User login
Vacuum extraction: Tips for achieving an optimal outcome
CASE: Is vacuum extraction right for this delivery?
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.

Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
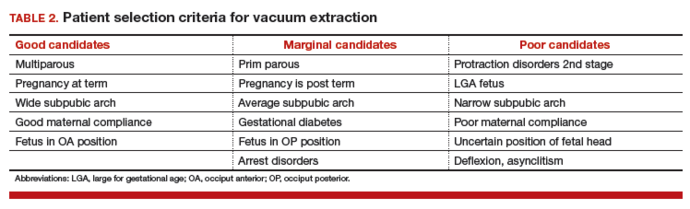
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).
Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
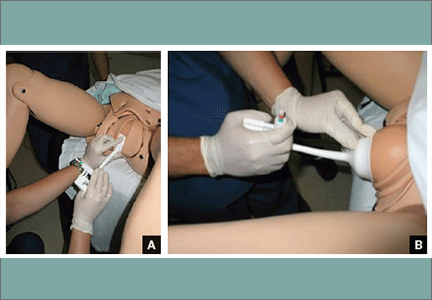
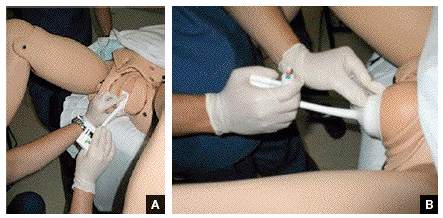
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).
Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).
In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
CASE: Is vacuum extraction right for this delivery?
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.

Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).

Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).

Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).

In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Is vacuum extraction right for this delivery?
A 41-year-old woman (G2P2002) is at term in her third pregnancy, and the fetus exhibits prolonged deceleration that does not resolve while the mother pushes from a +3 station. The fetus, estimated to weigh 8 lb, is in the occiput anterior (OA) position. The mother is willing to consider vaginal extraction, and you must weigh the factors that may influence successful delivery.
Vacuum extraction (VE) is an effective method to facilitate delivery. From 2007 to 2013, VE was used to facilitate about 3% of vaginal deliveries in the United States.1 By contrast, cesarean delivery rates over the same period averaged about 30%.2
Controversy exists on the pros and cons of operative vaginal deliveries versus cesarean delivery, as well as on the instruments and operational approaches used. While opinion tends to be resolute and influential, evidence remains inconclusive.
Multiple factors influence a decision on whether to choose VE. The clinician’s own bias regarding delivery routes and comfort level with performing VE are important. The patient, too, may have preconceived opinions about VE. Knowing the indications for VE and its benefits and risks (TABLE 1) can help the patient make an informed choice and the counseling on which will be needed in obtaining the patient’s informed consent. The expectations and desires of the patient in concert with the experience and skill of the clinician will serve to achieve the optimal decision.

Indications for VE
Maternal indications for the use of VE include prolongation or arrest of the second stage of labor. Another indication is the need to shorten the second stage due to a maternal cardiac or cardiovascular disorder or due to maternal exhaustion.
Fetal indications include nonreassuring fetal status or a need to correct for minor degrees of malposition (asynclitism, deflexion) that historically have been addressed with the use of obstetric forceps. VE delivery in these circumstances requires a very experienced and skilled operator.
Further selection criteria
Birthweight influences the consideration of VE. Low birthweight or prematurity are contraindications to the use of VE due to concerns about fetal/neonatal bleeding. Large fetuses will have issues with cephalopelvic disproportion, thus increasing the risk for 2 disorders: shoulder dystocia and fetal cranial bleeding.
Cranial bleeding, both intracranial and extracranial, can result in serious neonatal morbidity and mortality. Bleeding may occur spontaneously or with the use of VE. In using VE, force is transmitted to the fetal scalp. The scalp then has the tendency to pull on its contents and attachments—skull bones, brain, fluids, etc. The scalp attachments include vessels at right angles to the scalp, which may be traumatized or torn by the pulling force. This may lead to subgaleal hemorrhage, a collection of blood in the large potential space below the scalp and above the aponeurosis. Enough force may be generated to deform the intracranial contents and cause intracranial bleeding.
The likelihood of success with VE varies depending on maternal anatomy, the position of the fetal head, gestational age, and the presence or absence of gestational diabetes (TABLE 2).

Delivery by VE: Main considerations
After determining that a candidate is suitable for VE and obtaining informed consent, consider key operative factors:
- choice of extraction cup
- adequate anesthesia
- careful maternal positioning
- maternal bladder emptying
- review of fetal status.
Two major cup types are available: rigid and flexible.
Rigid plastic cup. This design is similar to the metal cup used by Malmström and attaches to the scalp via chignon formation. A variation of the rigid cup is the mityvac “M” that mimics the Malmström design but incorporates a semiflexible handle to facilitate proper cup placement and aid in the direction of pulling force.
Flexible cup. This type of cup flattens against the scalp with vacuum and may result in less minor scalp trauma than the rigid cup.
Greater force can be employed with rigid cup designs than with flexible cups, which can increase the chances of a successful delivery when the fetus is in the occiput posterior (OP) position. Flexible designs tend to cause less damage to the scalp than the rigid cup but are reported to have a higher failure rate.
Cardinal rule of any procedure. Prior to cup placement, remember this rule: abandon the procedure if it proves too difficult. Most deliveries will occur with 3 or 4 pulls.3 Difficulties include:
- failure to gain station with the initial pull
- repetitive cup pop-offs (3 or more)
- an excessive duration of the procedure (>10 minutes).
Less than optimal placement of the vacuum extractor will increase the risk of scalp trauma, particularly in nulliparous women.3
If the procedure is unsuccessful, the resulting options include cesarean delivery and expectant management.
Tip. Use both hands during the pull to more reliably detect a problem with cup attachment, thereby minimizing the possibility of detachment and subsequent scalp trauma (FIGURE).

Key points of technique
Perform a careful and thorough pelvic examination to determine fetal station, position, attitude, and synclitism.
The optimal cup placement is 2- to 3-cm proximal to the posterior fontanel or, alternatively, 5- to 6-cm distal to the anterior fontanel, assuming the fetal head is properly flexed.4 The correct point of flexion will result in the smallest diameter of the fetal head presenting to the birth canal and should minimize the force necessary to achieve delivery.
Use minimal vacuum to attach the cup to the fetal head. As the subsequent contraction develops, apply full vacuum with the hand device. Encourage maternal expulsive effort and use traction only in concert with pushing efforts. Three pushes facilitated with pulling may be achieved during a single contraction. Failure to bring about descent with the initial pull indicates potential failure with this approach and, in the absence of technical reasons for the failure, merits serious consideration of abandoning the procedure (TABLE 3).

In the event of failed delivery with VE, it is important to recognize that you should not make a second attempt with another instrument; the chance of success is low and the risk of injury is significantly increased.5
Carefully document the decision for VE and its implementation
The medical record is the most important witness to the event. Clearly record the following items, preferably as close in time to the decision/event as possible:
- the indication for the procedure
- the antecedent labor course
- maternal anesthesia
- personnel present
- instruments employed
- position and station of the fetal head
- force and duration of traction
- nature of the attempt
- immediate condition of the neonate, and any resuscitative efforts.
Closing reminders and advice
In preparing to discuss the patient’s preferences for delivery, understand clearly the risks and benefits of VE and develop a comfortable approach to sharing this information with your patient and her family. Also, if VE is selected, consider performing the procedure in the cesarean delivery room. This will serve to remind you to be mindful to abandon the procedure, if need be, at an appropriate point.
CASE: Resolved
You apply the vacuum extractor, and a small amount of vacuum demonstrates satisfactory attachment. On the second pull, the fetus easily delivers, and the Apgar scores are 8 and 8. The birthweight is 3,725 g. The vacuum-assisted delivery has resulted in the shortest delay in delivery and without adverse consequences for neonate or mother.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64(1):1–65.
- Committee on Practice Bulletins—Obstetrics; American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 154 Summary: operative vaginal delivery. Obstet Gynecol. 2015;126(5):1118–1119.
- Baskett TF, Fanning CA, Young DC. A prospective observational study of 1000 vacuum assisted deliveries with the OmniCup device. J Obstet Gynaecol Can. 2008;30(7):573–580.
- O’Grady JP. Instrumental delivery. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello LA, Giordano K, eds. Operative Obstetrics. 2nd ed. New York, New York: Cambridge University Press; 2008:475.
- Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341(23):1709–1714.
In this Article
- Patient selection criteria
- Key technique points
- Important documentation
3 clear dos, and 3 specific don'ts, of vacuum extraction
EHRs and medicolegal risk: How they help, when they could hurt
Survey: Many physicians plan to leave or scale down practice
Janelle Yates (February 2012)
Is private ObGyn practice on its way out?
Lucia DiVenere, MA (October 2011)
The medical record has evolved considerably since it originated in ancient Greece as a narrative of cure.1 For one thing, it’s now electronic. For another, it’s no longer a medical record but a health record. According to the US Department of Health and Human Services, the distinction is not a trivial one. A medical record is used by clinicians mostly for diagnosis and treatment, whereas the health record focuses on the total wellbeing of the patient.2 The medical record is used primarily within a practice. The electronic health record (EHR) reaches across borders to other offices, institutions, and clinicians.
Use of the EHR has been stimulated by the Health Information Technology for Economic and Clinical Health Act,3 which offers grants and incentives for “meaningful use” of electronic records.4 After 2014, medical practices that do not use EHRs will face a financial penalty that amounts to 2% of 2013 clinical revenue.
EHRs have been hailed as a panacea and derided as anathema. Whatever your perspective, there is no denying that they dramatically increase the immediate and easy availability of information and, therefore, influence decision-making in regard to medical care, cost-effectiveness, and patient safety. EHRs have the potential to improve communication, broaden access to information, and help guide clinical decision-making through the use of best-practice algorithms. When used properly—which means taking advantage of the EHR’s full potential and adapting to the way information is organized and analyzed—the EHR can reduce adverse events and help defend the appropriateness of the care provided. This lowers your medicolegal risk. When used improperly or haphazardly, they may increase that risk. In this article, we elaborate on both.
EHRs have many benefits
Improved communication. EHRs facilitate communication between healthcare providers. A primary care physician can access a consultant’s report practically as it is written. Providers also can carry on a dialogue electronically, planning together for care that will best serve the patient, with less redundancy and time.
The EHR also facilitates communication between physician and patient, allowing the physician to see the patient’s recent history and plan her management while speaking to her on the phone. Issues can be addressed with greater accuracy and expediency, leading to reduced anxiety for the patient and increased compliance.
Seamless integration. Information can be entered into the EHR and integrated into the full record more seamlessly than it is with written records. And data can be entered once and used many times.
Enhanced decision-making. Decision-making depends on careful analysis of a clinical scenario. Protocols, templates, and order sets embedded in the EHR can reduce medical errors by identifying scenarios for the physician to review.5,6
The EHR can also highlight adverse drug-drug interactions and help avoid potential allergic reactions. Murphy and colleagues reported a reduction of medical errors by utilizing a pharmacy-driven EHR component—a reduction from 90% to 47% on the surgical unit and from 57% to 33% on the medicine unit.7
Improved documentation. The EHR can enhance documentation by offering specific and detailed templates for informed consent, making it more comprehensive than a handwritten notation of the risks and benefits.
Decipherability is another strength of the EHR. Because physicians are notorious for poor handwriting skills, some hospitals now require a writing sample as part of their privileging process. The EHR avoids this issue entirely.8 Typos and grammatical errors are minimized by spellchecking and grammar-correcting programs written into the EHR.
Quality assurance. Timely evaluation of approaches to clinical care is available to physicians as well as hospitals that use EHRs.9 An individual physician can perform personal quality-assurance audits. And hospital management can gather cumulative statistics more quickly and easily.5,6,10,11
Patient data can be accessed independent of medical department, with lab tests, imaging studies, and pathology reports readily available for review. And accessibility is available regardless of geographic location.
EHRs are not perfect, and neither are their users. EHRs present the potential for problems related to absent or erroneous data entry, patient privacy issues, misunderstanding and misuse of software, and development of metadata.
With initial use, EHRs can create documentation gaps with the transition from paper to electronic records. In addition, inadequate provider training can create new error pathways, and a failure to use EHRs consistently can lead to loss of data and communication errors. These gaps and errors can increase medicolegal risk, as can the more extensive documentation often seen with early use, which creates more discoverable data. The temptation to cut and paste risks repeating earlier errors and omitting new information.
Another area of risk involves communication with the patient via email. A failure to reply could result in claims of negligence, and information overload could obscure pertinent pieces of information. And a departure from clinical decision support could be used by the patient to defend allegations of negligence.
With widespread use of EHRs, improved access to data could change the “duty” owed to the patient. In addition, clinical decision support embedded within the software could become the de facto “standard of care.”
The learning curve can be steep
The learning curve for EHRs may be steep and, at times, discouraging. One reason is that data are organized differently than in the conventional paper record, where information is read and analyzed in a progressive and stepwise manner, as in an analog or vertical system. The EHR is a digital format, so finding information requires digital (horizontal) inquiry. Information is, therefore, utilized in both horizontal and vertical formats in everyday situations. If data are entered incorrectly, all subsequent decisions could be flawed. And if the EHR suggests a plan, and that plan is not performed by the provider, the risk of liability could increase.
Inadvertent violation of the Health Insurance Portability and Accountability Act (HIPAA) with an EHR could increase medicolegal risk. For example, HIPAA allows for patients to make corrections to inaccurate information in their personal documents, but access by the patient could require the physician to review all records viewed by the patient after visit notes have been entered. This could drive up the cost of practice and reduce face-to-face time between physician and patient. Patients are not necessarily the best judges of which information is most important in their medical records.
Internet access raises concerns about the privacy of sensitive issues and misuse of information. Making a patient’s protected health information accessible electronically leaves physicians and hospitals at risk for a government fine or lawsuit. In several instances, the US Department of Health and Human Services (HHS) has levied fines against small practices and government agencies.
In one case, HHS fined Phoenix Cardiac Surgery in Phoenix, Arizona, $100,000 for posting surgery and appointment schedules on an Internet-based calendar that was accessible to the public.12 In another, HHS fined the Massachusetts Eye and Ear Infirmary in Boston $1.5 million after it reported the loss of an encrypted personal laptop containing the protected health information of patients and research subjects.13 The Alaska Department of Health and Social Services (DHSS) agreed to pay HHS $1.7 million after it reported the loss of a USB drive—possibly containing protected health information—from the vehicle of a DHSS employee.14
In traditional physician practices that employ handwritten records, the potential for compromise of patient information is limited. An organization may lose a few patient charts in the office and recover from the loss without incident. With the EHR, the loss poses a significant threat. The cases mentioned above were attributed to negligence or ignorance. The consequences could be worse if the compromise of EHR data is determined to be intentional. On September 4, 2010, hackers may have exposed the personal information of approximately 9,493 patients at Southwest Seattle Orthopaedics and Sports Medicine in Burien, Washington. Even with the best encryption technology, any electronic system remains vulnerable to external attack.
