User login
Distinguishing cellulitis from its mimics
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
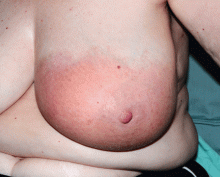
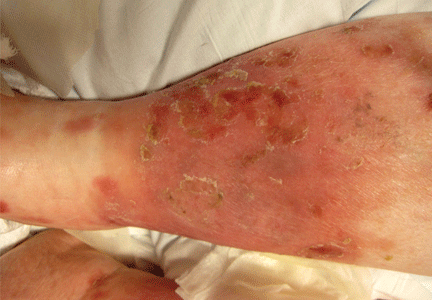
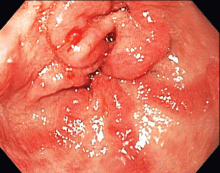
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
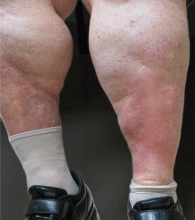
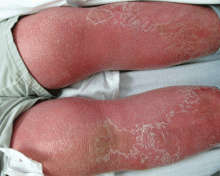
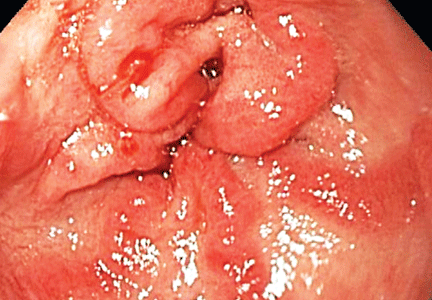
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
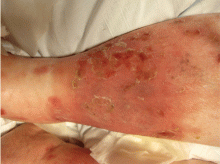
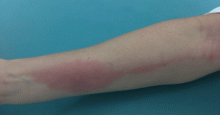
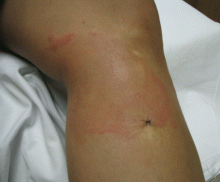
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
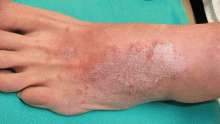
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
KEY POINTS
- Cellulitis is rarely bilateral.
- Patients with cellulitis often have systemic symptoms, such as fever and leukocytosis.
- A chronic course points to a diagnosis other than cellulitis.
- Plaques with a “bound-down” appearance or dark pigmentation point to a chronic disease rather than cellulitis.
- Stasis dermatitis is the most common mimic of cellulitis.
Superior vena cava syndrome
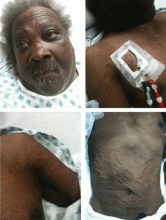
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
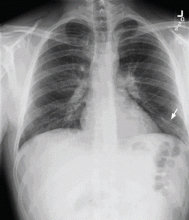
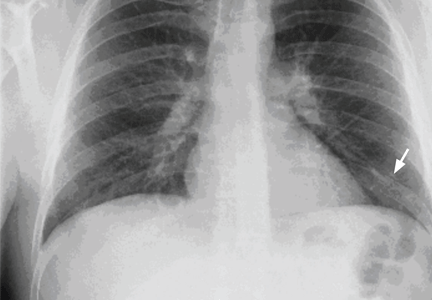
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
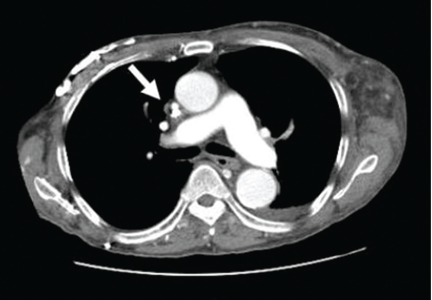
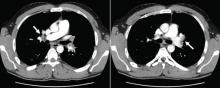
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
A 60-year-old man presented with progressive swelling of his face and neck, which had begun 2 weeks earlier. He denied any headache, lightheadedness, blurry vision, syncope, or change in his cognitive or memory function. A review of symptoms was unremarkable.
The patient had hypertension and end-stage renal disease, for which he was receiving hemodialysis via a catheter tunneled into his right internal jugular vein. He had undergone multiple unsuccessful attempts to create an arteriovenous fistula over the previous 2 years.
Doppler ultrasonography revealed chronic thrombosis and reverse flow in the right internal jugular vein and reverse flow in the right subclavian vein. These findings were consistent with central venous thrombosis and superior vena cava (SVC) syndrome.
Diagnosis: SVC syndrome secondary to intravascular thrombosis related to his central venous dialysis catheter.
SVC SYNDROME
The SVC is the major drainage vessel for venous blood from the head, neck, upper extremities, and upper thorax. Obstruction to its flow increases venous pressure, which results in interstitial edema and retrograde collateral flow.1
More than 80% of cases of SVC syndrome are caused by malignant lung tumors and lymphoma.
Nonmalignant causes include mediastinal fibrosis; vascular diseases (eg, aortic aneurysm, large-vessel vasculitis); infections such as histoplasmosis, tuberculosis, syphilis, and actinomycosis; benign mediastinal tumors such as teratoma, cystic hygroma, thymoma, and dermoid cyst; and thrombosis from central venous catheters, pacemaker leads, and guidewires.2–6 A recent report suggests that benign causes may now account for up to 40% of cases as a result of a rise in the use of indwelling central venous catheters and cardiac pacemakers during the past 2 decades, resulting in a higher incidence of SVC thrombosis.7
An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through different pathways. The most important pathway is the azygos venous system, which includes the azygos vein. Occlusion of the SVC at the level of the azygos vein contributes to the appearance of collateral veins on the chest and abdominal walls, and venous blood flows via these collaterals into the inferior vena cava.1,8,9
Different presentations
The diagnosis of SVC syndrome is often made on clinical grounds alone, ie, the combination of the clinical presentation and, often, a thoracic malignancy or contributing factors such as a central catheter.1
With slowly progressive obstruction of the SVC, the most common presenting symptoms include swelling of the face, neck, and both arms. On the other hand, adequate collateral drainage may develop,1 and patients may have minimal symptoms.
However, a rapid onset of SVC syndrome in the absence of collateral circulation will cause a more dramatic and life-threatening presentation, often with neurologic and respiratory sequelae such as cerebral and laryngeal edema and respiratory embarrassment, which were not present in our patient’s case.1,10–15 These serious complications are rare and are considered an acute emergency. In these cases, special attention to airway, breathing, and circulation (the “ABCs”) is essential, and endovascular repairs and stenting or open surgical reconstruction and alternate approaches for renal replacement therapy should be considered.1,12,13,15
CT is diagnostic and provides accurate information about the location of the obstruction and about other critical surrounding structures such as the lungs, mediastinum, and adjacent blood vessels.1,7,10,11 Our patient’s CT scan confirmed a significant stenosis of the SVC due to thrombosis, with no compression coming from the lungs or mediastinal structures.
Thrombolytic therapy in acute cases
In cases of acute thrombosis (with symptom onset less than 2 days previously), thrombolytic therapy followed by anticoagulation is recommended and may both cause the symptoms to regress within several days and allow the central catheter to be kept in.16 However, thrombolytic therapy is less effective in chronic thrombosis (with onset of symptoms more than 10 days previously).16
Vascular or surgical intervention is often needed to treat SVC syndrome related to dialysis access.
Most experts recommend anticoagulation after thrombosis to prevent disease progression and recurrence, although the benefit of either short-term or long-term anticoagulation therapy for this syndrome is unclear.16
Recommended treatments for cancer-related SVC syndrome include chemotherapy and radiation to shrink the tumor that is causing the obstruction. Tissue diagnosis is often necessary to direct treatment decisions.1 However, percutaneous angioplasty and the use of intravenous stents are becoming increasingly common and are simple, safe, and effective in rapidly relieving SVC syndrome caused by malignant diseases.1 A bypass of the SVC may be indicated in some cases.1 Adjunctive therapies include diuretics, corticosteroids, thrombolytics, anticoagulation, and elevating the head of the patient’s bed.1
CASE CONTINUED
Our patient was started on heparin intravenously for 7 days and long-term oral anticoagulant therapy with warfarin (Coumadin) to continue as long as the catheter was in place, with a target international normalized ratio between 2 and 2.5. He required no other interventions, and his dialysis catheter remained functioning. He was monitored in the hospital for 2 weeks, during which his symptoms gradually improved, with noticeable resolution of his facial swelling.
He was discharged home to continue on an oral anticoagulant and was then followed to monitor for a reappearance of the symptoms (which would force the removal of the catheter), and to pursue possible percutaneous angioplasty, stenting, or surgical reconstruction of the SVC if needed.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007; 356:1862–1869.
- Parish JM, Marschke RF, Dines DE, Lee RE. Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 1981; 56:407–413.
- Aurora R, Milite F, Vander Els NJ. Respiratory emergencies. Semin Oncol 2000; 27:256–269.
- Markman M. Diagnosis and management of superior vena cava syndrome. Cleve Clin J Med 1999; 66:59–61.
