User login
Systems biology – A primer
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.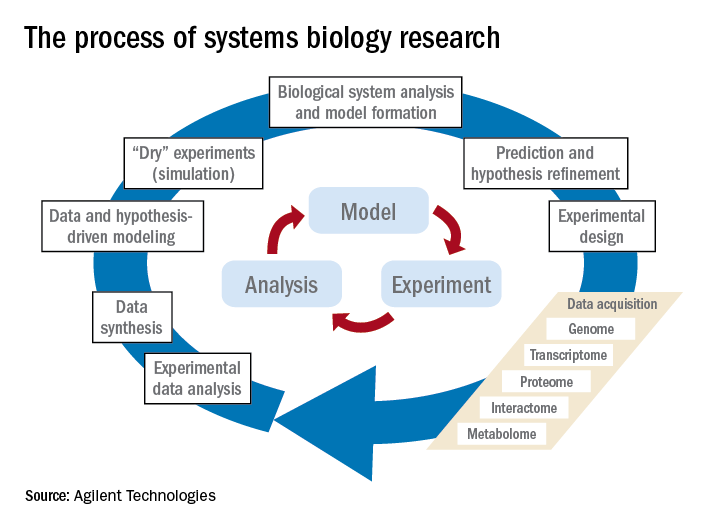
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Vaccine renaissance
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.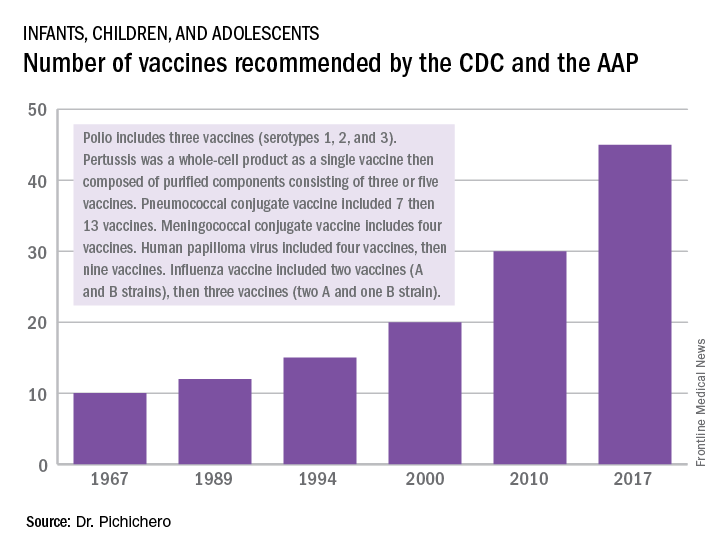
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at pdnews@frontlinemedcom.com.
Five-day treatment of ear infections
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?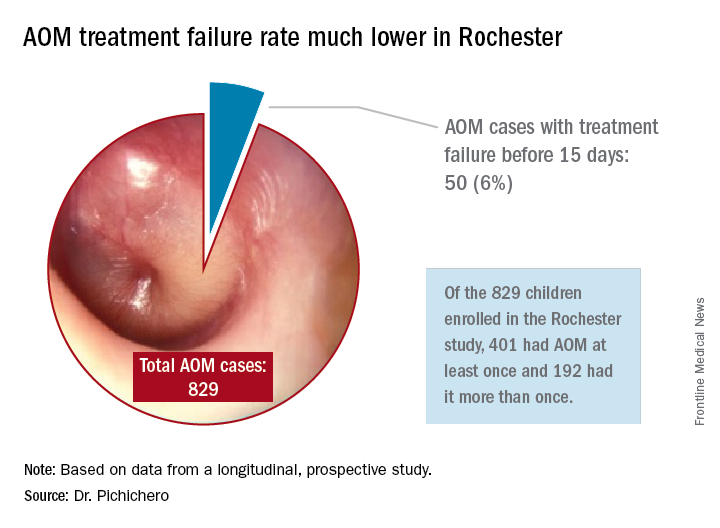
Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?
Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
In December 2016, the results of a randomized, controlled trial of 5-day vs. 10-day amoxicillin/clavulanate treatment of acute otitis media (AOM) in children aged 6-23 months was reported by Hoberman et al. in the New England Journal of Medicine (NEJM).1 Predefined criteria for clinical failure were used that considered both symptoms and signs of AOM, assessed on days 12-14 after start of treatment with 5 vs. 10 days of treatment with the antibiotic. The conclusion reached was clear: The clinical failure rate for the 5-day regimen was 34% vs. 16% in the 10-day group, supporting a preference for the 10-day treatment.