Metadata reveal how original data are used
Another concern regarding EHRs involves metadata—”data about data content.”15 Metadata is structured information that describes, locates, explains, or manages information. Metadata relevant to the EHR includes the data and time it was reviewed by the provider and whether it was manipulated in any way. Clearly, there is a potential for use and misuse by third-party reviewers.
Specialty-specific EHRs are recommended
Many ObGyns have found that most EHR systems are inadequate to the task of recording and analyzing information relevant to their specialty. Obstetric care is episodic and frequent. Data are added into the flow that must be considered at each visit, such as gestational age, fetal growth, labs (and normative values), prenatal diagnostic studies, and so on, representing both vertical and horizontal processing.16
The legal discovery process poses challenges that have not yet been resolved
The legal discovery process grants all parties to a lawsuit equal access to information. Under ideal circumstances, the EHR can provide comprehensive data more quickly than traditional records can. The problem is determining what constitutes relevant data and which party has the burden or benefit of making that decision. Uncontrolled access has the potential to violate privacy and privilege requirements.
Rules regarding discovery are still being debated in regard to their applicability to digital discovery.17 Even before a lawsuit is filed, the potential for “data mining” by third parties could lead to allegations of malpractice.
How to use EHRs responsibly without increasing risk
Good communication between patient and provider is paramount in the provision of quality medical care. Adherence to evidence-based standards with thorough documentation always serves the best interests of both patients and providers. The EHR can facilitate this process.
Our recommendations for appropriate use of your EHR include:
- Spend time learning the ins and outs of your particular EHR, and make sure your staff does the same. This will help reduce the likelihood that errors will be introduced into the record and ensure consistent use.
- Use individual sign-ons for anyone involved in data entry. This step facilitates the identification of users responsible for inaccurate use or errors, so that the situation can be addressed efficiently.
- Do not let third parties enter or manipulate data. This could jeopardize patient privacy, as well as the integrity of the record itself.
- Track all data entry on a regular basis. The frequency of tracking should be a function of routine as well as clinical circumstance. All new data from the previous interval should be reviewed at the time of the subsequent visit in order to direct care and ensure proper data entry.
Because of the considerable risk of liability claims in ObGyn practice, it is critical that the medical record accurately and precisely reflects the circumstances of each case. The EHR can be an effective and useful tool to document what occurred (and when) in a clinical scenario.18 As with all medical records, completeness and accuracy are the first and best defense against allegations of medical malpractice.
1. The Casebooks Project. History of Medical Record-keeping. http://www.magicandmedicine.hps.cam.ac.uk/on-astrological-medicine/further-reading/history-of-medical-record-keeping/. Accessed February 26 2013.
2. US Department of Health and Human Services. EMR vs EHR—What is the difference? Health IT Buzz. http://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/emr-vs-ehr-difference/. Accessed February 20, 2013.
3. Health Information Technology for Economic and Clinical Health Act of 2009. HITECH Act. Pub L No 111-5 Div A tit XIII Div B tit IV Feb 17 2009, 123 stat 226, 467. Codified in scattered sections of 42 USCA.
4. Mangalmurti S, Murtagh L, Mello M. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363(21):2060-2067.
5. Reid P, Compton D, Grossman J, et al. Building a Better Delivery System: A New Engineering/Healthcare Partnership. Committee on Engineering and the Health Care System, Institute of Medicine and the National Academy of Engineering. Washington, DC: National Academies Press; 2005.
6. Grossman J. Disruptive innovation in healthcare: challenges for engineering. The Bridge. 2008;38:10-16.
7. Murphy E, Oxencis C, Klauck J, et al. Medication reconciliation at an academic medical center; implementation of a comprehensive program from admission to discharge. Am J Health-System Pharmacy. 2009;66(23):2126-2131.
8. Schuler R. The smart grid: a bridge between emerging technologies society and the environment. The Bridge. 2010;40:42-49.
9. Haberman S, Feldman J, Merhi Z, et al. Effect of clinical decision support on documentation compliance in an electronic medical record. Obstet Gynecol. 2009;114(2 Pt 1):311-317.
10. Hasley S. Decision support and patient safety: the time has come. Am J Obstet Gynecol. 2011;204(6):461-465.
11. Lagrew D, Stutman H, Sicaeros L. Voluntary physician adoption of an inpatient electronic medical record by obstetrician-gynecologists. Am J Obstet Gynecol. 2008;198(6):690.e1-e6.
12. Dolan PL. $100,000 HIPAA fine designed to send message to small physician practices. American Medical News. 2012. http://www.ama-assn.org/amednews/2012/04/30/bisd0502.htm. Accessed February 26, 2013.
13. US Department of Health and Human Services. Massachusetts provider settles HIPAA case for $1.5 million [news release]. September 17 2012. http://www.hhs.gov/news/press/2012pres/09/20120917a.html. Accessed February 26, 2013.
14. US Department of Health and Human Services. Alaska settles HIPAA security case for $1,700,000 [news release]. June 26, 2012. http://www.hhs.gov/news/press/2012pres/06/20120626a.html. Accessed February 26, 2013.
15. National Information Standards Organization. Understanding Metadata. Bethesda MD: NISO Press; 2004. http://www.niso.org/publications/press/UnderstandingMetadata.pdf. Accessed February 26, 2013.
16. McCoy M, Diamond A, Strunk A. Special requirements of electronic medical record systems in obstetrics and gynecology. Obstet Gynecol. 2010;116(1):140-143.
17. The Berkman Center for Internet and Society at Harvard Law School. The Federal Rules of Civil Procedure: The Impact of Digital Discovery. http://cyber.law.harvard.edu/digitaldiscovery/digdisc_library_4.html. Accessed February 26 2013.
18. Quinn M, Kats A, Kleinman K, et al. The relationship between electronic health records and malpractice claims. Arch Intern Med. 2012;172(15):1187-1188.
Survey: Many physicians plan to leave or scale down practice
Janelle Yates (February 2012)
Is private ObGyn practice on its way out?
Lucia DiVenere, MA (October 2011)
The medical record has evolved considerably since it originated in ancient Greece as a narrative of cure.1 For one thing, it’s now electronic. For another, it’s no longer a medical record but a health record. According to the US Department of Health and Human Services, the distinction is not a trivial one. A medical record is used by clinicians mostly for diagnosis and treatment, whereas the health record focuses on the total wellbeing of the patient.2 The medical record is used primarily within a practice. The electronic health record (EHR) reaches across borders to other offices, institutions, and clinicians.
Use of the EHR has been stimulated by the Health Information Technology for Economic and Clinical Health Act,3 which offers grants and incentives for “meaningful use” of electronic records.4 After 2014, medical practices that do not use EHRs will face a financial penalty that amounts to 2% of 2013 clinical revenue.
EHRs have been hailed as a panacea and derided as anathema. Whatever your perspective, there is no denying that they dramatically increase the immediate and easy availability of information and, therefore, influence decision-making in regard to medical care, cost-effectiveness, and patient safety. EHRs have the potential to improve communication, broaden access to information, and help guide clinical decision-making through the use of best-practice algorithms. When used properly—which means taking advantage of the EHR’s full potential and adapting to the way information is organized and analyzed—the EHR can reduce adverse events and help defend the appropriateness of the care provided. This lowers your medicolegal risk. When used improperly or haphazardly, they may increase that risk. In this article, we elaborate on both.
EHRs have many benefits
Improved communication. EHRs facilitate communication between healthcare providers. A primary care physician can access a consultant’s report practically as it is written. Providers also can carry on a dialogue electronically, planning together for care that will best serve the patient, with less redundancy and time.
The EHR also facilitates communication between physician and patient, allowing the physician to see the patient’s recent history and plan her management while speaking to her on the phone. Issues can be addressed with greater accuracy and expediency, leading to reduced anxiety for the patient and increased compliance.
Seamless integration. Information can be entered into the EHR and integrated into the full record more seamlessly than it is with written records. And data can be entered once and used many times.
Enhanced decision-making. Decision-making depends on careful analysis of a clinical scenario. Protocols, templates, and order sets embedded in the EHR can reduce medical errors by identifying scenarios for the physician to review.5,6
The EHR can also highlight adverse drug-drug interactions and help avoid potential allergic reactions. Murphy and colleagues reported a reduction of medical errors by utilizing a pharmacy-driven EHR component—a reduction from 90% to 47% on the surgical unit and from 57% to 33% on the medicine unit.7
Improved documentation. The EHR can enhance documentation by offering specific and detailed templates for informed consent, making it more comprehensive than a handwritten notation of the risks and benefits.
Decipherability is another strength of the EHR. Because physicians are notorious for poor handwriting skills, some hospitals now require a writing sample as part of their privileging process. The EHR avoids this issue entirely.8 Typos and grammatical errors are minimized by spellchecking and grammar-correcting programs written into the EHR.
Quality assurance. Timely evaluation of approaches to clinical care is available to physicians as well as hospitals that use EHRs.9 An individual physician can perform personal quality-assurance audits. And hospital management can gather cumulative statistics more quickly and easily.5,6,10,11
Patient data can be accessed independent of medical department, with lab tests, imaging studies, and pathology reports readily available for review. And accessibility is available regardless of geographic location.
EHRs are not perfect, and neither are their users. EHRs present the potential for problems related to absent or erroneous data entry, patient privacy issues, misunderstanding and misuse of software, and development of metadata.
With initial use, EHRs can create documentation gaps with the transition from paper to electronic records. In addition, inadequate provider training can create new error pathways, and a failure to use EHRs consistently can lead to loss of data and communication errors. These gaps and errors can increase medicolegal risk, as can the more extensive documentation often seen with early use, which creates more discoverable data. The temptation to cut and paste risks repeating earlier errors and omitting new information.
Another area of risk involves communication with the patient via email. A failure to reply could result in claims of negligence, and information overload could obscure pertinent pieces of information. And a departure from clinical decision support could be used by the patient to defend allegations of negligence.
With widespread use of EHRs, improved access to data could change the “duty” owed to the patient. In addition, clinical decision support embedded within the software could become the de facto “standard of care.”
The learning curve can be steep
The learning curve for EHRs may be steep and, at times, discouraging. One reason is that data are organized differently than in the conventional paper record, where information is read and analyzed in a progressive and stepwise manner, as in an analog or vertical system. The EHR is a digital format, so finding information requires digital (horizontal) inquiry. Information is, therefore, utilized in both horizontal and vertical formats in everyday situations. If data are entered incorrectly, all subsequent decisions could be flawed. And if the EHR suggests a plan, and that plan is not performed by the provider, the risk of liability could increase.
Inadvertent violation of the Health Insurance Portability and Accountability Act (HIPAA) with an EHR could increase medicolegal risk. For example, HIPAA allows for patients to make corrections to inaccurate information in their personal documents, but access by the patient could require the physician to review all records viewed by the patient after visit notes have been entered. This could drive up the cost of practice and reduce face-to-face time between physician and patient. Patients are not necessarily the best judges of which information is most important in their medical records.
Internet access raises concerns about the privacy of sensitive issues and misuse of information. Making a patient’s protected health information accessible electronically leaves physicians and hospitals at risk for a government fine or lawsuit. In several instances, the US Department of Health and Human Services (HHS) has levied fines against small practices and government agencies.
In one case, HHS fined Phoenix Cardiac Surgery in Phoenix, Arizona, $100,000 for posting surgery and appointment schedules on an Internet-based calendar that was accessible to the public.12 In another, HHS fined the Massachusetts Eye and Ear Infirmary in Boston $1.5 million after it reported the loss of an encrypted personal laptop containing the protected health information of patients and research subjects.13 The Alaska Department of Health and Social Services (DHSS) agreed to pay HHS $1.7 million after it reported the loss of a USB drive—possibly containing protected health information—from the vehicle of a DHSS employee.14
In traditional physician practices that employ handwritten records, the potential for compromise of patient information is limited. An organization may lose a few patient charts in the office and recover from the loss without incident. With the EHR, the loss poses a significant threat. The cases mentioned above were attributed to negligence or ignorance. The consequences could be worse if the compromise of EHR data is determined to be intentional. On September 4, 2010, hackers may have exposed the personal information of approximately 9,493 patients at Southwest Seattle Orthopaedics and Sports Medicine in Burien, Washington. Even with the best encryption technology, any electronic system remains vulnerable to external attack.
Metadata reveal how original data are used
Another concern regarding EHRs involves metadata—”data about data content.”15 Metadata is structured information that describes, locates, explains, or manages information. Metadata relevant to the EHR includes the data and time it was reviewed by the provider and whether it was manipulated in any way. Clearly, there is a potential for use and misuse by third-party reviewers.
Specialty-specific EHRs are recommended
Many ObGyns have found that most EHR systems are inadequate to the task of recording and analyzing information relevant to their specialty. Obstetric care is episodic and frequent. Data are added into the flow that must be considered at each visit, such as gestational age, fetal growth, labs (and normative values), prenatal diagnostic studies, and so on, representing both vertical and horizontal processing.16
The legal discovery process poses challenges that have not yet been resolved
The legal discovery process grants all parties to a lawsuit equal access to information. Under ideal circumstances, the EHR can provide comprehensive data more quickly than traditional records can. The problem is determining what constitutes relevant data and which party has the burden or benefit of making that decision. Uncontrolled access has the potential to violate privacy and privilege requirements.
Rules regarding discovery are still being debated in regard to their applicability to digital discovery.17 Even before a lawsuit is filed, the potential for “data mining” by third parties could lead to allegations of malpractice.
How to use EHRs responsibly without increasing risk
Good communication between patient and provider is paramount in the provision of quality medical care. Adherence to evidence-based standards with thorough documentation always serves the best interests of both patients and providers. The EHR can facilitate this process.
Our recommendations for appropriate use of your EHR include:
- Spend time learning the ins and outs of your particular EHR, and make sure your staff does the same. This will help reduce the likelihood that errors will be introduced into the record and ensure consistent use.
- Use individual sign-ons for anyone involved in data entry. This step facilitates the identification of users responsible for inaccurate use or errors, so that the situation can be addressed efficiently.
