User login
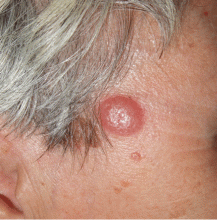
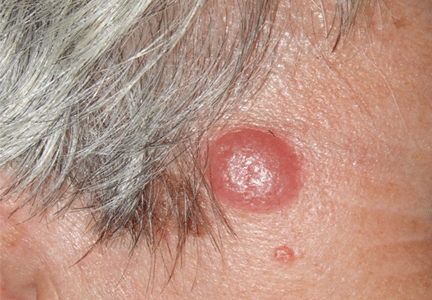
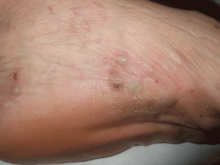
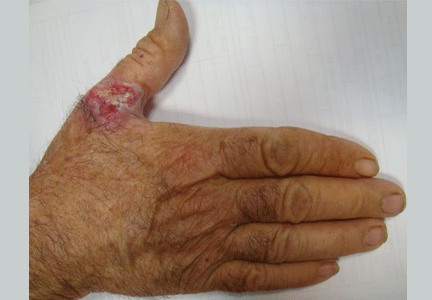
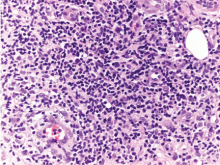
A nodule on a woman’s face
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
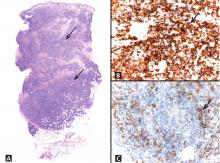
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
Q: Which is the most likely diagnosis?
- Basal cell carcinoma
- Squamous cell carcinoma
- Lymphocytoma cutis
- Amelanotic melanoma
- Pyogenic granuloma
A: The correct answer is lymphocytoma cutis. The differential diagnosis of a pink papule on the face of a middle-aged person includes nonmelanoma skin cancer, lymphoma, lymphocytoma cutis, metastatic disease, certain infections, Jessner lymphocytic infiltrate, connective tissue disease, and some adnexal tumors. Histologic study is a useful diagnostic aid in this context.
Basal cell carcinoma is the most common cutaneous malignant neoplasm, and although these tumors rarely metastasize, they are capable of gross tissue destruction, particularly those lesions arising on the face. Clinically, this tumor presents as a shiny, pearly nodule with telangiectasias on the surface, as in our patient, but skin biopsy shows large basaloid lobules of varying shape and size forming a relatively circumscribed mass with a “palisade” around the rim of the lobule.
Squamous cell carcinoma manifests as shallow ulcers, often with a keratinous crust and elevated, indurate borders, but also as plaques or nodules. The clinical diagnosis should be confirmed with skin biopsy, which reveals atypical keratinocytes extending from the epidermis to the dermis with dyskeratosis, intercellular bridges, variable central keratinization, and horn pearl formation, depending on the differentiation of the tumor.
Amelanotic melanoma is nonpigmented and appears as a pink nodule mimicking basal cell carcinoma or squamous cell carcinoma. Histologic study is necessary for the diagnosis, and shows an atypical proliferation of melanocytic cells in the epidermis and dermis.
Pyogenic granuloma is a very common benign vascular lesion considered to be a hyperplastic process or a vascular neoplasm. The lesion typically presents as a red or bluish papule or polyp that bleeds easily, and a reddish homogeneous area surrounded by a white “collarette” is found in most cases. Histologic features of an early lesion resemble granulation tissue and include lobules of capillaries and venules that often radiate from larger, more central vessels.
LYMPHOCYTOMA CUTIS: KEY FEATURES
Lymphocytoma cutis (pseudolymphoma) is a benign reactive polyclonal and inflammatory disorder that most frequently includes B lymphocytes, with a smaller population of T lymphocytes. It infiltrates the skin and resembles rudimentary germinal follicles, as in the present case. The lesion usually presents as an asymptomatic red-brown or violet papule or nodule, 3 mm to 5 cm in diameter, most often on the face, chest, or upper extremities.1 The lesion may be solitary, as in our patient, but lesions may also be grouped or numerous and widespread. It is three times more common in women than in men. It may resolve spontaneously, but it may also recur.
In Europe, lymphocytoma cutis occurs most often in B burgdorferi infection after a tick bite. Lymphocytoma cutis occurs in 1.3% of cases of B burgdorferi infection,2 although other infectious, physical, or chemical agents may produce the same reaction pattern. Tattooing (particularly red areas), acupuncture, vaccination, arthropod reactions, hyposensitization antigen reaction, and ingestion of drug have been implicated in this form of lymphoid hyperplasia.3,4
DIAGNOSTIC CHALLENGES
Lymphocytoma cutis can be challenging to diagnose, and although it can be suspected clinically, incisional biopsy is usually necessary in order to differentiate it from cutaneous B lymphoma.5
The infiltrate is predominantly nodular (> 90%) and located in the upper and mid dermis (“top heavy”) in lymphocytoma cutis, whereas it can be nodular or diffuse in cutaneous B lymphoma, with sharply demarcated borders that are convex rather than concave. Lymphoid follicles with germinal centers are sometimes present, and the interfollicular cellular population is polymorphic in lymphocytoma cutis (lymphocytes, plasma cells, histiocytes, eosinophils). In lymphocytoma cutis, cells express the phenotype of mature B lymphocytes (CD20, CD79a) and show regular and sharply demarcated networks of CD21+ follicular dendritic cells, whereas in cutaneous B lymphoma these networks are irregular. Light chains are usually polyclonal, although monoclonal populations of B cell in cases of cutaneous lymphocytoma cutis have been described. Extracutaneous involvement is possible in cutaneous B lymphoma but is usually absent in lymphocytoma cutis.