I was surprised. The clinical failure rate for the 5-day regimen seemed very high for treatment with amoxicillin/clavulanate. If it is 34% with amoxicillin/clavulanate, then what would it have been with amoxicillin, as recommended by the American Academy of Pediatrics?
So, why did the systematic review conclude that there was a minimal difference between shortened treatments and the standard 10-day when the NEJM study reported such a striking difference?
In Rochester, N.Y., we have been conducting a longitudinal, prospective study of AOM that is NIH-sponsored to better understand the immune response to AOM, especially in otitis-prone children.3,4 In that study we are treating all children aged 6-23 months with amoxicillin/clavulanate using the same dose as used in the study by Hoberman et al. We have two exceptions: If the child has a second AOM within 30 days of a prior episode or they have an eardrum rupture, we treat for 10 days.5 Our clinical failure rate is 6%. Why is the failure rate in Rochester so much lower than that in Pittsburgh and Bardstown, Ky., where the Hoberman et al. study was done?
One possibility is an important difference in our study design, compared with that of the NEJM study. All the children in our prospective study have a tympanocentesis to confirm the clinical diagnosis, and our research has shown that tympanocentesis results in immediate relief of ear pain and reduces the frequency of antibiotic treatment failure about twofold, compared with children diagnosed and treated by the same physicians in the same clinic practice.6 So, if the tympanocentesis is factored out of the equation, the Rochester clinical failure comes out to 14% for 5-day treatment. Why would the children in Rochester not getting a tympanocentesis, being treated with the same antibiotic, same dose, and same definition of clinical failure, during the same time frame, and having the same bacteria with the same antibiotic resistance rates have a clinical failure rate of 14%, compared with the 34% in the NEJM study?
Next question: How does a clinical failure rate of 34% fit according to past studies of shortened course antibiotic treatment of AOM? Besides the systematic review and meta-analysis noted above, in many countries outside the United States the 5-day regimen is standard, so, if health care providers were seeing a 34% failure rate, that would have been noticeable for sure.8 So, if health care providers were seeing a 34% failure rate, would that not have been noticeable? And would not a 16% failure rate, nearly 1 of 5 cases, be noticeable for children treated for 10 days?
Was there something different about the children who were in the Hoberman et al. study and the children treated in countries outside the United States and in our practice in Rochester? My group has collaborated and published on studies of AOM with the Pittsburgh and Kentucky groups, and we have not found significant site to site differences in outcomes, demonstrating that a population difference is unlikely.9-11
Next question: How does a clinical failure rate of 16% fit according to past studies of 10 days’ antibiotic treatment of AOM? It is on target with the meta-analysis and two other recent studies in the NEJM.12,13 However, if the failure rate was 16% with amoxicillin/clavulanate (which is effective against beta-lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, whereas amoxicillin is not), then the predicted failure rate with amoxicillin for 10 days should be double (34%) or triple (51%) had amoxicillin been used as recommended by the AAP in light of the bacterial resistance of otopathogens. That calculation is based on the prevalence of beta-lactamase–producing H. influenzae and M. catarrhalis in the Pittsburgh and Kentucky populations, the same prevalence seen in the Rochester population.” 14
So, I conclude that this wonderful study does not convince me to change my practice from standard use of 5-day amoxicillin/clavulanate treatment of AOM. Besides, outside of a study setting, most parents don’t give the full 10-day treatment. They stop when their child seems normal (a few days after starting treatment) and save the remainder of the medicine in the refrigerator for the next illness to save a trip to the doctor. Plus, in this column, I did not even get into the issue of disturbing the microbiome with longer courses of antibiotic treatment, a topic for a future discussion.
References
1. N Engl J Med. 2016 Dec 22;375(25):2446-56.
2. Cochrane Database Syst Rev. 2010 Sep 8;(9):CD001095.
3. Pediatr Infect Dis J. 2016 Sep;35(9):1027-32.
4. Pediatr Infect Dis J. 2016 Sep;35(9):1033-9.
5. Otolaryngol Head Neck Surg. 2001 Apr;124(4):381-7.
6. Pediatr Infect Dis J. 2013 May;32(5):473-8.
7. Pediatr Infect Dis J. 2006 Mar;25(3):211-8.
8. Pediatr Infect Dis J. 2000 Sep;19(9):929-37.
9. Pediatr Infect Dis J. 1999 Aug;18(8):741-4.
10. Clin Pediatr (Phila). 2008 Nov;47(9):901-6.
11. Drugs. 2012 Oct 22;72(15):1991-7.
12. N Engl J Med. 2011 Jan 13;364(2):105-15.
13. N Engl J Med. 2011 Jan 13;364(2):116-26.
14. Pediatr Infect Dis J. 2016 Aug;35(8):901-6.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
It’s in the nose
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at pdnews@frontlinemedcom.com.