- Do not let third parties enter or manipulate data. This could jeopardize patient privacy, as well as the integrity of the record itself.
- Track all data entry on a regular basis. The frequency of tracking should be a function of routine as well as clinical circumstance. All new data from the previous interval should be reviewed at the time of the subsequent visit in order to direct care and ensure proper data entry.
Because of the considerable risk of liability claims in ObGyn practice, it is critical that the medical record accurately and precisely reflects the circumstances of each case. The EHR can be an effective and useful tool to document what occurred (and when) in a clinical scenario.18 As with all medical records, completeness and accuracy are the first and best defense against allegations of medical malpractice.
Survey: Many physicians plan to leave or scale down practice
Janelle Yates (February 2012)
Is private ObGyn practice on its way out?
Lucia DiVenere, MA (October 2011)
The medical record has evolved considerably since it originated in ancient Greece as a narrative of cure.1 For one thing, it’s now electronic. For another, it’s no longer a medical record but a health record. According to the US Department of Health and Human Services, the distinction is not a trivial one. A medical record is used by clinicians mostly for diagnosis and treatment, whereas the health record focuses on the total wellbeing of the patient.2 The medical record is used primarily within a practice. The electronic health record (EHR) reaches across borders to other offices, institutions, and clinicians.
Use of the EHR has been stimulated by the Health Information Technology for Economic and Clinical Health Act,3 which offers grants and incentives for “meaningful use” of electronic records.4 After 2014, medical practices that do not use EHRs will face a financial penalty that amounts to 2% of 2013 clinical revenue.
EHRs have been hailed as a panacea and derided as anathema. Whatever your perspective, there is no denying that they dramatically increase the immediate and easy availability of information and, therefore, influence decision-making in regard to medical care, cost-effectiveness, and patient safety. EHRs have the potential to improve communication, broaden access to information, and help guide clinical decision-making through the use of best-practice algorithms. When used properly—which means taking advantage of the EHR’s full potential and adapting to the way information is organized and analyzed—the EHR can reduce adverse events and help defend the appropriateness of the care provided. This lowers your medicolegal risk. When used improperly or haphazardly, they may increase that risk. In this article, we elaborate on both.
EHRs have many benefits
Improved communication. EHRs facilitate communication between healthcare providers. A primary care physician can access a consultant’s report practically as it is written. Providers also can carry on a dialogue electronically, planning together for care that will best serve the patient, with less redundancy and time.
The EHR also facilitates communication between physician and patient, allowing the physician to see the patient’s recent history and plan her management while speaking to her on the phone. Issues can be addressed with greater accuracy and expediency, leading to reduced anxiety for the patient and increased compliance.
Seamless integration. Information can be entered into the EHR and integrated into the full record more seamlessly than it is with written records. And data can be entered once and used many times.
Enhanced decision-making. Decision-making depends on careful analysis of a clinical scenario. Protocols, templates, and order sets embedded in the EHR can reduce medical errors by identifying scenarios for the physician to review.5,6
The EHR can also highlight adverse drug-drug interactions and help avoid potential allergic reactions. Murphy and colleagues reported a reduction of medical errors by utilizing a pharmacy-driven EHR component—a reduction from 90% to 47% on the surgical unit and from 57% to 33% on the medicine unit.7
Improved documentation. The EHR can enhance documentation by offering specific and detailed templates for informed consent, making it more comprehensive than a handwritten notation of the risks and benefits.
Decipherability is another strength of the EHR. Because physicians are notorious for poor handwriting skills, some hospitals now require a writing sample as part of their privileging process. The EHR avoids this issue entirely.8 Typos and grammatical errors are minimized by spellchecking and grammar-correcting programs written into the EHR.
Quality assurance. Timely evaluation of approaches to clinical care is available to physicians as well as hospitals that use EHRs.9 An individual physician can perform personal quality-assurance audits. And hospital management can gather cumulative statistics more quickly and easily.5,6,10,11
Patient data can be accessed independent of medical department, with lab tests, imaging studies, and pathology reports readily available for review. And accessibility is available regardless of geographic location.
EHRs are not perfect, and neither are their users. EHRs present the potential for problems related to absent or erroneous data entry, patient privacy issues, misunderstanding and misuse of software, and development of metadata.
With initial use, EHRs can create documentation gaps with the transition from paper to electronic records. In addition, inadequate provider training can create new error pathways, and a failure to use EHRs consistently can lead to loss of data and communication errors. These gaps and errors can increase medicolegal risk, as can the more extensive documentation often seen with early use, which creates more discoverable data. The temptation to cut and paste risks repeating earlier errors and omitting new information.
Another area of risk involves communication with the patient via email. A failure to reply could result in claims of negligence, and information overload could obscure pertinent pieces of information. And a departure from clinical decision support could be used by the patient to defend allegations of negligence.
With widespread use of EHRs, improved access to data could change the “duty” owed to the patient. In addition, clinical decision support embedded within the software could become the de facto “standard of care.”
The learning curve can be steep
The learning curve for EHRs may be steep and, at times, discouraging. One reason is that data are organized differently than in the conventional paper record, where information is read and analyzed in a progressive and stepwise manner, as in an analog or vertical system. The EHR is a digital format, so finding information requires digital (horizontal) inquiry. Information is, therefore, utilized in both horizontal and vertical formats in everyday situations. If data are entered incorrectly, all subsequent decisions could be flawed. And if the EHR suggests a plan, and that plan is not performed by the provider, the risk of liability could increase.
Inadvertent violation of the Health Insurance Portability and Accountability Act (HIPAA) with an EHR could increase medicolegal risk. For example, HIPAA allows for patients to make corrections to inaccurate information in their personal documents, but access by the patient could require the physician to review all records viewed by the patient after visit notes have been entered. This could drive up the cost of practice and reduce face-to-face time between physician and patient. Patients are not necessarily the best judges of which information is most important in their medical records.
Internet access raises concerns about the privacy of sensitive issues and misuse of information. Making a patient’s protected health information accessible electronically leaves physicians and hospitals at risk for a government fine or lawsuit. In several instances, the US Department of Health and Human Services (HHS) has levied fines against small practices and government agencies.
In one case, HHS fined Phoenix Cardiac Surgery in Phoenix, Arizona, $100,000 for posting surgery and appointment schedules on an Internet-based calendar that was accessible to the public.12 In another, HHS fined the Massachusetts Eye and Ear Infirmary in Boston $1.5 million after it reported the loss of an encrypted personal laptop containing the protected health information of patients and research subjects.13 The Alaska Department of Health and Social Services (DHSS) agreed to pay HHS $1.7 million after it reported the loss of a USB drive—possibly containing protected health information—from the vehicle of a DHSS employee.14
In traditional physician practices that employ handwritten records, the potential for compromise of patient information is limited. An organization may lose a few patient charts in the office and recover from the loss without incident. With the EHR, the loss poses a significant threat. The cases mentioned above were attributed to negligence or ignorance. The consequences could be worse if the compromise of EHR data is determined to be intentional. On September 4, 2010, hackers may have exposed the personal information of approximately 9,493 patients at Southwest Seattle Orthopaedics and Sports Medicine in Burien, Washington. Even with the best encryption technology, any electronic system remains vulnerable to external attack.
Metadata reveal how original data are used
Another concern regarding EHRs involves metadata—”data about data content.”15 Metadata is structured information that describes, locates, explains, or manages information. Metadata relevant to the EHR includes the data and time it was reviewed by the provider and whether it was manipulated in any way. Clearly, there is a potential for use and misuse by third-party reviewers.
Specialty-specific EHRs are recommended
Many ObGyns have found that most EHR systems are inadequate to the task of recording and analyzing information relevant to their specialty. Obstetric care is episodic and frequent. Data are added into the flow that must be considered at each visit, such as gestational age, fetal growth, labs (and normative values), prenatal diagnostic studies, and so on, representing both vertical and horizontal processing.16
The legal discovery process poses challenges that have not yet been resolved
The legal discovery process grants all parties to a lawsuit equal access to information. Under ideal circumstances, the EHR can provide comprehensive data more quickly than traditional records can. The problem is determining what constitutes relevant data and which party has the burden or benefit of making that decision. Uncontrolled access has the potential to violate privacy and privilege requirements.
Rules regarding discovery are still being debated in regard to their applicability to digital discovery.17 Even before a lawsuit is filed, the potential for “data mining” by third parties could lead to allegations of malpractice.
How to use EHRs responsibly without increasing risk
Good communication between patient and provider is paramount in the provision of quality medical care. Adherence to evidence-based standards with thorough documentation always serves the best interests of both patients and providers. The EHR can facilitate this process.
Our recommendations for appropriate use of your EHR include:
- Spend time learning the ins and outs of your particular EHR, and make sure your staff does the same. This will help reduce the likelihood that errors will be introduced into the record and ensure consistent use.
- Use individual sign-ons for anyone involved in data entry. This step facilitates the identification of users responsible for inaccurate use or errors, so that the situation can be addressed efficiently.
- Do not let third parties enter or manipulate data. This could jeopardize patient privacy, as well as the integrity of the record itself.
- Track all data entry on a regular basis. The frequency of tracking should be a function of routine as well as clinical circumstance. All new data from the previous interval should be reviewed at the time of the subsequent visit in order to direct care and ensure proper data entry.
Because of the considerable risk of liability claims in ObGyn practice, it is critical that the medical record accurately and precisely reflects the circumstances of each case. The EHR can be an effective and useful tool to document what occurred (and when) in a clinical scenario.18 As with all medical records, completeness and accuracy are the first and best defense against allegations of medical malpractice.
1. The Casebooks Project. History of Medical Record-keeping. http://www.magicandmedicine.hps.cam.ac.uk/on-astrological-medicine/further-reading/history-of-medical-record-keeping/. Accessed February 26 2013.
2. US Department of Health and Human Services. EMR vs EHR—What is the difference? Health IT Buzz. http://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/emr-vs-ehr-difference/. Accessed February 20, 2013.
3. Health Information Technology for Economic and Clinical Health Act of 2009. HITECH Act. Pub L No 111-5 Div A tit XIII Div B tit IV Feb 17 2009, 123 stat 226, 467. Codified in scattered sections of 42 USCA.
4. Mangalmurti S, Murtagh L, Mello M. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363(21):2060-2067.
5. Reid P, Compton D, Grossman J, et al. Building a Better Delivery System: A New Engineering/Healthcare Partnership. Committee on Engineering and the Health Care System, Institute of Medicine and the National Academy of Engineering. Washington, DC: National Academies Press; 2005.
6. Grossman J. Disruptive innovation in healthcare: challenges for engineering. The Bridge. 2008;38:10-16.
7. Murphy E, Oxencis C, Klauck J, et al. Medication reconciliation at an academic medical center; implementation of a comprehensive program from admission to discharge. Am J Health-System Pharmacy. 2009;66(23):2126-2131.
8. Schuler R. The smart grid: a bridge between emerging technologies society and the environment. The Bridge. 2010;40:42-49.
9. Haberman S, Feldman J, Merhi Z, et al. Effect of clinical decision support on documentation compliance in an electronic medical record. Obstet Gynecol. 2009;114(2 Pt 1):311-317.
10. Hasley S. Decision support and patient safety: the time has come. Am J Obstet Gynecol. 2011;204(6):461-465.
11. Lagrew D, Stutman H, Sicaeros L. Voluntary physician adoption of an inpatient electronic medical record by obstetrician-gynecologists. Am J Obstet Gynecol. 2008;198(6):690.e1-e6.
12. Dolan PL. $100,000 HIPAA fine designed to send message to small physician practices. American Medical News. 2012. http://www.ama-assn.org/amednews/2012/04/30/bisd0502.htm. Accessed February 26, 2013.
13. US Department of Health and Human Services. Massachusetts provider settles HIPAA case for $1.5 million [news release]. September 17 2012. http://www.hhs.gov/news/press/2012pres/09/20120917a.html. Accessed February 26, 2013.
14. US Department of Health and Human Services. Alaska settles HIPAA security case for $1,700,000 [news release]. June 26, 2012. http://www.hhs.gov/news/press/2012pres/06/20120626a.html. Accessed February 26, 2013.
15. National Information Standards Organization. Understanding Metadata. Bethesda MD: NISO Press; 2004. http://www.niso.org/publications/press/UnderstandingMetadata.pdf. Accessed February 26, 2013.
16. McCoy M, Diamond A, Strunk A. Special requirements of electronic medical record systems in obstetrics and gynecology. Obstet Gynecol. 2010;116(1):140-143.
17. The Berkman Center for Internet and Society at Harvard Law School. The Federal Rules of Civil Procedure: The Impact of Digital Discovery. http://cyber.law.harvard.edu/digitaldiscovery/digdisc_library_4.html. Accessed February 26 2013.
18. Quinn M, Kats A, Kleinman K, et al. The relationship between electronic health records and malpractice claims. Arch Intern Med. 2012;172(15):1187-1188.
1. The Casebooks Project. History of Medical Record-keeping. http://www.magicandmedicine.hps.cam.ac.uk/on-astrological-medicine/further-reading/history-of-medical-record-keeping/. Accessed February 26 2013.
2. US Department of Health and Human Services. EMR vs EHR—What is the difference? Health IT Buzz. http://www.healthit.gov/buzz-blog/electronic-health-and-medical-records/emr-vs-ehr-difference/. Accessed February 20, 2013.
3. Health Information Technology for Economic and Clinical Health Act of 2009. HITECH Act. Pub L No 111-5 Div A tit XIII Div B tit IV Feb 17 2009, 123 stat 226, 467. Codified in scattered sections of 42 USCA.
4. Mangalmurti S, Murtagh L, Mello M. Medical malpractice liability in the age of electronic health records. N Engl J Med. 2010;363(21):2060-2067.
5. Reid P, Compton D, Grossman J, et al. Building a Better Delivery System: A New Engineering/Healthcare Partnership. Committee on Engineering and the Health Care System, Institute of Medicine and the National Academy of Engineering. Washington, DC: National Academies Press; 2005.
6. Grossman J. Disruptive innovation in healthcare: challenges for engineering. The Bridge. 2008;38:10-16.
7. Murphy E, Oxencis C, Klauck J, et al. Medication reconciliation at an academic medical center; implementation of a comprehensive program from admission to discharge. Am J Health-System Pharmacy. 2009;66(23):2126-2131.
8. Schuler R. The smart grid: a bridge between emerging technologies society and the environment. The Bridge. 2010;40:42-49.
9. Haberman S, Feldman J, Merhi Z, et al. Effect of clinical decision support on documentation compliance in an electronic medical record. Obstet Gynecol. 2009;114(2 Pt 1):311-317.