- Khanna S, Sniderman K, Simons M, Besley M, Uldall R. Superior vena cava stenosis associated with hemodialysis catheters. Am J Kidney Dis 1993; 21:278–281.
- Bertrand M, Presant CA, Klein L, Scott E. Iatrogenic superior vena cava syndrome. A new entity. Cancer 1984; 54:376–378.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006; 85:37–42.
- Plekker D, Ellis T, Irusen EM, Bolliger CT, Diacon AH. Clinical and radiological grading of superior vena cava obstruction. Respiration 2008; 76:69–75.
- Sheth S, Ebert MD, Fishman EK. Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol 2010; 194:W336–W346.
- DeMichele A, Glick J. Cancer-related emergencies. In:Lenhard R, Osteen R, Gansler T, eds. Clinical Oncology. Atlanta, GA: American Cancer Society; 2001:733–764.
- Chen JC, Bongard F, Klein SR. A contemporary perspective on superior vena cava syndrome. Am J Surg 1990; 160:207–211.
- Sheikh MA, Fernandez BB, Gray BH, Graham LM, Carman TL. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv 2005; 65:405–411.
- Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008; 6:1262–1266.
- Greenberg S, Kosinski R, Daniels J. Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 1991; 99:1298–1301.
- Molhem A, Sabry A, Bawadekji H, Al Saran K. Superior vena cava syndrome in hemodialysis patient. Saudi J Kidney Dis Transpl 2011; 22:381–386.
- Akoglu H, Yilmaz R, Peynircioglu B, et al. A rare complication of hemodialysis catheters: superior vena cava syndrome. Hemodial Int 2007; 11:385–391.
Hyperpigmentation and hypotension
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
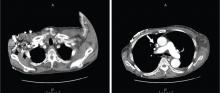
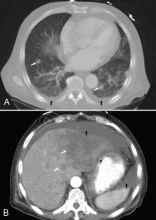
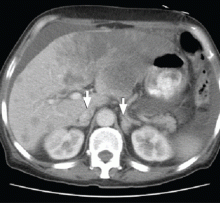
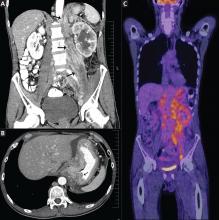
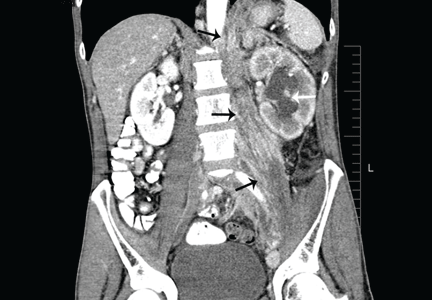
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
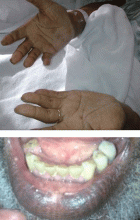
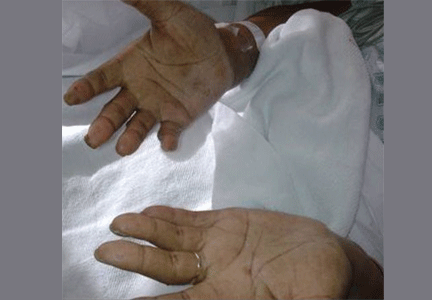
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
A 65-year-old man presents with a 2-month history of generalized weakness, dizziness, and blurred vision. His symptoms began gradually and have been progressing over the last few weeks, so that they now affect his ability to perform normal daily activities.
He has lost 20 lb and has become anorectic. He has no fever, night sweats, headache, cough, hemoptysis, or dyspnea. He has no history of abdominal pain, changes in bowel habits, nausea, vomiting, or urinary symptoms. He was admitted 6 weeks ago for the same symptoms; he was treated for hypotension and received intravenous (IV) fluids and electrolyte supplements for dehydration.
He has a history of hypertension, stroke, vascular dementia, and atrial fibrillation. He is taking warfarin (Coumadin), extended-release diltiazem (Cardizem), simvastatin (Zocor), and donepezil (Aricept). He underwent right hemicolectomy 5 years ago for a large tubular adenoma with high-grade dysplasia in the cecum.
Initial laboratory values are as follows:
- White blood cell count 7.4 × 109/L (reference range 4.5–11.0), with a normal differential
- Mild anemia, with a hemoglobin of 116 g/L (140–175)
- Activated partial thromboplastin time 59.9 sec (23.0–32.4)
- Serum sodium 135 mmol/L (136–142)
- Serum potassium 4.6 mmol/L (3.5–5.0)
- Aspartate aminotransferase 58 U/L (10–30)
- Alanine aminotransferase 16 U/L (10–40)
- Alkaline phosphatase 328 U/L (30–120)
- Urea, creatinine, and corrected calcium are normal.
Electrocardiography shows atrial fibrillation with low-voltage QRS complexes. Chest radiography is normal. A stool test is negative for occult blood. A workup for sepsis is negative.
Q: Which is the appropriate test at this point to determine the cause of the hypotension?
- Serum parathyroid-hormone-related protein
- Baseline serum cortisol, plasma adrenocorticotropic hormone (ACTH) levels, and an ACTH stimulation test with cosyntropin (Cortrosyn)
- Serum thyrotropin level
- Aspiration biopsy of subcutaneous fat with Congo red and immunostaining
- Late-night salivary cortisol
A: The correct next step is to measure baseline serum cortisol, to test ACTH levels, and to order an ACTH stimulation test with cosyntropin.
Primary adrenocortical insufficiency should be considered in patients with metastatic malignancy who present with peripheral vascular collapse, particularly when it is associated with cutaneous hyperpigmentation, chronic malaise, fatigue, weakness, anorexia, weight loss, hypoglycemia, and electrolyte disturbances such as hyponatremia and hyperkalemia.
Checking the baseline serum cortisol and ACTH levels and cosyntropin stimulation testing are vital steps in making an early diagnosis of primary adrenocortical insufficiency. Inappropriately low serum cortisol is highly suggestive of primary adrenal insufficiency, especially if accompanied by simultaneous elevation of the plasma ACTH level. The result of the ACTH stimulation test with cosyntropin is often confirmatory.
Measuring the serum parathyroid-hormone-related protein level is not indicated, since the patient has a normal corrected calcium. Patients with ectopic Cushing syndrome may present with weight loss due to underlying malignancy, but the presence of hypotension and a lack of hypokalemia makes such a diagnosis unlikely, and, therefore, measurement of late-night salivary cortisol is not the best answer. Amyloidosis, hypothyroidism, or hyperthyroidism are unlikely to have this patient’s presentation.
RESULTS OF FURTHER EVALUATION
Our patient’s ACTH serum level was elevated, and an ACTH stimulation test with cosyntropin confirmed the diagnosis of primary adrenal insufficiency.
CT of the abdomen failed to demonstrate primary tumors, but both adrenal glands were enlarged, likely from metastasis (Figure 4). His hypotension responded to treatment with hydrocortisone and fludrocortisone, and his symptoms resolved. No further testing or therapy was directed to the primary occult malignancy, as it was considered advanced. The prognosis was discussed with the patient, and he deferred any further management and was discharged to hospice care. He died a few months later.
PRIMARY ADRENOCORTICAL INSUFFICIENCY
Primary adrenocortical insufficiency is an uncommon disorder caused by destruction or dysfunction of the adrenal cortices. It is characterized by chronic deficiency of cortisol, aldosterone, and adrenal androgens. In the United States, nearly 6 million people are considered to have undiagnosed adrenal insufficiency, which is clinically significant only during times of physiologic stress.1
Primary adrenocortical insufficiency affects men and women equally. However, the idiopathic autoimmune form of adrenal insufficiency (Addison disease) is two to three times more common in women than in men.
If the condition is undiagnosed or ineffectively treated, the risk of significant morbidity and death is high. Symptoms and signs are nonspecific, and the onset is insidious.
Almost all patients with primary adrenal insufficiency have malaise, fatigue, anorexia, and weight loss. Vomiting, abdominal pain, and fever are more common during an adrenal crisis, when a patient with subclinical disease is subjected to major stress. Postural dizziness or syncope is a common result of volume depletion and hypotension.2–4 It is commonly accompanied by hyponatremia and hyperkalemia.
