259 vs 0.330 mm per day), and it is likely to break faster than it is growing in black versus white children (146.6 vs 13.13 total broken hairs).8 Reassurance that the hair is indeed growing and that the length will increase as the child matures is important. Avoidance of hairstyles that promote traction and use of hair extensions, as well as use of moisturizing shampoos and conditioners, may minimize breakage and support the growth of healthy hair.
Conclusion
Hair- and scalp-related disease in black adults and children is commonly encountered in dermatology practice. It is important to understand the intrinsic characteristics of facial and scalp hair as well as hair care practices in this patient population that differ from those of white and Asian populations, such as frequency of shampooing, products, and styling. Familiarity with these differences may aid in effective diagnosis, treatment, and hair care recommendations in patients with these conditions.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Hickman JG, Cardin C, Dawson TL, et al. Dandruff, part I: scalp disease prevalence in Caucasians, African Americans, and Chinese and the effects of shampoo frequency on scalp health. Poster presented at: 60th Annual Meeting of the American Academy of Dermatology; February 22-27, 2002; New Orleans, LA.
- Swee W, Klontz KC, Lambert LA. A nationwide outbreak of alopecia associated with the use of a hair-relaxing formulation. Arch Dermatol. 2000;136:1104-1108.
- Nicholson AG, Harland CC, Bull RH, et al. Chemically induced cosmetic alopecia. Br J Dermatol. 1993;128:537-541.
- Detwiler SP, Carson JL, Woosley JT, et al. Bubble hair. case caused by an overheating hair dryer and reproducibility in normal hair with heat. J Am Acad Dermatol. 1994;30:54-60.
- Khumalo NP, Dawber RP, Ferguson DJ. Apparent fragility of African hair is unrelated to the cystine-rich protein distribution: a cytochemical electron microscopic study. Exp Dermatol. 2005;14:311-314.
- Robbins C. Hair breakage during combing. I. pathways of breakage. J Cosmet Sci. 2006;57:233-243.
- Lewallen R, Francis S, Fisher B, et al. Hair care practices and structural evaluation of scalp and hair shaft parameter in African American and Caucasian women. J Cosmet Dermatol. 2015;14:216-223.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314.
- Franbourg A, Hallegot P, Baltenneck F, et al. Current research on ethnic hair. J Am Acad Dermatol. 2003;48(6 suppl):S115-S119.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38(suppl 1):24-27.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part II. disorders occurring predominately in skin of color. Am Fam Physician. 2013;87:859-865.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Dlova NC, Fabbrocini G, Lauro C, et al. Quality of life in South African black women with alopecia: a pilot study. Int J Dermatol. 2016;55:875-881.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- McDonald KA, Shelley AJ, Colantonio S, et al. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76:472-477.
- Miteva M, Tosti A. Dermatoscopic features of central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014;71:443-444.
- Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
- Silverberg NB. Scalp hyperkeratosis in children with skin of color: diagnostic and therapeutic considerations. Cutis. 2015;95:199-204, 207.
- Hubbard TW. The predictive value of symptoms in diagnosing childhood tinea capitis. Arch Pediatr Adolesc Med. 1999;153:1150-1153.
- Kakourou T, Uksal U; European Society for Pediatric Dermatology. Guidelines for the management of tinea capitis in children. Pediatr Dermatol. 2010;27:226-228.
- Sethi A, Antanya R. Systemic antifungal therapy for cutaneous infections in children. Pediatr Infect Dis J. 2006;25:643-644.
- Gupta AK. Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol 2000;39:302-304.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol. 2000;39:302-304.
One of the most common concerns among black patients is hair- and scalp-related disease. As increasing numbers of black patients opt to see dermatologists, it is imperative that all dermatologists be adequately trained to address the concerns of this patient population. When patients ask for help with common skin diseases of the hair and scalp, there are details that must be included in diagnosis, treatment, and hair care recommendations to reach goals for excellence in patient care. Herein, we provide must-know information to effectively approach this patient population.
Seborrheic Dermatitis
A study utilizing data from the National Ambulatory Medical Care Survey from 1993 to 2009 revealed seborrheic dermatitis (SD) as the second most common diagnosis for black patients who visit a dermatologist.1 Prevalence data from a population of 1408 white, black, and Chinese patients from the United States and China revealed scalp flaking in 81% to 95% of black patients, 66% to 82% in white patients, and 30% to 42% in Chinese patients.2 Seborrheic dermatitis has a notable prevalence in black women and often is considered normal by patients. It can be exacerbated by infrequent shampooing (ranging from once per month or longer in between shampoos) and the inappropriate use of hair oils and pomades; it also has been associated with hair breakage, lichen simplex chronicus, and folliculitis. Seborrheic dermatitis must be distinguished from other disorders including sarcoidosis, psoriasis, discoid lupus erythematosus, tinea capitis, and lichen simplex chronicus.