10. Hasley S. Decision support and patient safety: the time has come. Am J Obstet Gynecol. 2011;204(6):461-465.
11. Lagrew D, Stutman H, Sicaeros L. Voluntary physician adoption of an inpatient electronic medical record by obstetrician-gynecologists. Am J Obstet Gynecol. 2008;198(6):690.e1-e6.
12. Dolan PL. $100,000 HIPAA fine designed to send message to small physician practices. American Medical News. 2012. http://www.ama-assn.org/amednews/2012/04/30/bisd0502.htm. Accessed February 26, 2013.
13. US Department of Health and Human Services. Massachusetts provider settles HIPAA case for $1.5 million [news release]. September 17 2012. http://www.hhs.gov/news/press/2012pres/09/20120917a.html. Accessed February 26, 2013.
14. US Department of Health and Human Services. Alaska settles HIPAA security case for $1,700,000 [news release]. June 26, 2012. http://www.hhs.gov/news/press/2012pres/06/20120626a.html. Accessed February 26, 2013.
15. National Information Standards Organization. Understanding Metadata. Bethesda MD: NISO Press; 2004. http://www.niso.org/publications/press/UnderstandingMetadata.pdf. Accessed February 26, 2013.
16. McCoy M, Diamond A, Strunk A. Special requirements of electronic medical record systems in obstetrics and gynecology. Obstet Gynecol. 2010;116(1):140-143.
17. The Berkman Center for Internet and Society at Harvard Law School. The Federal Rules of Civil Procedure: The Impact of Digital Discovery. http://cyber.law.harvard.edu/digitaldiscovery/digdisc_library_4.html. Accessed February 26 2013.
18. Quinn M, Kats A, Kleinman K, et al. The relationship between electronic health records and malpractice claims. Arch Intern Med. 2012;172(15):1187-1188.
More strategies to avoid malpractice hazards on labor and delivery
Sound strategies to avoid malpractice hazards on labor and delivery
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
CASE 1: Pregestational diabetes, large baby, birth injury
A 31-year-old gravida 1 is admitted to labor and delivery. She is at 39-5/7 weeks’ gestation, dated by last menstrual period and early sonogram. The woman is a pregestational diabetic and uses insulin to control her blood glucose level.
Three weeks before admission, ultrasonography (US) revealed an estimated fetal weight of 3,650 g—at the 71st percentile for gestational age.
After an unremarkable course of labor, delivery is complicated by severe shoulder dystocia. The newborn has a birth weight of 4,985 g and sustains an Erb’s palsy-type injury. The mother develops a rectovaginal fistula after a fourth-degree tear.
In the first part of this article, we discussed how an allegation of malpractice can arise because of an unexpected event or outcome for a mother in your care, or her baby, apart from any specific clinical action you undertook. We offered an example: Counseling that you provide about options for prenatal care that falls short of full understanding by the patient.
In this article, we enter the realm of the hands-on practice of medicine and discuss causation: namely, the actions of a physician, in the course of managing labor and delivering a baby, that put that physician at risk of a charge of malpractice because the medical care 1) is inconsistent with current medical practice and thus 2) harmed mother or newborn.
Let’s return to the opening case above and discuss key considerations for the physician. Three more cases follow that, with analysis and recommendations.
Considerations in CASE 1
- A woman who has pregestational diabetes should receive ongoing counseling about the risks of fetal anomalies, macrosomia, and problems in the neonatal period. Be certain that she understands that these risks can be ameliorated, but not eliminated, with careful blood glucose control.
- The fetus of a diabetic gravida develops a relative decrease in the ratio of head circumference-to-abdominal circumference that predisposes it to shoulder dystocia. Cesarean delivery can decrease, but not eliminate, the risk of traumatic birth injury in a diabetic mother. (Of course, cesarean delivery will, on its own, substantially increase the risk of maternal morbidity—including at any subsequent cesarean delivery.)
What do they mean? terms and concepts intended to bolster your work and protect you
It’s not easy to define what constitutes “best care” in a given clinical circumstance. Generalizations are useful, but they may possess an inherent weakness: “Best practices,” “evidence-based care,” “standardization of care,” and “uniformity of care” usually apply more usefully to populations than individuals.
Such concepts derive from broader applications in economics, politics, and science. They are useful to define a reasonable spectrum of anticipated practices, and they certainly have an expanding role in the care of patients and in medical education (TABLE). Clinical guidelines serve as strategies that may be very helpful to the clinician. All of us understand and implement appropriate care in the great majority of clinical scenarios, but none of us are, or can be, expert in all situations. Referencing and using guidelines can fill a need for a functional starting point when expertise is lacking or falls short.
Best practices result from evidence-directed decision-making. This concept logically yields a desirable uniformity of practice. Although we all believe that our experience is our best teacher, we may best serve patients if we sample knowledge and wisdom from controlled clinical trials and from the experiences of others. What is accepted local practice must also be considered important when you devise a plan of care.1,2
A selected glossary of clinical care guidelines
| Term | What does it mean? |
|---|---|
| “Best practice” | A process or activity that is believed to be more effective at delivering a particular outcome than any other when applied to a particular condition or circumstance. The idea? With proper processes, checks, and testing, a desired outcome can be delivered with fewer problems and unforeseen complications than otherwise possible.5 |
| “Evidence-based care” | The best available process or activity arising from both 1) individual expertise and 2) best external evidence derived from systematic research.6 |
| “Standard of care” | A clinical practice to maximize success and minimize risk, applied to professional decision-making.7 |
| “Uniformity of practice” | Use of systematic, literature-based research findings to develop an approach that is efficacious and safe; that maximizes benefit; and that minimizes risk.8 |
Consider the management of breech presentation that is recognized at the 36th week antepartum visit: Discussion with the patient should include 1) reference to concerns with congenital anomalies and genetic syndromes, 2) in-utero growth and development, and 3) the delivery process. The management algorithm may include external cephalic version, elective cesarean delivery before onset of labor, or cesarean delivery after onset of labor. Each approach has advocates—based on expert opinion clinical trials.
Management options may vary from institution to institution, however, because of limited availability of certain services—such as the expertise required for a trial of external cephalic version, the availability of on-site cesarean delivery capabilities, and patient and clinician preferences.
Uniformity of care, based on best practices, can therefore simplify the care process and decrease the risk that may be associated with individual experience-based management. Adhering to a uniform practice augments the clinician’s knowledge and allows for enhanced nursing and therapeutic efficiency.
The greatest benefit of using an evidence-based, widely accepted approach, however, is the potential to diminish poor practice and consequent malpractice exposure for both clinicians and the hospital.
Note: Although your adherence to clinical guidelines, best practices, and uniformity of care ought to be consistent with established standards of care, don’t automatically consider any deviation a lapse or failing because it’s understood and accepted that some local variability exists in practice.
Prelude to birth: triage and admission Triage. Most women in labor arrive at the hospital or birthing center to an area set aside for labor and delivery triage. There, 1) recording of the chief complaint and vital signs and 2) completion of a brief history and physical generate a call to the clinician.
The record produced in triage should be scrutinized carefully for accuracy. Clarify, in as timely a manner as possible, any errors in:
- timing (possibly because of different clocks set to different times)
- the precise capture of the chief complaint
- reporting difficulty or ease in reaching the responsible clinician.
Whether these records are electronic or paper, an addendum marked with the time is always acceptable. Never attempt to correct a record! Always utilize a late entry or addendum.
Admission. After the patient is admitted, she generally undergoes an admission protocol, specific to the hospital, regarding her situation. This includes:
- the history
- special requests
- any previously agreed-on plan of care
- any problems that have developed since her last prenatal visit.
This protocol is generally completed by a nurse, resident, nurse practitioner, or physician assistant.
Hospitals generally request input from the attending physician on the specifics of the admission, based on those hospital protocols. There may be some room to individualize the admission process to labor and delivery.
4 pillars of care during labor
In general, labor is defined as progressive dilation of the cervix. Several parameters serve as guidelines regarding adequate progress through the various stages of labor.
Fetal monitoring. Continuous evaluation of the fetus during labor is a routine part of intrapartum care. Recording and observing the FHR tracing is an accepted—and expected—practice. Documentation of the FHR in the medical record is specifically required, and should include both the physician’s and nursing notes.
Anesthesia care. The patient’s preference and the availability of options allows for several accepted practices regarding anesthesia and analgesia during L & D. Does she want epidural anesthesia during labor, for example? Intravenous narcotics? Her choice is an important facet of your provision of care.
However, such choice requires the patient to give consent and to understand the risk-benefit equation. Documentation by nursing of the patient’s consent and understanding should be complete, including discussion and administration. Anesthesia staff should be clear, complete, and legible in making a record.
Neonatal care. If logistics permit, a member of the pediatrics service should be routinely available to see the newborn at delivery. The patient should view the pediatrician and obstetrician as partners working as a team for the benefit of the mother and her family. This can enhance the patient’s understanding and confidence about the well being of her baby.
Documentation. Although deficient documentation does not, itself, lead to a finding of malpractice, appropriate documentation plays an important role in demonstrating that clinical practices have addressed issues about both allegation and causation of potential adverse outcomes.
We cannot overemphasize that nursing documentation should complement and be consistent with notes made by the physician. That said, nursing notes are not a substitute for the physician’s notes. Practices that integrate the written comments of nursing and physician into a single set of progress notes facilitate this complementary interaction.
3 more clinical scenarios
CASE 2: Admitted at term with contractions
The initial exam determines that this 21-year-old gravida 1 is 2/80/-1. Re-examination in 3 hours finds her at 3-4/80/0.
She requests pain relief and states that she wants epidural anesthesia.
Evaluation 2 hours later suggests secondary arrest of dilation. Oxytocin is begun.
Soon after, late decelerations are observed on the FHR monitor.
Use of exogenous oxytocin in L & D is a double-edged sword: The drug can enhance the safety and efficacy of labor and delivery for mother and fetus, but using it in an unregulated manner (in terms of its indication and administration) can subject both to increased risk.
In fact, it is fair to say that the most widespread and potentially dangerous intervention during labor is the administration of oxytocin. Many expert opinions, guidelines, and strategies have been put forward about intrapartum use of oxytocin. These include consideration of:
- indications
- dosage (including the maximum)
- interval
- fetal response
- ultimately, the availability of a physician during administration to manage any problem that arises.
Considerations in CASE 2
- Always clearly indicate the reason for using oxytocin: Is this an induction? Or an augmentation? Was there evidence of fetal well-being, or non-reassurance, before oxytocin was administered? Certainly, there are circumstances in which either fetal status or non-progression of labor (or both) are an indication for oxytocin. A clear, concise, and properly timed progress note is always appropriate under these circumstances.
- Discuss treatment with the patient. Does she understand why this therapy is being recommended? Does she agree to its use? And does she understand what the alternatives are?
- Verify that nursing has accurately charted this process. Ensure that the nursing staff’s notes are complete and are consistent with yours.
- Simplify the entire process: Use premixed solution and protocol-driven orders. Know what the standards and protocols are in your department. Minimizing patient-to-patient variability should lessen the risk of error.
- Always be available in L & D for the first 30 minutes that oxytocin is being administered. If a problem with excessive uterine activity is going to occur, it is most likely to do so upon initial administration.
- Monitor the FHR continuously. At the first suggestion of a change in fetal status, discontinue oxytocin. Perform a pelvic exam to reassess the situation. Understand and apply appropriate inutero resuscitative measures (IV fluids, O2, change in maternal position). Depending on circumstances, you can consider a restart of oxytocin after the FHR returns to its pre-oxytocin pattern.
- Monitor uterine response to oxytocin. If the membranes are ruptured and if it is clinically feasible, an intrauterine pressure transducer will allow you to more objectively assess the uterine response to oxytocin and make decisions on that basis. Determine beforehand whether the patient is agreeable to this intervention.
- When oxytocin is used for augmentation, reassess labor within 4 hours of achieving a satisfactory pattern. If minimal progress is not made, assess the clinical situation to determine why oxytocin, at an adequate response level, has failed to return labor to a normal active phase slope. Are there minor degrees of malposition? Is there an element of cephalopelvic disproportion? Recall that progress in labor is dependent on multiple factors.
- Chart the process concurrently. Specify options for delivery before delivery.
CASE 3: Spontaneous delivery arrests after delivery of the head
The patient is a multipara with three prior normal vaginal deliveries. Her diabetic screen is negative. At admission, the estimated fetal weight was 3,628 g—in the same range as her other deliveries. A nuchal cord is absent.
After the patient assumes the McRobert’s position, delivery is accomplished with suprapubic pressure. Weakness is noted in the newborn’s right upper extremity. Birth weight is 3,515 g.
Maneuvers to manage shoulder dystocia should be part of all clinicians’ skill set. The sequence of those maneuvers, and their timing, are subject to some variation. Efficacy seems to be related most to recognizing and performing each maneuver properly.
Guidelines for managing shoulder dystocia should include reference to 1) the initial evaluation of the patient on admission to labor and delivery and 2) the delivery itself.3
Considerations in CASE 3
- Before you admit them to L & D, counsel patients who have diabetes, morbid obesity (body mass index >40), or birth trauma in a prior delivery, or who have had a prior large infant (>9 lb birth weight), about the risk of shoulder dystocia. Present possible alternatives, and draw the patient into the conversation.
- Consider delivering all women at term in the McRobert’s position, prophylactically.
- Always check for a nuchal cord after delivery of the head. If you find one, reduce it if possible. Take a few seconds and carefully assess the situation before you cut the umbilical cord.
- Lateral traction on the fetus’ head has the potential to cause tension on the brachial plexus, or make it worse. Gentle rotation of the head (<90 degrees) can move the shoulders into a more favorable location for delivery. Don’t rush—call for assistance! Continuously explain to the patient what you are doing; reassure her about the process.
- Use suprapubic pressure wisely. The anterior shoulder may be dislodged by direct downward force; suprapubic force in a lateral direction may also dislodge the shoulder. Apply force from above the patient’s pelvis. Your assistant will have the best mechanical advantage by standing on a stool.
- Is an episiotomy or episioproctotomy advantageous? In attempting to reach either the anterior or posterior shoulder vaginally, individualized assessment is called for.
- When the posterior shoulder cannot be satisfactorily engaged and moved, try doing so with the anterior shoulder. Insert your hand between the symphysis and the fetal head and place downward pressure on the head to dislodge it and complete the delivery.
- If it becomes necessary to attempt delivery by direct traction on the posterior hand or arm, try to avoid extension. Maintain flexion and move the upper extremity across the fetal chest before you attempt extension.