Lymphocytoma cutis typically involutes over a period of months, even with no treatment, as it did in our patient. Otherwise, there are different therapeutic options, including intralesional and topical corticosteroids, surgery, and cryosurgery.6 Photodynamic therapy with delta-aminolevulinic acid is an effective and safe modality for the treatment of lymphocytoma cutis and may be cosmetically beneficial.7
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.
- Ploysangam T, Breneman DL, Mutasim DF. Cutaneous pseudolymphomas. J Am Acad Dermatol 1998; 38:877–895.
- Albrecht S, Hofstadter S, Artsob H, Chaban O, From L. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol 1991; 24:621–625.
- Peretz E, Grunwald MH, Cagnano E, Halevy S. Follicular B-cell pseudolymphoma. Australas J Dermatol 2000; 41:48–49.
- Hermes B, Haas N, Grabbe J, Czarnetzki BM. Foreign-body granuloma and IgE-pseudolymphoma after multiple bee stings. Br J Dermatol 1994; 130:780–784.
- Kerl H, Fink-Puches R, Cerroni L. Diagnostic criteria of primary cutaneous B-cell lymphomas and pseudolymphomas. Keio J Med 2001; 50:269–273.
- Kuflik AS, Schwartz RA. Lymphocytoma cutis: a series of five patients successfully treated with cryosurgery. J Am Acad Dermatol 1992; 26:449–452.
- Takeda H, Kaneko T, Harada K, Matsuzaki Y, Nakano H, Hanada K. Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 2005; 211:264–266.
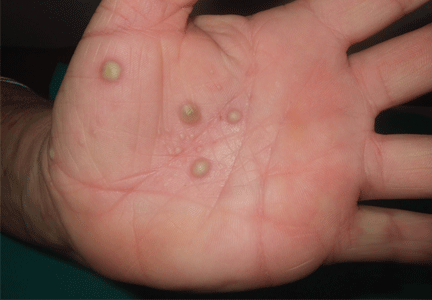
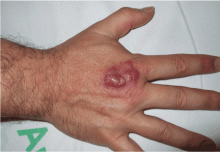
Palmoplantar eruption
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
A 38-year-old woman presents with recurrent asymptomatic lesions on the palms and soles and on the sides of both feet. The lesions have been developing for 2 months, unaccompanied by fever or other systemic symptoms.
Laboratory tests of C-reactive protein, erythrocyte sedimentation rate, viral serologies, and antinuclear antibodies are normal. A pustule culture is negative, and a cutaneous biopsy shows parakeratosis and elongation of rete ridges, neutrophils migrating from papillary capillaries to the epidermis, and spongiform Kogoj pustule.
Q: Which is the most likely diagnosis?
- Pustular psoriasis
- Impetigo contagiosa
- Syndrome of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO)
- Dyshidrotic eczema
- Acute exanthematous pustulosis (drug eruption)
A: SAPHO syndrome is the most likely diagnosis. The presence of pustules and aseptic osteitis of the anterior chest wall is compatible with SAPHO syndrome. Treatment with topical clobetasol propionate (Temovate) for pustules and NSAIDs for osteitis brought a good response.
Pustular psoriasis is an uncommon type of psoriasis characterized by erythema and pustules involving the flexural and anogenital areas. Cutaneous lesions of psoriasis vulgaris may be present before an acute pustular episode. Withdrawal of systemic corticosteroids in a patient with psoriasis has been reported as a precipitating factor.
Impetigo contagiosa is a superficial cutaneous infection characterized by an erythematous macule that evolves into a vesicle or pustule. These lesions are more common in children. Culture of the fluid usually reveals Staphylococcus aureus or S pyogenes.
Dyshidrotic eczema is a pruritic vesicular eruption of unknown cause on the palm and soles (bilateral and symmetric). The typical histologic findings are spongiotic and intraepidermal vesicles.
Acute exanthematous pustulosis is a drug-induced reaction characterized by confluent erythema, blisters, and pustules, mucous membrane erosions with fever, and lymphadenopathy. Cultures of the pustules are negative, and biopsy can help confirm the diagnosis of drug eruption.
SAPHO SYNDROME
SAPHO syndrome is a rare condition of unknown pathogenesis originally described by Chamot et al1 in 1987. The onset is usually in young adulthood, and is similar in men and women. It is characterized by synovitis, acne, pustulosis, hyperostosis, and osteitis. Of paramount importance is the finding of a non-infectious inflammatory osteitis in a patient with skin lesions.
Clinical findings: Pustules plus rheumatic pain
SAPHO syndrome must be suspected when a patient is affected by a pustular skin disease associated with rheumatic pain. If examination shows that the pain is caused by a sterile inflammation of bone or joints, the diagnosis tends to be confirmed.2
Osteoarticular involvement tends to be limited to the anterior chest wall. It may include aseptic osteitis, hyperostosis, and symmetrical arthritis. Peripheral and axial osteitis is one of the main characteristics of the syndrome and is found in around 90% of cases.
