User login
MRI Is an Important Tool in Identifying Silent JIA
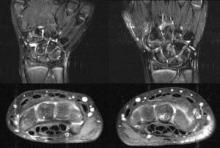
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
Magnetic resonance imaging is an important tool for detecting subclinical arthritis in children with juvenile idiopathic arthritis. In addition, MRI is a useful way to determine if there is disease persistence or silent progression before discontinuing treatment, according to a study of children who have clinically inactive JIA or are in remission on medication, according to Dr. Nikolay Tzaribachev.
All of the patients with clinically defined persistent oligoarticular juvenile idiopathic arthritis (JIA) were found to have polyarticular disease on MRI, according to Dr. Tzaribachev, head of the pediatric rheumatology department at the Center for Rheumatic Diseases in Bad Bramstedt, Germany.
"JIA tends to have an insidious onset and disease course. Subclinical JIA can elude the human tactile senses and clinical capacity to detect arthritis. In patients who are on drug treatment, symptoms may drop beyond clinical activity and disease may only become detectable by imaging techniques," said Dr. Tzaribachev in an interview.
"Remission criteria appear to be insufficient to detect the real extent of disease, which is of high importance to prevent joint damage, especially in growing children, who are supposed to become healthy adults.
"Furthermore, differentiation between oligo- and polyarticular disease at the first clinical evaluation after onset of symptoms is indispensable, since according to the ACR 2011 treatment guidelines [Arthritis Care Res. (Hoboken) 2011;63:465-82], oligo- and polyarticular disease have different treatment approaches," he noted.
During the last few years, silent arthritis in adults with RA has been detected only through the use of different imaging techniques, according to Dr. Tzaribachev. This fact led him and his colleagues to speculate that silent arthritis might also be the cause of the high percentage of disabilities in young adults with JIA, and that the clinical criteria for inactive disease and remission in JIA might be inadequate for detecting silent arthritis.
"We saw the huge discrepancy between clinical examination and MRI results, and understood the urgent need for more knowledge about the real extent of disease and silent disease progression, in order to protect the children from joint damage," said Dr. Tzaribachev.
The study included 21 patients with JIA (median age, 10.2 years at enrollment), who were on medications including NSAIDs, methotrexate, and/or tumor necrosis factor antagonists.
Clinically inactive disease or remission on medication were defined according to the Wallace criteria (J. Rheumatol. 2006;33:789-95). Patients underwent clinical examination, laboratory tests, and MRIs, and were asked to fill out a child health assessment questionnaire at every visit. The joints examined included wrists, knees, and ankles. Clinical and MRI exams and laboratory investigations were performed on the same day. Joint counts included those done clinically, as well as those based on MRI findings.
JIA subtype distribution included persistent oligoarticular (five patients), extended oligoarticular (four), polyarthritis (six), psoriatic arthritis (five), and undifferentiated arthritis (one). The median follow up time was 2.5 years.
Overall, 45 events were documented, 29 of which involved silent arthritis.
In all events, MRI revealed the following findings: 36 events with joint effusion, 39 with synovitis, 42 with synovial hypertrophy, 15 with bone marrow edema, 14 with osteitis, 15 with erosions, and 3 with tenosynovitis. All the silent arthritis events involved signs of arthritis that could be seen on MRI. Silent arthritis events were followed after a delay by clinical activity, according to the study.
In patients with JIA who present with clinically inactive disease or remission on medication, flares are preceded by arthritis detected on MRI. "In those cases, clinical examination and laboratory parameters remain normal despite ongoing disease activity, and remission criteria fail to show the real extent of disease," said Dr. Tzaribachev. "The lack of symptoms may also lead to silent disease progression, explaining the high percentage of physical disabilities in young adults with JIA. Imaging seems to be an important tool for defining disease activity, which should be included in the remission criteria and performed before treatment discontinuation."
The JIA classification probably needs to be redefined and remission criteria need to be reviewed to allow for the use of MRI and ultrasound, said Dr. Tzaribachev. Imaging techniques and protocols also need to be improved to become more child friendly, he noted. In addition, more studies are needed to define normal findings with respect to the growing musculoskeletal system in children and to allow for differentiation from mild pathology, especially for new imaging techniques like Xiralite. "Last, but not least, as in adult rheumatology, imaging should probably find its place in all clinical trials of children with inflammatory arthritis," he said.