Hyperpigmentation is the most characteristic physical finding and is caused by an ACTH-mediated increase in melanin content in the skin.2,4,5 The resulting brown hyperpigmentation is most obvious in areas exposed to sunlight (face, neck, backs of hands), and in areas exposed to chronic friction or pressure, such as the elbows, knees, knuckles, waist, and shoulders (brassiere straps).4 Pigmentation is also prominent in the palmar creases, areolae, axillae, perineum, surgical scars, and umbilicus. Other patterns of hyperpigmentation are patchy pigmentation on the inner surface of lips, the buccal mucosa, under the tongue, and on the hard palate.3,5 The hyperpigmentation begins to fade within several days and largely disappears after a few months of adequate glucocorticoid therapy.4
In the United States, 80% of cases of primary adrenocortical insufficiency are caused by autoimmune adrenal destruction. The remainder are caused by infectious diseases (eg, tuberculosis, fungal infection, cytomegalovirus infection, and Mycobacterium aviumintracellulare infection in the context of human immunodeficiency virus infection), by infiltration of the adrenal glands by metastatic cancer, by adrenal hemorrhage, or by drugs such as ketoconazole, fluconazole (Diflucan), metyrapone (Metopirone), mitotane (Lysodren), and etomidate (Amidate).4,6
Adrenal metastatic disease
Infiltration of the adrenal glands by metastatic cancer is not uncommon, probably because of their rich sinusoidal blood supply, and the adrenals are the fourth most common site of metastasis. Common primary tumors are lung, breast, melanoma, gastric, esophageal, and colorectal cancers, while metastasis due to an undetermined primary tumor is the least common.7
Clinically evident adrenal insufficiency produced by metastatic carcinoma is uncommon because most of the adrenal cortex must be destroyed before hypofunction becomes evident.7–9
Malignancy rarely presents first as adrenal insufficiency caused by metastatic infiltration.10
Hormonal therapy may significantly improve symptoms and quality of life in patients with metastatic adrenal insufficiency.8,11
DIAGNOSIS AND MANAGEMENT
Once primary adrenal insufficiency is suspected, prompt diagnosis and treatment are essential. A low plasma cortisol level (< 3 μg/dL) at 8 am is highly suggestive of adrenal insufficiency if exposure to exogenous glucocorticoids has been excluded (including oral, inhaled, and injected),12,13 especially if accompanied by simultaneous elevation of the plasma ACTH level (usually > 200 pg/mL). An 8 am cortisol concentration above 15 μg/dL makes adrenal insufficiency highly unlikely, but levels between 3 and 15 μg/dL are nondiagnostic and need to be further evaluated by an ACTH stimulation test with cosyntropin.4,7
Imaging in primary adrenal insufficiency may be considered when the condition is not clearly autoimmune.14 Abdominal CT is the ideal imaging test for detecting abnormal adrenal glands. CT shows small, noncalcified adrenals in autoimmune Addison disease. It demonstrates enlarged adrenals in about 85% of cases caused by metastatic or granulomatous disease; and calcification is noted in cases of tuberculous adrenal disease.4
Management involves treating the underlying cause and starting hormone replacement therapy. Hormonal therapy consists of corticosteroids and mineralocorticoids; hydrocortisone is the drug of choice and is usually given with fludrocortisone acetate, which has a potent sodium-retaining effect. In the presence of a stressor (fever, surgery, severe illness), the dose of hydrocortisone should be doubled (> 50 mg hydrocortisone per day) for at least 3 to 5 days.2,4
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
- Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol 2009; 160:233–237.
- Oelkers W. Adrenal insufficiency. N Engl J Med 1996; 335:1206–1212.
- Redman BG, Pazdur R, Zingas AP, Loredo R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987; 60:103–107.
- Berger M., Hypofunction of the adrenal cortex in infancy. Manit Med Rev 1949; 29:132.
- Stulberg DL, Clark N, Tovey D. Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, café au lait macules, diffuse hyperpigmentation, sun exposure, and phototoxic reactions. Am Fam Physician 2003; 68:1955–1960.
- Zelissen PM, Bast EJ, Croughs RJ. Associated autoimmunity in Addison’s disease. J Autoimmun 1995; 8:121–130.
- Lutz A, Stojkovic M, Schmidt M, Arlt W, Allolio B, Reincke M. Adrenocortical function in patients with macrometastases of the adrenal gland. Eur J Endocrinol 2000; 143:91–97.
- Kung AW, Pun KK, Lam K, Wang C, Leung CY. Addisonian crisis as presenting feature in malignancies. Cancer 1990; 65:177–179.
- Cedermark BJ, Sjöberg HE. The clinical significance of metastases to the adrenal glands. Surg Gynecol Obstet 1981; 152:607–610.
- Rosenthal FD, Davies MK, Burden AC. Malignant disease presenting as Addison’s disease. Br Med J 1978; 1:1591–1592.
- Seidenwurm DJ, Elmer EB, Kaplan LM, Williams EK, Morris DG, Hoffman AR. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984; 54:552–557.
- Santiago AH, Ratzan S. Acute adrenal crisis in an asthmatic child treated with inhaled fluticasone proprionate. Int J Pediatr Endocrinol 2010; 2010. pii:749239.
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J 2008; 32:1047–1052.
- Mohammad K, Sadikot RT. Adrenal insufficiency as a presenting manifestation of nonsmall cell lung cancer. South Med J 2009; 102:665–667.
Factor V Leiden: How great is the risk of venous thromboembolism?
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
A 29-year-old white man with no chronic medical problems presents to the emergency department with shortness of breath, left-sided pleuritic chest pain, cough, and hemoptysis. These symptoms began abruptly 1 day ago and have persisted. He also has mild pain and swelling in both calves. He denies having any fever, night sweats, or chills. On further questioning, he reports having taken a long, nonstop driving trip that lasted 8 hours 1 week ago.
His medical history is negative, and he specifically reports no history of deep venous thrombosis or pulmonary embolism. He underwent appendectomy 10 years ago but has had no other operations. He does not take any medications. His family history is noncontributory and is negative for venous thromboembolism. He smokes and uses alcohol occasionally but not illicit drugs.
Examination. He appears to be in considerable distress because of his chest pain. His temperature is 100.4°F (38.0°C), blood pressure 125/70 mm Hg, heart rate 125 beats per minute, respiratory rate 26 breaths per minute, oxygen saturation 92% on room air, and body mass index 19 kg/m2.
Chest examination reveals diminished vesicular breathing in the left base, which is normal to percussion without added sounds. Both calves are swollen and tender to palpation without skin discoloration. The rest of his examination is normal.
Laboratory values:
- White blood cell count 9.3 × 109/L (reference range 4.5–11.0)
- Hemoglobin 15.9 g/dL (14.0–17.5)
- Platelets 205 × 109/L (150–350)
- Sodium 140 mEq/L (136–142)
- Potassium 3.9 mEq/L (3.5–5.0)
- Chloride 108 mEq/L (96–106)
- Bicarbonate 23 mEq/L (21–28)
- Blood urea nitrogen 14 mg/dL (8–23)
- Creatinine 0.9 mg/dL (0.6–1.2)
- Glucose 95 mg/dL (70–110)
- International normalized ratio (INR) 0.90 (0.00–1.2)
- Partial thromboplastin time 27.5 seconds (24.6–31.8)
- Creatine phosphokinase 205 U/L (39–308)
- Troponin T < 0.015 ng/mL (0.01–0.045).
Pulmonary embolism is diagnosed
Factor V Leiden is diagnosed, and the patient recovers with treatment
Anticoagulation is started in the emergency department.
Given this patient’s young age and clot burden, a hypercoagulable state is suspected. Thrombophilia screening is performed, with tests for the factor V Leiden mutation, the prothrombin G20210A mutation, and antiphospholipid and lupus anticoagulant antibodies. The rest of the thrombophilia panel, including antithrombin III, factor VIII, protein C, and protein S, is deferred because the levels of these substances would be expected to change during the acute thrombosis.
The direct test for factor V Leiden mutation is positive for the heterozygous type. The test for the prothrombin G20210A mutation is negative, and his antiphospholipid antibody levels, including the lupus anticoagulant titer, are within normal limits.
The patient is kept on a standard regimen of unfractionated heparin, overlapped with warfarin (Coumadin) until his INR is 2.0 to 3.0 on 2 consecutive days. His hospital course is uneventful and his condition gradually improves.
He is discharged home to continue on oral anticoagulation for 6 months with a target INR of 2.0 to 3.0. Two weeks after completing his anticoagulation therapy, his levels of antithrombin III, factor VIII, protein C, and protein S are all within normal limits.
FACTOR V LEIDEN IS COMMON
Factor V Leiden is the most common inherited thrombophilia, with a prevalence of 3% to 7% in the general US population,1 approximately 5% in whites, 2.2% in Hispanics, and 1.2% in blacks.2 Its prevalence in patients with venous thromboembolism, however, is 50%.1,3 The annual incidence of venous thromboembolism in patients with factor V Leiden is 0.5%.4,5
MORE COAGULATION, LESS ANTICOAGULATION
Factor V has a critical position in both the coagulant and anticoagulant pathways. Factor V Leiden results in a hypercoagulable state by both increasing coagulation and decreasing anticoagulation.