Cutaneous manifestations are present in two-thirds of patients and consist chiefly of severe acne (acne fulminans, acne conglobata, and hidradenitis suppurativa), pustular psoriasis, and palmoplantar pustulosis. Neutrophilic dermatoses associated with this syndrome include Sweet syndrome and pyoderma gangrenosum. Acne lesions are usually seen in men, whereas palmoplantar pustulosis is seen in women,3 often accompanying osteoarticular manifestations.
Radiologic findings in the spine are spondylodiskitis, osteosclerosis, sacroiliac joint involvement, and paravertebral ossification. In anterior chest wall hyperostosis, common findings are bone hypertrophy and sclerosis with a soft-tissue component. Laboratory test results are uncharacteristic, with variable signs of inflammation, and the C-reactive protein and sedimentation rate are usually elevated in the absence of leukocytosis.
Pathogenesis remains elusive
The pathogenesis of this syndrome remains elusive. Since SAPHO syndrome usually involves the axial skeleton, some investigators have suggested a possible link between the SAPHO syndrome and the seronegative spondyloarthopathies.3 It has also been related to an infection by Propionibacterium acnes and Corynebacterium species,4 which have been isolated in cultures of bone and skin lesions. However, the fact that these bacteria are contaminant agents makes their involvement in the pathogenesis of this syndrome unlikely. More recently, high concentrations of tumor necrosis factor alpha in bone specimens of patients with SAPHO syndrome have been reported, thus highlighting the central role of this cytokine in maintaining inflammation.
Treatment is to relieve symptoms
Since understanding of the pathogenesis of SAPHO syndrome is limited, a wide range of therapies has been used,5 mostly to relieve symptoms. These include NSAIDs; steroids; antibiotics; bisphosphonates such as pamidronate (Aredia) and zoledronic acid (Reclast); and immunosuppressors and immunomodulators such as methotrexate (Trexall), leflunomide (Arava), sulfasalazine (Azulfidine), cyclosporine (Sandimmune). The results with these therapies have been quite varied. Good response has been reported with tumor necrosis factor alpha blockers—infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira).6
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
- Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases [in French]. Rev Rhum Mal Osteoartic 1987; 54:187–196.
- Benhamou CL, Chamot AM, Kahn MF. Synovitis-acnepustulosis hyperostosis-osteomyelitis syndrome (SAPHO). A new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988; 6:109–112.
- Hayem G, Bouchaud-Chabot A, Benali K, et al. SAPHO syndrome: a long-term follow-up study of 120 cases. Semin Arthritis Rheum 1999; 29:159–171.
- Moll C, Hernández MV, Cañete JD, et al. Ilium osteitis as the main manifestation of the SAPHO syndrome: response to infliximab therapy and review of the literature. Semin Arthritis Rheum 2008; 37:299–306.
- Olivieri I, Padula A, Palazzi C. Pharmacological management of SAPHO syndrome. Expert Opin Investig Drugs 2006; 15:1229–1233.
- Arias-Santiago S, Sanchez-Cano D, Callejas-Rubio JL, Fernández-Pugnaire MA, Ortego-Centeno N. Adalimumab treatment for SAPHO syndrome. Acta Derm Venereol 2010; 90:301–302.
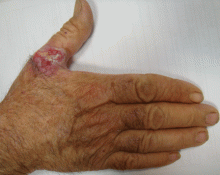
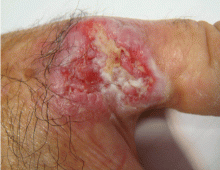
An ulcerated plaque on the hand
Q: Which is the most likely diagnosis?
- Blastomycosis
- Keratoacanthoma
- Basal cell carcinoma
- Squamous cell carcinoma
- Extramammary Paget disease
A: Squamous cell carcinoma is the most likely diagnosis. The lesions may take the form of a patch, plaque, or nodule, sometimes with scaling or an ulcerated center. The borders often are irregular, fleshy, and bleed easily.
Blastomycosis is a rare fungal infection caused by inhaling the spores of the fungus Blastomyces dermatitidis. Skin features begin as papules, pustules, or subcutaneous nodules. Over a period of months, lesions heal to form raised wart-like scars, which are often irreversible.
Keratoacanthoma is a benign nonmelanomatous cancer that grows rapidly and remits spontaneously. This diagnosis is unlikely in our patient, whose lesion evolved over 2 years.
Basal cell carcinoma has a waxy, translucent, or pearly appearance, without a keratotic component. Telangiectasias are common. Unlike squamous cell carcinomas, the edges of basal cell lesions are clear rather than fleshy.
Extramammary Paget disease usually manifests as intense pruritus in affected areas, which appear as chronic intertrigo or nonresolving eczema. The dorsum of the hand is a very rare location for this disease. Given our patient’s presentation, extrammary Paget disease is an unlikely diagnosis.
CHRONIC SUN EXPOSURE IS A KEY RISK FACTOR
Our patient has worked outdoors for many years, and chronic sun exposure is a key risk factor for squamous cell carcinoma.1
Squamous cell carcinoma is a nonmelanomatous skin cancer arising from the more superficial layers of keratinocytes. Unlike basal cell carcinoma, it can metastasize,2 so the diagnosis must be made as soon as possible.
