User login
White Concretions on the Hair Shaft
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
The Diagnosis: White Piedra
A fungal culture demonstrated a filamentous fungus that was identified as Trichosporon inkin via DNA sequencing, which confirmed the diagnosis of white piedra (WP).
Piedra refers to a group of fungal infections presenting as gritty nodules adherent to the hair shaft.1 It is further categorized into black piedra, which occurs more commonly in tropical climates and is caused by Piedraia hortae, and WP, which occurs in tropical and temperate climates and is caused by the Trichosporon genus.1-3 Among the Trichosporon genus, clinical manifestations have varied based on species; for example, T inkin commonly causes genital WP, Trichosporon ovoides commonly causes scalp WP, and Trichosporon asahii and Trichosporon mucoides have been described to cause systemic fungal infections in immunocompromised hosts.1,4 Scalp WP most commonly occurs in children and young adults, and females are at greater risk than males.1,2,5,6
Clinically, WP presents with pale irregular nodules along the hair shaft that are not fluorescent on Wood lamp examination.1,6,7 Nodules are soft and easily detached from the hair shaft, unlike the hard, tightly adherent nodules seen in black piedra.1,7 White piedra affects hair in a variety of areas including the scalp, beard, eyebrows, eyelashes, axillae, and genitals.1,7 Affected hair may become brittle and break at points of invasion.1 Alternatively, WP may resemble tinea capitis with scalp hyperkeratosis and alopecia, though tinea typically affects the base of the hair shaft.1 Immunocompromised patients can develop disseminated WP, and cases of progressive pneumonia, lung abscess, peritonitis, vascular access infection, and endocarditis have been reported.2
Diagnosis of WP is made through a combination of clinical findings and culture of infected hair. Potassium hydroxide preparation demonstrates sleevelike concretions formed of masses of septate hyphae with dense zones of arthrospores and blastospores.1,2 Culture on Sabouraud agar demonstrates creamy colonies that develop a dull, gray, wrinkled surface.1,2 Differential diagnosis includes pediculosis; however, the concretions of WP are circumferential around the hair shaft on microscopy.1 Notably, a case of concomitant WP and pediculosis has been reported.8 In cases of potential pediculosis resistant to therapy, consider hair casts, which are asymptomatic, white, cylindrical concretions that encircle the hair without adherence and can therefore be differentiated from pediculosis via dermoscopy.9 Because this phenomenon is more commonly observed in preadolescent girls, it is hypothesized that scalp inflammation due to traction from hairstyles or atopic dermatitis contributes to the development of hair casts.9,10 Thus, when a potassium hydroxide mount is equivocal for nits and dermoscopy demonstrates concretions that completely encircle the hair shaft, it is important to perform a microbiologic culture to rule out piedra of the hair or scalp. Other differential diagnoses include tinea capitis, black piedra, trichobacteriosis, and hair shaft abnormalities.
Transmission of WP is thought to result from a combination of poor hygiene; humidity due to climate; personal care practices such as habitually tying wet hair, applying hair oils and conditioners, or covering hair according to social customs; and close contact with an infected individual.1,3,6 Long scalp hair potentially correlates with increased risk.1,6 Finally, WP has been described in animals and has been isolated from soil, vegetable matter, and water.3,10
Treatment of WP generally involves removal of infected hair, antifungal agents, and improved hygienic habits to avoid relapses. The American Academy of Dermatology’s Guidelines/Outcomes Committee recommends complete removal of infected hair; however, patients may desire hair-preserving treatments.11 Kiken et al1 reported success with the combination of an oral azole antifungal agent for 3 weeks to 1 month and an antifungal shampoo for 2 to 3 months. The authors proposed that oral medication eliminates scalp carriage while antifungal shampoo eliminates hair shaft concretions.1
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
1. Kiken DA, Sekaran A, Antaya RJ, et al. White piedra in children. J Am Acad Dermatol. 2006;55:956-961.
2. Bonifaz A, Gómez-Daza F, Paredes V, et al. Tinea versicolor, tinea nigra, white piedra, and black piedra. Clin Dermatol. 2010;28:140-145.
3. Shivaprakash MR, Singh G, Gupta P, et al. Extensive white piedra of the scalp caused by Trichosporon inkin: a case report and review of literature. Mycopathologia. 2011;172:481-486.