Although there is a paucity of literature on the treatment of SD in black patients, components of treatment are similar to those recommended for other populations. Black women are advised to carefully utilize antidandruff shampoos containing zinc pyrithione, selenium sulfide, or tar to avoid hair shaft damage and dryness. Ketoconazole shampoo rarely is recommended and may be more appropriately used in men and boys, as hair fragility is less of a concern for them. The shampoo should be applied directly to the scalp rather than the hair shafts to minimize dryness, with no particular elongated contact time needed for these medicated shampoos to be effective. Because conditioners can wash off the active ingredients in therapeutic shampoos, antidandruff conditioners are recommended. Potent or ultrapotent topical corticosteroids applied to the scalp 3 to 4 times weekly initially will control the symptoms of itching as well as scaling, and mid-potency topical corticosteroid oil may be used at weekly intervals.
Hairline and facial involvement of SD often co-occurs, and low-potency topical steroids may be applied to the affected areas twice daily for 3 to 4 weeks, which may be repeated for flares. Topical calcineurin inhibitors or antifungal creams such as ketoconazole or econazole may then provide effective control. Encouraging patients to increase shampooing to once weekly or every 2 weeks and discontinue use of scalp pomades and oils also is recommended. Patients must know that an itchy scaly scalp represents a treatable disorder.
Acquired Trichorrhexis Nodosa
Hair fragility and breakage is common and multifactorial in black patients. Hair shaft breakage can occur on the vertex scalp in central centrifugal cicatricial alopecia (CCCA), with random localized breakage due to scratching in SD. Heat, hair colorants, and chemical relaxers may result in diffuse damage and breakage.3 Sodium-, potassium-, and guanine hydroxide–containing chemical relaxers change the physical properties of the hair by rearranging disulfide bonds. They remove the monomolecular layer of fatty acids covalently bound to the cuticle that help prevent penetration of water into the hair shaft. Additionally, chemical relaxers weaken the hair shaft and decrease tensile strength.
Unlike hair relaxers, colorants are less likely to lead to catastrophic hair breakage after a single use and require frequent use, which leads to cumulative damage. Thermal straightening is another cause of hair-shaft weakening in black patients.4,5 Flat irons and curling irons can cause substantially more damage than blow-dryers due to the amount of heat generated. Flat irons may reach a high temperature of 230ºC (450ºF) as compared to 100°C (210°F) for a blow-dryer. Even the simple act of combing the hair can cause hair breakage, as demonstrated in African volunteers whose hair remained short in contrast to white and Asian volunteers, despite the fact that they had not cut their hair for 1 or more years.6,7 These volunteers had many hair strand knots that led to breakage during combing and hair grooming.6
There is no known prevalence data for acquired trichorrhexis nodosa, though a study of 30 white and black women demonstrated that broken hairs were significantly increased in black women (P=.0001).8 Another study by Hall et al9 of 103 black women showed that 55% of the women reported breakage of hair shafts with normal styling. Khumalo et al6 investigated hair shaft fragility and reported no trichothiodystrophy; the authors concluded that the cause of the hair fragility likely was physical trauma or an undiscovered structural abnormality. Franbourg et al10 examined the structure of hair fibers in white, Asian, and black patients and found no differences, but microfractures were only present in black patients and were determined to be the cause of hair breakage. These studies underscore the need for specific questioning of the patient on hair care including combing, washing, drying, and using products and chemicals.
The approach to the treatment of hair breakage involves correcting underlying abnormalities (eg, iron deficiency, hypothyroidism, nutritional deficiencies). Patients should “give their hair a rest” by discontinuing use of heat, colorants, and chemical relaxers. For patients who are unable to comply, advising them to stop these processes for 6 to 12 months will allow for repair of the hair shaft. To minimize damage from colorants, recommend semipermanent, demipermanent, or temporary dyes. Patients should be counseled to stop bleaching their hair or using permanent colorants. The use of heat protectant products on the hair before styling as well as layering moisturizing regimens starting with a moisturizing shampoo followed by a leave-in, dimethicone-containing conditioner marketed for dry damaged hair is suggested. Dimethicone thinly coats the hair shaft to restore hydrophobicity, smoothes cuticular scales, decreases frizz, and protects the hair from damage. Use of a 2-in-1 shampoo and conditioner containing anionic surfactants and wide-toothed, smooth (no jagged edges in the grooves) combs along with rare brushing are recommended. The hair may be worn in its natural state, but straightening with heat should be avoided. Air drying the hair can minimize breakage, but if thermal styling is necessary, patients should turn the temperature setting of the flat or curling iron down. Protective hair care practices may include placing a loosely sewn-in hair weave that will allow for good hair care, wearing loose braids, or using a wig. Serial trimming of the hair every 6 to 8 weeks is recommended. Improvement may take time, and patients should be advised of this timeline to prevent frustration.
