User login
Role of 3D Printing and Modeling to Aid in Neuroradiology Education for Medical Trainees
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
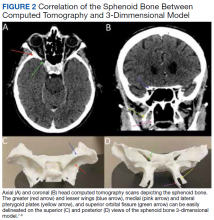
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
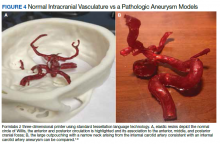
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
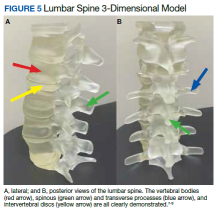
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
Applications of 3-dimensional (3D) printing in medical imaging and health care are expanding. 3D printing may serve a variety of roles and is used increasingly in the context of presurgical planning, as specific medical models may be created using individual patient imaging data.1 These patient-specific models may assist in medical trainee education, decrease operating room time, improve patient education for potential planned surgery, and guide clinicians for optimizing therapy.1,2 This article discusses the utility of 3D printing at a single institution to serve in enhancing specifically neuroradiology education.
Background
As digital imaging and 3D printing have increased in popularity, the potential application of using imaging data to guide patient therapy has shown significant promise. Computed tomography (CT) is a commonly used modality that can be used to create 3D anatomical models, as it is frequently used in the medical setting, demonstrates excellent resolution to the millimeter scale, and can readily pinpoint pathology on imaging.
Image Acquisition
CT scans can be rapidly obtained, which adds significant value, particularly in the context of point-of-care 3D printing. Another modality commonly used for 3D printing is magnetic resonance imaging (MRI), which unlike CT, does not expose the patient to ionizing radiation. The 3D printing process is initiated with patient-specific CT or MRI data stored in the digital imaging and communications in medicine (DICOM) format, which is the international standard for communication and management of medical imaging information and related data. DICOM allows for faster and robust collaboration among imaging professionals.3
Image Processing
To print 3D anatomical models, patient-specific data must be converted from DICOM into standard tessellation language (STL) format, which can be created and edited with a variety of softwares.3 At James A. Haley Veterans’ Hospital in Tampa, Florida, we use an image processing package that includes the Materialise 3-matic and interactive medical image control system. Image quality is essential; therefore, careful attention to details such as pixel dimensions, slice thickness, and slice increments must be considered.3,4
An STL file creates a 3D image from triangle approximations. The entire 3D shape will be made of numerous large or small triangles, depending on the slice thickness, therefore, quality of the original radiologic image. The size and position of the triangles used to make the model can be varied to approximate the object’s shape. The smaller the triangles, the better the image quality and vice versa. This concept is analogous to approximating a circle using straight lines of equal length—more, smaller lines will result in better approximation of a circle (Figure 1).5,6 Similarly, using smaller triangles allows for better approximation of the image. As the human body is a complex structure, mimicking the body requires a system able to create nongeometrical shapes, which is made possible via these triangle approximations in a 3D STL file.
The creation of an STL file from DICOM data starts with a threshold-based segmentation process followed by additional fine-tuning and edits, and ends in the creation of a 3D part. The initial segmentation can be created with the threshold tool, using a Hounsfield unit range based on the area of interest desired (eg, bone, blood, fat). This is used to create an initial mask, which can be further optimized. The region grow tool allows the user to focus the segmentation by discarding areas that are not directly connected to the region of interest. In contrast, the split mask tool divides areas that are connected. Next, fine-tuning the segmentation using tools such as multiple slice edit helps to optimize the model. After all edits are made, the calculate part tool converts the mask into a 3D component that can be used in downstream applications. For the purposes of demonstration and proof of concept, the models provided in this article were created via open-source hardware designs under free or open licenses.7-9
3D Printing in Neuroradiology Education
Neuroradiologists focus on diagnosing pathology related to the brain, head and neck, and spine. CT and MRI scans are the primary modalities used to diagnose these conditions. 3D printing is a useful tool for the trainee who wishes to fully understand neuroanatomy and obtain further appreciation of imaging pathology as it relates to 3D anatomy. Head and neck imaging are a complex subdiscipline of neuroradiology that often require further training beyond radiology residency. A neuroradiology fellowship that focuses on head and neck imaging extends the training.
3D printing has the potential to improve the understanding of various imaging pathologies by providing the trainee with a more in-depth appreciation of the anterior, middle, and posterior cranial fossa, the skull base foramina (ie, foramen ovale, spinosum, rotundum), and complex 3D areas, such as the pterygopalatine fossa, which are all critical areas to investigate on imaging. Figure 2 highlights how a complex anatomical structure, such as the sphenoid bone when printed in 3D, can be correlated with CT cross-sectional images to supplement the educational experience.
Furthermore, the various lobes, sulci, and gyri of the brain and cerebellum and how they interrelate to nearby vasculature and bony structures can be difficult to conceptualize for early trainees. A 3D-printed cerebellum and its relation to the brainstem is illustrated in Figure 3A. Additional complex head and neck structures of the middle ear membranous and bony labyrinth and ossicles and multiple views of the mandible are shown in Figures 3B through 3E.
3D printing in the context of neurovascular pathology holds great promise, particularly as these models may provide the trainee, patient, and proceduralist essential details such as appearance and morphology of an intracranial aneurysm, relationship and size of the neck of aneurysm, incorporation of vessels emanating from the aneurysmal sac, and details of the dome of the aneurysm. For example, the normal circle of Willis in Figure 4A is juxtaposed with an example of a saccular internal carotid artery aneurysm (Figure 4B).