4. Goldberg LJ, Wise EM, Miller NS. White piedra caused by Trichosporon inkin: a report of two cases in a northern climate. Br J Dermatol. 2015;173:866-868.
5. Schwartz RA. Superficial fungal infections. Lancet. 2004;364:1173-1182.
6. Fischman O, Bezerra FC, Francisco EC, et al. Trichosporon inkin: an uncommon agent of scalp white piedra. report of four cases in Brazilian children. Mycopathologia. 2014;178:85-89.
7. Pontes ZB, Ramos AL, Lima Ede O, et al. Clinical and mycological study of scalp white piedra in the State of Paraíba, Brazil. Mem Inst Oswaldo Cruz. 2002;97:747-750.
8. Marques SA, Richini-Pereira VB, Camargo RM. White piedra and pediculosis capitis in the same patient. An Bras Dermatol. 2012;87:786-787.
9. Gnarra M, Saraceni P, Rossi A, et al. Challenging diagnosis of peripillous sheaths. Pediatr Dermatol. 2014;31:E112-E113.
10. França K, Villa RT, Silva IR, et al. Hair casts or pseudonits. Int J Trichology. 2011;3:121-122.
11. Guidelines of care for superficial mycotic infections of the skin: piedra. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:122-124.
A 35-year-old woman presented with possible nits on the hair of 1 year’s duration. She was previously evaluated by several outside medical providers and was unsuccessfully treated with topical and systemic medications for pediculosis. She reported sporadic scalp pruritus but denied hair loss, breakage, close contacts with similar symptoms, or recent travel outside the United States. She was otherwise healthy and was not taking any medications. Physical examination revealed small 1- to 2-mm, generalized, somewhat detachable, white concretions randomly distributed on the hair shafts. No broken hairs were observed. The eyebrows, eyelash hairs, and surrounding skin were normal. Potassium hydroxide mount was equivocal for nits.
Red-Brown Plaque on the Leg
The Diagnosis: Wells Syndrome
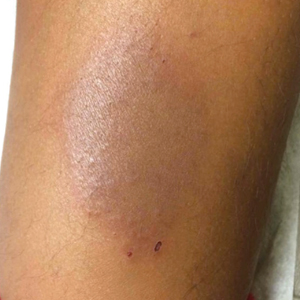
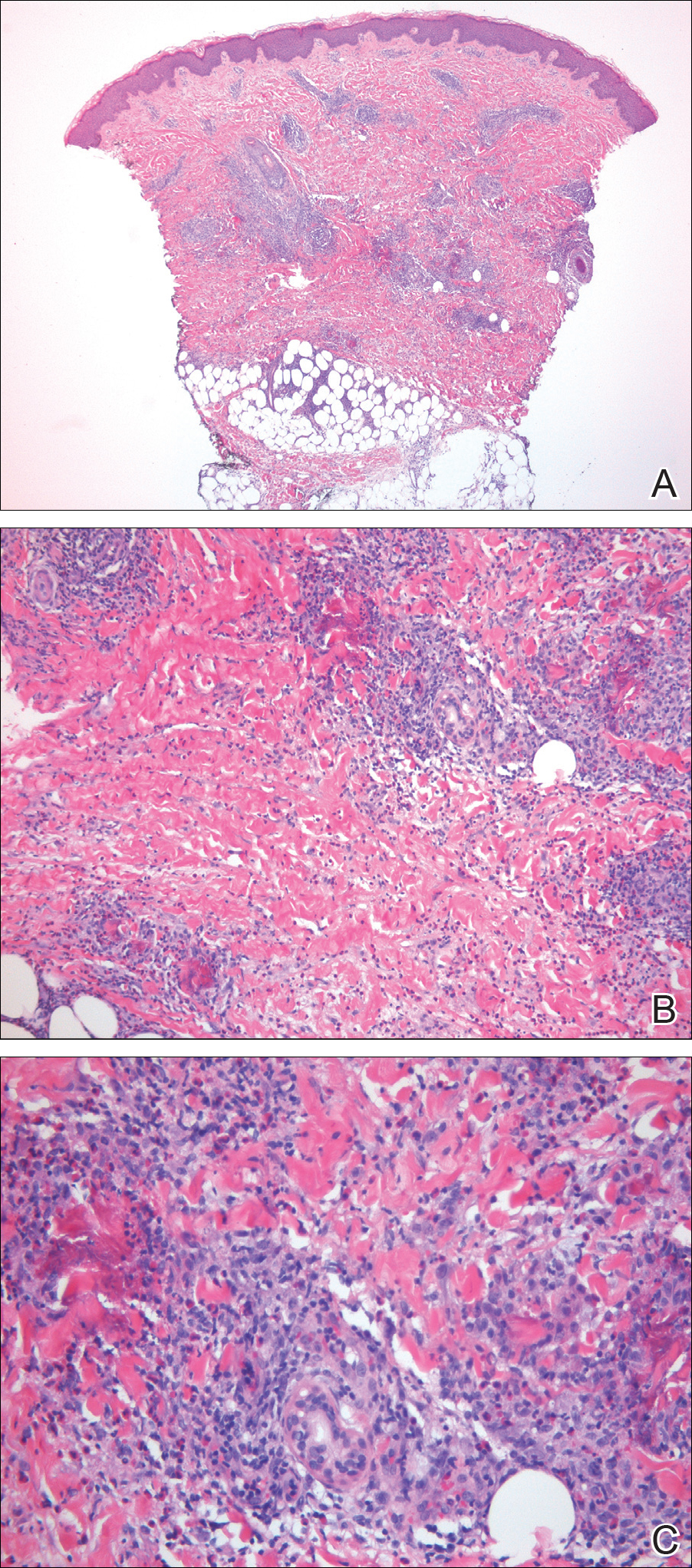
A punch biopsy taken from the perimeter of the lesion demonstrated mild spongiosis overlying a dense nodular to diffuse infiltrate of lymphocytes, neutrophils, and numerous eosinophils, some involving underlying fat lobules (Figure, A and B). In some areas, eosinophilic degeneration of collagen bundles surrounded by a rim of histiocytes, "flame features," were observed (Figure C). The clinical and histological features were consistent with Wells syndrome (WS), also known as eosinophilic cellulitis. Given the localized mild nature of the disease, the patient was started on a midpotency topical corticosteroid.