Dr. Tzaribachev has received a research grant from Pfizer on temporomandibular joint arthritis in patients with JIA. His coauthors reported no disclosures.
FROM THE ANNUAL EUROPEAN CONGRESS OF RHEUMATOLOGY
Beware Cardiac Arrest Soon After Pneumonia Admission
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
FROM THE INTERNATIONAL CONFERENCE OF THE AMERICAN THORACIC SOCIETY
Major Finding: A total of 12% of in-hospital cardiac events within 72 hours of hospital admission occurred in patients who had pneumonia as a preexisting condition, and 19.3% of the events in pneumonia patients occurred outside an intensive care or step-down unit.
Data Source: A retrospective analysis of 5,367 in-hospital cardiac events from the American Heart Association’s Get With The Guidelines–Resuscitation database.
Disclosures: Dr. Carr had no disclosures.
Beware Cardiac Arrest Soon After Pneumonia Admission
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
FROM THE INTERNATIONAL CONFERENCE OF THE AMERICAN THORACIC SOCIETY
Beware Cardiac Arrest Soon After Pneumonia Admission
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
DENVER – Patients with pneumonia may be at risk of sudden cardiovascular collapse within the first 72 hours after admission to the hospital, according to the preliminary findings of a large retrospective analysis.
In addition, almost one in five of those in-hospital cardiac arrests (IHCA) occurred outside of the intensive care unit, and many of the patients were not receiving critical care interventions prior to the cardiac arrest, the study investigators found.
The findings "may indicate that current triage practices or other processes of care are inadequate," the researchers noted (Am. J. Respir. Crit. Care Med. 2011;183:A6339).
The new study is the first of its kind to analyze the characteristics of in-hospital cardiac arrest among pneumonia patients, Dr. Gordon E. Carr, the study’s lead author, said during a briefing at an international conference of the American Thoracic Society.
The sudden and rapid decline in pneumonia patients "is a problem that may happen in 1 in 10 patients but hasn’t received due attention, in part because it’s hard to study," said Dr. Carr, pulmonary and critical care fellow at the University of Chicago Medical Center. "There’s an unmet need to know more about what’s going on with these patients."
More than 1 million patients with pneumonia are admitted to hospitals each year, and 3.4% of in-hospital patient deaths are due to pneumonia, according to national data.
Patients with pneumonia are at risk of following a progressive pathway of severe sepsis, septic shock, and multiple organ failure before having a cardiac arrest, Dr. Carr noted. However, some patients go from developing severe infection straight to cardiopulmonary collapse, without developing severe sepsis or septic shock. Several clinical and epidemiologic studies have shown that not all patients with sepsis go down the typical progressive pathway (Curr. Opin. Anaesthesiol. 2008;21:128-40).
Dr. Carr and his colleagues at the University of Chicago Medical Center conducted the retrospective analysis using the American Heart Association’s Get With The Guidelines Resuscitation database, formerly known as the National Registry of Cardiopulmonary Resuscitation. The data covered 9 years and included 10% of hospitals (approximately 500) in North America.
The team analyzed 166,919 cardiopulmonary arrest events, 44,416 of which occurred within 72 hours after admission. They focused on 5,367 events in which patients had pneumonia as a preexisting condition (12% of the 44,416 events) prior to having their first pulseless event within 72 hours of hospital admission.
The median time from hospital admission to IHCA was 20.7 hours. Only 14.7% of patients with pneumonia and IHCA survived to hospital discharge, according to the analysis. Also, 19.3% of the IHCA events occurred in a general inpatient area, while 77.2% of IHCA events occurred in an intensive care or step-down unit.
At the time of IHCA, 40% of the patients were receiving mechanical ventilation, 12.2% had a central venous catheter in place, and 36.3% were receiving continuous infusions of vasoactive medications.
The analysis showed that arrhythmia was the most common cause of IHCA (65%) among that group of patients, followed by respiratory insufficiency (53.9%), and hypotension/hypoperfusion (49.8%).
In addition, the majority of the rhythms were not "shockable," said Dr. Carr, including pulseless electrical activity (45.2% of cases), asystole (38.4%), and ventricular fibrillation or tachycardia (16.4%).
The study had several limitations, Dr. Carr said. It is based on a large database, and "any huge set of data is going to have the inherent problem in terms of bias," he said. Also, the researchers couldn’t adjust for the severity of pneumonia, and they had no information on the processes of care, such as antibiotic administration.