- Repeat these maneuvers a second time before you attempt cephalic replacement or other maneuvers. Remember to move with deliberate speed to lessen the risk of making the injury worse. Have pediatric support present. Continue speaking with and reassuring the patient.
- Under anesthesia in the operating room, perform a hysterotomy incision. With an assistant working through the vagina, combine the forces available to complete the delivery.
- After delivery is complete, take time to write a note. (Speak with the patient and her family first, however.) Read the notes written by nursing. If they are not available when you write your note, mention that. Add a second note later, when nursing notes become available.
CASE 4: Meconium-stained fluid
A 35-year-old multigravida is 6 to 7 cm dilated. Her membranes have just spontaneously ruptured; you note copious meconium-stained fluid. The FHR demonstrates recurrent variable decelerations; baseline fetal heart rate remains normal.
The description and implications of various FHR patterns are important when documenting the fetal metabolic state during the birth process. Current guidelines have attempted to simplify, standardize, and clarify the interpretation of the FHR tracing.4
Considerations in CASE 4
- Explain the situation to the patient. Perform a pelvic examination. If possible, wait for nursing assistance to ensure accurate documentation.
- Reassure the patient; help her move to a lateral position. Observe the FHR monitor for a response.
- Administer supplemental O2. Increase IV fluids to facilitate utero-placental perfusion.
- If useful or necessary, consider attaching a fetal scalp electrode to better delineate fetal status.
- When the FHR returns to baseline state (before spontaneous rupture of membranes), perform vibro-acoustic stimulation as a test to support fetal well-being.
- Engage the patient and her family in a discussion about the sequence of events. Depending on the acuity of the situation, allow her to voice her concerns and reiterate what has occurred, and what will occur.
- Outline a plan of management to the patient—verbally and in the record—with clear reference to events that have occurred. Then, stick to that plan!
- Carefully review corresponding nursing notes. Always write your own assessment of events and actions.
Summing up: three “keepers”
First, the cornerstones of your effort to reduce malpractice risk are 1) thoughtful and informed discussion with the patient and 2) clear, concise documentation.
Second, don’t expect to be able to eliminate unnecessary or inappropriate allegations of medical malpractice; the best you can do is limit them.
Third, and most important, remember: The knowledgeable clinician you strive to be will make appropriate judgments in a timely fashion and will take appropriate actions to provide good medical care.
We want to hear from you! Tell us what you think.
1. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
2. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199(2):105.e1-e7.
3. Crofts JF, Fox F, Ellis D. Observations from 450 shoulder dystocia simulations: lessons for skills training. Obstet Gynecol. 2008;112(4):906-912.
4. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring. Obstet Gynecol. 2008;112(3):661-666.
5. Best practice. Web site. 2010. http://www.businessdictionary.com/definition/best-practice.html. Accessed December 17, 2010.
6. Sackett DL, Rosenberg WC, Gray JA, Haynes BR, Richardson WS. Evidence based medicine: what it is and what it isn’t 1996;312(7023):71-72.
7. Hayes EJ, Weinstein L. Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol. 2008;198(6):622.e1-7.
8. Proctor SJ, Taylor PR. A practical guide to continuous population-based data collection (PACE): a process facilitating uniformity of care and research into practice. 2000;93(2):67-73.
9. Cohen W, Friedman EA. Management of Labor. Baltimore, MD: University Park Press; 1983.
Sound strategies to avoid malpractice hazards on labor and delivery
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
CASE 1: Pregestational diabetes, large baby, birth injury
A 31-year-old gravida 1 is admitted to labor and delivery. She is at 39-5/7 weeks’ gestation, dated by last menstrual period and early sonogram. The woman is a pregestational diabetic and uses insulin to control her blood glucose level.
Three weeks before admission, ultrasonography (US) revealed an estimated fetal weight of 3,650 g—at the 71st percentile for gestational age.
After an unremarkable course of labor, delivery is complicated by severe shoulder dystocia. The newborn has a birth weight of 4,985 g and sustains an Erb’s palsy-type injury. The mother develops a rectovaginal fistula after a fourth-degree tear.
In the first part of this article, we discussed how an allegation of malpractice can arise because of an unexpected event or outcome for a mother in your care, or her baby, apart from any specific clinical action you undertook. We offered an example: Counseling that you provide about options for prenatal care that falls short of full understanding by the patient.
In this article, we enter the realm of the hands-on practice of medicine and discuss causation: namely, the actions of a physician, in the course of managing labor and delivering a baby, that put that physician at risk of a charge of malpractice because the medical care 1) is inconsistent with current medical practice and thus 2) harmed mother or newborn.
Let’s return to the opening case above and discuss key considerations for the physician. Three more cases follow that, with analysis and recommendations.
Considerations in CASE 1
- A woman who has pregestational diabetes should receive ongoing counseling about the risks of fetal anomalies, macrosomia, and problems in the neonatal period. Be certain that she understands that these risks can be ameliorated, but not eliminated, with careful blood glucose control.
- The fetus of a diabetic gravida develops a relative decrease in the ratio of head circumference-to-abdominal circumference that predisposes it to shoulder dystocia. Cesarean delivery can decrease, but not eliminate, the risk of traumatic birth injury in a diabetic mother. (Of course, cesarean delivery will, on its own, substantially increase the risk of maternal morbidity—including at any subsequent cesarean delivery.)
What do they mean? terms and concepts intended to bolster your work and protect you
It’s not easy to define what constitutes “best care” in a given clinical circumstance. Generalizations are useful, but they may possess an inherent weakness: “Best practices,” “evidence-based care,” “standardization of care,” and “uniformity of care” usually apply more usefully to populations than individuals.
Such concepts derive from broader applications in economics, politics, and science. They are useful to define a reasonable spectrum of anticipated practices, and they certainly have an expanding role in the care of patients and in medical education (TABLE). Clinical guidelines serve as strategies that may be very helpful to the clinician. All of us understand and implement appropriate care in the great majority of clinical scenarios, but none of us are, or can be, expert in all situations. Referencing and using guidelines can fill a need for a functional starting point when expertise is lacking or falls short.
Best practices result from evidence-directed decision-making. This concept logically yields a desirable uniformity of practice. Although we all believe that our experience is our best teacher, we may best serve patients if we sample knowledge and wisdom from controlled clinical trials and from the experiences of others. What is accepted local practice must also be considered important when you devise a plan of care.1,2
A selected glossary of clinical care guidelines
| Term | What does it mean? |
|---|---|
| “Best practice” | A process or activity that is believed to be more effective at delivering a particular outcome than any other when applied to a particular condition or circumstance. The idea? With proper processes, checks, and testing, a desired outcome can be delivered with fewer problems and unforeseen complications than otherwise possible.5 |
| “Evidence-based care” | The best available process or activity arising from both 1) individual expertise and 2) best external evidence derived from systematic research.6 |
| “Standard of care” | A clinical practice to maximize success and minimize risk, applied to professional decision-making.7 |
| “Uniformity of practice” | Use of systematic, literature-based research findings to develop an approach that is efficacious and safe; that maximizes benefit; and that minimizes risk.8 |
Consider the management of breech presentation that is recognized at the 36th week antepartum visit: Discussion with the patient should include 1) reference to concerns with congenital anomalies and genetic syndromes, 2) in-utero growth and development, and 3) the delivery process. The management algorithm may include external cephalic version, elective cesarean delivery before onset of labor, or cesarean delivery after onset of labor. Each approach has advocates—based on expert opinion clinical trials.
Management options may vary from institution to institution, however, because of limited availability of certain services—such as the expertise required for a trial of external cephalic version, the availability of on-site cesarean delivery capabilities, and patient and clinician preferences.
Uniformity of care, based on best practices, can therefore simplify the care process and decrease the risk that may be associated with individual experience-based management. Adhering to a uniform practice augments the clinician’s knowledge and allows for enhanced nursing and therapeutic efficiency.
The greatest benefit of using an evidence-based, widely accepted approach, however, is the potential to diminish poor practice and consequent malpractice exposure for both clinicians and the hospital.
Note: Although your adherence to clinical guidelines, best practices, and uniformity of care ought to be consistent with established standards of care, don’t automatically consider any deviation a lapse or failing because it’s understood and accepted that some local variability exists in practice.
Prelude to birth: triage and admission Triage. Most women in labor arrive at the hospital or birthing center to an area set aside for labor and delivery triage. There, 1) recording of the chief complaint and vital signs and 2) completion of a brief history and physical generate a call to the clinician.
The record produced in triage should be scrutinized carefully for accuracy. Clarify, in as timely a manner as possible, any errors in:
- timing (possibly because of different clocks set to different times)
- the precise capture of the chief complaint
- reporting difficulty or ease in reaching the responsible clinician.
Whether these records are electronic or paper, an addendum marked with the time is always acceptable. Never attempt to correct a record! Always utilize a late entry or addendum.
Admission. After the patient is admitted, she generally undergoes an admission protocol, specific to the hospital, regarding her situation. This includes:
- the history
- special requests
- any previously agreed-on plan of care
- any problems that have developed since her last prenatal visit.
This protocol is generally completed by a nurse, resident, nurse practitioner, or physician assistant.
Hospitals generally request input from the attending physician on the specifics of the admission, based on those hospital protocols. There may be some room to individualize the admission process to labor and delivery.
4 pillars of care during labor
In general, labor is defined as progressive dilation of the cervix. Several parameters serve as guidelines regarding adequate progress through the various stages of labor.
Fetal monitoring. Continuous evaluation of the fetus during labor is a routine part of intrapartum care. Recording and observing the FHR tracing is an accepted—and expected—practice. Documentation of the FHR in the medical record is specifically required, and should include both the physician’s and nursing notes.
Anesthesia care. The patient’s preference and the availability of options allows for several accepted practices regarding anesthesia and analgesia during L & D. Does she want epidural anesthesia during labor, for example? Intravenous narcotics? Her choice is an important facet of your provision of care.
However, such choice requires the patient to give consent and to understand the risk-benefit equation. Documentation by nursing of the patient’s consent and understanding should be complete, including discussion and administration. Anesthesia staff should be clear, complete, and legible in making a record.
Neonatal care. If logistics permit, a member of the pediatrics service should be routinely available to see the newborn at delivery. The patient should view the pediatrician and obstetrician as partners working as a team for the benefit of the mother and her family. This can enhance the patient’s understanding and confidence about the well being of her baby.
Documentation. Although deficient documentation does not, itself, lead to a finding of malpractice, appropriate documentation plays an important role in demonstrating that clinical practices have addressed issues about both allegation and causation of potential adverse outcomes.
We cannot overemphasize that nursing documentation should complement and be consistent with notes made by the physician. That said, nursing notes are not a substitute for the physician’s notes. Practices that integrate the written comments of nursing and physician into a single set of progress notes facilitate this complementary interaction.
3 more clinical scenarios
CASE 2: Admitted at term with contractions
The initial exam determines that this 21-year-old gravida 1 is 2/80/-1. Re-examination in 3 hours finds her at 3-4/80/0.
She requests pain relief and states that she wants epidural anesthesia.
Evaluation 2 hours later suggests secondary arrest of dilation. Oxytocin is begun.
Soon after, late decelerations are observed on the FHR monitor.
Use of exogenous oxytocin in L & D is a double-edged sword: The drug can enhance the safety and efficacy of labor and delivery for mother and fetus, but using it in an unregulated manner (in terms of its indication and administration) can subject both to increased risk.
In fact, it is fair to say that the most widespread and potentially dangerous intervention during labor is the administration of oxytocin. Many expert opinions, guidelines, and strategies have been put forward about intrapartum use of oxytocin. These include consideration of:
- indications
- dosage (including the maximum)
- interval
- fetal response
- ultimately, the availability of a physician during administration to manage any problem that arises.
Considerations in CASE 2
- Always clearly indicate the reason for using oxytocin: Is this an induction? Or an augmentation? Was there evidence of fetal well-being, or non-reassurance, before oxytocin was administered? Certainly, there are circumstances in which either fetal status or non-progression of labor (or both) are an indication for oxytocin. A clear, concise, and properly timed progress note is always appropriate under these circumstances.
- Discuss treatment with the patient. Does she understand why this therapy is being recommended? Does she agree to its use? And does she understand what the alternatives are?
- Verify that nursing has accurately charted this process. Ensure that the nursing staff’s notes are complete and are consistent with yours.
- Simplify the entire process: Use premixed solution and protocol-driven orders. Know what the standards and protocols are in your department. Minimizing patient-to-patient variability should lessen the risk of error.
- Always be available in L & D for the first 30 minutes that oxytocin is being administered. If a problem with excessive uterine activity is going to occur, it is most likely to do so upon initial administration.
- Monitor the FHR continuously. At the first suggestion of a change in fetal status, discontinue oxytocin. Perform a pelvic exam to reassess the situation. Understand and apply appropriate inutero resuscitative measures (IV fluids, O2, change in maternal position). Depending on circumstances, you can consider a restart of oxytocin after the FHR returns to its pre-oxytocin pattern.
- Monitor uterine response to oxytocin. If the membranes are ruptured and if it is clinically feasible, an intrauterine pressure transducer will allow you to more objectively assess the uterine response to oxytocin and make decisions on that basis. Determine beforehand whether the patient is agreeable to this intervention.
- When oxytocin is used for augmentation, reassess labor within 4 hours of achieving a satisfactory pattern. If minimal progress is not made, assess the clinical situation to determine why oxytocin, at an adequate response level, has failed to return labor to a normal active phase slope. Are there minor degrees of malposition? Is there an element of cephalopelvic disproportion? Recall that progress in labor is dependent on multiple factors.
- Chart the process concurrently. Specify options for delivery before delivery.
CASE 3: Spontaneous delivery arrests after delivery of the head
The patient is a multipara with three prior normal vaginal deliveries. Her diabetic screen is negative. At admission, the estimated fetal weight was 3,628 g—in the same range as her other deliveries. A nuchal cord is absent.
After the patient assumes the McRobert’s position, delivery is accomplished with suprapubic pressure. Weakness is noted in the newborn’s right upper extremity. Birth weight is 3,515 g.
Maneuvers to manage shoulder dystocia should be part of all clinicians’ skill set. The sequence of those maneuvers, and their timing, are subject to some variation. Efficacy seems to be related most to recognizing and performing each maneuver properly.
Guidelines for managing shoulder dystocia should include reference to 1) the initial evaluation of the patient on admission to labor and delivery and 2) the delivery itself.3
Considerations in CASE 3
- Before you admit them to L & D, counsel patients who have diabetes, morbid obesity (body mass index >40), or birth trauma in a prior delivery, or who have had a prior large infant (>9 lb birth weight), about the risk of shoulder dystocia. Present possible alternatives, and draw the patient into the conversation.