This mutation causes factor V to be resistant to being cleaved and inactivated by activated protein C, a condition known as APC resistance. As a result, more factor Va is available within the prothrombinase complex, increasing coagulation by increased generation of thrombin.6–8
Furthermore, a cofactor formed by cleavage of factor V at position 506 is thought to support activated protein C in degrading factor VIIIa (in the tenase complex), along with protein S. People with factor V Leiden lack this cleavage product and thus have less anticoagulant activity from activated protein C. The increased coagulation and decreased anticoagulation appear to contribute equally to the hypercoagulable state in factor V Leiden-associated APC resistance.9–11
Heterozygosity for the factor V Leiden mutation accounts for 90% to 95% of cases of APC resistance. A much smaller number of people are homozygous for it.1
People who are homozygous for factor V Leiden are at higher risk of venous thromboembolism than those who are heterozygous for it, since the latter group’s blood contains both factor V Leiden and normal factor V. The normal factor V allows anticoagulation via the second pathway of inactivation of factor VIIIa by activated protein C, giving some protection against thrombosis. In people who are homozygous for factor V Leiden, the lack of normal factor V acting as an anticoagulant protein results in a higher thrombotic risk.9–11
Other factor V mutations may also cause APC resistance
Although factor V Leiden is the only genetic defect for which a causal relationship with APC resistance has been clearly determined, other, rarer hereditary factor V mutations or polymorphisms have been described, such as factor V Cambridge (Arg306Thr)12 and factor V Hong Kong (Arg306Gly).13 These mutations may result in APC resistance, but their clinical association with thrombosis is less clear.14 Factor V Liverpool (Ile359Thr) is associated with a higher risk of thrombosis, apparently because of reduced APC-mediated inactivation of factor Va and because it is a poor cofactor with activated protein C for the inactivation of factor VIIIa.15
An R2 haplotype has also been described in association with APC resistance.16,17 The phenomenon may be due to a reduction in activated protein C cofactor activity.9 However, not all studies have been convincing regarding the role of this haplotype in clinical disease.18 Coinheritance of this haplotype with factor V Leiden may increase the risk of venous thromboembolism above that associated with factor V Leiden alone.19
Although factor V Leiden is the most common cause of inherited APC resistance, other changes in hemostasis cause acquired APC resistance and may contribute to the thrombotic tendency in these patients.20–22 The most common causes of acquired APC resistance include elevated factor VIII levels,23–25 pregnancy,26–28 use of oral contraceptives,29,30 and antiphospholipid antibodies.31
USUALLY MANIFESTS AS DEEP VEIN THROMBOSIS
Factor V Leiden usually manifests as deep vein thrombosis with or without pulmonary embolism, but thrombosis in unusual locations also occurs.32
The risk of a first episode of venous thromboembolism is two to five times higher with heterozygous factor V Leiden. However, even though the relative risk is high, the absolute risk is low. Furthermore, despite the higher risk of venous thrombosis, there is no evidence that heterozygosity for factor V Leiden increases the overall mortality rate.4,33–36
In people with homozygous factor V Leiden or with combined inherited thrombophilias, the risk of venous thromboembolism is increased to a greater degree: it is 20 to 50 times higher.7,8,37–39 However, whether the risk of death is higher is not clear.
VENOUS THROMBOEMBOLISM IS MULTIFACTORIAL
The pathogenesis of venous thromboembolism is multifactorial and involves an interaction between inherited and acquired factors. Very often, people with factor V Leiden have additional risk factors that contribute to the development of venous clots, and it is very unusual for them to have thrombosis in the absence of these additional factors.
These factors include older age, surgery, obesity, prolonged travel, immobility, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy. They increase the risk of venous thrombosis in normal individuals as well, but more so in people with factor V Leiden.40–43
Testing for other known causes of thrombophilia may also be pursued. These include elevated homocysteine levels, the factor II (prothrombin) G20210A mutation, anticardiolipin antibody, lupus anticoagulant, and deficiencies of antithrombin III, protein C, and protein S.
Factor V Leiden by itself does not appear to increase the risk of arterial thrombosis, ie, heart attack and stroke.33,38,44–46
Family history: A risk indicator for venous thrombosis
Family history is an important indicator of risk for a first venous thromboembolic event, regardless of other risk factors identified. The risk of a first event is two to three times higher in people with a family history of thrombosis in a first-degree relative. The risk is four times higher when multiple family members are affected, at least one of them before age 50.47
In people with genetic thrombophilia, the risk of thrombosis (especially unprovoked thrombosis at a young age) is also higher in those with a strong family history than in those without a family history. In those with factor V Leiden, the risk of venous thromboembolism is three to four times higher if there is a positive family history. The risk is five times higher in carriers of factor V Leiden with a family history of venous thromboembolism before age 50, and 13 times higher in those with more than one affected family member.47
Possible shared environmental factors or coinheritance of other unidentified genetic factors may also contribute to the higher susceptibility in thrombosis-prone families.
TESTING FOR APC RESISTANCE AND FACTOR V LEIDEN
The factor V Leiden mutation can be detected directly by genetic testing of peripheral blood mononuclear cells. This method is relatively time-consuming and expensive, however.
At present, the most cost-effective approach is to test first for APC resistance using a second-generation coagulation assay—the modified APC sensitivity test. In this clot-based method, the patient’s sample is prediluted with factor V-deficient plasma to eliminate the effect of lupus anticoagulants and factor deficiencies that could prolong the baseline clotting time, and heparin is inactivated by polybrene. Then either an augmented partial-thromboplastin-time-based assay or a tissue-factor-dependent factor V assay is performed.
This test is nearly 100% sensitive and specific for factor V Leiden, in contrast to the first-generation, or classic, APC sensitivity test, which lacked specificity and sensitivity for it.9–11,48–60 This modification also permits testing of patients receiving anticoagulants or who have abnormal augmented partial thromboplastin times due to coagulation factor deficiencies.
A positive result on the modified APC sensitivity test should be confirmed by a direct genetic test for the factor V Leiden mutation. An APC resistance assay is unnecessary if a direct genetic test is used initially.
HOW LONG TO GIVE ANTICOAGULATION AFTER VENOUS THROMBOEMBOLISM?
Patients who have had an episode of venous thromboembolism have to be treated with anticoagulants.
In general, the initial management of venous thromboembolism in patients with heritable thrombophilias is no different from that in any other patient with a clot. Anticoagulants such as warfarin are given at a target INR of 2.5 (range 2.0–3.0).32 The duration of treatment is based on the risk factors that resulted in the thrombotic event.
After a first event, some authorities recommend anticoagulant therapy for 6 months.32 A shorter period (3 months) is recommended if there is a transient risk factor (eg, surgery, oral contraceptive use, travel, pregnancy, the puerperium) and the thrombosis is confined to distal veins (eg, the calf veins).32
Factor V Leiden does not necessarily increase the risk of recurrent events in patients who have a transient risk factor. Therefore, people who are heterozygous for this mutation do not usually need to be treated lifelong with anticoagulants if they have had only one episode of deep vein thrombosis or pulmonary embolism, given the risk of bleeding associated with anticoagulation, unless they have additional risk factors.
Conditions in which indefinite anticoagulation may be required after careful consideration of the risks and benefits are:
- Life-threatening events such as near-fatal pulmonary embolism
- Cerebral or visceral vein thrombosis
- Recurrent thrombotic events
- Additional persistent risk factors (eg, active malignant neoplasm, extremity paresis, and antiphospholipid antibodies)
- Combined thrombophilias (eg, combined heterozygosity for factor V Leiden and the prothrombin G20210A mutation)
- Homozygosity for factor V Leiden.32,46,48
Factor V Leiden by itself or combined with other thrombophilic abnormalities is not associated with a higher risk of recurrent venous thromboembolism during warfarin therapy (a possible exception is the combination of factor V Leiden plus antiphospholipid antibodies).32,34 Furthermore, current evidence suggests that no thrombophilic defect is a clinically important risk factor for recurrent venous thromboembolism after anticoagulant therapy is stopped. All these facts indicate that clinical factors are probably more important than laboratory abnormalities in determining the duration of anticoagulation therapy.32,35,36,61–63
PRIMARY PROPHYLAXIS IN PATIENTS WITH FACTOR V LEIDEN
Factor V Leiden is only one of many risk factors for deep vein thrombosis or pulmonary embolism. If carriers of factor V Leiden have never had a blood clot, then they are not routinely treated with an anticoagulant. Rather, they should be counseled about reducing or eliminating other factors that may add to their risk of developing a clot in the future.
Usually, the effect of risk factors is additive: the more risk factors present, the higher the risk.46,50 Sometimes, however, the effect of multiple risk factors is more than additive.
Some risk factors, such as genetics or age, are not alterable, but many can be controlled by medications or lifestyle modifications. Therefore, general measures and precautions are recommended to minimize the risk of thrombosis. For example:
Losing weight (if the patient is overweight) is an important intervention for risk reduction, since obesity is probably the most common modifiable risk factor for developing blood clots.
Avoiding long periods of immobility is recommended. For example, if the patient is taking a long car ride (more than 2 hours), then stopping every few hours and walking around for a few minutes is a good way to keep the blood circulating. If the patient has a desk job, getting up and walking around the office periodically is advised. On long airplane trips, a walk in the aisle every so often and preventing dehydration by drinking plenty of fluids and avoiding alcohol are recommended.