The key to diagnosis is a high degree of suspicion. Patients usually present with chronic sun-damaged skin with lesions as actinic keratoses or keratin horns. Squamous cell carcinoma appears as a thick, adherent scale that does not heal and may intermittently bleed. Because it spreads into the dermis, it can appear like an ulcer, with hard, raised edges, as in our patient.
TREATMENT AND PREVENTION
The standard effective treatment is complete surgical excision.1 Nonsurgical treatments include curettage plus cautery, cryosurgery, radiotherapy, photodynamic therapy, and imiquimod (Aldara) 5% cream. Minimizing sun exposure and regular checkups are important preventive measures.
Our patient underwent total surgical excision of the lesion. Due to the size of the lesion, a skin graft was required and was obtained from the abdomen. No relapse was observed after 1 year of follow-up.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med 2001; 344:975–983.
- Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
Q: Which is the most likely diagnosis?
- Blastomycosis
- Keratoacanthoma
- Basal cell carcinoma
- Squamous cell carcinoma
- Extramammary Paget disease
A: Squamous cell carcinoma is the most likely diagnosis. The lesions may take the form of a patch, plaque, or nodule, sometimes with scaling or an ulcerated center. The borders often are irregular, fleshy, and bleed easily.
Blastomycosis is a rare fungal infection caused by inhaling the spores of the fungus Blastomyces dermatitidis. Skin features begin as papules, pustules, or subcutaneous nodules. Over a period of months, lesions heal to form raised wart-like scars, which are often irreversible.
Keratoacanthoma is a benign nonmelanomatous cancer that grows rapidly and remits spontaneously. This diagnosis is unlikely in our patient, whose lesion evolved over 2 years.
Basal cell carcinoma has a waxy, translucent, or pearly appearance, without a keratotic component. Telangiectasias are common. Unlike squamous cell carcinomas, the edges of basal cell lesions are clear rather than fleshy.
Extramammary Paget disease usually manifests as intense pruritus in affected areas, which appear as chronic intertrigo or nonresolving eczema. The dorsum of the hand is a very rare location for this disease. Given our patient’s presentation, extrammary Paget disease is an unlikely diagnosis.
CHRONIC SUN EXPOSURE IS A KEY RISK FACTOR
Our patient has worked outdoors for many years, and chronic sun exposure is a key risk factor for squamous cell carcinoma.1
Squamous cell carcinoma is a nonmelanomatous skin cancer arising from the more superficial layers of keratinocytes. Unlike basal cell carcinoma, it can metastasize,2 so the diagnosis must be made as soon as possible.
The key to diagnosis is a high degree of suspicion. Patients usually present with chronic sun-damaged skin with lesions as actinic keratoses or keratin horns. Squamous cell carcinoma appears as a thick, adherent scale that does not heal and may intermittently bleed. Because it spreads into the dermis, it can appear like an ulcer, with hard, raised edges, as in our patient.
TREATMENT AND PREVENTION
The standard effective treatment is complete surgical excision.1 Nonsurgical treatments include curettage plus cautery, cryosurgery, radiotherapy, photodynamic therapy, and imiquimod (Aldara) 5% cream. Minimizing sun exposure and regular checkups are important preventive measures.
Our patient underwent total surgical excision of the lesion. Due to the size of the lesion, a skin graft was required and was obtained from the abdomen. No relapse was observed after 1 year of follow-up.
Q: Which is the most likely diagnosis?
- Blastomycosis
- Keratoacanthoma
- Basal cell carcinoma
- Squamous cell carcinoma
- Extramammary Paget disease
A: Squamous cell carcinoma is the most likely diagnosis. The lesions may take the form of a patch, plaque, or nodule, sometimes with scaling or an ulcerated center. The borders often are irregular, fleshy, and bleed easily.
Blastomycosis is a rare fungal infection caused by inhaling the spores of the fungus Blastomyces dermatitidis. Skin features begin as papules, pustules, or subcutaneous nodules. Over a period of months, lesions heal to form raised wart-like scars, which are often irreversible.
Keratoacanthoma is a benign nonmelanomatous cancer that grows rapidly and remits spontaneously. This diagnosis is unlikely in our patient, whose lesion evolved over 2 years.
Basal cell carcinoma has a waxy, translucent, or pearly appearance, without a keratotic component. Telangiectasias are common. Unlike squamous cell carcinomas, the edges of basal cell lesions are clear rather than fleshy.
Extramammary Paget disease usually manifests as intense pruritus in affected areas, which appear as chronic intertrigo or nonresolving eczema. The dorsum of the hand is a very rare location for this disease. Given our patient’s presentation, extrammary Paget disease is an unlikely diagnosis.
CHRONIC SUN EXPOSURE IS A KEY RISK FACTOR
Our patient has worked outdoors for many years, and chronic sun exposure is a key risk factor for squamous cell carcinoma.1
Squamous cell carcinoma is a nonmelanomatous skin cancer arising from the more superficial layers of keratinocytes. Unlike basal cell carcinoma, it can metastasize,2 so the diagnosis must be made as soon as possible.
The key to diagnosis is a high degree of suspicion. Patients usually present with chronic sun-damaged skin with lesions as actinic keratoses or keratin horns. Squamous cell carcinoma appears as a thick, adherent scale that does not heal and may intermittently bleed. Because it spreads into the dermis, it can appear like an ulcer, with hard, raised edges, as in our patient.