- Consider delivering all women at term in the McRobert’s position, prophylactically.
- Always check for a nuchal cord after delivery of the head. If you find one, reduce it if possible. Take a few seconds and carefully assess the situation before you cut the umbilical cord.
- Lateral traction on the fetus’ head has the potential to cause tension on the brachial plexus, or make it worse. Gentle rotation of the head (<90 degrees) can move the shoulders into a more favorable location for delivery. Don’t rush—call for assistance! Continuously explain to the patient what you are doing; reassure her about the process.
- Use suprapubic pressure wisely. The anterior shoulder may be dislodged by direct downward force; suprapubic force in a lateral direction may also dislodge the shoulder. Apply force from above the patient’s pelvis. Your assistant will have the best mechanical advantage by standing on a stool.
- Is an episiotomy or episioproctotomy advantageous? In attempting to reach either the anterior or posterior shoulder vaginally, individualized assessment is called for.
- When the posterior shoulder cannot be satisfactorily engaged and moved, try doing so with the anterior shoulder. Insert your hand between the symphysis and the fetal head and place downward pressure on the head to dislodge it and complete the delivery.
- If it becomes necessary to attempt delivery by direct traction on the posterior hand or arm, try to avoid extension. Maintain flexion and move the upper extremity across the fetal chest before you attempt extension.
- Repeat these maneuvers a second time before you attempt cephalic replacement or other maneuvers. Remember to move with deliberate speed to lessen the risk of making the injury worse. Have pediatric support present. Continue speaking with and reassuring the patient.
- Under anesthesia in the operating room, perform a hysterotomy incision. With an assistant working through the vagina, combine the forces available to complete the delivery.
- After delivery is complete, take time to write a note. (Speak with the patient and her family first, however.) Read the notes written by nursing. If they are not available when you write your note, mention that. Add a second note later, when nursing notes become available.
CASE 4: Meconium-stained fluid
A 35-year-old multigravida is 6 to 7 cm dilated. Her membranes have just spontaneously ruptured; you note copious meconium-stained fluid. The FHR demonstrates recurrent variable decelerations; baseline fetal heart rate remains normal.
The description and implications of various FHR patterns are important when documenting the fetal metabolic state during the birth process. Current guidelines have attempted to simplify, standardize, and clarify the interpretation of the FHR tracing.4
Considerations in CASE 4
- Explain the situation to the patient. Perform a pelvic examination. If possible, wait for nursing assistance to ensure accurate documentation.
- Reassure the patient; help her move to a lateral position. Observe the FHR monitor for a response.
- Administer supplemental O2. Increase IV fluids to facilitate utero-placental perfusion.
- If useful or necessary, consider attaching a fetal scalp electrode to better delineate fetal status.
- When the FHR returns to baseline state (before spontaneous rupture of membranes), perform vibro-acoustic stimulation as a test to support fetal well-being.
- Engage the patient and her family in a discussion about the sequence of events. Depending on the acuity of the situation, allow her to voice her concerns and reiterate what has occurred, and what will occur.
- Outline a plan of management to the patient—verbally and in the record—with clear reference to events that have occurred. Then, stick to that plan!
- Carefully review corresponding nursing notes. Always write your own assessment of events and actions.
Summing up: three “keepers”
First, the cornerstones of your effort to reduce malpractice risk are 1) thoughtful and informed discussion with the patient and 2) clear, concise documentation.
Second, don’t expect to be able to eliminate unnecessary or inappropriate allegations of medical malpractice; the best you can do is limit them.
Third, and most important, remember: The knowledgeable clinician you strive to be will make appropriate judgments in a timely fashion and will take appropriate actions to provide good medical care.
We want to hear from you! Tell us what you think.
Sound strategies to avoid malpractice hazards on labor and delivery
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
CASE 1: Pregestational diabetes, large baby, birth injury
A 31-year-old gravida 1 is admitted to labor and delivery. She is at 39-5/7 weeks’ gestation, dated by last menstrual period and early sonogram. The woman is a pregestational diabetic and uses insulin to control her blood glucose level.
Three weeks before admission, ultrasonography (US) revealed an estimated fetal weight of 3,650 g—at the 71st percentile for gestational age.
After an unremarkable course of labor, delivery is complicated by severe shoulder dystocia. The newborn has a birth weight of 4,985 g and sustains an Erb’s palsy-type injury. The mother develops a rectovaginal fistula after a fourth-degree tear.
In the first part of this article, we discussed how an allegation of malpractice can arise because of an unexpected event or outcome for a mother in your care, or her baby, apart from any specific clinical action you undertook. We offered an example: Counseling that you provide about options for prenatal care that falls short of full understanding by the patient.
In this article, we enter the realm of the hands-on practice of medicine and discuss causation: namely, the actions of a physician, in the course of managing labor and delivering a baby, that put that physician at risk of a charge of malpractice because the medical care 1) is inconsistent with current medical practice and thus 2) harmed mother or newborn.
Let’s return to the opening case above and discuss key considerations for the physician. Three more cases follow that, with analysis and recommendations.
Considerations in CASE 1
- A woman who has pregestational diabetes should receive ongoing counseling about the risks of fetal anomalies, macrosomia, and problems in the neonatal period. Be certain that she understands that these risks can be ameliorated, but not eliminated, with careful blood glucose control.
- The fetus of a diabetic gravida develops a relative decrease in the ratio of head circumference-to-abdominal circumference that predisposes it to shoulder dystocia. Cesarean delivery can decrease, but not eliminate, the risk of traumatic birth injury in a diabetic mother. (Of course, cesarean delivery will, on its own, substantially increase the risk of maternal morbidity—including at any subsequent cesarean delivery.)
What do they mean? terms and concepts intended to bolster your work and protect you
It’s not easy to define what constitutes “best care” in a given clinical circumstance. Generalizations are useful, but they may possess an inherent weakness: “Best practices,” “evidence-based care,” “standardization of care,” and “uniformity of care” usually apply more usefully to populations than individuals.
Such concepts derive from broader applications in economics, politics, and science. They are useful to define a reasonable spectrum of anticipated practices, and they certainly have an expanding role in the care of patients and in medical education (TABLE). Clinical guidelines serve as strategies that may be very helpful to the clinician. All of us understand and implement appropriate care in the great majority of clinical scenarios, but none of us are, or can be, expert in all situations. Referencing and using guidelines can fill a need for a functional starting point when expertise is lacking or falls short.
Best practices result from evidence-directed decision-making. This concept logically yields a desirable uniformity of practice. Although we all believe that our experience is our best teacher, we may best serve patients if we sample knowledge and wisdom from controlled clinical trials and from the experiences of others. What is accepted local practice must also be considered important when you devise a plan of care.1,2
A selected glossary of clinical care guidelines
| Term | What does it mean? |
|---|---|
| “Best practice” | A process or activity that is believed to be more effective at delivering a particular outcome than any other when applied to a particular condition or circumstance. The idea? With proper processes, checks, and testing, a desired outcome can be delivered with fewer problems and unforeseen complications than otherwise possible.5 |
| “Evidence-based care” | The best available process or activity arising from both 1) individual expertise and 2) best external evidence derived from systematic research.6 |
| “Standard of care” | A clinical practice to maximize success and minimize risk, applied to professional decision-making.7 |
| “Uniformity of practice” | Use of systematic, literature-based research findings to develop an approach that is efficacious and safe; that maximizes benefit; and that minimizes risk.8 |
Consider the management of breech presentation that is recognized at the 36th week antepartum visit: Discussion with the patient should include 1) reference to concerns with congenital anomalies and genetic syndromes, 2) in-utero growth and development, and 3) the delivery process. The management algorithm may include external cephalic version, elective cesarean delivery before onset of labor, or cesarean delivery after onset of labor. Each approach has advocates—based on expert opinion clinical trials.
Management options may vary from institution to institution, however, because of limited availability of certain services—such as the expertise required for a trial of external cephalic version, the availability of on-site cesarean delivery capabilities, and patient and clinician preferences.
Uniformity of care, based on best practices, can therefore simplify the care process and decrease the risk that may be associated with individual experience-based management. Adhering to a uniform practice augments the clinician’s knowledge and allows for enhanced nursing and therapeutic efficiency.
The greatest benefit of using an evidence-based, widely accepted approach, however, is the potential to diminish poor practice and consequent malpractice exposure for both clinicians and the hospital.
Note: Although your adherence to clinical guidelines, best practices, and uniformity of care ought to be consistent with established standards of care, don’t automatically consider any deviation a lapse or failing because it’s understood and accepted that some local variability exists in practice.
Prelude to birth: triage and admission Triage. Most women in labor arrive at the hospital or birthing center to an area set aside for labor and delivery triage. There, 1) recording of the chief complaint and vital signs and 2) completion of a brief history and physical generate a call to the clinician.
The record produced in triage should be scrutinized carefully for accuracy. Clarify, in as timely a manner as possible, any errors in:
- timing (possibly because of different clocks set to different times)
- the precise capture of the chief complaint
- reporting difficulty or ease in reaching the responsible clinician.
Whether these records are electronic or paper, an addendum marked with the time is always acceptable. Never attempt to correct a record! Always utilize a late entry or addendum.
Admission. After the patient is admitted, she generally undergoes an admission protocol, specific to the hospital, regarding her situation. This includes:
- the history
- special requests
- any previously agreed-on plan of care
- any problems that have developed since her last prenatal visit.
This protocol is generally completed by a nurse, resident, nurse practitioner, or physician assistant.
Hospitals generally request input from the attending physician on the specifics of the admission, based on those hospital protocols. There may be some room to individualize the admission process to labor and delivery.
4 pillars of care during labor
In general, labor is defined as progressive dilation of the cervix. Several parameters serve as guidelines regarding adequate progress through the various stages of labor.
Fetal monitoring. Continuous evaluation of the fetus during labor is a routine part of intrapartum care. Recording and observing the FHR tracing is an accepted—and expected—practice. Documentation of the FHR in the medical record is specifically required, and should include both the physician’s and nursing notes.
Anesthesia care. The patient’s preference and the availability of options allows for several accepted practices regarding anesthesia and analgesia during L & D. Does she want epidural anesthesia during labor, for example? Intravenous narcotics? Her choice is an important facet of your provision of care.
However, such choice requires the patient to give consent and to understand the risk-benefit equation. Documentation by nursing of the patient’s consent and understanding should be complete, including discussion and administration. Anesthesia staff should be clear, complete, and legible in making a record.
Neonatal care. If logistics permit, a member of the pediatrics service should be routinely available to see the newborn at delivery. The patient should view the pediatrician and obstetrician as partners working as a team for the benefit of the mother and her family. This can enhance the patient’s understanding and confidence about the well being of her baby.
Documentation. Although deficient documentation does not, itself, lead to a finding of malpractice, appropriate documentation plays an important role in demonstrating that clinical practices have addressed issues about both allegation and causation of potential adverse outcomes.
We cannot overemphasize that nursing documentation should complement and be consistent with notes made by the physician. That said, nursing notes are not a substitute for the physician’s notes. Practices that integrate the written comments of nursing and physician into a single set of progress notes facilitate this complementary interaction.
3 more clinical scenarios
CASE 2: Admitted at term with contractions
The initial exam determines that this 21-year-old gravida 1 is 2/80/-1. Re-examination in 3 hours finds her at 3-4/80/0.
She requests pain relief and states that she wants epidural anesthesia.
Evaluation 2 hours later suggests secondary arrest of dilation. Oxytocin is begun.
Soon after, late decelerations are observed on the FHR monitor.
Use of exogenous oxytocin in L & D is a double-edged sword: The drug can enhance the safety and efficacy of labor and delivery for mother and fetus, but using it in an unregulated manner (in terms of its indication and administration) can subject both to increased risk.
In fact, it is fair to say that the most widespread and potentially dangerous intervention during labor is the administration of oxytocin. Many expert opinions, guidelines, and strategies have been put forward about intrapartum use of oxytocin. These include consideration of:
- indications
- dosage (including the maximum)
- interval
- fetal response
- ultimately, the availability of a physician during administration to manage any problem that arises.
Considerations in CASE 2
- Always clearly indicate the reason for using oxytocin: Is this an induction? Or an augmentation? Was there evidence of fetal well-being, or non-reassurance, before oxytocin was administered? Certainly, there are circumstances in which either fetal status or non-progression of labor (or both) are an indication for oxytocin. A clear, concise, and properly timed progress note is always appropriate under these circumstances.
- Discuss treatment with the patient. Does she understand why this therapy is being recommended? Does she agree to its use? And does she understand what the alternatives are?
- Verify that nursing has accurately charted this process. Ensure that the nursing staff’s notes are complete and are consistent with yours.
- Simplify the entire process: Use premixed solution and protocol-driven orders. Know what the standards and protocols are in your department. Minimizing patient-to-patient variability should lessen the risk of error.
- Always be available in L & D for the first 30 minutes that oxytocin is being administered. If a problem with excessive uterine activity is going to occur, it is most likely to do so upon initial administration.
- Monitor the FHR continuously. At the first suggestion of a change in fetal status, discontinue oxytocin. Perform a pelvic exam to reassess the situation. Understand and apply appropriate inutero resuscitative measures (IV fluids, O2, change in maternal position). Depending on circumstances, you can consider a restart of oxytocin after the FHR returns to its pre-oxytocin pattern.
- Monitor uterine response to oxytocin. If the membranes are ruptured and if it is clinically feasible, an intrauterine pressure transducer will allow you to more objectively assess the uterine response to oxytocin and make decisions on that basis. Determine beforehand whether the patient is agreeable to this intervention.
- When oxytocin is used for augmentation, reassess labor within 4 hours of achieving a satisfactory pattern. If minimal progress is not made, assess the clinical situation to determine why oxytocin, at an adequate response level, has failed to return labor to a normal active phase slope. Are there minor degrees of malposition? Is there an element of cephalopelvic disproportion? Recall that progress in labor is dependent on multiple factors.
- Chart the process concurrently. Specify options for delivery before delivery.
CASE 3: Spontaneous delivery arrests after delivery of the head
The patient is a multipara with three prior normal vaginal deliveries. Her diabetic screen is negative. At admission, the estimated fetal weight was 3,628 g—in the same range as her other deliveries. A nuchal cord is absent.
After the patient assumes the McRobert’s position, delivery is accomplished with suprapubic pressure. Weakness is noted in the newborn’s right upper extremity. Birth weight is 3,515 g.