Given the preliminary nature of the data, it is hard to draw firm conclusions, he noted. However, the study highlights the need for more research on cardiac arrest in pneumonia patients.
The take-away message for physicians is "to be alert to the possibility of abrupt collapse in pneumonia patients," and monitor those patients with comorbidities carefully, Dr. Carr cautioned.
Dr. Carr had no disclosures.
FROM AN INTERNATIONAL CONFERENCE OF THE AMERICAN THORACIC SOCIETY
Major Finding: A total of 12% of in-hospital cardiac events within 72 hours of hospital admission occurred in patients who had pneumonia as a preexisting condition, and 19.3% of the events in pneumonia patients occurred outside an intensive care or step-down unit.
Data Source: A retrospective analysis of 5,367 in-hospital cardiac events from the American Heart Association’s Get With The Guidelines–Resuscitation database.
Disclosures: Dr. Carr had no disclosures.
FDA Clears Quick Test Distinguishing MRSA From MSSA
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Clears Quick Test Distinguishing MRSA From MSSA
The Food and Drug Administration has cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The May 6 clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration has cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The May 6 clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration has cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The May 6 clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Clears Quick Test Distinguishing MRSA From MSSA
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
FROM THE FOOD AND DRUG ADMINISTRATION
FDA Clears Quick Test Distinguishing MRSA From MSSA
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
The Food and Drug Administration on May 6 cleared the KeyPath MRSA/MSSA blood culture test, which can quickly determine whether the Staphylococcus aureus infection is methicillin resistant (MRSA) or methicillin susceptible (MSSA).
"This not only saves time in diagnosing potentially life-threatening infections but also allows health care professionals to optimize treatment and start appropriate contact precautions to prevent the spread of the organism," Alberto Gutierrez, Ph.D., director of the Office of In Vitro Diagnostics Device Evaluation and Safety in the FDA’s Center for Devices and Radiological Health, said in a statement.
The test determines MRSA or MSSA infections within 5 ½ hours directly from gram-positive blood cultures. The test does not require any specific or additional instruments aside from blood culture equipment, according to the FDA.
Identifying whether an S. aureus infection is MRSA or MSSA currently takes a day or more, depending on the organism and whether the laboratory is on site or not, said Dr. Donna E. Sweet, professor of internal medicine at the University of Kansas, Wichita. "So, this is good news," she said in an interview.
"Any time you can know [the results] sooner than later, then it decreases [patients’] exposure to more potent antibiotics. A quick test will help you narrow your antibiotic focus more quickly and target the bug more quickly," said Dr. Sweet, who is not affiliated with the test or its manufacturer.
The clearance was based on a clinical study of 1,116 blood samples at four major U.S. hospital centers. The test’s accuracy was 98.9% for detecting MRSA (178/180) and 99.4% for MSSA (153/154).
National statistics show that the rate of MRSA infections in the United States is falling. However, it remains an "important public health problem," according to the Centers for Disease Control and Prevention.
The test is manufactured by MicroPhage Inc.
FROM THE FOOD AND DRUG ADMINISTRATION
Race, Ethnicity Affect Adherence to Gestational Weight Gain Recommendations
WASHINGTON – Adherence to gestational weight gain recommendations and prepregnancy body mass index varies significantly depending on race and ethnicity, Erica Holland reported.
In addition, black women were at the greatest risk of prepregnancy overweight or obesity while Asian women were at the greatest risk of being underweight. Meanwhile, the majority of black, white, and Hispanic women overgained weight during pregnancy.
When adjusted for age, marital status, and several other factors, Asian, black, and Hispanic women had significantly decreased odds of gaining excessive weight compared with white women, even though the majority of black and Hispanic women gained weight excessively during pregnancy, according to study results presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Black women were 1.56 times more likely to be overweight and 1.61 times more likely to be obese prior to pregnancy than their white counterparts. Hispanic women were 1.28 times more likely to be overweight, and 1.23 times more likely to be obese compared with white women. Asian women were 2.25 times more likely to be underweight and less likely to be overweight or obese compared with their white counterparts.
The findings have opened the door for further research on maternal and neonatal outcomes based on race and ethnicity and on gestational weight gain (GWG) adherence, which could in turn change recommendations, said Ms. Holland, a third-year medical student at the University of Massachusetts, Worcester, who presented the study findings.