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at pdnews@frontlinemedcom.com.
There is a lot more going on in the nose besides air going in and out. The nose is where it all begins for pathogenesis for all respiratory infections. The interplay between the commensal microbes, the potential pathogens, innate immunity, and adaptive immunity is much more complex than was previously understood. So what is new?
In our research on acute otitis media, we swab and wash out noses of children aged 6-36 months to isolate the potential pathogens Streptococcus pneumoniae, nontypeable Haemophilus influenza, Moraxella catarrhalis, Staphylococcus aureus, and Group A streptococci. We isolate one or more of these bacteria from most of the children even though they are well. We observe perhaps a half-dozen other species of bacteria on the culture plate. Mostly, we isolate S. pneumoniae, nontypeable H. influenza, or M. catarrhalis and alpha-hemolytic streptococci and corynebacterium species.
We have recently begun to investigate the other microbiota in the nose and found they are indeed plentiful. In a recent screening of a half-dozen children, we found hundreds of different microbes in their noses, so cultures and standard molecular detection methods were just touching the surface. I was asked recently at a medical conference – the American Academy of Pediatrics– Orange County, California, annual CME course – at which I spoke on this topic what I thought would be the most-important area of research in the next decade. I responded, the microbiome. The microbiome is indeed a hot topic. Research over the last decade suggests that 90% of all diseases can be traced in some way to disturbances in the microbiome. What I mean by microbiome is “the totality of microorganisms and their collective genetic material present in or on the human body.” The term is often used interchangeably with “microbiota,” although the former refers to genes of microbes and the latter refers to the microbes themselves. What I mean by “disturbance” is excessive use of antibiotics.
How many microbes are in the nose? We don’t know. But if the gut is any indication, there are thousands of microbes in the nose because the gut has more than 10,000 different microbes. Recognizing that there are hundreds of microbes in the nose and from time to time children get colonized by potential pathogens that can cause otitis media, sinusitis, or pneumonia, how does pathogenesis get started? It starts with a respiratory virus infection. The bacteria need help from the viruses to cause disease. The viruses cause damage to the epithelial cells of the nose, and this gives the bacteria more places to attach when they divide so the amount of bacteria increases exponentially. As the viruses replicate, they more effectively slow down cilia beating, and the nasal mucus thickens. This, too, helps the bacteria and viruses attach to and penetrate epithelial cells in the nose and increase in density on the surface of the cells and inside the cells. The viruses divert and/or suppress the innate immune system, represented by neutrophils that migrate to the nose and discharge their intracellular contents to turn nasal mucus yellow and green. The viruses even down-modulate the adaptive immune system in clever ways that result in fewer potentially protective cytotoxic lymphocytes that kill viruses making their way to the nose, and fewer T cells that discharge cytokines that promote a necessary inflammatory response to clear both bacteria and viruses from the nose and fewer B cells that become plasma cells and release antibodies into the nose.
When the bacteria with potential to cause diseases reach a “pathogenic threshold,” they move, along with mucus, into the middle ear, the sinuses, or down the throat to the lungs, usually with the accompanying respiratory virus. There pathogenesis continues in the otherwise sterile and protected sanctuary of these interconnected respiratory sites. A few days later, we as clinicians observe the symptoms and signs of otitis media, sinusitis, or pneumonia.
What can we do to help the nose? Mostly, we should do no harm, and that has been our failing for decades since the introduction of antibiotics. The allure of antibiotics is great because they have indeed saved many lives and shortened many illnesses when appropriately used. However, too often clinicians have seen patients with yellow and green nasal mucus (or any increased nasal mucus) and diagnosed “a bacterial infection” and prescribed antibiotics. And too often clinicians have seen patients with an annoying cough (or any cough) and diagnosed “a bacterial chest infection” and prescribed antibiotics. The clinicians thought it was the right thing to do because they wanted to help their patient. And they did not want them to come back in a few days with persistence or worsening of symptoms, or worse, seek care from other health care providers elsewhere. So they gave antibiotics.