TREATMENT AND PREVENTION
The standard effective treatment is complete surgical excision.1 Nonsurgical treatments include curettage plus cautery, cryosurgery, radiotherapy, photodynamic therapy, and imiquimod (Aldara) 5% cream. Minimizing sun exposure and regular checkups are important preventive measures.
Our patient underwent total surgical excision of the lesion. Due to the size of the lesion, a skin graft was required and was obtained from the abdomen. No relapse was observed after 1 year of follow-up.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med 2001; 344:975–983.
- Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med 2001; 344:975–983.
- Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
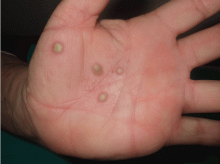
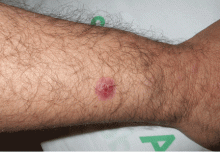
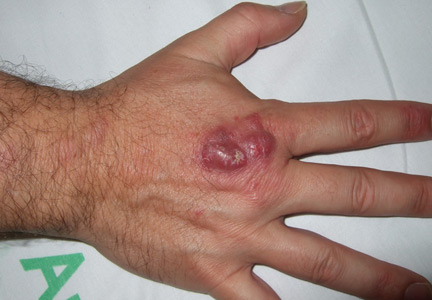
Painful red nodule on the right hand
A 46-year-old healthy man presents with a 15-day history of a tender subcutaneous nodule on the dorsum of the right hand that appeared after cleaning his aquarium. He has no fever or systemic symptoms. For 2 weeks he has been taking amoxicillin-clavulanate (Augmentin) and metronidazole (Flagyl), but without an adequate response.
Q: Which is the most likely diagnosis?
- Tuberculosis verrucosa cutis
- Sporotrichosis
- Nontuberculous mycobacterial infection
- Leishmaniasis
- Nocardiosis
A: The correct answer is nontuberculous mycobacterial infection. Mycobacterium marinum was subsequently isolated from culture (Löwenstein-Jensen medium) of wound drainage.
Tuberculosis verrucosa cutis is an indolent, warty plaque that occurs after direct inoculation of M tuberculosis into the skin of someone previously infected with M tuberculosis. It can result from accidental exposure to tuberculous tissue in high-risk groups such as laboratory workers, physicians, and pathologists.
Sporothrix schenckii is the dimorphic fungus that causes sporotrichosis. Activities associated with acquisition of sporotrichosis include gardening (rose-gardener's disease), landscaping, farming, berry-picking, horticulture, and carpentry. The characteristic infection involves suppurating subcutaneous nodules that progress proximally along lymphatic channels (lymphocutaneous sporotrichosis).
Leishmaniasis is a disease caused by the protozoa of the Leishmania species, which is transmitted by the bite of a female sandfly. Initially, the lesion is a small, red papule up to 2 cm in diameter. Over several weeks, the papule becomes darker, develops a crust in the center, and eventually ulcerates to present a typical appearance of an ulcer with raised edges and surrounding dusky red skin.
Nocardia is a genus of filamentous grampositive bacteria that stains acid-fast, just as M marinum does. Nocardia asteroides primarily affects the lungs and can disseminate systemically. However, Nocardia brasiliensis is often associated with sporotrichoid-spreading subcutaneous nodules.
CLINICAL PRESENTATION AND DIAGNOSIS
M marinum is a genus of nontuberculous mycobacteria that usually causes disease in fish but can cause human skin infection by penetrating through a break in the skin. It can spread to deeper structures, resulting in tenosynovitis, arthritis, or osteomyelitis.1 The disease caused by M marinum is sometimes called “swimming pool granuloma” or “fish-tank granuloma.”2
In this case, the patient probably acquired the infection while cleaning his home aquarium,3 so asking about contact with pet fish is very important for the clinical diagnosis.
Lesions are usually localized to the site of the inoculation, with a predilection for areas predisposed to trauma, such as the hands and the fingers, after an incubation period of 2 to 8 weeks. Lesions are self-limited and usually appear as pruriginous bluish-red papules or pustules that may increase in size to form a verrucous or violaceous plaque or nodule. Superficial lesions often undergo central ulceration. Disseminated infection is rare and occurs mainly in patients who are immunocompromised because of renal transplant, systemic lupus erythematosus, chronic steroid therapy, or anti-tumor necrosis factor alpha therapy.4 In such cases, infection can be life-threatening.
On histopathologic study, a nonspecific inflammation consisting of a mixed infiltrate with lymphocytes, neutrophils, and histiocytes is usually observed. Sometimes a granulomatous inflammatory infiltrate mimicking tuberculoid granuloma may be noted. M marinum is an aerobic, nonmotile acid-fast bacillus. It grows at 30°C to 32°C on Löwenstein-Jensen medium in 2 to 5 weeks, but cultures are rarely positive. Polymerase chain reaction and enzyme-linked immunosorbent assays can help identify the organism. Other diagnoses to be considered are leishmaniasis, tuberculosis verrucosa cutis, blastomycosis, histoplasmosis, and sporotrichosis.