She speculated that the variation could be caused by various factors such as cultural differences, maybe a genetic component, and disparities in weight gain advice given to women based on their race.
Evidence suggests GWG nonadherence is a risk factor for adverse birth outcomes. The Institute of Medicine updated its recommendations for GWG in 2009, giving a range based on the mother’s body mass index (BMI). Women at a normal weight for their height (BMI of 18.5-24.9) should gain 25-35 pounds during pregnancy, underweight women (BMI less than 18.5) should gain 28-40 pounds, and overweight women (BMI of 25-29.9) should gain 15-25 pounds, according to the 2009 IOM guidelines. The recommendations, however, are not tailored based on race and ethnicity, Ms. Holland said in her presentation.
To find the association of race and ethnicity with prepregnancy BMI and GWG adherence, researchers conducted a retrospective study using automated labor and delivery records of 11,992 women with a mean age of 29 years. In total, 70% of the women were multigravida, 69% were white, 18% Hispanic, 9% black, and 5% Asian; 91% of the women delivered full-term.
In total, 3.8% of the population was underweight and 21% were obese before pregnancy. A quarter gained weight properly during pregnancy, said Ms. Holland, but 68% overgained weight during pregnancy.
Prior to pregnancy, 2% of black women were underweight, 39% were normal weight, and 59% were overweight or obese. The statistics were, respectively, 3%, 51%, and 46% for white women; 9%, 73%, and 18% for Asian women; and 5%, 47%, and 48% for Hispanic women.
For GWG, 20% of black women undergained, 27% appropriately gained and 54% over gained. The statistics were 15%, 26%, and 60% for white women; 22%, 38%, and 41% for Asian women; and 20%, 27%, and 54% for Hispanic women.
Ms. Holland said that while both undergaining and overgaining are risk factors for adverse birth outcomes, overgaining has been more prevalent due the obesity epidemic.
"As mounting evidence suggests that GWG nonadherence is a risk for adverse birth outcomes, appropriate counseling and interventions to optimize gain are proving more important. Further study is needed to understand how recommendations can be tailored to meet specific needs of different racial/ethnic groups," she said.
Ms. Holland said she had no relevant financial disclosures.
WASHINGTON – Adherence to gestational weight gain recommendations and prepregnancy body mass index varies significantly depending on race and ethnicity, Erica Holland reported.
In addition, black women were at the greatest risk of prepregnancy overweight or obesity while Asian women were at the greatest risk of being underweight. Meanwhile, the majority of black, white, and Hispanic women overgained weight during pregnancy.
When adjusted for age, marital status, and several other factors, Asian, black, and Hispanic women had significantly decreased odds of gaining excessive weight compared with white women, even though the majority of black and Hispanic women gained weight excessively during pregnancy, according to study results presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Black women were 1.56 times more likely to be overweight and 1.61 times more likely to be obese prior to pregnancy than their white counterparts. Hispanic women were 1.28 times more likely to be overweight, and 1.23 times more likely to be obese compared with white women. Asian women were 2.25 times more likely to be underweight and less likely to be overweight or obese compared with their white counterparts.
The findings have opened the door for further research on maternal and neonatal outcomes based on race and ethnicity and on gestational weight gain (GWG) adherence, which could in turn change recommendations, said Ms. Holland, a third-year medical student at the University of Massachusetts, Worcester, who presented the study findings.
She speculated that the variation could be caused by various factors such as cultural differences, maybe a genetic component, and disparities in weight gain advice given to women based on their race.
Evidence suggests GWG nonadherence is a risk factor for adverse birth outcomes. The Institute of Medicine updated its recommendations for GWG in 2009, giving a range based on the mother’s body mass index (BMI). Women at a normal weight for their height (BMI of 18.5-24.9) should gain 25-35 pounds during pregnancy, underweight women (BMI less than 18.5) should gain 28-40 pounds, and overweight women (BMI of 25-29.9) should gain 15-25 pounds, according to the 2009 IOM guidelines. The recommendations, however, are not tailored based on race and ethnicity, Ms. Holland said in her presentation.
To find the association of race and ethnicity with prepregnancy BMI and GWG adherence, researchers conducted a retrospective study using automated labor and delivery records of 11,992 women with a mean age of 29 years. In total, 70% of the women were multigravida, 69% were white, 18% Hispanic, 9% black, and 5% Asian; 91% of the women delivered full-term.