Well, the paradigm has changed. It is now clearly known that antibiotics can be harmful mainly by damaging the normal, healthy microbiome. The change in healthy homeostasis of the microbiome wrought by antibiotics is greatest in newborns, especially premature newborns, then next worst for infants, and then next worst for young children. These are the age groups where antibiotics are prescribed most frequently! And everyone needs to stop writing those prescriptions for runny noses, yellow and green mucus in the nose, and coughs. All of us need to prescribe antibiotics only when there is an accurate diagnosis of otitis media or sinusitis or bronchopneumonia or lobar pneumonia. And when we do prescribe the antibiotics ,we need to give them for as short a time as possible. But that is a topic for another column.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he has no relevant financial disclosures, and that his research is supported by a grant from the National Institutes of Health National Institute of Deafness and Communication Disorders. Email him at pdnews@frontlinemedcom.com.
Summer colds
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Most viral infections in summer months are caused by enteroviruses. We studied illnesses in about 400 kids aged 4-18 years seen in private pediatric practice and were surprised by what we found.
Our impression was that summer colds lasted for a shorter time span than winter colds. What we found was that the median duration of illness was about 8 days. Among the various syndromes, the most common was stomatitis (viral blisters in the throat), accounting for 58% of all cases seen. A flulike illness with fever, myalgias, and malaise was second most common (28% of cases), followed by hand/foot/mouth syndrome (8%), pleurodynia (3%), fever with viral rash (3%), and aseptic meningitis (1%). Most of the cases occurred among children 4-12 years old.
The most prevalent symptoms were fever, headache, sore throat, tiredness, muscle aches, and crankiness. Fever was present in about 85% of cases of children with stomatitis, in 95% of cases with myalgias and malaise, but in only 50% of cases of hand/foot/mouth. Headache was very common as well, occurring in about 40% of children with stomatitis, 70% of children with myalgias and malaise, and in 30% of children with hand/foot/mouth.
Illness within a household was quite common. About 50% of the children who came for care had a sibling or parent ill with a summer cold. However, while the symptoms of the family members often were the same as the child who presented for care, that was not always the case. As anticipated, most illness within a household occurred within a 2-week time span. Hand/foot/mouth was most easily recognized by parents to have spread among their children. When a parent became ill, it was almost always the mother because she was almost always the primary parent caretaker.
Summer colds took a toll on families in terms of loss of work by parents. Most of the children were ill enough to stay out of day care or school for about 2-4 days. Virtually all the children with hand/foot/mouth and stomatitis with classic viral blister lesions had a single visit to the pediatric practice, and very limited or no tests done or medications prescribed other than acetaminophen or ibuprofen. But for the children with higher fevers without hand/foot/mouth or stomatitis, the costs of care escalated as tests were much more often performed (CBC, chest x-ray), and medications prescribed (antibiotics for uncertain diagnosis in the context of high fever), and occasional referrals made to the emergency department for further work-up (100% of cases of aseptic meningitis and 50% of cases of pleurodynia).
Overall, summer colds are not so insignificant as presumed at first glance. What interests me now is why summer colds so infrequently are followed by an acute otitis media or sinusitis, whereas winter colds caused by respiratory syncytial virus, influenza, and rhinoviruses are followed by an acute otitis media in about one-third of cases. A new study is underway!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no disclosures.
Why 10 days of antibiotics for infections is not magic
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
In the United States, we treat almost all infections for 10 days. Why? In France, most infections are treated for 8 days. In the U.K., most infections are treated for 5 days. In many other countries, infections are treated until symptomatic improvement occurs. Can everyone outside the United States be wrong? What is the evidence base for the various recommended durations? Moreover, what is the harm in treating for longer than necessary?
The U.S. tradition of 10 days’ treatment for infections arose from the 1940 trials of injectable penicillin for prevention of acute rheumatic fever in military recruits who had group A streptococcal pharyngitis. Injections of penicillin G mixed in peanut oil produced therapeutic levels of penicillin for about 3 days. Soldiers who received three sequential injections had the lowest occurrence of rheumatic fever; two injections were not as good and four injections did not add to the prevention rate. So three injections meant 9 days’ treatment; 9 days was rounded up to 10 days, and there you have it.