Wearing elastic stockings with a graduated elastic pressure may prevent deep venous thrombosis from developing on long flights.63–65
Staying active and getting regular exercise through such activities as walking, bicycling, or swimming are helpful.
Avoiding smoking is critical.50,63
Thromboprophylaxis is recommended for most acutely ill hospitalized patients, especially those confined to bed with additional risk factors. Guidelines for prophylaxis are based on an individualized risk assessment and not on thrombophilia status. Prophylactic anticoagulation is routinely recommended for patients undergoing major high-risk surgery, such as an orthopedic, urologic, gynecologic, or bariatric procedure. Any excess thrombotic risk conferred by thrombophilia is likely small compared with the risk of surgery, and recommendations on the duration and intensity of thromboprophylaxis are not based on thrombophilic status.46,48
Education. Pain, swelling, redness of a limb, unexplained shortness of breath, and chest pain are the most common symptoms of deep vein thrombosis and pulmonary embolism.46,50 It is crucial to teach patients with factor V Leiden to recognize these symptoms and to seek early medical attention in case they experience any of them.
SCREENING FAMILY MEMBERS FOR THE FACTOR V LEIDEN MUTATION
Factor V Leiden by itself is a relatively mild thrombophilic defect that does not cause thrombosis in all carriers, and there is no evidence that early diagnosis reduces rates of morbidity or mortality. Therefore, routine screening of all asymptomatic relatives of affected patients with venous thrombosis is not recommended. Rather, the decision to screen should be made on an individual basis.50,66
Screening may be beneficial in selected cases, especially when patients have a strong family history of recurrent venous thrombosis at a young age (younger than 50 years) and the family member has additional risk factors for venous thromboembolism such as oral contraception or is planning for pregnancy.32,48,49,66
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet 1995; 346:1133–1134.
- Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA 1997; 277:1305–1307.
- Rosendaal FR, Koster T, Vandenbroucke JP, Reitsma PH. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995; 85:1504–1508.
- Stolz E, Kemkes-Matthes B, Pötzsch B, et al. Screening for thrombophilic risk factors among 25 German patients with cerebral venous thrombosis. Acta Neurol Scand 2000; 102:31–36.
- Langlois NJ, Wells PS. Risk of venous thromboembolism in relatives of symptomatic probands with thrombophilia: a systematic review. Thromb Haemost 2003; 90:17–26.
- Juul K, Tybjaerg-Hansen A, Mortensen J, Lange P, Vestbo J, Nordestgaard BG. Factor V leiden homozygosity, dyspnea, and reduced pulmonary function. Arch Intern Med 2005; 165:2032–2036.
- Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994; 369:64–67.
- Dahlbäck B. New molecular insights into the genetics of thrombophilia. Resistance to activated protein C caused by Arg506 to Gln mutation in factor V as a pathogenic risk factor for venous thrombosis. Thromb Haemost 1995; 74:139–148.
- Castoldi E, Brugge JM, Nicolaes GA, Girelli D, Tans G, Rosing J. Impaired APC cofactor activity of factor V plays a major role in the APC resistance associated with the factor V Leiden (R506Q) and R2 (H1299R) mutations. Blood 2004; 103:4173–4179.
- Dahlback B. Anticoagulant factor V and thrombosis risk (editorial). Blood 2004; 103:3995.
- Simioni P, Castoldi E, Lunghi B, Tormene D, Rosing J, Bernardi F. An underestimated combination of opposites resulting in enhanced thrombotic tendency. Blood 2005; 106:2363–2365.
- Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306-->Thr) associated with resistance to activated protein C. Blood 1998; 91:1140–1144.
- Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood 1998; 91:1135–1139.
- Liang R, Lee CK, Wat MS, Kwong YL, Lam CK, Liu HW. Clinical significance of Arg306 mutations of factor V gene. Blood 1998; 92:2599–2600.
- Steen M, Norstrøm EA, Tholander AL, et al. Functional characterization of factor V-Ile359Thr: a novel mutation associated with thrombosis. Blood 2004; 103:3381–3387.
- Bernardi F, Faioni EM, Castoldi E, et al. A factor V genetic component differing from factor V R506Q contributes to the activated protein C resistance phenotype. Blood 1997; 90:1552–1557.
- Lunghi B, Castoldi E, Mingozzi F, Bernardi F. A new factor V gene polymorphism (His 1254 Arg) present in subjects of African origin mimics the R2 polymorphism (His 1299 Arg). Blood 1998; 91:364–365.
- Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost 2000; 83:204–208.
- Faioni EM, Franchi F, Bucciarelli P, et al. Coinheritance of the HR2 haplotype in the factor V gene confers an increased risk of venous thromboembolism to carriers of factor V R506Q (factor V Leiden). Blood 1999; 94:3062–3066.
- Clark P, Walker ID. The phenomenon known as acquired activated protein C resistance. Br J Haematol 2001; 115:767–773.
- Tosetto A, Simioni M, Madeo D, Rodeghiero F. Intraindividual consistency of the activated protein C resistance phenotype. Br J Haematol 2004; 126:405–409.
- de Visser MC, Rosendaal FR, Bertina RM. A reduced sensitivity for activated protein C in the absence of factor V Leiden increases the risk of venous thrombosis. Blood 1999; 93:1271–1276.
- Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost 2000; 83:5–9.
- Kamphuisen PW, Eikenboom JC, Bertina RM. Elevated factor VIII levels and the risk of thrombosis. Arterioscler Thromb Vasc Biol 2001; 21:731–738.
- Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155.
- Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost 1998; 79:1166–1170.
- Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol 1995; 90:725–727.
- Mathonnet F, de Mazancourt P, Bastenaire B, et al. Activated protein C sensitivity ratio in pregnant women at delivery. Br J Haematol 1996; 92:244–246.
- Post MS, Rosing J, Van Der Mooren MJ, et al; Ageing Women’ and the Institute for Cardiovascular Research-Vrije Universiteit (ICaRVU). Increased resistance to activated protein C after short-term oral hormone replacement therapy in healthy post-menopausal women. Br J Haematol 2002; 119:1017–1023.
- Olivieri O, Friso S, Manzato F, et al. Resistance to activated protein C in healthy women taking oral contraceptives. Br J Haematol 1995; 91:465–470.
- Bokarewa MI, Blombäck M, Egberg N, Rosén S. A new variant of interaction between phospholipid antibodies and the protein C system. Blood Coagul Fibrinolysis 1994; 5:37–41.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- van Stralen KJ, Doggen CJ, Bezemer ID, Pomp ER, Lisman T, Rosendaal FR. Mechanisms of the factor V Leiden paradox. Arterioscler Thromb Vasc Biol 2008; 28:1872–1877.
- Agaoglu N, Mustafa NA, Turkyilmaz S. Prothrombotic disorders in patients with mesenteric vein thrombosis. J Invest Surg 2003; 16:299–304.
- El-Karaksy H, El-Koofy N, El-Hawary M, et al. Prevalence of factor V Leiden mutation and other hereditary thrombophilic factors in Egyptian children with portal vein thrombosis: results of a single-center case-control study. Ann Hematol 2004; 83:712–715.
- Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost 1998; 80:607–609.
- Turkstra F, Karemaker R, Kuijer PM, Prins MH, Büller HR. Is the prevalence of the factor V Leiden mutation in patients with pulmonary embolism and deep vein thrombosis really different? Thromb Haemost 1999; 81:345–348.
- Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med 1995; 332:912–917.
- Manten B, Westendorp RG, Koster T, Reitsma PH, Rosendaal FR. Risk factor profiles in patients with different clinical manifestations of venous thromboembolism: a focus on the factor V Leiden mutation. Thromb Haemost 1996; 76:510–513.
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005; 293:715–722.
- Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Büller HR, Vandenbroucke JP. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet 1995; 346:1593–1596.
- Murphy PT. Factor V Leiden and venous thromboembolism. Ann Intern Med 2004; 141:483–484.
- Nizankowska-Mogilnicka E, Adamek L, Grzanka P, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J 2003; 21:25–30.
- Arsov T, Miladinova D, Spiroski M. Factor V Leiden is associated with higher risk of deep venous thrombosis of large blood vessels. Croat Med J 2006; 47:433–439.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood 2000; 96:3329–3333.
- Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation 2003; 107:e94–e97.
- Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med 2009; 169:610–615.
- Press RD, Bauer KA, Kujovich JL, Heit JA. Clinical utility of factor V leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med 2002; 126:1304–1318.
- Gadelha T, Roldán V, Lecumberri R, et al; RIETE Investigators. Clinical characteristics of patients with factor V Leiden or prothrombin G20210A and a first episode of venous thromboembolism. Findings from the RIETE Registry. Thromb Res 2010; 126:283–286.