TREATMENT
Treatment of M marinum infection is not based on any specific criteria. Spontaneous resolution in 24 to 36 months has been described. 5 Antibiotics are the first-line therapy, and sometimes surgical treatment may be necessary for deeper infection with necrotic tissue. Cryotherapy, electrode therapy, and irradiation have been reported to be effective.
Few studies have been conducted to determine the first-line antibiotic treatment for M marinum infection. In this case, treatment with sulfamethoxazole-trimethoprim6 (Bactrim) resulted in resolution of the lesions in 6 months. Other antibiotics often used as monotherapy are tetracycline, minocycline (Minocin), and doxycycline (Vibramycin) 100 mg twice daily for 3 months.7 However, treatment failure has been described with many antibiotics.
Multidrug therapy is usually prescribed to minimize the risk of resistance. A combination of clarithromycin (Biaxin), streptomycin, and ethionamide (Trecator) has been prescribed successfully.3 Another combined regimen—clarithromycin 500 mg twice a day, rifampin (Rifadin) 600 mg daily, and ethambutol (Myambutol) 25 mg/kg daily—has been shown to be effective.5 Also, rifampin 600 mg daily and ethambutol 15 to 25 mg/kg/day were effective in a patient with a sporotrichoid M marinum infection associated with infliximab (Remicade). 8 The duration of treatment ranged between 2 and 6 months, or up to 2 months after the disappearance of the cutaneous lesions.
Clinical awareness of M marinum infection is important so that the diagnosis can be made and appropriate therapy can be initiated promptly, because some cases with disseminated disease and serious complications have been described.9
- Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol 2006; 33:817–819.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis 2003; 37:390–397.
- Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases. J Am Acad Dermatol 2007; 57:413–420.
- Streit M, Böhlen LM, Hunziker T, et al. Disseminated Mycobacterium marinum infection with extensive cutaneous eruption and bacteremia in an immunocompromised patient. Eur J Dermatol 2006; 16:79–83.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther 2004; 17:491–498.
- Alinovi A, Vecchini F, Bassissi P. Sporothricoid mycobacterial infection. A case report. Acta Derm Venereol 1993; 73:146–147.
- Mahaisavariya P, Chaiprasert A, Khemngern S, et al. Nontuberculous mycobacterial skin infections: clinical and bacteriological studies. J Med Assoc Thai 2003; 86:52–60.
- Rallis E, Koumantaki-Mathioudaki E, Frangoulis E, Chatziolou E, Katsambas A. Severe sporotrichoid fish tank granuloma following infliximab therapy. Am J Clin Dermatol 2007; 8:385–388.
- Imakado S, Kojima Y, Hiyoshi T, Morimoto S. Disseminated Mycobacterium marinum infection in a patient with diabetic nephropathy. Diabetes Res Clin Pract 2009; 83:e35–e36.
A 46-year-old healthy man presents with a 15-day history of a tender subcutaneous nodule on the dorsum of the right hand that appeared after cleaning his aquarium. He has no fever or systemic symptoms. For 2 weeks he has been taking amoxicillin-clavulanate (Augmentin) and metronidazole (Flagyl), but without an adequate response.
Q: Which is the most likely diagnosis?
- Tuberculosis verrucosa cutis
- Sporotrichosis
- Nontuberculous mycobacterial infection
- Leishmaniasis
- Nocardiosis
A: The correct answer is nontuberculous mycobacterial infection. Mycobacterium marinum was subsequently isolated from culture (Löwenstein-Jensen medium) of wound drainage.
Tuberculosis verrucosa cutis is an indolent, warty plaque that occurs after direct inoculation of M tuberculosis into the skin of someone previously infected with M tuberculosis. It can result from accidental exposure to tuberculous tissue in high-risk groups such as laboratory workers, physicians, and pathologists.
Sporothrix schenckii is the dimorphic fungus that causes sporotrichosis. Activities associated with acquisition of sporotrichosis include gardening (rose-gardener's disease), landscaping, farming, berry-picking, horticulture, and carpentry. The characteristic infection involves suppurating subcutaneous nodules that progress proximally along lymphatic channels (lymphocutaneous sporotrichosis).
Leishmaniasis is a disease caused by the protozoa of the Leishmania species, which is transmitted by the bite of a female sandfly. Initially, the lesion is a small, red papule up to 2 cm in diameter. Over several weeks, the papule becomes darker, develops a crust in the center, and eventually ulcerates to present a typical appearance of an ulcer with raised edges and surrounding dusky red skin.
Nocardia is a genus of filamentous grampositive bacteria that stains acid-fast, just as M marinum does. Nocardia asteroides primarily affects the lungs and can disseminate systemically. However, Nocardia brasiliensis is often associated with sporotrichoid-spreading subcutaneous nodules.
CLINICAL PRESENTATION AND DIAGNOSIS
M marinum is a genus of nontuberculous mycobacteria that usually causes disease in fish but can cause human skin infection by penetrating through a break in the skin. It can spread to deeper structures, resulting in tenosynovitis, arthritis, or osteomyelitis.1 The disease caused by M marinum is sometimes called “swimming pool granuloma” or “fish-tank granuloma.”2
In this case, the patient probably acquired the infection while cleaning his home aquarium,3 so asking about contact with pet fish is very important for the clinical diagnosis.