A variety of conditions can affect the bony spine from degenerative, trauma, neoplastic, and inflammatory etiologies. A CT scan of the spine is readily used to detect these different conditions and often is used in the initial evaluation of trauma as indicated in the American College of Radiology appropriateness criteria.10 In addition, MRI is used to evaluate the spinal cord and to further define spinal stenosis as well as evaluate radiculopathy. An appreciation of the bony and soft tissue structures within the spine can be garnered with the use of 3D models (Figure 5).
Trainees can further their understanding of approaches in spinal procedures, including lumbar puncture, myelography, and facet injections. A variety of approaches to access the spinal canal have been documented, such as interspinous, paraspinous, and interlaminar oblique; 3D-printed models can aid in practicing these procedures.11 For example, a water-filled tube can be inserted into the vertebral canal to provide realistic tactile feedback for simulation of a lumbar puncture. An appreciation of the 3D anatomy can guide the clinician on the optimal approach, which can help limit time and potentially improve outcomes.
Future Directions
Artificial Intelligence (AI) offers the ability to teach computers to perform tasks that ordinarily require human intelligence. In the context of 3D printing, the ability to use AI to readily convert and process DICOM data into printable STL models holds significant promise. Currently, the manual conversion of a DICOM file into a segmented 3D model may take several days, necessitating a number of productive hours even from the imaging and engineering champion. If machines could aid in this process, the ability to readily scale clinical 3D printing and promote widespread adoption would be feasible. Several studies already are looking into this concept to determine how deep learning networks may automatically recognize lesions on medical imaging to assist a human operator, potentially cutting hours from the clinical 3D printing workflow.12,13
Furthermore, there are several applications for AI in the context of 3D printing upstream or before the creation of a 3D model. A number of AI tools are already in use at the CT and MRI scanner. Current strategies leverage deep learning and advances in neural networks to improve image quality and create thin section DICOM data, which can be converted into printable 3D files. Additionally, the ability to automate tasks using AI can improve production capacity by assessing material costs and ensuring cost efficiency, which will be critical as point-of-care 3D printing develops widespread adoption. AI also can reduce printing errors by using automated adaptive feedback, using machine learning to search for possible print errors, and sending feedback to the computer to ensure appropriate settings (eg, temperature settings/environmental conditions).
Conclusions
Based on this single-institution experience, 3D-printed complex neuroanatomical structures seems feasible and may enhance resident education and patient safety. Interested trainees may have the opportunity to learn and be involved in the printing process of new and innovative ideas. Further studies may involve printing various pathologic processes and applying these same steps and principles to other subspecialties of radiology. Finally, AI has the potential to advance the 3D printing process in the future.
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010
1. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5(4):335-341. doi:10.1007/s11548-010-0476-x
2. Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7(6):668-677. doi:10.21037/qims.2017.11.02
3. Hwang JJ, Jung Y-H, Cho B-H. The need for DICOM encapsulation of 3D scanning STL data. Imaging Sci Dent. 2018;48(4):301-302. doi:10.5624/isd.2018.48.4.301
4. Whyms BJ, Vorperian HK, Gentry LR, Schimek EM, Bersu ET, Chung MK. The effect of computed tomographic scanner parameters and 3-dimensional volume rendering techniques on the accuracy of linear, angular, and volumetric measurements of the mandible. Oral Surg Oral Med, Oral Pathol Oral Radiol. 2013;115(5):682-691. doi:10.1016/j.oooo.2013.02.008
5. Materialise Cloud. Triangle reduction. Accessed May 20, 2021. https://cloud.materialise.com/tools/triangle-reduction
6. Comaneanu RM, Tarcolea M, Vlasceanu D, Cotrut MC. Virtual 3D reconstruction, diagnosis and surgical planning with Mimics software. Int J Nano Biomaterials. 2012;4(1);69-77.
7. Thingiverse: Digital designs for physical objects. Accessed May 20, 2021. https://www.thingiverse.com
8. Cults. Download for free 3D models for 3D printers. Accessed May 20, 2021. https://cults3d.com/en
9. yeggi. Search engine for 3D printer models. Accessed May 20, 2021. https://www.yeggi.com
10. Expert Panel on Neurological Imaging and Musculoskeletal Imaging; Beckmann NM, West OC, Nunez D, et al. ACR appropriateness criteria suspected spine trauma. J Am Coll Radiol. 2919;16(5):S264-285. doi:10.1016/j.jacr.2019.02.002
11. McKinney AM. Normal variants of the lumbar and sacral spine. In: Atlas of Head/Neck and Spine Normal Imaging Variants. Springer; 2018:263-321.
12. Sollini M, Bartoli F, Marciano A, et al. Artificial intelligence and hybrid imaging: the best match for personalized medicine in oncology. Eur J Hybrid Imaging. 2020;4(1):24. doi:10.1186/s41824-020-00094-8
13. Küstner T, Hepp T, Fischer M, et al. Fully automated and standardized segmentation of adipose tissue compartments via deep learning in 3D whole-body MRI of epidemiologic cohort studies. Radiol Artif Intell.2020;2(6):e200010. doi:10.1148/ryai.2020200010