- Severinsen MT, Overvad K, Johnsen SP, et al. Genetic susceptibility, smoking, obesity and risk of venous thromboembolism. Br J Haematol 2010; 149:273–279.
- Kujovich JL. Factor V Leiden thrombophilia. Genet Med 2011; 13:1–16.
- Lijfering WM, Brouwer JL, Veeger NJ, et al. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood 2009; 113:5314–5322.
- Kearon C, Julian JA, Kovacs MJ, et al; ELATE Investigators. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood 2008; 112:4432–4436.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006; 166:729–736.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005; 293:2352–2361.
- Strobl FJ, Hoffman S, Huber S, Williams EC, Voelkerding KV. Activated protein C resistance assay performance: improvement by sample dilution with factor V-deficient plasma. Arch Pathol Lab Med 1998; 122:430–433.
- Legnani C, Palareti G, Biagi R, et al. Activated protein C resistance: a comparison between two clotting assays and their relationship to the presence of the factor V Leiden mutation. Br J Haematol 1996; 93:694–699.
- Gouault-Heilmann M, Leroy-Matheron C. Factor V Leiden-dependent APC resistance: improved sensitivity and specificity of the APC resistance test by plasma dilution in factor V-depleted plasma. Thromb Res 1996; 82:281–283.
- Svensson PJ, Zöller B, Dahlbäck B. Evaluation of original and modified APC-resistance tests in unselected outpatients with clinically suspected thrombosis and in healthy controls. Thromb Haemost 1997; 77:332–335.
- Tripodi A, Negri B, Bertina RM, Mannucci PM. Screening for the FV:Q506 mutation—evaluation of thirteen plasma-based methods for their diagnostic efficacy in comparison with DNA analysis. Thromb Haemost 1997; 77:436–439.
- Wåhlander K, Larson G, Lindahl TL, et al. Factor V Leiden (G1691A) and prothrombin gene G20210A mutations as potential risk factors for venous thromboembolism after total hip or total knee replacement surgery. Thromb Haemost 2002; 87:580–585.
- Joseph JE, Low J, Courtenay B, Neil MJ, McGrath M, Ma D. A single-centre prospective study of clinical and haemostatic risk factors for venous thromboembolism following lower limb arthroplasty. Br J Haematol 2005; 129:87–92.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):381S–453S.
- Brenner B. Prophylaxis for travel-related thrombosis? Yes. J Thromb Haemost 2004; 2:2089–2091.
- Gavish I, Brenner B. Air travel and the risk of thromboembolism. Intern Emerg Med 2011; 6:113–116.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
KEY POINTS
- The pathogenesis of venous thromboembolism is complex and multifactorial, often reflecting the interplay between environmental, clinical, and genetic factors.
- Factor V Leiden increases the risk of venous thromboembolism but by itself does not appear to increase the risk of arterial thrombosis.
- Often, people with factor V Leiden may have additional risk factors that increase the rate of venous clots, such as older age, surgery, obesity, immobility, prolonged travel, hospitalization, oral contraceptive use, hormonal replacement therapy, pregnancy, and malignancy.
- General measures and precautions are needed to minimize the risk of venous thromboembolism in people with the factor V Leiden mutation, especially when modifiable factors are present, such as obesity and long periods of immobilization.
Should target natriuretic peptide levels be used for outpatient management of chronic heart failure?
In the last few years, a number of randomized controlled trials have explored the value of using target levels of natriuretic peptides such as brain-type natriuretic peptide (BNP) and N-terminal BNP in the outpatient management of heart failure. Unfortunately, the results have been inconclusive.
RATIONALE FOR TARGETING NATRIURETIC PEPTIDE LEVELS
Heart failure causes devastating morbidity and death, yet its management is guided more often by subjective than by objective data.1 In other chronic conditions such as hypertension, diabetes mellitus, and hyperlipidemia, numerical targets for blood pressure, hemoglobin A1c, and low-density lipoprotein cholesterol levels are used to guide medical therapy, and lower rates of both morbidity and death have resulted.1 Extensive efforts have been undertaken to use natriuretic peptide levels to similarly guide heart failure therapy and improve outcomes.
LIMITATIONS TO TARGETING NATRIURETIC PEPTIDES
The relationship between natriuretic peptide levels and patient symptoms1 and outcomes2 is neither predictable nor linear, although the association between these levels and outcomes is stronger at the extremes, ie, at very low and very high levels.
Moreover, baseline levels vary significantly among people and within the same person, affected by factors such as genetic polymorphisms, 3 age, sex,4 body mass index,5 and other diseases, such as renal insufficiency.6
In addition, natriuretic peptide levels behave differently depending on the type of heart failure, rising much higher in systolic heart failure than in diastolic heart failure.7
ESTABLISHED USES OF MEASURING NATRIURETIC PEPTIDE LEVELS
Measuring natriuretic peptide levels has proven useful in diagnosing heart failure and in risk stratification of heart failure patients. BNP levels of less than 100 pg/mL practically exclude the diagnosis of heart failure (negative predictive value 89%),8 as do N-terminal BNP levels less than 300 pg/mL (negative predictive value 99%).9 Changes from baseline levels during acute hospitalization correlate with heart failure mortality rates, while elevated levels at discharge are associated with a higher risk of heart failure death and of readmission.10,11
NATRIURETIC PEPTIDES TO GUIDE THERAPY
Of the seven published clinical trials of therapy guided by natriuretic peptide levels, three were positive, three were negative, and one had mixed results.
Three positive trials
The Christchurch, New Zealand, trial12 (with 69 patients) found that there were fewer total cardiovascular events (death, hospital admission, or heart failure decompensation) at 9.5 months in the group randomized to receive treatment guided by the N-terminal BNP concentration than in the control group (19 vs 54, P = .02).
The STARS-BNP trial (Systolic Heart Failure Treatment Supported by BNP),13 with 220 patients, showed a significant reduction in the rate of deaths from heart failure and of readmission at 15 months in patients receiving BNP-guided treatment compared with controls (24% vs 52%, P < .001).
The PROTECT trial (Pro-B Type Natriuretic Peptide Outpatient Tailored Chronic Heart Failure Therapy),14 with 151 patients enrolled, showed a significant reduction in a composite of cardiovascular events (worsening heart failure, hospitalization for heart failure, acute coronary syndromes, ventricular arrhythmias, cerebral ischemia, and cardiovascular death) with N-terminal BNP guidance compared with standard care at a mean of 10 months of follow-up (58 events vs 100 events, P = .009). It also showed significant improvements in quality of life, left ventricular ejection fraction, and both left ventricular end-systolic and end-diastolic volume indexes with therapy guided by N-terminal BNP measurement. Moreover, therapy guided by N-terminal BNP was not associated with higher rates of renal dysfunction from more aggressive diuretic use.
Three negative trials
Conversely, three trials did not find significant reductions in rates of death or hospitalization-free survival between groups:
The STARBRITE trial (Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Versus the Clinical Congestion Score) (N = 130)15
The BATTLESCARRED trial (NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death) (N = 364)16
The PRIMA trial (Can Pro-brain-natriuretic Peptide Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality?) (N = 345).17
One trial with mixed results
The TIME-CHF (Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure),18 the largest of these trials to date (N = 499), did not show a survival benefit, but it did show a lower rate of hospitalization due to heart failure in the group receiving treatment guided by N-terminal BNP levels than in controls. Also, this study found that in the subset of patients younger than 75 years, therapy guided by N-terminal BNP levels reduced the risk of death and hospitalization from heart failure.
Why the different results in these studies?
Several reasons can be invoked to explain the heterogeneity of results in the studies mentioned above. Most importantly, the small sample sizes in these trials may have prevented differences from reaching statistical significance. Also, the inclusion criteria and methods varied considerably, with different natriuretic peptide targets, doses of medications, and treatment strategies.
WHAT IS THE CONCLUSION?
Although there are data to suggest that serial natriuretic peptide guidance can reduce the rates of hospitalization and death from heart failure in patients under age 75, there is not enough evidence to recommend routine measurements for the outpatient management of heart failure.
A 2009 focused update to the joint American College of Cardiology and American Heart Association 2005 guidelines19 concluded that using natriuretic peptide levels to guide heart failure therapy is not well established (class 2b, level of evidence C).
Measurement of natriuretic peptides can be useful in evaluating and risk-stratifying patients presenting in the urgent care setting in whom the clinical diagnosis of heart failure is uncertain. These measurements are to be viewed as part of the total evaluation but are not to be used in isolation to confirm or exclude the presence of heart failure or to monitor the patient for decompensation.
Natriuretic peptide measurement is not a substitute for the information derived from a good history (dyspnea, orthopnea, paroxysmal nocturnal dyspnea) and physical examination (eg, weight, jugular venous distention, crackles, a third heart sound, edema).
The consensus opinion remains that the favorable outcomes with natriuretic peptide guidance in clinical trials were due to better adherence and continuous up-titration of medications to maximally tolerated target doses of angiotensin-converting enzyme inhibitors and beta-blockers, in addition to closer follow-up of patients in those groups.20 This can be done without serial natriuretic peptide measurements.