Acne Keloidalis Nuchae
Acne keloidalis nuchae (AKN) is characterized by papules and pustules located on the occipital scalp and/or the nape of the neck, which may result in keloidal papules and plaques. The etiology is unknown, but ingrown hairs, genetics, trauma, infection, inflammation, and androgen hormones have been proposed to play a role.11 Although AKN may occur in black women, it is primarily a disorder in black men. The diagnosis is made based primarily on clinical findings, and a history of short haircuts may support the diagnosis. Treatment is tailored to the severity of the disease (Table 1). Avoidance of short haircuts and irritation from shirt collars may be helpful. Patients should be advised that the condition is controllable but not curable.

Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB) is characterized by papules and pustules in the beard region that may result in postinflammatory hyperpigmentation, keloidal scar formation, and/or linear scarring. The coarse curled hairs characteristic of black men penetrate the follicle before exiting the skin and penetrate the skin after exiting the follicle, resulting in inflammation. Shaving methods and genetics also may contribute to the development of PFB. As with AKN, diagnosis is made clinically and does not require a skin biopsy. Important components of the patient’s history that should be obtained are hair removal practices and the use of over-the-counter products (eg, shave [pre and post] moisturizers, exfoliants, shaving creams or gels, keratin-softening agents containing α- or β-hydroxy acids). A bacterial culture may be appropriate if a notable pustular component is present. The patient should be advised to discontinue shaving if possible, which may require a physician’s letter explaining the necessity to the patient’s employer. Pseudofolliculitis barbae often can be prevented or lessened with the right hair removal strategy. Because there is not one optimal hair removal strategy that suits every patient, encourage the patient to experiment with different hair removal techniques, from depilatories to electric shavers, foil-guard razors, and multiple-blade razors. Preshave hydration and postshave moisturiza-tion also should be encouraged.12 Benzoyl peroxide–containing shave gels and cleansers, as well as moisturizers containing glycolic, salicylic, and phytic acids, may minimize ingrown hairs, papules, and inflammation.
Other useful topical agents include eflornithine hydrochloride to decrease hair growth, retinoids to soften hair fibers, mild topical steroids to reduce inflammation, and/or topical erythromycin or clindamycin if pustules are present.13 Oral antibiotics such as doxycycline, minocycline, or erythromycin can be added for more severe cases of inflammation or infection. Procedural interventions include laser hair removal to prevent PFB and intralesional triamcinolone 10 to 40 mg/cc every 4 to 6 weeks, with the total volume depending on the size and number of lesions.
Alopecia
Alopecia is the sixth most common diagnosis seen in black patients visiting a dermatologist.14 The physician’s response to the patient’s chief concern of hair loss is key to building a relationship of confidence and trust. Trivializing the concern or dismissing it will undermine the physician-patient relationship. A survey by Gathers and Mahan15 revealed that 68% of patients thought that physicians did not understand their hair.
Hair loss negatively impacts quality of life, and a study of 50 black South African women with alopecia demonstrated a notable disease burden. Factors with the highest impact were those related to self-image, relationships, and interactions with others.16
It is not unusual for black women to have multiple types of alopecia identified in one biopsy specimen. Wohltmann and Sperling17 demonstrated 2 or more different types of alopecia in more than 10% of biopsy specimens of alopecia, including CCCA, androgenetic alopecia, end-stage traction alopecia, telogen effluvium, and tinea capitis. A complete history, physical examination, and appropriate procedures (eg, hair pull test, dermatoscopic examination and scalp biopsy) likely will yield an accurate diagnosis. Table 2 highlights important questions that should be asked about the patient’s history.

Physical examination of the scalp including dermatoscopic examination and a hair pull test as well as an evaluation of other hair-bearing areas may suggest a diagnosis that can be confirmed with a scalp biopsy.18,19 Selection of a biopsy site at the periphery of the alopecic area that includes hair and consultation with a dermatopathologist familiar with features of CCCA, traction, and traumatic alopecia are important for making an accurate diagnosis.
Tinea Capitis in Black Pediatric Patients
Tinea capitis, a fungal infection of the scalp and hair, is one of the most common issues in children with skin of color. Clinical presentation may include widely distributed scaling, annular scaly plaques, annular patches of alopecia studded with black dots (broken hairs), and/or annular inflammatory plaques. Although scalp hyperkeratosis often is a hallmark of pediatric tinea capitis, it is not diagnostic. The differential diagnosis of pediatric scalp hyperkeratosis/scaling includes tinea capitis, SD, atopic dermatitis, psoriasis, and sebopsoriasis.20,21 Clues to accurate diagnosis of tinea capitis may be found by examination of the adult who combs the child’s hair, as erythematous annular scaly plaques representing tinea corporis may be observed on the forearms or thighs. Although the thighs are a seemingly unusual location, the frequent practice of the child sitting on the floor between the legs of the adult during hairstyling provides a point of contact for the transmission of tinea from the child’s scalp to the thighs or forearms of the adult. Once tinea capitis is clinically suspected, the diagnosis is confirmed by a fungal culture. Adequate sampling is obtained by clipping hairs in an area of scaling for submission and vigorously rubbing the area of black dots or hyperkeratosis with a cotton swab.