We have come a long way since the 1940s. For strep throat, we now have three approved antibiotics for 5 days’ treatment: cefdinir, cefpodoxime proxetil, and azithromycin, all evidence based and U.S. Food and Drug Administration approved. One large study was done in the 1980s with cefadroxil for 5 days, and that duration was as effective in strep eradication as was 10 days, but the company never pursued the 5-day indication.
The optimal duration of antibiotic treatment is generally considered to be 10 days in the United States, however, there is scant evidence base for that recommendation. The recent American Academy of Pediatrics/American Academy of Family Physicians guidelines endorse 10 days of treatment duration as the standard for most acute otitis media (AOM) (Pediatrics 2013;131[3]:e964-99), but acknowledge that shorter treatment regimens may be as effective. Specifically, the guideline states: “A 7-day course of oral antibiotic appears to be equally effective in children 2- to 5 years of age with mild to moderate AOM. For children 6 years and older with mild to moderate AOM symptoms, a 5- to 7-day course is adequate treatment.” A systematic analysis and a meta-analysis have concluded that 5 days’ duration of antibiotics is as effective as 10 days’ treatment for all children over age 2 years and only marginally inferior to 10 days for children under the age of 2 years old (Cochrane Database Syst Rev. 2010;[9]:CD001095).
Thirty years ago, our group and others began to do studies involving “double tympanocentesis,” where an ear tap was done at time of diagnosis and again 3-5 days later to prove bacterial cure for various antibiotics that were in trials. We learned that if the organism was sensitive to the antibiotic chosen, then it was dead by days 3-5. Most of the failures were due to resistant bacteria. So treating longer was not going to help. It was time to change the antibiotic if clinical improvement had not occurred. Our group published a study 15 years ago of 2,172 children comparing 5-, 7-, and 10-days’ treatment of AOM, and concluded that 5 days’ treatment was equivalent to 7- and 10-days of treatment for all ages unless the child had a perforated tympanic membrane or the child had been treated for AOM within the preceding month since recently treated AOM was associated with more frequent causation of AOM by resistant bacteria and with a continued inflamed middle ear mucosa (Otolaryngol Head Neck Surg. 2001 Apr;124[4]:381-7). Since then we have treated all children with ear infections for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins unless the eardrum had perforated or the child had a recurrent AOM within the prior 30 days. That is a lot of patients in 15 years, and the results have been just as good as when we used 10 days as standard.
Acute sinusitis is another interesting story. The AAP guideline states: “The optimal duration of antimicrobial therapy for patients with acute bacterial sinusitis has not received systematic study. Recommendations based on clinical observation varied widely, from 1- to 28 days (Pediatrics. 2013 Jul;132[1]:e262-80). The prior AAP guideline endorsed “antibiotic therapy be continued for 7 days after the patient becomes free of symptoms and signs (Pediatrics. 2001 Sep;108[3]:798-808). Our group reasoned that the etiology and pathogenesis of sinusitis and AOM are identical, involving ascension of a bacterial inoculum from the nasopharynx via the osteomeatal complex to the sinuses just like ascension of infection via the eustachian tube to the middle ear. Therefore, beginning 25 years ago, we began to treat all children with sinus infections for 5 days, including amoxicillin and amoxicillin/clavulanate, as well as various cephalosporins. Again, that is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about community-acquired pneumonia? The Infectious Disease Society of America (IDSA) guideline states: “Treatment courses of 10 days [of antibiotics] have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis” (Clin Infect Dis. 2011 Oct;53[7]:617-30). Our group reasoned that antibiotics reach higher levels in the lungs than they do in the closed space of the middle ear or sinuses. Therefore, beginning 25 years ago, we began to treat all children with bronchopneumonia and lobar pneumonia for 5 days, including amoxicillin and amoxicillin/clavulanate as well as various cephalosporins and azithromycin. That is a lot of patients, and the results have been just as good as when we used 10 days as standard.
What about skin and soft tissue infections? The IDSA guideline states that the duration of treatment for impetigo is 7 days, for cellulitis is 5 days, and for furuncles and carbuncles no duration is stated, but they allow no antibiotics be used at all if the patient is not febrile and white blood cell count is not elevated after incision and drainage (Clin Infect Dis. 2014 Jul 15;59[2]:e10-52).