- Bonow RO, Bennett S, Casey DE, et al; American College of Cardiology; American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures); Heart Failure Society of America. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol 2005; 46:1144–1178.
- Packer M. Should B-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation 2003; 108:2950–2953.
- Takeishi Y, Toriyama S, Takabatake N, et al. Linkage disequilibrium analyses of natriuretic peptide precursor B locus reveal risk haplotype conferring high plasma BNP levels. Biochem Biophys Res Commun 2007; 362:480–484.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004; 109:594–600.
- Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol 2006; 47:91–97.
- Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47:742–748.
- Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161–167.
- Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95:948–954.
- Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004; 110:2168–2174.
- Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004; 43:635–641.
- Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000; 355:1126–1130.
- Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol 2007; 49:1733–1739.
- Januzzi JL, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881–1891.
- Shah MR, Claise KA, Bowers MT, et al. Testing new targets of therapy in advanced heart failure: the design and rationale of the Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: BRain NatrIuretic Peptide Versus the Clinical CongesTion ScorE (STARBRITE) trial. Am Heart J 2005; 150:893–898.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 2010; 56:2090–2100.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:e391–e479.
- O’Donoghue M, Braunwald E. Natriuretic peptides in heart failure: should therapy be guided by BNP levels? Nat Rev Cardiol 2010; 7:13–20.
In the last few years, a number of randomized controlled trials have explored the value of using target levels of natriuretic peptides such as brain-type natriuretic peptide (BNP) and N-terminal BNP in the outpatient management of heart failure. Unfortunately, the results have been inconclusive.
RATIONALE FOR TARGETING NATRIURETIC PEPTIDE LEVELS
Heart failure causes devastating morbidity and death, yet its management is guided more often by subjective than by objective data.1 In other chronic conditions such as hypertension, diabetes mellitus, and hyperlipidemia, numerical targets for blood pressure, hemoglobin A1c, and low-density lipoprotein cholesterol levels are used to guide medical therapy, and lower rates of both morbidity and death have resulted.1 Extensive efforts have been undertaken to use natriuretic peptide levels to similarly guide heart failure therapy and improve outcomes.
LIMITATIONS TO TARGETING NATRIURETIC PEPTIDES
The relationship between natriuretic peptide levels and patient symptoms1 and outcomes2 is neither predictable nor linear, although the association between these levels and outcomes is stronger at the extremes, ie, at very low and very high levels.
Moreover, baseline levels vary significantly among people and within the same person, affected by factors such as genetic polymorphisms, 3 age, sex,4 body mass index,5 and other diseases, such as renal insufficiency.6
In addition, natriuretic peptide levels behave differently depending on the type of heart failure, rising much higher in systolic heart failure than in diastolic heart failure.7
ESTABLISHED USES OF MEASURING NATRIURETIC PEPTIDE LEVELS
Measuring natriuretic peptide levels has proven useful in diagnosing heart failure and in risk stratification of heart failure patients. BNP levels of less than 100 pg/mL practically exclude the diagnosis of heart failure (negative predictive value 89%),8 as do N-terminal BNP levels less than 300 pg/mL (negative predictive value 99%).9 Changes from baseline levels during acute hospitalization correlate with heart failure mortality rates, while elevated levels at discharge are associated with a higher risk of heart failure death and of readmission.10,11
NATRIURETIC PEPTIDES TO GUIDE THERAPY
Of the seven published clinical trials of therapy guided by natriuretic peptide levels, three were positive, three were negative, and one had mixed results.
Three positive trials
The Christchurch, New Zealand, trial12 (with 69 patients) found that there were fewer total cardiovascular events (death, hospital admission, or heart failure decompensation) at 9.5 months in the group randomized to receive treatment guided by the N-terminal BNP concentration than in the control group (19 vs 54, P = .02).
The STARS-BNP trial (Systolic Heart Failure Treatment Supported by BNP),13 with 220 patients, showed a significant reduction in the rate of deaths from heart failure and of readmission at 15 months in patients receiving BNP-guided treatment compared with controls (24% vs 52%, P < .001).
The PROTECT trial (Pro-B Type Natriuretic Peptide Outpatient Tailored Chronic Heart Failure Therapy),14 with 151 patients enrolled, showed a significant reduction in a composite of cardiovascular events (worsening heart failure, hospitalization for heart failure, acute coronary syndromes, ventricular arrhythmias, cerebral ischemia, and cardiovascular death) with N-terminal BNP guidance compared with standard care at a mean of 10 months of follow-up (58 events vs 100 events, P = .009). It also showed significant improvements in quality of life, left ventricular ejection fraction, and both left ventricular end-systolic and end-diastolic volume indexes with therapy guided by N-terminal BNP measurement. Moreover, therapy guided by N-terminal BNP was not associated with higher rates of renal dysfunction from more aggressive diuretic use.
Three negative trials
Conversely, three trials did not find significant reductions in rates of death or hospitalization-free survival between groups:
The STARBRITE trial (Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Versus the Clinical Congestion Score) (N = 130)15
The BATTLESCARRED trial (NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death) (N = 364)16
The PRIMA trial (Can Pro-brain-natriuretic Peptide Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality?) (N = 345).17
One trial with mixed results
The TIME-CHF (Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure),18 the largest of these trials to date (N = 499), did not show a survival benefit, but it did show a lower rate of hospitalization due to heart failure in the group receiving treatment guided by N-terminal BNP levels than in controls. Also, this study found that in the subset of patients younger than 75 years, therapy guided by N-terminal BNP levels reduced the risk of death and hospitalization from heart failure.
Why the different results in these studies?
Several reasons can be invoked to explain the heterogeneity of results in the studies mentioned above. Most importantly, the small sample sizes in these trials may have prevented differences from reaching statistical significance. Also, the inclusion criteria and methods varied considerably, with different natriuretic peptide targets, doses of medications, and treatment strategies.
WHAT IS THE CONCLUSION?
Although there are data to suggest that serial natriuretic peptide guidance can reduce the rates of hospitalization and death from heart failure in patients under age 75, there is not enough evidence to recommend routine measurements for the outpatient management of heart failure.
A 2009 focused update to the joint American College of Cardiology and American Heart Association 2005 guidelines19 concluded that using natriuretic peptide levels to guide heart failure therapy is not well established (class 2b, level of evidence C).
Measurement of natriuretic peptides can be useful in evaluating and risk-stratifying patients presenting in the urgent care setting in whom the clinical diagnosis of heart failure is uncertain. These measurements are to be viewed as part of the total evaluation but are not to be used in isolation to confirm or exclude the presence of heart failure or to monitor the patient for decompensation.
Natriuretic peptide measurement is not a substitute for the information derived from a good history (dyspnea, orthopnea, paroxysmal nocturnal dyspnea) and physical examination (eg, weight, jugular venous distention, crackles, a third heart sound, edema).
The consensus opinion remains that the favorable outcomes with natriuretic peptide guidance in clinical trials were due to better adherence and continuous up-titration of medications to maximally tolerated target doses of angiotensin-converting enzyme inhibitors and beta-blockers, in addition to closer follow-up of patients in those groups.20 This can be done without serial natriuretic peptide measurements.
In the last few years, a number of randomized controlled trials have explored the value of using target levels of natriuretic peptides such as brain-type natriuretic peptide (BNP) and N-terminal BNP in the outpatient management of heart failure. Unfortunately, the results have been inconclusive.
RATIONALE FOR TARGETING NATRIURETIC PEPTIDE LEVELS
Heart failure causes devastating morbidity and death, yet its management is guided more often by subjective than by objective data.1 In other chronic conditions such as hypertension, diabetes mellitus, and hyperlipidemia, numerical targets for blood pressure, hemoglobin A1c, and low-density lipoprotein cholesterol levels are used to guide medical therapy, and lower rates of both morbidity and death have resulted.1 Extensive efforts have been undertaken to use natriuretic peptide levels to similarly guide heart failure therapy and improve outcomes.
LIMITATIONS TO TARGETING NATRIURETIC PEPTIDES
The relationship between natriuretic peptide levels and patient symptoms1 and outcomes2 is neither predictable nor linear, although the association between these levels and outcomes is stronger at the extremes, ie, at very low and very high levels.
Moreover, baseline levels vary significantly among people and within the same person, affected by factors such as genetic polymorphisms, 3 age, sex,4 body mass index,5 and other diseases, such as renal insufficiency.6
In addition, natriuretic peptide levels behave differently depending on the type of heart failure, rising much higher in systolic heart failure than in diastolic heart failure.7
ESTABLISHED USES OF MEASURING NATRIURETIC PEPTIDE LEVELS
Measuring natriuretic peptide levels has proven useful in diagnosing heart failure and in risk stratification of heart failure patients. BNP levels of less than 100 pg/mL practically exclude the diagnosis of heart failure (negative predictive value 89%),8 as do N-terminal BNP levels less than 300 pg/mL (negative predictive value 99%).9 Changes from baseline levels during acute hospitalization correlate with heart failure mortality rates, while elevated levels at discharge are associated with a higher risk of heart failure death and of readmission.10,11
NATRIURETIC PEPTIDES TO GUIDE THERAPY
Of the seven published clinical trials of therapy guided by natriuretic peptide levels, three were positive, three were negative, and one had mixed results.