Hubbard22 shed light on the decision to treat tinea capitis empirically or await the culture results. One hundred consecutive children (98 were black) presented with the constellation of scalp alopecia, scaling, pruritus, and occipital lymphadenopathy. Sixty-eight of those children had positive fungal cultures, and of them, 60 had both occipital lymphadenopathy and scaling and 55 had both occipital lymphadenopathy and alopecia.22 Thus, occipital lymphadenopathy in conjunction with alopecia and/or scaling is predictive of tinea capitis in this population and suggests that the initiation of treatment prior to confirmative culture results is appropriate.
The mainstay of treatment for tinea capitis is griseofulvin, but it is often underdosed and not continued for an adequate period of time to ensure clearance of the infection. Griseofulvin microsize (125 mg/5 mL) at the dosage of 20 to 25 mg/kg once daily for 8 to 12 weeks is recommended instead of a lower-dosed 4- to 6-week course.23,24
Options for treating a child with residual disease include increasing and/or extending the griseofulvin dosage, encouraging ingestion of fatty foods to enhance absorption, dividing the dosage of griseofulvin from once daily to twice daily, changing therapy to oral terbinafine due to resistance to griseofulvin, examining siblings as a source of reinfection, and reviewing the positive fungal culture report to distinguish Trichophyton tonsurans versus Microsporum canis as the causative agent and adjust treatment accordingly. Although griseofulvin is the first-line treatment for M canis, terbinafine, which is approved for children 4 years and older for tineacapitis, is most efficacious for T tonsurans.25 Treatment with terbinafine is weight based and should extend for 2 to 4 weeksfor T tonsurans and 8 to 12 weeks for M canis.
Antifungal shampoos may help reduce household spread of tinea and decrease transmissible fungal spores, but they may cause hair dryness and breakage.26,27 Antifungal shampoos can be applied directly onto the scalp for a 5- to 10-minute contact time and rinsed, and then the hair should be shampooed with a moisturizing shampoo followed by a moisturizing conditioner. Hair conditioners may decrease household spread of tinea capitis and should be used by the patient and other members of the household.28 Infection control may be enhanced by advising parents to dispose of hair pomades and washing hair accessories, combs, and brushes in hot soapy water, preferably in the dishwasher.
Hair Growth
The inability of the hair of black children to grow long is a common concern for parents of toddlers and preschool-aged children. Although the hair does grow, it grows more slowly than hair in white children (0.259 vs 0.330 mm per day), and it is likely to break faster than it is growing in black versus white children (146.6 vs 13.13 total broken hairs).8 Reassurance that the hair is indeed growing and that the length will increase as the child matures is important. Avoidance of hairstyles that promote traction and use of hair extensions, as well as use of moisturizing shampoos and conditioners, may minimize breakage and support the growth of healthy hair.
Conclusion
Hair- and scalp-related disease in black adults and children is commonly encountered in dermatology practice. It is important to understand the intrinsic characteristics of facial and scalp hair as well as hair care practices in this patient population that differ from those of white and Asian populations, such as frequency of shampooing, products, and styling. Familiarity with these differences may aid in effective diagnosis, treatment, and hair care recommendations in patients with these conditions.
One of the most common concerns among black patients is hair- and scalp-related disease. As increasing numbers of black patients opt to see dermatologists, it is imperative that all dermatologists be adequately trained to address the concerns of this patient population. When patients ask for help with common skin diseases of the hair and scalp, there are details that must be included in diagnosis, treatment, and hair care recommendations to reach goals for excellence in patient care. Herein, we provide must-know information to effectively approach this patient population.
Seborrheic Dermatitis
A study utilizing data from the National Ambulatory Medical Care Survey from 1993 to 2009 revealed seborrheic dermatitis (SD) as the second most common diagnosis for black patients who visit a dermatologist.1 Prevalence data from a population of 1408 white, black, and Chinese patients from the United States and China revealed scalp flaking in 81% to 95% of black patients, 66% to 82% in white patients, and 30% to 42% in Chinese patients.2 Seborrheic dermatitis has a notable prevalence in black women and often is considered normal by patients. It can be exacerbated by infrequent shampooing (ranging from once per month or longer in between shampoos) and the inappropriate use of hair oils and pomades; it also has been associated with hair breakage, lichen simplex chronicus, and folliculitis. Seborrheic dermatitis must be distinguished from other disorders including sarcoidosis, psoriasis, discoid lupus erythematosus, tinea capitis, and lichen simplex chronicus.