So what is the harm to longer courses of antibiotics? As I have written in this column recently, we have learned a lot about the importance of our gut microbiome. The resident flora of our gut modulates our immune system favorably. Disturbing our gut flora with antibiotics is potentially harmful because the antibiotics often kill many species of healthy gut flora and cause disequilibrium of the flora, resulting in diminished innate immunity responses. Shorter treatment courses with antibiotics cause less disturbance of the healthy gut flora.
The rest of the world cannot all be wrong and the United States all right regarding the duration of antibiotic treatment for common infections. Moreover, in an era of evidence-based medicine, it is necessary to make changes from tradition. The evidence is there to recommend that 5 days’ treatment become the standard for treatment with selected cephalosporins as approved by the FDA – for AOM, for sinusitis, for community-acquired pneumonia, and for skin and soft tissue infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said that he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Fewer doses of PCV13 could save money – but at what cost?
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Streptococcus pneumoniae is the most common bacterial cause of pneumonia, sinusitis, and acute otitis media (AOM). It also causes invasive pneumococcal disease (IPD), such as bacteremia and meningitis, and it is the leading cause of vaccine-preventable death in children younger than 5 years of age. Pneumococcal conjugate vaccines (PCVs) are effective in infants and young children against IPD, non-IPD, and the acquisition of vaccine serotype nasopharyngeal carriage (contagion). PCV7 was licensed and introduced in 2000 on a schedule that matched the schedule of other routine infant immunizations of three primary doses at 2, 4, and 6 months, and a booster at 12-15 months. Later in 2010, PCV13 was licensed on that same “3+1” schedule. Different pneumococcal vaccination schedules are recommended across Europe and other countries, after consideration of the epidemiology, disease burden, immunogenicity of the vaccine, its compatibility with other vaccines, and its cost. The World Health Organization recently updated its PCV policy to support the use of three doses on either 3+0 or 2+1 schedules. Most European countries have adopted the 2+1 schedule used for routine infant immunizations.
In light of the escalating costs of providing current vaccines, and the anticipated need for additional vaccines, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) has convened a working group to evaluate the transition from a 3+1 to a 2+1 schedule for PCV administration to infants and children. This is not a trivial decision. In the United States, cost must be considered in the context of an additional focus on non-IPD disease prevention, especially AOM, where serotypes and immune protection levels differ from IPD. A 2+1 schedule may be effective to prevent IPD, compared with a 3+1 schedule, but its impact on non-IPD may be compromised, especially for AOM, for some serotypes of pneumococci, and for control of nasopharyngeal carriage.
Immunogenicity studies show that antibody responses from a vaccine regimen consisting of two doses in the primary series are less immunogenic, compared with those for a three-dose regimen, yet both regimens are effective for the prevention of IPD. Immunogenicity data that support the use of reduced-dose schedules for most, but not all, vaccine serotypes, were based on IPD. The degree to which higher antibody concentrations are important for protecting against nonbacteremic pneumonia, sinusitis, and AOM, and for preventing nasopharyngeal carriage, is not established.
However, clinical outcomes since the introduction of PCVs indicate that the true threshold will vary by serotype and host and disease condition, with higher concentrations required for certain serotypes, in immunologically less mature hosts, and in mucosal infections like nonbacteremic pneumonia, sinusitis, and AOM, compared with IPD. Also, higher IgG levels clearly are important in protecting against nasopharyngeal colonization, thereby conferring herd immunity, prolonging individual protection, and possibly correlating at the individual level with disease protection. Studies that evaluated the correlation of antibody concentration and protection against nasopharyngeal colonization have shown that a greater than 10-fold higher antibody concentration is needed, compared with levels in blood, to protect against IPD. Similarly protection against AOM require higher levels of antibody than are needed to protect against IPD, as evidenced by the lower efficacy of PCVs against AOM, compared with IPD.
Epidemiology and risk factors differ among countries of the world. Therefore, even among developed countries, there is a need for caution in accepting that what works in one country will work as well in another. For example, attendance at day care is the highest risk factor for both IPD and non-IPD. In the United States, we have many types of day care, including relatively large day care centers, and many infants enter day care at 2 months of age. In other developed countries, the size of day care centers is much smaller, and children may not enter day care until 1 or even 2 years of age. Those differences may have implications for protective efficacy with a reduced-dose vaccine schedule.
Siblings under the age of 8 years are also at significant risk. Again, the family size may differ among developed countries. Breastfeeding is protective for pneumococcal infections. Breastfeeding duration may differ among countries. The theme of this concern is apparent: Even evidence of adequate protection with a reduced-dose schedule in Finland, France, Germany, the United Kingdom, or elsewhere should not be interpreted to be completely applicable to the United States.