Wells syndrome is a rare inflammatory condition characterized by clinical polymorphism, suggestive histologic findings, and a recurrent course.1,2 This condition is especially rare in children.3,4 Caputo et al1 described 7 variants in their case series of 19 patients: classic plaque-type variant (the most common clinical presentation in children); annular granuloma-like (the most common clinical presentation in adults); urticarialike; bullous; papulonodular; papulovesicular; and fixed drug eruption-like. Wells syndrome is thought to result from excess production of IL-5 in response to a hypersensitivity reaction to an exogenous or endogenous circulating antigen.3,4 Increased levels of IL-5 enhance eosinophil accumulation in the skin, degranulation, and subsequent tissue destruction.3,4 Reported triggers include insect bites, viral and bacterial infections, drug eruptions, recent vaccination, and paraphenylenediamine in henna tattoos.3-7 Additionally, WS has been reported in the setting of gastrointestinal pathologies, such as celiac disease and ulcerative colitis, and with asthma exacerbations.8,9 However, in half of pediatric cases, no trigger can be identified.7
Clinically, WS presents with pruritic, mildly tender plaques.7 Lesions may be localized or diffuse and range from mild annular or circinate plaques with infiltrated borders to cellulitic-appearing lesions that are occasionally associated with bullae.5,6 Patients often report prodromal symptoms of burning and pruritus.5,6 Lesions rapidly progress over 2 to 3 days, pass through a blue grayish discoloration phase, and gradually resolve over 2 to 8 weeks.5,6,10 Although patients generally heal without scarring, WS lesions have been described to resolve with atrophy and hyperpigmentation resembling morphea.5-7 Additionally, patients typically experience a relapsing remitting course over months to years with eventual spontaneous resolution.1,5 Patients also may experience systemic symptoms including fever, lymphadenopathy, and arthralgia, though they do not develop more widespread systemic manifestations.2,3,7
Diagnosis of WS is based on clinicopathologic correlation. Histopathology of WS lesions demonstrates 3 phases. The acute phase demonstrates edema of the superficial and mid dermis with a dense dermal eosinophilic infiltrate.1,6,10 The subacute granulomatous phase demonstrates flame figures in the dermis.1,2,6,7,10 Flame figures consist of palisading groups of eosinophils and histiocytes around a core of degenerating basophilic collagen bundles associated with major basic protein.1,2,6,7,10 Finally, in the resolution phase, eosinophils gradually disappear while histiocytes and giant cells persist, forming microgranulomas.1,2,10 Notably, no vasculitis is observed and direct immunofluorescence is negative.3,7 Although flame figures are suggestive of WS, they are not pathognomonic and are observed in other conditions including Churg-Strauss syndrome, parasitic and fungal infections, herpes gestationis, bullous pemphigoid, and follicular mucinosis.2,5