Although there is a paucity of literature on the treatment of SD in black patients, components of treatment are similar to those recommended for other populations. Black women are advised to carefully utilize antidandruff shampoos containing zinc pyrithione, selenium sulfide, or tar to avoid hair shaft damage and dryness. Ketoconazole shampoo rarely is recommended and may be more appropriately used in men and boys, as hair fragility is less of a concern for them. The shampoo should be applied directly to the scalp rather than the hair shafts to minimize dryness, with no particular elongated contact time needed for these medicated shampoos to be effective. Because conditioners can wash off the active ingredients in therapeutic shampoos, antidandruff conditioners are recommended. Potent or ultrapotent topical corticosteroids applied to the scalp 3 to 4 times weekly initially will control the symptoms of itching as well as scaling, and mid-potency topical corticosteroid oil may be used at weekly intervals.
Hairline and facial involvement of SD often co-occurs, and low-potency topical steroids may be applied to the affected areas twice daily for 3 to 4 weeks, which may be repeated for flares. Topical calcineurin inhibitors or antifungal creams such as ketoconazole or econazole may then provide effective control. Encouraging patients to increase shampooing to once weekly or every 2 weeks and discontinue use of scalp pomades and oils also is recommended. Patients must know that an itchy scaly scalp represents a treatable disorder.
Acquired Trichorrhexis Nodosa
Hair fragility and breakage is common and multifactorial in black patients. Hair shaft breakage can occur on the vertex scalp in central centrifugal cicatricial alopecia (CCCA), with random localized breakage due to scratching in SD. Heat, hair colorants, and chemical relaxers may result in diffuse damage and breakage.3 Sodium-, potassium-, and guanine hydroxide–containing chemical relaxers change the physical properties of the hair by rearranging disulfide bonds. They remove the monomolecular layer of fatty acids covalently bound to the cuticle that help prevent penetration of water into the hair shaft. Additionally, chemical relaxers weaken the hair shaft and decrease tensile strength.
Unlike hair relaxers, colorants are less likely to lead to catastrophic hair breakage after a single use and require frequent use, which leads to cumulative damage. Thermal straightening is another cause of hair-shaft weakening in black patients.4,5 Flat irons and curling irons can cause substantially more damage than blow-dryers due to the amount of heat generated. Flat irons may reach a high temperature of 230ºC (450ºF) as compared to 100°C (210°F) for a blow-dryer. Even the simple act of combing the hair can cause hair breakage, as demonstrated in African volunteers whose hair remained short in contrast to white and Asian volunteers, despite the fact that they had not cut their hair for 1 or more years.6,7 These volunteers had many hair strand knots that led to breakage during combing and hair grooming.6
There is no known prevalence data for acquired trichorrhexis nodosa, though a study of 30 white and black women demonstrated that broken hairs were significantly increased in black women (P=.0001).8 Another study by Hall et al9 of 103 black women showed that 55% of the women reported breakage of hair shafts with normal styling. Khumalo et al6 investigated hair shaft fragility and reported no trichothiodystrophy; the authors concluded that the cause of the hair fragility likely was physical trauma or an undiscovered structural abnormality. Franbourg et al10 examined the structure of hair fibers in white, Asian, and black patients and found no differences, but microfractures were only present in black patients and were determined to be the cause of hair breakage. These studies underscore the need for specific questioning of the patient on hair care including combing, washing, drying, and using products and chemicals.
The approach to the treatment of hair breakage involves correcting underlying abnormalities (eg, iron deficiency, hypothyroidism, nutritional deficiencies). Patients should “give their hair a rest” by discontinuing use of heat, colorants, and chemical relaxers. For patients who are unable to comply, advising them to stop these processes for 6 to 12 months will allow for repair of the hair shaft. To minimize damage from colorants, recommend semipermanent, demipermanent, or temporary dyes. Patients should be counseled to stop bleaching their hair or using permanent colorants. The use of heat protectant products on the hair before styling as well as layering moisturizing regimens starting with a moisturizing shampoo followed by a leave-in, dimethicone-containing conditioner marketed for dry damaged hair is suggested. Dimethicone thinly coats the hair shaft to restore hydrophobicity, smoothes cuticular scales, decreases frizz, and protects the hair from damage. Use of a 2-in-1 shampoo and conditioner containing anionic surfactants and wide-toothed, smooth (no jagged edges in the grooves) combs along with rare brushing are recommended. The hair may be worn in its natural state, but straightening with heat should be avoided. Air drying the hair can minimize breakage, but if thermal styling is necessary, patients should turn the temperature setting of the flat or curling iron down. Protective hair care practices may include placing a loosely sewn-in hair weave that will allow for good hair care, wearing loose braids, or using a wig. Serial trimming of the hair every 6 to 8 weeks is recommended. Improvement may take time, and patients should be advised of this timeline to prevent frustration.
Acne Keloidalis Nuchae
Acne keloidalis nuchae (AKN) is characterized by papules and pustules located on the occipital scalp and/or the nape of the neck, which may result in keloidal papules and plaques. The etiology is unknown, but ingrown hairs, genetics, trauma, infection, inflammation, and androgen hormones have been proposed to play a role.11 Although AKN may occur in black women, it is primarily a disorder in black men. The diagnosis is made based primarily on clinical findings, and a history of short haircuts may support the diagnosis. Treatment is tailored to the severity of the disease (Table 1). Avoidance of short haircuts and irritation from shirt collars may be helpful. Patients should be advised that the condition is controllable but not curable.