Whether reduced-dose schedules can provide equivalent protection against vaccine type IPD equivalent to a 3+1 schedule for all serotypes and for non-IPD when introduced into a national immunization program is unclear. Do we have enough data to inform the decision process, and specifically do we have a clear understanding of the full impact of reduced-dose schedules on non-IPD relative to 3+1? How would you vote?
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Pfizer, which makes PCV vaccine, has funded an investigator-initiated grant and a postmarketing study to Dr. Pichichero’s institution, and he is the primary investigator of both grants.
Microbiome and innate immunity in the respiratory tract – a primer
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at pdnews@frontlinemedcom.com.
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at pdnews@frontlinemedcom.com.
The pathogenesis of respiratory infections such as acute otitis media (AOM), sinusitis, and pneumonia involves complex interactions among bacteria, respiratory viruses, and host immune responses.
My clinical and laboratory group and others have described respiratory infections as resulting from the growth of a single otopathogen, such as Streptococcus pneumoniae (Spn), nontypeable Haemophilus influenzae (NTHi), or Moraxella catarrhalis (Mcat) in the nasopharynx (NP) followed by ascension to the middle ear, sinuses, or descent to the lungs. Recent research from my group and others has resulted in a shift from a single pathogen focus toward consideration of respiratory infections as a polymicrobial disease. Bacterial and viral interactions are critical in respiratory infection pathogenesis. Commensal bacteria can alter virulence of bacterial pathogens and interfere with antibiotic treatment.
The traditional view of the immune system is that it is an assembly of human tissues, cells, and molecules that work to eliminate pathogens. Recent discoveries indicate that commensals play a central role in regulating human immune responses. Thus, the key questions in the field are:
1) How do members of the NP microbiome and innate immune responses maintain health in young children over time?
2) Do specific deleterious members of the NP microbiome alter host innate immune responses in a manner that predisposes to respiratory infections?
3) How does the microbiome and innate response in the NP differ when recovery, relapse of infection, or persistent infection occurs?
Virtually all young children are colonized by Spn, NTHi, or Mcat during the first 3 years of life. My group and others have shown that competitive interactions among bacteria influence whether these potential pathogens successfully colonize and cause respiratory infections. Recent studies have demonstrated that hundreds of different bacterial species colonize the upper respiratory tract. Diverse communities have been shown to be more stable and resistant to invasion by foreign species. Data from cross-sectional studies demonstrate that specific commensals, including Dolosigranulum, Corynebacterium, and Lactococcus, are associated with decreased risk of respiratory infections. Prior studies have been limited by the use of culture-based methods or have been cross sectional in design. Therefore, the optimal levels of diversity and NP commensals critical for maintaining health in the upper respiratory tract of children are currently unknown and under study by my group and others. Studies that utilize high-throughput culture-independent molecular detection methods are now used to identify optimal levels of diversity and commensal members of the microbiome critical for maintaining health homeostasis.
The innate immune system constitutes the first line of defense against respiratory pathogen colonization and respiratory virus infection. It relies on pattern recognition receptors on innate immune cells to detect evolutionarily conserved pathogen-associated molecular patterns expressed on pathogen surfaces. Toll-like receptors (TLRs) are crucial in the innate immune response; TLR 3, 7, and 8 recognize respiratory infection-associated viral pathogens. TLR2, 4, and 5 recognize respiratory infection-associated bacterial pathogens, and TLR9 and TLR13 recognize both viral and bacterial pathogens. The activation of TLRs triggers signaling cascades and regulates the expression of a wide range of cytokines leading to antimicrobial and inflammatory responses. Cytokines (there are dozens) associated with the pathogenesis, development, severity, and clinical outcomes of respiratory infections identify hypotheses that our group is exploring to expand our understanding of how innate responses might be manipulated to favor the child host. Importantly, it has already been shown that cytokine profiles differ in the NP depending on the number and type of bacteria and viruses involved.