Wells syndrome is a self-resolving and benign condition.1,10 Physicians are recommended to gather a complete history including review of medications and vaccinations; a history of insect bites, infections, and asthma; laboratory workup consisting of a complete blood cell count with differential and stool samples for ova and parasites; and a skin biopsy if the diagnosis is unclear.7 Identification and treatment of underlying causes often results in resolution.6 Systemic corticosteroids frequently are used in both adult and pediatric patients, though practitioners should consider alternative treatments when recurrences occur to avoid steroid side effects.3,6 Midpotency topical corticosteroids present a safe alternative to systemic corticosteroids in the pediatric population, especially in cases of localized WS without systemic symptoms.3 Other medications reported in the literature include cyclosporine, dapsone, antimalarial medications, and azathioprine.6 Despite appropriate therapy, patients and physicians should anticipate recurrence over months to years.1,6
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Smith SM, Kiracofe EA, Clark LN, et al. Idiopathic hypereosinophilic syndrome with cutaneous manifestations and flame figures: a spectrum of eosinophilic dermatoses whose features overlap with Wells' syndrome. Am J Dermatopathol. 2015;37:910-914.
- Gilliam AE, Bruckner AL, Howard RM, et al. Bullous "cellulitis" with eosinophilia: case report and review of Wells' syndrome in childhood. Pediatrics. 2005;116:E149-E155.
- Nacaroglu HT, Celegen M, Karkıner CS, et al. Eosinophilic cellulitis (Wells' syndrome) caused by a temporary henna tattoo. Postepy Dermatol Alergol. 2014;31:322-324.
- Heelan K, Ryan JF, Shear NH, et al. Wells syndrome (eosinophilic cellulitis): proposed diagnostic criteria and a literature review of the drug-induced variant. J Dermatol Case Rep. 2013;7:113-120.
- Sinno H, Lacroix JP, Lee J, et al. Diagnosis and management of eosinophilic cellulitis (Wells' syndrome): a case series and literature review. Can J Plast Surg. 2012;20:91-97.
- Cherng E, McClung AA, Rosenthal HM, et al. Wells' syndrome associated with parvovirus in a 5-year-old boy. Pediatr Dermatol. 2012;29:762-764.
- Eren M, Açikalin M. A case report of Wells' syndrome in a celiac patient. Turk J Gastroenterol. 2010;21:172-174.
- Cruz MJ, Mota A, Baudrier T, et al. Recurrent Wells' syndrome associated with allergic asthma exacerbation. Cutan Ocul Toxicol. 2012;31:154-156.
- Van der Straaten S, Wojciechowski M, Salgado R, et al. Eosinophilic cellulitis or Wells' syndrome in a 6-year-old child. Eur J Pediatr. 2006;165:197-198.
The Diagnosis: Wells Syndrome
A punch biopsy taken from the perimeter of the lesion demonstrated mild spongiosis overlying a dense nodular to diffuse infiltrate of lymphocytes, neutrophils, and numerous eosinophils, some involving underlying fat lobules (Figure, A and B). In some areas, eosinophilic degeneration of collagen bundles surrounded by a rim of histiocytes, "flame features," were observed (Figure C). The clinical and histological features were consistent with Wells syndrome (WS), also known as eosinophilic cellulitis. Given the localized mild nature of the disease, the patient was started on a midpotency topical corticosteroid.