Pseudofolliculitis Barbae
Pseudofolliculitis barbae (PFB) is characterized by papules and pustules in the beard region that may result in postinflammatory hyperpigmentation, keloidal scar formation, and/or linear scarring. The coarse curled hairs characteristic of black men penetrate the follicle before exiting the skin and penetrate the skin after exiting the follicle, resulting in inflammation. Shaving methods and genetics also may contribute to the development of PFB. As with AKN, diagnosis is made clinically and does not require a skin biopsy. Important components of the patient’s history that should be obtained are hair removal practices and the use of over-the-counter products (eg, shave [pre and post] moisturizers, exfoliants, shaving creams or gels, keratin-softening agents containing α- or β-hydroxy acids). A bacterial culture may be appropriate if a notable pustular component is present. The patient should be advised to discontinue shaving if possible, which may require a physician’s letter explaining the necessity to the patient’s employer. Pseudofolliculitis barbae often can be prevented or lessened with the right hair removal strategy. Because there is not one optimal hair removal strategy that suits every patient, encourage the patient to experiment with different hair removal techniques, from depilatories to electric shavers, foil-guard razors, and multiple-blade razors. Preshave hydration and postshave moisturiza-tion also should be encouraged.12 Benzoyl peroxide–containing shave gels and cleansers, as well as moisturizers containing glycolic, salicylic, and phytic acids, may minimize ingrown hairs, papules, and inflammation.
Other useful topical agents include eflornithine hydrochloride to decrease hair growth, retinoids to soften hair fibers, mild topical steroids to reduce inflammation, and/or topical erythromycin or clindamycin if pustules are present.13 Oral antibiotics such as doxycycline, minocycline, or erythromycin can be added for more severe cases of inflammation or infection. Procedural interventions include laser hair removal to prevent PFB and intralesional triamcinolone 10 to 40 mg/cc every 4 to 6 weeks, with the total volume depending on the size and number of lesions.
Alopecia
Alopecia is the sixth most common diagnosis seen in black patients visiting a dermatologist.14 The physician’s response to the patient’s chief concern of hair loss is key to building a relationship of confidence and trust. Trivializing the concern or dismissing it will undermine the physician-patient relationship. A survey by Gathers and Mahan15 revealed that 68% of patients thought that physicians did not understand their hair.
Hair loss negatively impacts quality of life, and a study of 50 black South African women with alopecia demonstrated a notable disease burden. Factors with the highest impact were those related to self-image, relationships, and interactions with others.16
It is not unusual for black women to have multiple types of alopecia identified in one biopsy specimen. Wohltmann and Sperling17 demonstrated 2 or more different types of alopecia in more than 10% of biopsy specimens of alopecia, including CCCA, androgenetic alopecia, end-stage traction alopecia, telogen effluvium, and tinea capitis. A complete history, physical examination, and appropriate procedures (eg, hair pull test, dermatoscopic examination and scalp biopsy) likely will yield an accurate diagnosis. Table 2 highlights important questions that should be asked about the patient’s history.

Physical examination of the scalp including dermatoscopic examination and a hair pull test as well as an evaluation of other hair-bearing areas may suggest a diagnosis that can be confirmed with a scalp biopsy.18,19 Selection of a biopsy site at the periphery of the alopecic area that includes hair and consultation with a dermatopathologist familiar with features of CCCA, traction, and traumatic alopecia are important for making an accurate diagnosis.
Tinea Capitis in Black Pediatric Patients
Tinea capitis, a fungal infection of the scalp and hair, is one of the most common issues in children with skin of color. Clinical presentation may include widely distributed scaling, annular scaly plaques, annular patches of alopecia studded with black dots (broken hairs), and/or annular inflammatory plaques. Although scalp hyperkeratosis often is a hallmark of pediatric tinea capitis, it is not diagnostic. The differential diagnosis of pediatric scalp hyperkeratosis/scaling includes tinea capitis, SD, atopic dermatitis, psoriasis, and sebopsoriasis.20,21 Clues to accurate diagnosis of tinea capitis may be found by examination of the adult who combs the child’s hair, as erythematous annular scaly plaques representing tinea corporis may be observed on the forearms or thighs. Although the thighs are a seemingly unusual location, the frequent practice of the child sitting on the floor between the legs of the adult during hairstyling provides a point of contact for the transmission of tinea from the child’s scalp to the thighs or forearms of the adult. Once tinea capitis is clinically suspected, the diagnosis is confirmed by a fungal culture. Adequate sampling is obtained by clipping hairs in an area of scaling for submission and vigorously rubbing the area of black dots or hyperkeratosis with a cotton swab.