Lesions are usually localized to the site of the inoculation, with a predilection for areas predisposed to trauma, such as the hands and the fingers, after an incubation period of 2 to 8 weeks. Lesions are self-limited and usually appear as pruriginous bluish-red papules or pustules that may increase in size to form a verrucous or violaceous plaque or nodule. Superficial lesions often undergo central ulceration. Disseminated infection is rare and occurs mainly in patients who are immunocompromised because of renal transplant, systemic lupus erythematosus, chronic steroid therapy, or anti-tumor necrosis factor alpha therapy.4 In such cases, infection can be life-threatening.
On histopathologic study, a nonspecific inflammation consisting of a mixed infiltrate with lymphocytes, neutrophils, and histiocytes is usually observed. Sometimes a granulomatous inflammatory infiltrate mimicking tuberculoid granuloma may be noted. M marinum is an aerobic, nonmotile acid-fast bacillus. It grows at 30°C to 32°C on Löwenstein-Jensen medium in 2 to 5 weeks, but cultures are rarely positive. Polymerase chain reaction and enzyme-linked immunosorbent assays can help identify the organism. Other diagnoses to be considered are leishmaniasis, tuberculosis verrucosa cutis, blastomycosis, histoplasmosis, and sporotrichosis.
TREATMENT
Treatment of M marinum infection is not based on any specific criteria. Spontaneous resolution in 24 to 36 months has been described. 5 Antibiotics are the first-line therapy, and sometimes surgical treatment may be necessary for deeper infection with necrotic tissue. Cryotherapy, electrode therapy, and irradiation have been reported to be effective.
Few studies have been conducted to determine the first-line antibiotic treatment for M marinum infection. In this case, treatment with sulfamethoxazole-trimethoprim6 (Bactrim) resulted in resolution of the lesions in 6 months. Other antibiotics often used as monotherapy are tetracycline, minocycline (Minocin), and doxycycline (Vibramycin) 100 mg twice daily for 3 months.7 However, treatment failure has been described with many antibiotics.
Multidrug therapy is usually prescribed to minimize the risk of resistance. A combination of clarithromycin (Biaxin), streptomycin, and ethionamide (Trecator) has been prescribed successfully.3 Another combined regimen—clarithromycin 500 mg twice a day, rifampin (Rifadin) 600 mg daily, and ethambutol (Myambutol) 25 mg/kg daily—has been shown to be effective.5 Also, rifampin 600 mg daily and ethambutol 15 to 25 mg/kg/day were effective in a patient with a sporotrichoid M marinum infection associated with infliximab (Remicade). 8 The duration of treatment ranged between 2 and 6 months, or up to 2 months after the disappearance of the cutaneous lesions.
Clinical awareness of M marinum infection is important so that the diagnosis can be made and appropriate therapy can be initiated promptly, because some cases with disseminated disease and serious complications have been described.9
A 46-year-old healthy man presents with a 15-day history of a tender subcutaneous nodule on the dorsum of the right hand that appeared after cleaning his aquarium. He has no fever or systemic symptoms. For 2 weeks he has been taking amoxicillin-clavulanate (Augmentin) and metronidazole (Flagyl), but without an adequate response.
Q: Which is the most likely diagnosis?
- Tuberculosis verrucosa cutis
- Sporotrichosis
- Nontuberculous mycobacterial infection
- Leishmaniasis
- Nocardiosis
A: The correct answer is nontuberculous mycobacterial infection. Mycobacterium marinum was subsequently isolated from culture (Löwenstein-Jensen medium) of wound drainage.
Tuberculosis verrucosa cutis is an indolent, warty plaque that occurs after direct inoculation of M tuberculosis into the skin of someone previously infected with M tuberculosis. It can result from accidental exposure to tuberculous tissue in high-risk groups such as laboratory workers, physicians, and pathologists.
Sporothrix schenckii is the dimorphic fungus that causes sporotrichosis. Activities associated with acquisition of sporotrichosis include gardening (rose-gardener's disease), landscaping, farming, berry-picking, horticulture, and carpentry. The characteristic infection involves suppurating subcutaneous nodules that progress proximally along lymphatic channels (lymphocutaneous sporotrichosis).
Leishmaniasis is a disease caused by the protozoa of the Leishmania species, which is transmitted by the bite of a female sandfly. Initially, the lesion is a small, red papule up to 2 cm in diameter. Over several weeks, the papule becomes darker, develops a crust in the center, and eventually ulcerates to present a typical appearance of an ulcer with raised edges and surrounding dusky red skin.
Nocardia is a genus of filamentous grampositive bacteria that stains acid-fast, just as M marinum does. Nocardia asteroides primarily affects the lungs and can disseminate systemically. However, Nocardia brasiliensis is often associated with sporotrichoid-spreading subcutaneous nodules.
CLINICAL PRESENTATION AND DIAGNOSIS
M marinum is a genus of nontuberculous mycobacteria that usually causes disease in fish but can cause human skin infection by penetrating through a break in the skin. It can spread to deeper structures, resulting in tenosynovitis, arthritis, or osteomyelitis.1 The disease caused by M marinum is sometimes called “swimming pool granuloma” or “fish-tank granuloma.”2
In this case, the patient probably acquired the infection while cleaning his home aquarium,3 so asking about contact with pet fish is very important for the clinical diagnosis.