Wells syndrome is a rare inflammatory condition characterized by clinical polymorphism, suggestive histologic findings, and a recurrent course.1,2 This condition is especially rare in children.3,4 Caputo et al1 described 7 variants in their case series of 19 patients: classic plaque-type variant (the most common clinical presentation in children); annular granuloma-like (the most common clinical presentation in adults); urticarialike; bullous; papulonodular; papulovesicular; and fixed drug eruption-like. Wells syndrome is thought to result from excess production of IL-5 in response to a hypersensitivity reaction to an exogenous or endogenous circulating antigen.3,4 Increased levels of IL-5 enhance eosinophil accumulation in the skin, degranulation, and subsequent tissue destruction.3,4 Reported triggers include insect bites, viral and bacterial infections, drug eruptions, recent vaccination, and paraphenylenediamine in henna tattoos.3-7 Additionally, WS has been reported in the setting of gastrointestinal pathologies, such as celiac disease and ulcerative colitis, and with asthma exacerbations.8,9 However, in half of pediatric cases, no trigger can be identified.7
Clinically, WS presents with pruritic, mildly tender plaques.7 Lesions may be localized or diffuse and range from mild annular or circinate plaques with infiltrated borders to cellulitic-appearing lesions that are occasionally associated with bullae.5,6 Patients often report prodromal symptoms of burning and pruritus.5,6 Lesions rapidly progress over 2 to 3 days, pass through a blue grayish discoloration phase, and gradually resolve over 2 to 8 weeks.5,6,10 Although patients generally heal without scarring, WS lesions have been described to resolve with atrophy and hyperpigmentation resembling morphea.5-7 Additionally, patients typically experience a relapsing remitting course over months to years with eventual spontaneous resolution.1,5 Patients also may experience systemic symptoms including fever, lymphadenopathy, and arthralgia, though they do not develop more widespread systemic manifestations.2,3,7
Diagnosis of WS is based on clinicopathologic correlation. Histopathology of WS lesions demonstrates 3 phases. The acute phase demonstrates edema of the superficial and mid dermis with a dense dermal eosinophilic infiltrate.1,6,10 The subacute granulomatous phase demonstrates flame figures in the dermis.1,2,6,7,10 Flame figures consist of palisading groups of eosinophils and histiocytes around a core of degenerating basophilic collagen bundles associated with major basic protein.1,2,6,7,10 Finally, in the resolution phase, eosinophils gradually disappear while histiocytes and giant cells persist, forming microgranulomas.1,2,10 Notably, no vasculitis is observed and direct immunofluorescence is negative.3,7 Although flame figures are suggestive of WS, they are not pathognomonic and are observed in other conditions including Churg-Strauss syndrome, parasitic and fungal infections, herpes gestationis, bullous pemphigoid, and follicular mucinosis.2,5
Wells syndrome is a self-resolving and benign condition.1,10 Physicians are recommended to gather a complete history including review of medications and vaccinations; a history of insect bites, infections, and asthma; laboratory workup consisting of a complete blood cell count with differential and stool samples for ova and parasites; and a skin biopsy if the diagnosis is unclear.7 Identification and treatment of underlying causes often results in resolution.6 Systemic corticosteroids frequently are used in both adult and pediatric patients, though practitioners should consider alternative treatments when recurrences occur to avoid steroid side effects.3,6 Midpotency topical corticosteroids present a safe alternative to systemic corticosteroids in the pediatric population, especially in cases of localized WS without systemic symptoms.3 Other medications reported in the literature include cyclosporine, dapsone, antimalarial medications, and azathioprine.6 Despite appropriate therapy, patients and physicians should anticipate recurrence over months to years.1,6
The Diagnosis: Wells Syndrome
A punch biopsy taken from the perimeter of the lesion demonstrated mild spongiosis overlying a dense nodular to diffuse infiltrate of lymphocytes, neutrophils, and numerous eosinophils, some involving underlying fat lobules (Figure, A and B). In some areas, eosinophilic degeneration of collagen bundles surrounded by a rim of histiocytes, "flame features," were observed (Figure C). The clinical and histological features were consistent with Wells syndrome (WS), also known as eosinophilic cellulitis. Given the localized mild nature of the disease, the patient was started on a midpotency topical corticosteroid.