In total, 3.8% of the population was underweight and 21% were obese before pregnancy. A quarter gained weight properly during pregnancy, said Ms. Holland, but 68% overgained weight during pregnancy.
Prior to pregnancy, 2% of black women were underweight, 39% were normal weight, and 59% were overweight or obese. The statistics were, respectively, 3%, 51%, and 46% for white women; 9%, 73%, and 18% for Asian women; and 5%, 47%, and 48% for Hispanic women.
For GWG, 20% of black women undergained, 27% appropriately gained and 54% over gained. The statistics were 15%, 26%, and 60% for white women; 22%, 38%, and 41% for Asian women; and 20%, 27%, and 54% for Hispanic women.
Ms. Holland said that while both undergaining and overgaining are risk factors for adverse birth outcomes, overgaining has been more prevalent due the obesity epidemic.
"As mounting evidence suggests that GWG nonadherence is a risk for adverse birth outcomes, appropriate counseling and interventions to optimize gain are proving more important. Further study is needed to understand how recommendations can be tailored to meet specific needs of different racial/ethnic groups," she said.
Ms. Holland said she had no relevant financial disclosures.
WASHINGTON – Adherence to gestational weight gain recommendations and prepregnancy body mass index varies significantly depending on race and ethnicity, Erica Holland reported.
In addition, black women were at the greatest risk of prepregnancy overweight or obesity while Asian women were at the greatest risk of being underweight. Meanwhile, the majority of black, white, and Hispanic women overgained weight during pregnancy.
When adjusted for age, marital status, and several other factors, Asian, black, and Hispanic women had significantly decreased odds of gaining excessive weight compared with white women, even though the majority of black and Hispanic women gained weight excessively during pregnancy, according to study results presented at the annual meeting of the American College of Obstetricians and Gynecologists.
Black women were 1.56 times more likely to be overweight and 1.61 times more likely to be obese prior to pregnancy than their white counterparts. Hispanic women were 1.28 times more likely to be overweight, and 1.23 times more likely to be obese compared with white women. Asian women were 2.25 times more likely to be underweight and less likely to be overweight or obese compared with their white counterparts.
The findings have opened the door for further research on maternal and neonatal outcomes based on race and ethnicity and on gestational weight gain (GWG) adherence, which could in turn change recommendations, said Ms. Holland, a third-year medical student at the University of Massachusetts, Worcester, who presented the study findings.
She speculated that the variation could be caused by various factors such as cultural differences, maybe a genetic component, and disparities in weight gain advice given to women based on their race.
Evidence suggests GWG nonadherence is a risk factor for adverse birth outcomes. The Institute of Medicine updated its recommendations for GWG in 2009, giving a range based on the mother’s body mass index (BMI). Women at a normal weight for their height (BMI of 18.5-24.9) should gain 25-35 pounds during pregnancy, underweight women (BMI less than 18.5) should gain 28-40 pounds, and overweight women (BMI of 25-29.9) should gain 15-25 pounds, according to the 2009 IOM guidelines. The recommendations, however, are not tailored based on race and ethnicity, Ms. Holland said in her presentation.
To find the association of race and ethnicity with prepregnancy BMI and GWG adherence, researchers conducted a retrospective study using automated labor and delivery records of 11,992 women with a mean age of 29 years. In total, 70% of the women were multigravida, 69% were white, 18% Hispanic, 9% black, and 5% Asian; 91% of the women delivered full-term.
In total, 3.8% of the population was underweight and 21% were obese before pregnancy. A quarter gained weight properly during pregnancy, said Ms. Holland, but 68% overgained weight during pregnancy.
Prior to pregnancy, 2% of black women were underweight, 39% were normal weight, and 59% were overweight or obese. The statistics were, respectively, 3%, 51%, and 46% for white women; 9%, 73%, and 18% for Asian women; and 5%, 47%, and 48% for Hispanic women.
For GWG, 20% of black women undergained, 27% appropriately gained and 54% over gained. The statistics were 15%, 26%, and 60% for white women; 22%, 38%, and 41% for Asian women; and 20%, 27%, and 54% for Hispanic women.
Ms. Holland said that while both undergaining and overgaining are risk factors for adverse birth outcomes, overgaining has been more prevalent due the obesity epidemic.