Lesions are usually localized to the site of the inoculation, with a predilection for areas predisposed to trauma, such as the hands and the fingers, after an incubation period of 2 to 8 weeks. Lesions are self-limited and usually appear as pruriginous bluish-red papules or pustules that may increase in size to form a verrucous or violaceous plaque or nodule. Superficial lesions often undergo central ulceration. Disseminated infection is rare and occurs mainly in patients who are immunocompromised because of renal transplant, systemic lupus erythematosus, chronic steroid therapy, or anti-tumor necrosis factor alpha therapy.4 In such cases, infection can be life-threatening.
On histopathologic study, a nonspecific inflammation consisting of a mixed infiltrate with lymphocytes, neutrophils, and histiocytes is usually observed. Sometimes a granulomatous inflammatory infiltrate mimicking tuberculoid granuloma may be noted. M marinum is an aerobic, nonmotile acid-fast bacillus. It grows at 30°C to 32°C on Löwenstein-Jensen medium in 2 to 5 weeks, but cultures are rarely positive. Polymerase chain reaction and enzyme-linked immunosorbent assays can help identify the organism. Other diagnoses to be considered are leishmaniasis, tuberculosis verrucosa cutis, blastomycosis, histoplasmosis, and sporotrichosis.
TREATMENT
Treatment of M marinum infection is not based on any specific criteria. Spontaneous resolution in 24 to 36 months has been described. 5 Antibiotics are the first-line therapy, and sometimes surgical treatment may be necessary for deeper infection with necrotic tissue. Cryotherapy, electrode therapy, and irradiation have been reported to be effective.
Few studies have been conducted to determine the first-line antibiotic treatment for M marinum infection. In this case, treatment with sulfamethoxazole-trimethoprim6 (Bactrim) resulted in resolution of the lesions in 6 months. Other antibiotics often used as monotherapy are tetracycline, minocycline (Minocin), and doxycycline (Vibramycin) 100 mg twice daily for 3 months.7 However, treatment failure has been described with many antibiotics.
Multidrug therapy is usually prescribed to minimize the risk of resistance. A combination of clarithromycin (Biaxin), streptomycin, and ethionamide (Trecator) has been prescribed successfully.3 Another combined regimen—clarithromycin 500 mg twice a day, rifampin (Rifadin) 600 mg daily, and ethambutol (Myambutol) 25 mg/kg daily—has been shown to be effective.5 Also, rifampin 600 mg daily and ethambutol 15 to 25 mg/kg/day were effective in a patient with a sporotrichoid M marinum infection associated with infliximab (Remicade). 8 The duration of treatment ranged between 2 and 6 months, or up to 2 months after the disappearance of the cutaneous lesions.
Clinical awareness of M marinum infection is important so that the diagnosis can be made and appropriate therapy can be initiated promptly, because some cases with disseminated disease and serious complications have been described.9
- Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol 2006; 33:817–819.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis 2003; 37:390–397.
- Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases. J Am Acad Dermatol 2007; 57:413–420.
- Streit M, Böhlen LM, Hunziker T, et al. Disseminated Mycobacterium marinum infection with extensive cutaneous eruption and bacteremia in an immunocompromised patient. Eur J Dermatol 2006; 16:79–83.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther 2004; 17:491–498.
- Alinovi A, Vecchini F, Bassissi P. Sporothricoid mycobacterial infection. A case report. Acta Derm Venereol 1993; 73:146–147.
- Mahaisavariya P, Chaiprasert A, Khemngern S, et al. Nontuberculous mycobacterial skin infections: clinical and bacteriological studies. J Med Assoc Thai 2003; 86:52–60.
- Rallis E, Koumantaki-Mathioudaki E, Frangoulis E, Chatziolou E, Katsambas A. Severe sporotrichoid fish tank granuloma following infliximab therapy. Am J Clin Dermatol 2007; 8:385–388.
- Imakado S, Kojima Y, Hiyoshi T, Morimoto S. Disseminated Mycobacterium marinum infection in a patient with diabetic nephropathy. Diabetes Res Clin Pract 2009; 83:e35–e36.
- Lam A, Toma W, Schlesinger N. Mycobacterium marinum arthritis mimicking rheumatoid arthritis. J Rheumatol 2006; 33:817–819.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis 2003; 37:390–397.
- Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases. J Am Acad Dermatol 2007; 57:413–420.
- Streit M, Böhlen LM, Hunziker T, et al. Disseminated Mycobacterium marinum infection with extensive cutaneous eruption and bacteremia in an immunocompromised patient. Eur J Dermatol 2006; 16:79–83.
- Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther 2004; 17:491–498.
- Alinovi A, Vecchini F, Bassissi P. Sporothricoid mycobacterial infection. A case report. Acta Derm Venereol 1993; 73:146–147.
- Mahaisavariya P, Chaiprasert A, Khemngern S, et al. Nontuberculous mycobacterial skin infections: clinical and bacteriological studies. J Med Assoc Thai 2003; 86:52–60.
- Rallis E, Koumantaki-Mathioudaki E, Frangoulis E, Chatziolou E, Katsambas A. Severe sporotrichoid fish tank granuloma following infliximab therapy. Am J Clin Dermatol 2007; 8:385–388.
- Imakado S, Kojima Y, Hiyoshi T, Morimoto S. Disseminated Mycobacterium marinum infection in a patient with diabetic nephropathy. Diabetes Res Clin Pract 2009; 83:e35–e36.