Wells syndrome is a rare inflammatory condition characterized by clinical polymorphism, suggestive histologic findings, and a recurrent course.1,2 This condition is especially rare in children.3,4 Caputo et al1 described 7 variants in their case series of 19 patients: classic plaque-type variant (the most common clinical presentation in children); annular granuloma-like (the most common clinical presentation in adults); urticarialike; bullous; papulonodular; papulovesicular; and fixed drug eruption-like. Wells syndrome is thought to result from excess production of IL-5 in response to a hypersensitivity reaction to an exogenous or endogenous circulating antigen.3,4 Increased levels of IL-5 enhance eosinophil accumulation in the skin, degranulation, and subsequent tissue destruction.3,4 Reported triggers include insect bites, viral and bacterial infections, drug eruptions, recent vaccination, and paraphenylenediamine in henna tattoos.3-7 Additionally, WS has been reported in the setting of gastrointestinal pathologies, such as celiac disease and ulcerative colitis, and with asthma exacerbations.8,9 However, in half of pediatric cases, no trigger can be identified.7
Clinically, WS presents with pruritic, mildly tender plaques.7 Lesions may be localized or diffuse and range from mild annular or circinate plaques with infiltrated borders to cellulitic-appearing lesions that are occasionally associated with bullae.5,6 Patients often report prodromal symptoms of burning and pruritus.5,6 Lesions rapidly progress over 2 to 3 days, pass through a blue grayish discoloration phase, and gradually resolve over 2 to 8 weeks.5,6,10 Although patients generally heal without scarring, WS lesions have been described to resolve with atrophy and hyperpigmentation resembling morphea.5-7 Additionally, patients typically experience a relapsing remitting course over months to years with eventual spontaneous resolution.1,5 Patients also may experience systemic symptoms including fever, lymphadenopathy, and arthralgia, though they do not develop more widespread systemic manifestations.2,3,7
Diagnosis of WS is based on clinicopathologic correlation. Histopathology of WS lesions demonstrates 3 phases. The acute phase demonstrates edema of the superficial and mid dermis with a dense dermal eosinophilic infiltrate.1,6,10 The subacute granulomatous phase demonstrates flame figures in the dermis.1,2,6,7,10 Flame figures consist of palisading groups of eosinophils and histiocytes around a core of degenerating basophilic collagen bundles associated with major basic protein.1,2,6,7,10 Finally, in the resolution phase, eosinophils gradually disappear while histiocytes and giant cells persist, forming microgranulomas.1,2,10 Notably, no vasculitis is observed and direct immunofluorescence is negative.3,7 Although flame figures are suggestive of WS, they are not pathognomonic and are observed in other conditions including Churg-Strauss syndrome, parasitic and fungal infections, herpes gestationis, bullous pemphigoid, and follicular mucinosis.2,5
Wells syndrome is a self-resolving and benign condition.1,10 Physicians are recommended to gather a complete history including review of medications and vaccinations; a history of insect bites, infections, and asthma; laboratory workup consisting of a complete blood cell count with differential and stool samples for ova and parasites; and a skin biopsy if the diagnosis is unclear.7 Identification and treatment of underlying causes often results in resolution.6 Systemic corticosteroids frequently are used in both adult and pediatric patients, though practitioners should consider alternative treatments when recurrences occur to avoid steroid side effects.3,6 Midpotency topical corticosteroids present a safe alternative to systemic corticosteroids in the pediatric population, especially in cases of localized WS without systemic symptoms.3 Other medications reported in the literature include cyclosporine, dapsone, antimalarial medications, and azathioprine.6 Despite appropriate therapy, patients and physicians should anticipate recurrence over months to years.1,6
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Smith SM, Kiracofe EA, Clark LN, et al. Idiopathic hypereosinophilic syndrome with cutaneous manifestations and flame figures: a spectrum of eosinophilic dermatoses whose features overlap with Wells' syndrome. Am J Dermatopathol. 2015;37:910-914.
- Gilliam AE, Bruckner AL, Howard RM, et al. Bullous "cellulitis" with eosinophilia: case report and review of Wells' syndrome in childhood. Pediatrics. 2005;116:E149-E155.
- Nacaroglu HT, Celegen M, Karkıner CS, et al. Eosinophilic cellulitis (Wells' syndrome) caused by a temporary henna tattoo. Postepy Dermatol Alergol. 2014;31:322-324.
- Heelan K, Ryan JF, Shear NH, et al. Wells syndrome (eosinophilic cellulitis): proposed diagnostic criteria and a literature review of the drug-induced variant. J Dermatol Case Rep. 2013;7:113-120.
- Sinno H, Lacroix JP, Lee J, et al. Diagnosis and management of eosinophilic cellulitis (Wells' syndrome): a case series and literature review. Can J Plast Surg. 2012;20:91-97.