Maneuvers to manage shoulder dystocia should be part of all clinicians’ skill set. The sequence of those maneuvers, and their timing, are subject to some variation. Efficacy seems to be related most to recognizing and performing each maneuver properly.
Guidelines for managing shoulder dystocia should include reference to 1) the initial evaluation of the patient on admission to labor and delivery and 2) the delivery itself.3
Considerations in CASE 3
- Before you admit them to L & D, counsel patients who have diabetes, morbid obesity (body mass index >40), or birth trauma in a prior delivery, or who have had a prior large infant (>9 lb birth weight), about the risk of shoulder dystocia. Present possible alternatives, and draw the patient into the conversation.
- Consider delivering all women at term in the McRobert’s position, prophylactically.
- Always check for a nuchal cord after delivery of the head. If you find one, reduce it if possible. Take a few seconds and carefully assess the situation before you cut the umbilical cord.
- Lateral traction on the fetus’ head has the potential to cause tension on the brachial plexus, or make it worse. Gentle rotation of the head (<90 degrees) can move the shoulders into a more favorable location for delivery. Don’t rush—call for assistance! Continuously explain to the patient what you are doing; reassure her about the process.
- Use suprapubic pressure wisely. The anterior shoulder may be dislodged by direct downward force; suprapubic force in a lateral direction may also dislodge the shoulder. Apply force from above the patient’s pelvis. Your assistant will have the best mechanical advantage by standing on a stool.
- Is an episiotomy or episioproctotomy advantageous? In attempting to reach either the anterior or posterior shoulder vaginally, individualized assessment is called for.
- When the posterior shoulder cannot be satisfactorily engaged and moved, try doing so with the anterior shoulder. Insert your hand between the symphysis and the fetal head and place downward pressure on the head to dislodge it and complete the delivery.
- If it becomes necessary to attempt delivery by direct traction on the posterior hand or arm, try to avoid extension. Maintain flexion and move the upper extremity across the fetal chest before you attempt extension.
- Repeat these maneuvers a second time before you attempt cephalic replacement or other maneuvers. Remember to move with deliberate speed to lessen the risk of making the injury worse. Have pediatric support present. Continue speaking with and reassuring the patient.
- Under anesthesia in the operating room, perform a hysterotomy incision. With an assistant working through the vagina, combine the forces available to complete the delivery.
- After delivery is complete, take time to write a note. (Speak with the patient and her family first, however.) Read the notes written by nursing. If they are not available when you write your note, mention that. Add a second note later, when nursing notes become available.
CASE 4: Meconium-stained fluid
A 35-year-old multigravida is 6 to 7 cm dilated. Her membranes have just spontaneously ruptured; you note copious meconium-stained fluid. The FHR demonstrates recurrent variable decelerations; baseline fetal heart rate remains normal.
The description and implications of various FHR patterns are important when documenting the fetal metabolic state during the birth process. Current guidelines have attempted to simplify, standardize, and clarify the interpretation of the FHR tracing.4
Considerations in CASE 4
- Explain the situation to the patient. Perform a pelvic examination. If possible, wait for nursing assistance to ensure accurate documentation.
- Reassure the patient; help her move to a lateral position. Observe the FHR monitor for a response.
- Administer supplemental O2. Increase IV fluids to facilitate utero-placental perfusion.
- If useful or necessary, consider attaching a fetal scalp electrode to better delineate fetal status.
- When the FHR returns to baseline state (before spontaneous rupture of membranes), perform vibro-acoustic stimulation as a test to support fetal well-being.
- Engage the patient and her family in a discussion about the sequence of events. Depending on the acuity of the situation, allow her to voice her concerns and reiterate what has occurred, and what will occur.
- Outline a plan of management to the patient—verbally and in the record—with clear reference to events that have occurred. Then, stick to that plan!
- Carefully review corresponding nursing notes. Always write your own assessment of events and actions.
Summing up: three “keepers”
First, the cornerstones of your effort to reduce malpractice risk are 1) thoughtful and informed discussion with the patient and 2) clear, concise documentation.
Second, don’t expect to be able to eliminate unnecessary or inappropriate allegations of medical malpractice; the best you can do is limit them.
Third, and most important, remember: The knowledgeable clinician you strive to be will make appropriate judgments in a timely fashion and will take appropriate actions to provide good medical care.
We want to hear from you! Tell us what you think.
1. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
2. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199(2):105.e1-e7.
3. Crofts JF, Fox F, Ellis D. Observations from 450 shoulder dystocia simulations: lessons for skills training. Obstet Gynecol. 2008;112(4):906-912.
4. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring. Obstet Gynecol. 2008;112(3):661-666.
5. Best practice. Web site. 2010. http://www.businessdictionary.com/definition/best-practice.html. Accessed December 17, 2010.
6. Sackett DL, Rosenberg WC, Gray JA, Haynes BR, Richardson WS. Evidence based medicine: what it is and what it isn’t 1996;312(7023):71-72.
7. Hayes EJ, Weinstein L. Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol. 2008;198(6):622.e1-7.
8. Proctor SJ, Taylor PR. A practical guide to continuous population-based data collection (PACE): a process facilitating uniformity of care and research into practice. 2000;93(2):67-73.
9. Cohen W, Friedman EA. Management of Labor. Baltimore, MD: University Park Press; 1983.
1. Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112(6):1279-1283.
2. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199(2):105.e1-e7.
3. Crofts JF, Fox F, Ellis D. Observations from 450 shoulder dystocia simulations: lessons for skills training. Obstet Gynecol. 2008;112(4):906-912.
4. Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring. Obstet Gynecol. 2008;112(3):661-666.
5. Best practice. Web site. 2010. http://www.businessdictionary.com/definition/best-practice.html. Accessed December 17, 2010.
6. Sackett DL, Rosenberg WC, Gray JA, Haynes BR, Richardson WS. Evidence based medicine: what it is and what it isn’t 1996;312(7023):71-72.
7. Hayes EJ, Weinstein L. Improving patient safety and uniformity of care by a standardized regimen for the use of oxytocin. Am J Obstet Gynecol. 2008;198(6):622.e1-7.
8. Proctor SJ, Taylor PR. A practical guide to continuous population-based data collection (PACE): a process facilitating uniformity of care and research into practice. 2000;93(2):67-73.
9. Cohen W, Friedman EA. Management of Labor. Baltimore, MD: University Park Press; 1983.
Sound strategies to avoid malpractice hazards on labor and delivery
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.
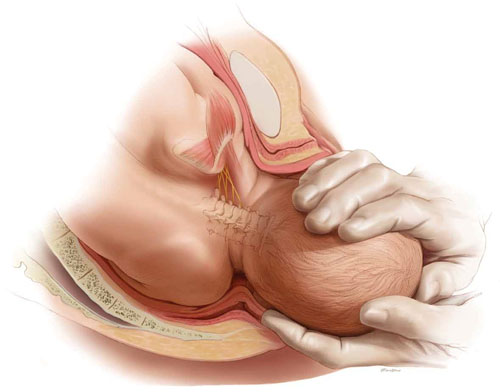
Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.

Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
CASE: Is TOLAC feasible?
Your patient is a 33-year-old gravida 3, para 2002, with a previous cesarean delivery who was admitted to labor and delivery with premature ruptured membranes at term. She is not contracting. Fetal status is reassuring.
Her obstetric history is of one normal, spontaneous delivery followed by one cesarean delivery, both occurring at term.
She wants to know if she can safely undergo a trial of labor, or if she must have a repeat cesarean delivery. How should you counsel her?
At the start of any discussion about how to reduce your risk of being sued for malpractice because of your work as an obstetrician, in particular during labor and delivery, two distinct, underlying avenues of concern need to be addressed. Before moving on to discuss strategy, then, let’s consider what they are and how they arise: Allegation (perception). You are at risk of an allegation of malpractice (or of a perception of malpractice) because of an unexpected event or outcome for mother or baby. Allegation and perception can arise apart from any specific clinical action you undertook, or did not undertake. An example? Counseling about options for care that falls short of full understanding by the patient.
Allegation and perception are the subjects of this first installment of our two-part article on strategies for avoiding claims of malpractice in L & D that begin with the first prenatal visit.
Causation. Your actions—what you do in the course of providing prenatal care and delivering a baby—put you at risk of a charge of malpractice when you have provided medical care that 1) is inconsistent with current medical practice and thus 2) harmed the mother or newborn.
For a medical malpractice case to go forward, it must meet a well-defined paradigm that teases apart components of causation, beginning with your duty to the patient (TABLE 1).
TABLE 1 Signposts in the medical malpractice paradigm
| When the clinical issue at hand is … | … Then the legal term is … |
|---|---|
| A health-care professional’s obligation to provide care | “Duty” |
| A deviation in the care that was provided | “Standard of care” |
| An allegation that a breach in the standard of care resulted in injury | “Proximate cause” |
| An assertion or finding that an injury is “compensable” | “Damages” |
| Source: Yale New Haven Medical Center, 1997.5 | |
Allegation of malpractice arises from a range of sources, as we’ll discuss, but it is causation that reflects the actual, hands-on practice of medicine. We’ll examine strategies for avoiding charges of causation in the second part of this article.
(For now, we’ll just note that a recent excellent review of intrapartum interventions and their basis in evidence1 offers a model for evaluating a number of widely utilized practices in obstetrics. The goal, of course, is to minimize bad outcomes that follow from causation. Regrettably, that evidence-based approach is a limited one, because of a paucity of adequately controlled studies about OB practice.)
CASE: Continued
You consider your patient’s comment that she would like to avoid a repeat cesarean delivery, and advise her that she may safely attempt vaginal birth.
When spontaneous labor does not occur in 6 hours, oxytocin is administered. She dilates to 9 cm and begins to push spontaneously.
The fetal heart rate then drops to 70/min; fetal station, which had been +2, is now -1. A Stat cesarean delivery is performed. Uterine rupture with partial fetal expulsion is found. Apgar scores are 1, 3, and 5 at 1, 5, and 10 minutes.
Your patient requires a hysterectomy to control bleeding.
Some broad considerations for the physician arising from this CASE
- The counseling that you provide to a patient should be nondirective; it should include your opinion, however, about the best option available to her. Insert yourself into this hypothetical case, for discussion’s sake: Did you provide that important opinion to her?
- You must make certain that she clearly understands the risks and benefits of a procedure or other action, and the available alternatives. Did you undertake a check of her comprehension, given the anxiety and confusion of the moment?
- When an adverse outcome ensues—however unlikely it was to occur—it is necessary for you to review the circumstances with the patient as soon as clinically possible. Did you “debrief” and counsel her before and after the hysterectomy?
No more “perfect outcomes”: Our role changed, so did our risk
From the moment an OB patient enters triage, until her arrival home with her infant, this crucial period of her life is colored by concern, curiosity, myth, and fear.
Every woman anticipates the birth of a healthy infant. In an earlier era, the patient and her family relied on the sage advice of their physician to ensure this outcome. To an extent, physicians themselves reinforced this reliance, embracing the notion that they were, in fact, able to provide such a perfect outcome.
With advances that have been made in reproductive medicine, pregnancy has become more readily available to women with increasingly advanced disease; this has made labor and delivery more challenging to them and to their physicians. Realistically, our role as physicians is now better expressed as providing advice to help a woman achieve the best possible outcome, recognizing her individual clinical circumstances, instead of ensuring a perfect outcome.

Every woman anticipates the birth of a healthy baby. But the role of the OB is better expressed as helping her achieve the best possible outcome, not a perfect outcome. ABOVE: Shoulder dystocia is one of the most treacherous and frightening—and litigated—complications of childbirth, yet it is, for the most part, unpredictable and unpreventable in the course of even routine delivery.
Key concept #1
COMMUNICATION
Communication is central to patients’ comprehension about the care that you provide to them. But to enter a genuine dialogue with a patient under your care, and with her family, can challenge your communication skills.
First, you need written and verbal skills. Second, you need to know how to read visual cues.
Third, the messages that you deliver to the patient are influenced by:
- your style of communication
- your cultural background
- the setting in which you’re providing care (office, hospital).
Where are such skills developed? For one, biopsychosocial models that are employed in medical student education and resident training aid the physician in developing appropriate communication skills.
But training alone cannot overcome the fact that communication is a double-sided activity: Patients bring many of their own variables to a dialogue. How patients understand and interact with you—and with other providers and the health-care system—is not, therefore, directly or strictly within your sphere of influence.
Yet your sensitivity to a patient’s issues can go a long way toward ameliorating her misconceptions and prejudices. Here are several suggestions, developed by others, to optimize patients’ understanding of their care2,3:
- Apply what’s known as flip default. Assume the patient does not understand the information that you’re providing. Ask her to repeat your instructions back to you (as is done with a verbal order in the hospital).
- Manage face-to-face time effectively. Don’t attempt to teach a patient everything about her care at once. Focus on the critical aspects of her case and on providing understanding; use a strategy of sequential learning.
- Reduce the “overwhelm” factor. Periodically, stop and ask the patient if she has questions. Don’t wait until the end of the appointment to do this.
- Eliminate jargon. When you notify a patient about the results of testing, for example, clarify what the results say about her health and mean for her care. Do so in plain language.
- Recognize her preconceptions. Discuss any psychosocial issues head on with the patient. Use an interpreter or a social worker, or counselors from other fields, as appropriate.
Remember: All health-care personnel need to understand the importance of making the patient comfortable in the often foreign, and sometimes sterile, milieu of the medical office and hospital.
Key concept #2
TRUST
Trust between patient and clinician is, we believe, the most basic necessity for ameliorating allegations of malpractice—secondary only, perhaps, to your knowledge of medicine.
Trust can be enhanced by interactions that demonstrate to both parties the advisability of working together to resolve a problem. Any aspect of the physician-patient interaction that is potentially adversarial does not serve the interests of either.
We encourage you to construct a communication bridge, so to speak, with your patient. Begin by:
- introducing yourself to her and explaining your role in her care
- making appropriate eye contact with her
- maintaining a positive attitude
- dressing appropriately
- making her feel that she is your No. 1 priority.
There is more.
Recognize the duality of respect
- Ask the patient how she wishes to be addressed
- Ask about her belief system
- Explain the specifics of her care without arrogance.
Engender trust
- Be honest with her
- Be on her side
- Take time with her
- Allow her the right that she has to select from the options or to refuse treatment
- Disclose to the patient your status as a student or resident, if that is your rank.
Recognize the benefits of partnership
Forging a partnership with the patient:
- improves the accuracy of information
- eases ongoing communication
- facilitates informed consent
- provides an opportunity for you to educate her.