Hubbard22 shed light on the decision to treat tinea capitis empirically or await the culture results. One hundred consecutive children (98 were black) presented with the constellation of scalp alopecia, scaling, pruritus, and occipital lymphadenopathy. Sixty-eight of those children had positive fungal cultures, and of them, 60 had both occipital lymphadenopathy and scaling and 55 had both occipital lymphadenopathy and alopecia.22 Thus, occipital lymphadenopathy in conjunction with alopecia and/or scaling is predictive of tinea capitis in this population and suggests that the initiation of treatment prior to confirmative culture results is appropriate.
The mainstay of treatment for tinea capitis is griseofulvin, but it is often underdosed and not continued for an adequate period of time to ensure clearance of the infection. Griseofulvin microsize (125 mg/5 mL) at the dosage of 20 to 25 mg/kg once daily for 8 to 12 weeks is recommended instead of a lower-dosed 4- to 6-week course.23,24
Options for treating a child with residual disease include increasing and/or extending the griseofulvin dosage, encouraging ingestion of fatty foods to enhance absorption, dividing the dosage of griseofulvin from once daily to twice daily, changing therapy to oral terbinafine due to resistance to griseofulvin, examining siblings as a source of reinfection, and reviewing the positive fungal culture report to distinguish Trichophyton tonsurans versus Microsporum canis as the causative agent and adjust treatment accordingly. Although griseofulvin is the first-line treatment for M canis, terbinafine, which is approved for children 4 years and older for tineacapitis, is most efficacious for T tonsurans.25 Treatment with terbinafine is weight based and should extend for 2 to 4 weeksfor T tonsurans and 8 to 12 weeks for M canis.
Antifungal shampoos may help reduce household spread of tinea and decrease transmissible fungal spores, but they may cause hair dryness and breakage.26,27 Antifungal shampoos can be applied directly onto the scalp for a 5- to 10-minute contact time and rinsed, and then the hair should be shampooed with a moisturizing shampoo followed by a moisturizing conditioner. Hair conditioners may decrease household spread of tinea capitis and should be used by the patient and other members of the household.28 Infection control may be enhanced by advising parents to dispose of hair pomades and washing hair accessories, combs, and brushes in hot soapy water, preferably in the dishwasher.
Hair Growth
The inability of the hair of black children to grow long is a common concern for parents of toddlers and preschool-aged children. Although the hair does grow, it grows more slowly than hair in white children (0.259 vs 0.330 mm per day), and it is likely to break faster than it is growing in black versus white children (146.6 vs 13.13 total broken hairs).8 Reassurance that the hair is indeed growing and that the length will increase as the child matures is important. Avoidance of hairstyles that promote traction and use of hair extensions, as well as use of moisturizing shampoos and conditioners, may minimize breakage and support the growth of healthy hair.
Conclusion
Hair- and scalp-related disease in black adults and children is commonly encountered in dermatology practice. It is important to understand the intrinsic characteristics of facial and scalp hair as well as hair care practices in this patient population that differ from those of white and Asian populations, such as frequency of shampooing, products, and styling. Familiarity with these differences may aid in effective diagnosis, treatment, and hair care recommendations in patients with these conditions.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Hickman JG, Cardin C, Dawson TL, et al. Dandruff, part I: scalp disease prevalence in Caucasians, African Americans, and Chinese and the effects of shampoo frequency on scalp health. Poster presented at: 60th Annual Meeting of the American Academy of Dermatology; February 22-27, 2002; New Orleans, LA.
- Swee W, Klontz KC, Lambert LA. A nationwide outbreak of alopecia associated with the use of a hair-relaxing formulation. Arch Dermatol. 2000;136:1104-1108.
- Nicholson AG, Harland CC, Bull RH, et al. Chemically induced cosmetic alopecia. Br J Dermatol. 1993;128:537-541.
- Detwiler SP, Carson JL, Woosley JT, et al. Bubble hair. case caused by an overheating hair dryer and reproducibility in normal hair with heat. J Am Acad Dermatol. 1994;30:54-60.
- Khumalo NP, Dawber RP, Ferguson DJ. Apparent fragility of African hair is unrelated to the cystine-rich protein distribution: a cytochemical electron microscopic study. Exp Dermatol. 2005;14:311-314.
- Robbins C. Hair breakage during combing. I. pathways of breakage. J Cosmet Sci. 2006;57:233-243.
- Lewallen R, Francis S, Fisher B, et al. Hair care practices and structural evaluation of scalp and hair shaft parameter in African American and Caucasian women. J Cosmet Dermatol. 2015;14:216-223.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314.
- Franbourg A, Hallegot P, Baltenneck F, et al. Current research on ethnic hair. J Am Acad Dermatol. 2003;48(6 suppl):S115-S119.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38(suppl 1):24-27.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part II. disorders occurring predominately in skin of color. Am Fam Physician. 2013;87:859-865.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Dlova NC, Fabbrocini G, Lauro C, et al. Quality of life in South African black women with alopecia: a pilot study. Int J Dermatol. 2016;55:875-881.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- McDonald KA, Shelley AJ, Colantonio S, et al. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76:472-477.