- Cherng E, McClung AA, Rosenthal HM, et al. Wells' syndrome associated with parvovirus in a 5-year-old boy. Pediatr Dermatol. 2012;29:762-764.
- Eren M, Açikalin M. A case report of Wells' syndrome in a celiac patient. Turk J Gastroenterol. 2010;21:172-174.
- Cruz MJ, Mota A, Baudrier T, et al. Recurrent Wells' syndrome associated with allergic asthma exacerbation. Cutan Ocul Toxicol. 2012;31:154-156.
- Van der Straaten S, Wojciechowski M, Salgado R, et al. Eosinophilic cellulitis or Wells' syndrome in a 6-year-old child. Eur J Pediatr. 2006;165:197-198.
- Caputo R, Marzano AV, Vezzoli P, et al. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161.
- Smith SM, Kiracofe EA, Clark LN, et al. Idiopathic hypereosinophilic syndrome with cutaneous manifestations and flame figures: a spectrum of eosinophilic dermatoses whose features overlap with Wells' syndrome. Am J Dermatopathol. 2015;37:910-914.
- Gilliam AE, Bruckner AL, Howard RM, et al. Bullous "cellulitis" with eosinophilia: case report and review of Wells' syndrome in childhood. Pediatrics. 2005;116:E149-E155.
- Nacaroglu HT, Celegen M, Karkıner CS, et al. Eosinophilic cellulitis (Wells' syndrome) caused by a temporary henna tattoo. Postepy Dermatol Alergol. 2014;31:322-324.
- Heelan K, Ryan JF, Shear NH, et al. Wells syndrome (eosinophilic cellulitis): proposed diagnostic criteria and a literature review of the drug-induced variant. J Dermatol Case Rep. 2013;7:113-120.
- Sinno H, Lacroix JP, Lee J, et al. Diagnosis and management of eosinophilic cellulitis (Wells' syndrome): a case series and literature review. Can J Plast Surg. 2012;20:91-97.
- Cherng E, McClung AA, Rosenthal HM, et al. Wells' syndrome associated with parvovirus in a 5-year-old boy. Pediatr Dermatol. 2012;29:762-764.
- Eren M, Açikalin M. A case report of Wells' syndrome in a celiac patient. Turk J Gastroenterol. 2010;21:172-174.
- Cruz MJ, Mota A, Baudrier T, et al. Recurrent Wells' syndrome associated with allergic asthma exacerbation. Cutan Ocul Toxicol. 2012;31:154-156.
- Van der Straaten S, Wojciechowski M, Salgado R, et al. Eosinophilic cellulitis or Wells' syndrome in a 6-year-old child. Eur J Pediatr. 2006;165:197-198.
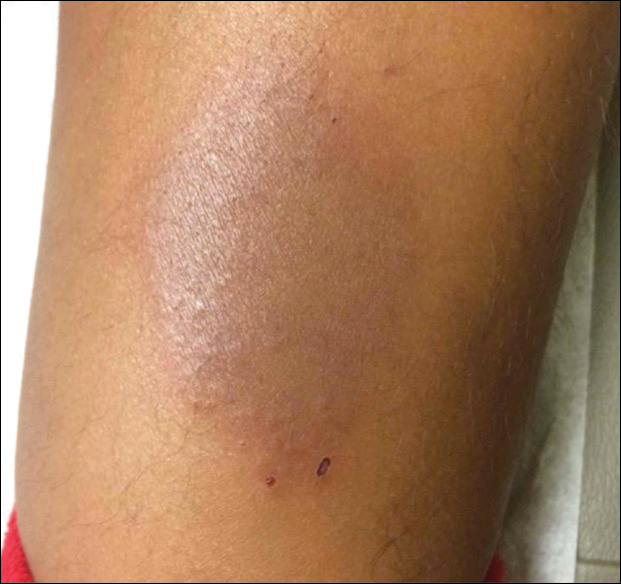
A healthy 7-year-old boy presented with an enlarging hyperpigmented plaque on the anterior aspect of the lower left leg of 2 months' duration. His mother reported onset following a mosquito bite. Clotrimazole was used without improvement. His mother denied recent travel, similar lesions in close contacts, fever, asthma, and arthralgia. Physical examination revealed a 5.2 ×3-cm nonscaly, red-brown, ovoid, thin plaque with a slightly raised border.