My group recently has shown that serum IL-10 levels are significantly higher in AOM from Spn than are the levels associated with NTHi and Mcat, suggesting use of detection of this cytokine as a serum biomarker. Others have shown that the levels of IL-1-beta, TNF-alpha, IL-6, IL-8, IL-10, and IL-17a in middle ear fluids from children with recurrent AOM correlate significantly with higher bacterial load (and worse disease). Previous studies on cytokine responses associated with AOM have focused on limited numbers of cytokines and have not examined any relationship with commensals of the NP microbiome. Moreover, the subset of children who experience excessively frequent respiratory infections likely have disturbances in their microbiome (made worse with antibiotics) and innate immune response. Because of our growing knowledge about the microbiome and innate immune response, I see a compelling need to assess interactions of the NP microbiome and innate immune responses in children that are associated with sustained health and control of respiratory infections.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said the work was supported by a National Institutes of Health grant, and he had no relevant conflicts of interest. E-mail him at pdnews@frontlinemedcom.com.
It’s time to take a stand against vaccine refusers
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
The challenges in primary care are many, and one of increasing importance is what to say to vaccine refusers. After much debate and thoughtful discussion, my medical partner, Dr. Janet Casey at Legacy Pediatrics, decided that the practice would refuse to care for the refusers.
Over the years, I have accepted such patients into my practice and worked with them to gain their confidence and debunk the many myths about the safety of vaccination that are so visible on the Internet. The approach worked well, and by the time the children were 1 year of age, I cannot remember but a handful of parents who did not come around to realize that it was best to vaccinate. However, with the recent measles outbreak at Disneyland in California, pertussis at epidemic proportions in pockets of the United States and elsewhere in the world, and the antivaccine voices gaining more and more attention, I agree, it is time to take a stand.
When a family brings their unvaccinated or undervaccinated child into the waiting room of a physician’s practice, that family is potentially exposing others in that waiting room to serious infectious diseases – that is not fair. In the waiting room may well be a patient who is on chemotherapy or immunotherapy or otherwise immunocompromised, and he relies on the “herd immunity” achieved by vaccinations of those who can safely be vaccinated for individual protection and public health. Those patients who have weakened immune systems did not choose to have their medical condition, whereas the vaccine refusers are choosing not to vaccinate their child (or typically themselves as well). And the reasons they are choosing not to vaccinate are based on misrepresentation of medical facts, fabrications of safety concerns, long ago disproven speculations by well-meaning and not so well-meaning physicians and scientists, pseudoscience published in pseudoscientific journals, and/or general distrust of the federal government that mandates vaccinations for the good of the public health.
My personal experience with vaccine scares dates back to a time when whole-cell pertussis vaccine was the only pertussis vaccine available. I was a medical student, resident, and then an infectious diseases fellow during the escalating debate about the significant side effects of vaccines. I joined in the chorus of voices questioning the need for clear data on the problem, and then the pursuit of a safer acellular pertussis vaccine. The physician community and the public were ready for change, and the National Institutes of Health took the lead in organizing multiple studies and clinical trials leading to eventual replacement of the whole cell pertussis vaccine with the current acellular vaccines.
Much more recently, at the request of National Institutes of Health, I led studies of the safety of thimerosal preservative in multidose vaccine vials that appeared in the Lancet (2002;360:1737-41); Pediatrics (2008;121:e208-14) and the Journal of Pediatrics (2009;155:495-9). Using the data from those three studies, the World Health Organization (WHO), the United Nations, the Institute of Medicine, and other organizations were able to see that the metabolism and elimination from the body of ethylmercury in thimerosal was dramatically faster, compared with methylmercury in fish. Therefore, the presumption of possible accumulation of mercury in the body of infants receiving vaccines from multidose vials when such vaccines were closely spaced was disproven by scientific data.
In plain language, there was never a known risk from thimerosal, but a premature, hurried decision was made to mandate removal of thimerosal from vaccines given to children in the United States and western Europe; thereby the myth lives on that thimerosal is not safe. Yet thimerosal is safe, and the WHO continues to advocate use of thimerosal in multidose vaccine vials. Nevertheless, I have been criticized personally on the Internet for this work. The accusation is that I, the rest of the scientists who participated in the study, and the NIH oversight were biased because our academic institutions had previously received funding from vaccine companies to perform clinical and translational research. I received many hate e-mails and even a death threat.
To close this column with a sense of humor, I suggest you Google the responses by U.S. presidential hopefuls on their stand with regard to vaccine refusers. The comments, then the reversal and “corrections” to their comments is amusing. The presidential hopefuls quickly recognized that the right to choose may not be the best policy for the public health of American citizens. Refusing to vaccinate a child potentially harms the child and may harm others!
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures. E-mail him at pdnews@frontlinemedcom.com.
ID CONSULT: Influenza virus and pneumococci dance together
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.