"As mounting evidence suggests that GWG nonadherence is a risk for adverse birth outcomes, appropriate counseling and interventions to optimize gain are proving more important. Further study is needed to understand how recommendations can be tailored to meet specific needs of different racial/ethnic groups," she said.
Ms. Holland said she had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS
Major Finding: In terms of gestational weight gain, 20% of black women undergained, 27% appropriately gained and 54% overgained. The statistics were, respectively, 15%, 26%, and 60% for white women; 22%, 38%, and 41% for Asian women; and 20%, 27%, and 54 for Hispanic women.
Data Source: A retrospective study using automated labor and delivery records of 11,992 women.
Disclosures: Ms. Holland said that she had no relevant financial disclosures.
Screening for Alcohol Use Disorders Important, Simple
WASHINGTON – When it comes to screening patients for alcohol use disorders, the small check box on the patient history form sometimes fails to tell physicians what they need to know about the patient’s alcohol use, experts said at the annual meeting of the American Society of Addiction Medicine.
That’s partly why Dr. Keith A. Nichols screens all of his adolescent and adult patients for alcohol use disorders beyond the patient history form in his private family practice in upstate New York. Often, Dr. Nichols’ questions lead to a conversation.
"Do not stop at taking history," Dr. Nichols said. "Delve into and find out if there’s a problem. Don’t take the person’s snap response. People in general aren’t offended if you ask them. In fact, you get a lot of people who are grateful if you help them."
Because of the key role alcohol plays in a variety of problems, diseases, and injuries, early identification is critical, said John P. Allen, Ph.D. Why? "Because the chances of treating problem drinking are likely most favorable before the drinking becomes more ingrained," said Dr. Allen, associate chief consultant for addictive disorders at the Veterans Health Administration’s (VHA’s) Office of Mental Health Services.
The importance of primary care as an entry point for treating these patients is well established. For example, screening and behavioral counseling interventions in primary care settings to reduce alcohol misuse are top recommendations of the U.S. Preventive Services Task Force. At the VHA, more than 96% of the patients are screened for alcohol use disorders. In addition, roughly two-thirds of patients with a diagnosis of substance use disorder at the VHA are treated in primary care or in general mental health services.
Providers at the VHA use a simple screening instrument called the Alcohol Use Disorders Identification Test (AUDIT-C), which includes three questions inquiring about the frequency of drinking, the usual level of alcohol consumption, and the frequency of very heavy alcohol use.
Despite physicians’ awareness of the importance of screening and probing for alcohol use disorders, one problem that might explain the failure of screening to become routine could be that physicians "aren’t sure what to do once they obtain a history" that suggests a problem, Dr. Nichols said. "I think there might be a concern that it’s not a priority for the patient, and the doctor might not want to go there."
For many physicians faced with 15-minute limits for each patient visit in order to make a viable practice, the biggest challenge is time. And the problem doesn’t end there. "Even if you have enough time to figure out the problem, there’s not enough time to delve into its origins," said Dr. Jose M. Partida Corona, an internist who practices in Las Vegas.
Other barriers also interfere, such as lack of an integrated system or access to specialty care.
Yet, screening advocates stress the importance of taking that first step.
"It’s important for health care providers to ask about drinking, because it plays a huge role in many physical and mental health problems," Dr. Partida Corona said. "And obviously, treatment for them will be very different if alcohol is playing a significant role."
Dr. Nichols, Dr. Allen, and Dr. Partida Corona reported no conflicts of interest.
WASHINGTON – When it comes to screening patients for alcohol use disorders, the small check box on the patient history form sometimes fails to tell physicians what they need to know about the patient’s alcohol use, experts said at the annual meeting of the American Society of Addiction Medicine.
That’s partly why Dr. Keith A. Nichols screens all of his adolescent and adult patients for alcohol use disorders beyond the patient history form in his private family practice in upstate New York. Often, Dr. Nichols’ questions lead to a conversation.
"Do not stop at taking history," Dr. Nichols said. "Delve into and find out if there’s a problem. Don’t take the person’s snap response. People in general aren’t offended if you ask them. In fact, you get a lot of people who are grateful if you help them."
Because of the key role alcohol plays in a variety of problems, diseases, and injuries, early identification is critical, said John P. Allen, Ph.D. Why? "Because the chances of treating problem drinking are likely most favorable before the drinking becomes more ingrained," said Dr. Allen, associate chief consultant for addictive disorders at the Veterans Health Administration’s (VHA’s) Office of Mental Health Services.