- Miteva M, Tosti A. Dermatoscopic features of central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014;71:443-444.
- Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
- Silverberg NB. Scalp hyperkeratosis in children with skin of color: diagnostic and therapeutic considerations. Cutis. 2015;95:199-204, 207.
- Hubbard TW. The predictive value of symptoms in diagnosing childhood tinea capitis. Arch Pediatr Adolesc Med. 1999;153:1150-1153.
- Kakourou T, Uksal U; European Society for Pediatric Dermatology. Guidelines for the management of tinea capitis in children. Pediatr Dermatol. 2010;27:226-228.
- Sethi A, Antanya R. Systemic antifungal therapy for cutaneous infections in children. Pediatr Infect Dis J. 2006;25:643-644.
- Gupta AK. Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol 2000;39:302-304.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol. 2000;39:302-304.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Hickman JG, Cardin C, Dawson TL, et al. Dandruff, part I: scalp disease prevalence in Caucasians, African Americans, and Chinese and the effects of shampoo frequency on scalp health. Poster presented at: 60th Annual Meeting of the American Academy of Dermatology; February 22-27, 2002; New Orleans, LA.
- Swee W, Klontz KC, Lambert LA. A nationwide outbreak of alopecia associated with the use of a hair-relaxing formulation. Arch Dermatol. 2000;136:1104-1108.
- Nicholson AG, Harland CC, Bull RH, et al. Chemically induced cosmetic alopecia. Br J Dermatol. 1993;128:537-541.
- Detwiler SP, Carson JL, Woosley JT, et al. Bubble hair. case caused by an overheating hair dryer and reproducibility in normal hair with heat. J Am Acad Dermatol. 1994;30:54-60.
- Khumalo NP, Dawber RP, Ferguson DJ. Apparent fragility of African hair is unrelated to the cystine-rich protein distribution: a cytochemical electron microscopic study. Exp Dermatol. 2005;14:311-314.
- Robbins C. Hair breakage during combing. I. pathways of breakage. J Cosmet Sci. 2006;57:233-243.
- Lewallen R, Francis S, Fisher B, et al. Hair care practices and structural evaluation of scalp and hair shaft parameter in African American and Caucasian women. J Cosmet Dermatol. 2015;14:216-223.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314.
- Franbourg A, Hallegot P, Baltenneck F, et al. Current research on ethnic hair. J Am Acad Dermatol. 2003;48(6 suppl):S115-S119.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Gray J, McMichael AJ. Pseudofolliculitis barbae: understanding the condition and the role of facial grooming. Int J Cosmet Sci. 2016;38(suppl 1):24-27.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part II. disorders occurring predominately in skin of color. Am Fam Physician. 2013;87:859-865.
- Davis SA, Naarahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Gathers RC, Mahan MG. African American women, hair care and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Dlova NC, Fabbrocini G, Lauro C, et al. Quality of life in South African black women with alopecia: a pilot study. Int J Dermatol. 2016;55:875-881.
- Wohltmann WE, Sperling L. Histopathologic diagnosis of multifactorial alopecia. J Cutan Pathol. 2016;43:483-491.
- McDonald KA, Shelley AJ, Colantonio S, et al. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76:472-477.
- Miteva M, Tosti A. Dermatoscopic features of central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2014;71:443-444.
- Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
- Silverberg NB. Scalp hyperkeratosis in children with skin of color: diagnostic and therapeutic considerations. Cutis. 2015;95:199-204, 207.
- Hubbard TW. The predictive value of symptoms in diagnosing childhood tinea capitis. Arch Pediatr Adolesc Med. 1999;153:1150-1153.
- Kakourou T, Uksal U; European Society for Pediatric Dermatology. Guidelines for the management of tinea capitis in children. Pediatr Dermatol. 2010;27:226-228.
- Sethi A, Antanya R. Systemic antifungal therapy for cutaneous infections in children. Pediatr Infect Dis J. 2006;25:643-644.
- Gupta AK. Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol 2000;39:302-304.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Greer DL. Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int J Dermatol. 2000;39:302-304.
Practice Points
- Instruct patients with acquired trichorrhexis nodosa to discontinue use of heat, colorants, and chemical relaxers on their hair.
- Create a contract with your seborrheic dermatitis patients to have them shampoo at least weekly or every 2 weeks.
- For children with treated tinea capitis that has not completely resolved, increase or extend the griseofulvin dosage, encourage ingestion of fatty foods to enhance absorption, and divide dosage of griseofulvin from once to twice daily.
- Selection of a biopsy site at the periphery of an alopecic area that includes hair and hair follicles and evaluation by a dermatopathologist familiar with the features of central centrifugal cicatricial, traction, and traumatic alopecias will ensure an accurate diagnosis of alopecia.