TABLE 2 When building trust, both patient and physician
are charged with responsibilities
| In regard to … | The patient’s responsibility is to … | The physician’s responsibility is to … |
|---|---|---|
| Gathering an honest and complete medical history | Know and report | Question completely |
| Being adherent to prescribed care | Follow through | Make reasonable demands |
| Making decisions about care | Ask questions and actively participate in choices Make realistic requests | Be knowledgeable about available alternatives Individualize options |
Key concept #3
SHARED RESPONSIBILITY
Patient and physician both have responsibilities that are important to achieving an optimal outcome; so does the hospital (TABLE 2 and TABLE 3). Both patient and physician should practice full disclosure throughout the course of care; this will benefit both of you.4 Here are a few select examples.
TABLE 3 Relative degrees of responsibility for a good outcome
vary across interested parties, but none are exempt
| Area of emphasis | Hospital’s responsibility | Physician’s responsibility | Patient’s responsibility |
|---|---|---|---|
| Creating a positive environment for care | 3+ | 2+ | 1+ |
| Providing clear communication | 3+ | 3+ | 3+ |
| Obtaining informed consent | 3+ | 3+ | 3+ |
| Making reasonable requests | 1+ | 1+ | 3+ |
| Compliance | 3+ | 3+ | 3+ |
| Key to the relative scale: 1+: at the least, minimally responsible; 2+: at the least, somewhat responsible; 3+, responsible to the greatest degree. | |||
The importance of the intake form
At the outset of OB care, in most practices, the patient provides the initial detailed medical history by completing a form in the waiting room. In reviewing and completing this survey with her during the appointment, pay particular attention to those questions for which the response has been left blank.
Patients need to understand that key recommendations about their care, and a proper analysis of their concerns, are based on the information that they provide on this survey. In our practices, we find that patients answer most of these early questions without difficulty—even inquiries of a personal nature, such as the number of prior pregnancies, or drug, alcohol, and smoking habits—as long as they understand why it’s in their best interests for you to have this information. If they leave a question blank and you do not follow up verbally, you may have lost invaluable information that can affect the outcome of her pregnancy.
What should you do when, occasionally, a patient refuses to answer one of your questions? We recommend that you record her refusal on the form itself, where the note remains part of the record.
Keep in mind that all necessary and useful information about a patient may not be available, or may not be appropriate to consider, at the initial prenatal visit. In that case, you have an ongoing opportunity—at subsequent visits during the pregnancy—to develop her full medical profile and algorithm.
The necessity of adherence
It almost goes without saying: To provide the care that our patients need, we sometimes require the unpleasant of them—to undergo evaluations, or testing, or to take medications that may be inconvenient or costly.
After you explain the specific course of care to a patient—whether you’re ordering a test or writing a prescription—your follow-up must include notation in the record of adherence. The fact is that both of you share responsibility for having her understand the importance of adherence to your instructions and the consequences of limited adherence or nonadherence.
Recall one of the lessons from the case that introduced this article: For the patient to make an informed decision about her care, the clinician must have thorough knowledge of 1) the risks and benefits of whatever intervention is being proposed in the particular clinical scenario and 2) the available alternatives. It is key that you communicate your risk-benefit assessment accurately to the patient.
Follow-up
Sometimes, new medical problems arise during subsequent prenatal visits. Follow-up appointments also provide an opportunity for you to expand your attention to problems identified earlier. Regardless of what the patient reported about her history and current health at the initial prenatal visit, listen for her to bring new issues to light for resolution later in the pregnancy that will have an impact on L & D. Again, it goes without saying but needs to be said: The OB clinician needs to have whatever skills are necessary to 1) fully evaluate the progress of a pregnancy and 2) make recommendations for care in light of changes in the status of mother and fetus along the way.
TABLE 4 Examples of the cardinal rule of “Be specific”
when you document care
| Instead of noting … | … Use alternative wording |
|---|---|
| “Mild vaginal bleeding” | “Vaginal bleeding requiring two pads an hour” |
| “Gentle traction” | “The shoulders were rotated before assisting the patient’s expulsive efforts” |
| “Patient refuses…” [or “declines…”] | “Patient voiced the nature of the problem and the alternatives that i have explained to her” |
| “Expedited cesarean section” | “The time from decision to incision was 35 minutes” |
Basic principles of documentation
The medical record is the best witness to interactions between a physician and a patient. In the record, we’re required to write a “5-C” description of events—namely, one that is:
- correct
- comprehensive
- conscientious
- clear
- contemporaneous.
Avoid medical jargon in the record. Be careful not to use vague terminology or descriptions, such as “mild vaginal bleeding,” “gentle traction,” or “patient refuses and accepts the consequences.” Specificity is the key to accuracy with respect to documentation (TABLE 4).
Editor’s note: Part 2 of this article will appear in the January 2011 issue of OBG Management. The authors’ analysis of L & D malpractice claims moves to a discussion of causation—by way of 4 troubling cases.
You’ll find a rich, useful archive of expert analysis of your professional liability and malpractice risk, at www.obgmanagement.com
• 10 keys to defending (or, better, keeping clear of) a shoulder dystocia suit
Andrew K. Worek, Esq (March 2008)
• After a patient’s unexpected death, First Aid for the emotionally wounded
Ronald A. Chez, MD, and Wayne Fortin, MS (April 2010)
• Afraid of getting sued? A plaintiff attorney offers counsel (but no sympathy)
Janelle Yates, Senior Editor, with Lewis Laska, JD, PhD (October 2009)
• Can a change in practice patterns reduce the number of OB malpractice claims?
Jason K. Baxter, MD, MSCP, and Louis Weinstein, MD (April 2009)
• Strategies for breaking bad news to patients
Barry Bub, MD (September 2008)
• Stuff of nightmares: Criminal prosecution for malpractice
Gary Steinman, MD, PhD (August 2008)
• Deposition Dos and Don’ts: How to answer 8 tricky questions
James L. Knoll, IV, MD, and Phillip J. Resnick, MD (May 2008)
• Playing high-stakes poker: Do you fight—or settle—that malpractice lawsuit?
Jeffrey Segal, MD (April 2008)
We want to hear from you! Tell us what you think.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
1. Berghella V, Baxter JK, Chauhan SP. Evidence-based labor and delivery management. Am J Obstet Gynecol. 2008;199(5):445-454.
2. Paasche-Orlow MK, Riekert KA, Bilderback A, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172:980-986.
3. Huvane K. Health literacy: reading is just the beginning. Focus on multicultural healthcare. 2007;3(4):16-19.
4. Giordano K. Legal Principles. In: O’Grady JP, Gimovsky ML, Bayer-Zwirello L, Giordano K, eds. Operative Obstetrics. 2nd ed. New York: Cambridge University Press; 2008.
5. The Four Elements of Medical Malpractice Yale New Haven Medical Center: Issues in Risk Management. 1997.
Reducing the medicolegal risk of vacuum extraction
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
Focus on Gynecologic Infections: Clindamycin treatment of bacterial vaginosis reduces preterm deliveries
Objective
To investigate whether treating abnormal genital tract flora with clindamycin vaginal cream in gravidas before 20 weeks’ gestation prevents preterm delivery.
Methods and results
This randomized, double-blind, placebo-controlled tricenter study included 409 women with abnormal genital tract flora presenting to antenatal care clinics at 13 to 20 weeks’ gestation. Infection or colonization consistent with bacterial vaginosis was defined as decreased lactobacilli and increased numbers of “other” bacterial morphotypes and was identified by Gram stain performed on secretions obtained from the upper portion of the vagina. Women who tested positive for infection or colonization were treated with a 3-day course of vaginal clindamycin cream (n = 208) or placebo (n = 201).
If infection or colonization persisted 3 weeks later, a second, 7-day course of the drug or placebo was given, in accordance with the original randomization.
Compared with controls, women treated with clindamycin had a statistically significant reduction in the incidence of preterm delivery (4% versus 10%, P = .03). Consequently, admission to the neonatal intensive care unit also was significantly reduced among babies born to women in the treatment group.
Expert commentary
Preterm delivery remains the bane of the obstetrician’s existence. Treatment of clinically evident preterm labor can delay delivery sufficiently to allow for administration of antenatal steroids, but only rarely is established labor abolished.
Given our limited effectiveness in combating premature labor, one alternative is identifying the woman at risk. Unfortunately, the majority of pregnancies complicated by preterm delivery have no obvious risk factors. The study by Lamont et al is important because it describes a means of identifying and successfully treating infection that might otherwise remain undiagnosed until preterm labor becomes established and essentially untreatable. Indeed, the essence of a good screening method is its ability to identify risk in those who exhibit no ostensible risk—that is, the population at large.
While this study is one of many to consider the role of bacterial vaginosis in preterm labor, the use of a Gram stain to identify the abnormal bacterial morphology is clever and deserves consideration. Once risk is identified, the next logical step is finding a means to facilitate its reduction—and the study succeeds here as well. If the risk of preterm delivery can be suitably diminished—as it was in the women given clindamycin—the potential to lower the preterm delivery rate is greater than with traditional interventions for clinically apparent preterm labor.
My practice is inner city, where preterm deliveries occur for a variety of reasons and the degree of prematurity is on the severe end of the scale. Thus, an approach that clearly lowers admissions to the neonatal intensive care unit would be valuable. In my opinion, this approach is worth a trial.
Bottom line
In the low-risk population studied here, identifying infection by Gram stain and treating it with intravaginal clindamycin cream had a marked impact on the goal of reducing preterm delivery. This is an elegant application of a simple, direct, and inexpensive means to a most valued end.
Objective
To investigate whether treating abnormal genital tract flora with clindamycin vaginal cream in gravidas before 20 weeks’ gestation prevents preterm delivery.
Methods and results
This randomized, double-blind, placebo-controlled tricenter study included 409 women with abnormal genital tract flora presenting to antenatal care clinics at 13 to 20 weeks’ gestation. Infection or colonization consistent with bacterial vaginosis was defined as decreased lactobacilli and increased numbers of “other” bacterial morphotypes and was identified by Gram stain performed on secretions obtained from the upper portion of the vagina. Women who tested positive for infection or colonization were treated with a 3-day course of vaginal clindamycin cream (n = 208) or placebo (n = 201).
If infection or colonization persisted 3 weeks later, a second, 7-day course of the drug or placebo was given, in accordance with the original randomization.
Compared with controls, women treated with clindamycin had a statistically significant reduction in the incidence of preterm delivery (4% versus 10%, P = .03). Consequently, admission to the neonatal intensive care unit also was significantly reduced among babies born to women in the treatment group.
Expert commentary
Preterm delivery remains the bane of the obstetrician’s existence. Treatment of clinically evident preterm labor can delay delivery sufficiently to allow for administration of antenatal steroids, but only rarely is established labor abolished.
Given our limited effectiveness in combating premature labor, one alternative is identifying the woman at risk. Unfortunately, the majority of pregnancies complicated by preterm delivery have no obvious risk factors. The study by Lamont et al is important because it describes a means of identifying and successfully treating infection that might otherwise remain undiagnosed until preterm labor becomes established and essentially untreatable. Indeed, the essence of a good screening method is its ability to identify risk in those who exhibit no ostensible risk—that is, the population at large.
While this study is one of many to consider the role of bacterial vaginosis in preterm labor, the use of a Gram stain to identify the abnormal bacterial morphology is clever and deserves consideration. Once risk is identified, the next logical step is finding a means to facilitate its reduction—and the study succeeds here as well. If the risk of preterm delivery can be suitably diminished—as it was in the women given clindamycin—the potential to lower the preterm delivery rate is greater than with traditional interventions for clinically apparent preterm labor.
My practice is inner city, where preterm deliveries occur for a variety of reasons and the degree of prematurity is on the severe end of the scale. Thus, an approach that clearly lowers admissions to the neonatal intensive care unit would be valuable. In my opinion, this approach is worth a trial.
Bottom line
In the low-risk population studied here, identifying infection by Gram stain and treating it with intravaginal clindamycin cream had a marked impact on the goal of reducing preterm delivery. This is an elegant application of a simple, direct, and inexpensive means to a most valued end.
Objective
To investigate whether treating abnormal genital tract flora with clindamycin vaginal cream in gravidas before 20 weeks’ gestation prevents preterm delivery.
Methods and results
This randomized, double-blind, placebo-controlled tricenter study included 409 women with abnormal genital tract flora presenting to antenatal care clinics at 13 to 20 weeks’ gestation. Infection or colonization consistent with bacterial vaginosis was defined as decreased lactobacilli and increased numbers of “other” bacterial morphotypes and was identified by Gram stain performed on secretions obtained from the upper portion of the vagina. Women who tested positive for infection or colonization were treated with a 3-day course of vaginal clindamycin cream (n = 208) or placebo (n = 201).
If infection or colonization persisted 3 weeks later, a second, 7-day course of the drug or placebo was given, in accordance with the original randomization.
Compared with controls, women treated with clindamycin had a statistically significant reduction in the incidence of preterm delivery (4% versus 10%, P = .03). Consequently, admission to the neonatal intensive care unit also was significantly reduced among babies born to women in the treatment group.
Expert commentary
Preterm delivery remains the bane of the obstetrician’s existence. Treatment of clinically evident preterm labor can delay delivery sufficiently to allow for administration of antenatal steroids, but only rarely is established labor abolished.
Given our limited effectiveness in combating premature labor, one alternative is identifying the woman at risk. Unfortunately, the majority of pregnancies complicated by preterm delivery have no obvious risk factors. The study by Lamont et al is important because it describes a means of identifying and successfully treating infection that might otherwise remain undiagnosed until preterm labor becomes established and essentially untreatable. Indeed, the essence of a good screening method is its ability to identify risk in those who exhibit no ostensible risk—that is, the population at large.
While this study is one of many to consider the role of bacterial vaginosis in preterm labor, the use of a Gram stain to identify the abnormal bacterial morphology is clever and deserves consideration. Once risk is identified, the next logical step is finding a means to facilitate its reduction—and the study succeeds here as well. If the risk of preterm delivery can be suitably diminished—as it was in the women given clindamycin—the potential to lower the preterm delivery rate is greater than with traditional interventions for clinically apparent preterm labor.
My practice is inner city, where preterm deliveries occur for a variety of reasons and the degree of prematurity is on the severe end of the scale. Thus, an approach that clearly lowers admissions to the neonatal intensive care unit would be valuable. In my opinion, this approach is worth a trial.
Bottom line
In the low-risk population studied here, identifying infection by Gram stain and treating it with intravaginal clindamycin cream had a marked impact on the goal of reducing preterm delivery. This is an elegant application of a simple, direct, and inexpensive means to a most valued end.