The importance of primary care as an entry point for treating these patients is well established. For example, screening and behavioral counseling interventions in primary care settings to reduce alcohol misuse are top recommendations of the U.S. Preventive Services Task Force. At the VHA, more than 96% of the patients are screened for alcohol use disorders. In addition, roughly two-thirds of patients with a diagnosis of substance use disorder at the VHA are treated in primary care or in general mental health services.
Providers at the VHA use a simple screening instrument called the Alcohol Use Disorders Identification Test (AUDIT-C), which includes three questions inquiring about the frequency of drinking, the usual level of alcohol consumption, and the frequency of very heavy alcohol use.
Despite physicians’ awareness of the importance of screening and probing for alcohol use disorders, one problem that might explain the failure of screening to become routine could be that physicians "aren’t sure what to do once they obtain a history" that suggests a problem, Dr. Nichols said. "I think there might be a concern that it’s not a priority for the patient, and the doctor might not want to go there."
For many physicians faced with 15-minute limits for each patient visit in order to make a viable practice, the biggest challenge is time. And the problem doesn’t end there. "Even if you have enough time to figure out the problem, there’s not enough time to delve into its origins," said Dr. Jose M. Partida Corona, an internist who practices in Las Vegas.
Other barriers also interfere, such as lack of an integrated system or access to specialty care.
Yet, screening advocates stress the importance of taking that first step.
"It’s important for health care providers to ask about drinking, because it plays a huge role in many physical and mental health problems," Dr. Partida Corona said. "And obviously, treatment for them will be very different if alcohol is playing a significant role."
Dr. Nichols, Dr. Allen, and Dr. Partida Corona reported no conflicts of interest.
WASHINGTON – When it comes to screening patients for alcohol use disorders, the small check box on the patient history form sometimes fails to tell physicians what they need to know about the patient’s alcohol use, experts said at the annual meeting of the American Society of Addiction Medicine.
That’s partly why Dr. Keith A. Nichols screens all of his adolescent and adult patients for alcohol use disorders beyond the patient history form in his private family practice in upstate New York. Often, Dr. Nichols’ questions lead to a conversation.
"Do not stop at taking history," Dr. Nichols said. "Delve into and find out if there’s a problem. Don’t take the person’s snap response. People in general aren’t offended if you ask them. In fact, you get a lot of people who are grateful if you help them."
Because of the key role alcohol plays in a variety of problems, diseases, and injuries, early identification is critical, said John P. Allen, Ph.D. Why? "Because the chances of treating problem drinking are likely most favorable before the drinking becomes more ingrained," said Dr. Allen, associate chief consultant for addictive disorders at the Veterans Health Administration’s (VHA’s) Office of Mental Health Services.
The importance of primary care as an entry point for treating these patients is well established. For example, screening and behavioral counseling interventions in primary care settings to reduce alcohol misuse are top recommendations of the U.S. Preventive Services Task Force. At the VHA, more than 96% of the patients are screened for alcohol use disorders. In addition, roughly two-thirds of patients with a diagnosis of substance use disorder at the VHA are treated in primary care or in general mental health services.
Providers at the VHA use a simple screening instrument called the Alcohol Use Disorders Identification Test (AUDIT-C), which includes three questions inquiring about the frequency of drinking, the usual level of alcohol consumption, and the frequency of very heavy alcohol use.
Despite physicians’ awareness of the importance of screening and probing for alcohol use disorders, one problem that might explain the failure of screening to become routine could be that physicians "aren’t sure what to do once they obtain a history" that suggests a problem, Dr. Nichols said. "I think there might be a concern that it’s not a priority for the patient, and the doctor might not want to go there."
For many physicians faced with 15-minute limits for each patient visit in order to make a viable practice, the biggest challenge is time. And the problem doesn’t end there. "Even if you have enough time to figure out the problem, there’s not enough time to delve into its origins," said Dr. Jose M. Partida Corona, an internist who practices in Las Vegas.
Other barriers also interfere, such as lack of an integrated system or access to specialty care.
Yet, screening advocates stress the importance of taking that first step.
"It’s important for health care providers to ask about drinking, because it plays a huge role in many physical and mental health problems," Dr. Partida Corona said. "And obviously, treatment for them will be very different if alcohol is playing a significant role."
Dr. Nichols, Dr. Allen, and Dr. Partida Corona reported no conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF ADDICTION MEDICINE