Three positive trials
The Christchurch, New Zealand, trial12 (with 69 patients) found that there were fewer total cardiovascular events (death, hospital admission, or heart failure decompensation) at 9.5 months in the group randomized to receive treatment guided by the N-terminal BNP concentration than in the control group (19 vs 54, P = .02).
The STARS-BNP trial (Systolic Heart Failure Treatment Supported by BNP),13 with 220 patients, showed a significant reduction in the rate of deaths from heart failure and of readmission at 15 months in patients receiving BNP-guided treatment compared with controls (24% vs 52%, P < .001).
The PROTECT trial (Pro-B Type Natriuretic Peptide Outpatient Tailored Chronic Heart Failure Therapy),14 with 151 patients enrolled, showed a significant reduction in a composite of cardiovascular events (worsening heart failure, hospitalization for heart failure, acute coronary syndromes, ventricular arrhythmias, cerebral ischemia, and cardiovascular death) with N-terminal BNP guidance compared with standard care at a mean of 10 months of follow-up (58 events vs 100 events, P = .009). It also showed significant improvements in quality of life, left ventricular ejection fraction, and both left ventricular end-systolic and end-diastolic volume indexes with therapy guided by N-terminal BNP measurement. Moreover, therapy guided by N-terminal BNP was not associated with higher rates of renal dysfunction from more aggressive diuretic use.
Three negative trials
Conversely, three trials did not find significant reductions in rates of death or hospitalization-free survival between groups:
The STARBRITE trial (Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Versus the Clinical Congestion Score) (N = 130)15
The BATTLESCARRED trial (NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death) (N = 364)16
The PRIMA trial (Can Pro-brain-natriuretic Peptide Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality?) (N = 345).17
One trial with mixed results
The TIME-CHF (Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure),18 the largest of these trials to date (N = 499), did not show a survival benefit, but it did show a lower rate of hospitalization due to heart failure in the group receiving treatment guided by N-terminal BNP levels than in controls. Also, this study found that in the subset of patients younger than 75 years, therapy guided by N-terminal BNP levels reduced the risk of death and hospitalization from heart failure.
Why the different results in these studies?
Several reasons can be invoked to explain the heterogeneity of results in the studies mentioned above. Most importantly, the small sample sizes in these trials may have prevented differences from reaching statistical significance. Also, the inclusion criteria and methods varied considerably, with different natriuretic peptide targets, doses of medications, and treatment strategies.
WHAT IS THE CONCLUSION?
Although there are data to suggest that serial natriuretic peptide guidance can reduce the rates of hospitalization and death from heart failure in patients under age 75, there is not enough evidence to recommend routine measurements for the outpatient management of heart failure.
A 2009 focused update to the joint American College of Cardiology and American Heart Association 2005 guidelines19 concluded that using natriuretic peptide levels to guide heart failure therapy is not well established (class 2b, level of evidence C).
Measurement of natriuretic peptides can be useful in evaluating and risk-stratifying patients presenting in the urgent care setting in whom the clinical diagnosis of heart failure is uncertain. These measurements are to be viewed as part of the total evaluation but are not to be used in isolation to confirm or exclude the presence of heart failure or to monitor the patient for decompensation.
Natriuretic peptide measurement is not a substitute for the information derived from a good history (dyspnea, orthopnea, paroxysmal nocturnal dyspnea) and physical examination (eg, weight, jugular venous distention, crackles, a third heart sound, edema).
The consensus opinion remains that the favorable outcomes with natriuretic peptide guidance in clinical trials were due to better adherence and continuous up-titration of medications to maximally tolerated target doses of angiotensin-converting enzyme inhibitors and beta-blockers, in addition to closer follow-up of patients in those groups.20 This can be done without serial natriuretic peptide measurements.
- Bonow RO, Bennett S, Casey DE, et al; American College of Cardiology; American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures); Heart Failure Society of America. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol 2005; 46:1144–1178.
- Packer M. Should B-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation 2003; 108:2950–2953.
- Takeishi Y, Toriyama S, Takabatake N, et al. Linkage disequilibrium analyses of natriuretic peptide precursor B locus reveal risk haplotype conferring high plasma BNP levels. Biochem Biophys Res Commun 2007; 362:480–484.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004; 109:594–600.
- Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol 2006; 47:91–97.
- Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47:742–748.
- Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161–167.
- Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95:948–954.
- Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004; 110:2168–2174.
- Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004; 43:635–641.
- Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000; 355:1126–1130.
- Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol 2007; 49:1733–1739.
- Januzzi JL, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881–1891.
- Shah MR, Claise KA, Bowers MT, et al. Testing new targets of therapy in advanced heart failure: the design and rationale of the Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: BRain NatrIuretic Peptide Versus the Clinical CongesTion ScorE (STARBRITE) trial. Am Heart J 2005; 150:893–898.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 2010; 56:2090–2100.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:e391–e479.
- O’Donoghue M, Braunwald E. Natriuretic peptides in heart failure: should therapy be guided by BNP levels? Nat Rev Cardiol 2010; 7:13–20.
- Bonow RO, Bennett S, Casey DE, et al; American College of Cardiology; American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures); Heart Failure Society of America. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol 2005; 46:1144–1178.
- Packer M. Should B-type natriuretic peptide be measured routinely to guide the diagnosis and management of chronic heart failure? Circulation 2003; 108:2950–2953.
- Takeishi Y, Toriyama S, Takabatake N, et al. Linkage disequilibrium analyses of natriuretic peptide precursor B locus reveal risk haplotype conferring high plasma BNP levels. Biochem Biophys Res Commun 2007; 362:480–484.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004; 109:594–600.
- Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol 2006; 47:91–97.
- Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006; 47:742–748.
- Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161–167.
- Januzzi JL, Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol 2005; 95:948–954.
- Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation 2004; 110:2168–2174.
- Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 2004; 43:635–641.
- Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000; 355:1126–1130.
- Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol 2007; 49:1733–1739.
- Januzzi JL, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881–1891.
- Shah MR, Claise KA, Bowers MT, et al. Testing new targets of therapy in advanced heart failure: the design and rationale of the Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: BRain NatrIuretic Peptide Versus the Clinical CongesTion ScorE (STARBRITE) trial. Am Heart J 2005; 150:893–898.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro-B-type natriuretic peptide-guided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol 2010; 56:2090–2100.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119:e391–e479.
- O’Donoghue M, Braunwald E. Natriuretic peptides in heart failure: should therapy be guided by BNP levels? Nat Rev Cardiol 2010; 7:13–20.
A less common source of dyspnea in scleroderma
A 48-year-old man reports progressive exercise intolerance, shortness of breath, fatigue, and melena over the past month. He has a long history of Raynaud phenomenon, and 5 months ago he developed severe sclerodactyly in both hands, diagnosed as limited cutaneous systemic sclerosis (scleroderma).
He has no chest pain, swelling of the lower limbs, change in weight, cough, fever, chills, or sick contacts, and he has not traveled recently.
His symptoms began as fatigue and shortness of breath, which worsened until he began having episodes of abdominal pain with melena and dizzy spills, although he never passed out.
He is currently taking long-term low-dose prednisone and mycophenolate mofetil (Cell-Cept) for the systemic sclerosis, and omeprazole (Prilosec) for gastroesophageal reflux. His father had lupus, and his grandmother had colon cancer.
An outpatient workup for sclerosis-related lung and heart involvement is negative. The workup includes computed tomography of the chest, pulmonary function tests, and Doppler echocardiography.
He is afebrile, with a blood pressure of 105/60 mm Hg and a pulse of 98. His cardiopulmonary examination results are normal. He has mild epigastric tenderness without rebound or guarding. His hemoglobin concentration at the time of hospital admission is 7.8 g/dL, down from 14.5 g/dL recorded when limited cutaneous systemic sclerosis was diagnosed. Iron studies reveal iron deficiency.
The antral ectasia is treated with argon plasma coagulation during the endoscopic examination.
Afterward, the patient's hemoglobin stabilizes, and the melena resolves. He is discharged on an oral proton pump inhibitor, with instructions to follow up for another endoscopic session in 1 month.
GASTROINTESTINAL FEATURES OF SYSTEMIC SCLEROSIS
Sclerodermal disorders have diverse manifestations that always include characteristic cutaneous signs. While there are several well-recognized symptomatic conditions commonly associated with scleroderma, attention must also be paid to the less common causes of these symptoms. Scleroderma has gastrointestinal complications that can easily be missed and may not respond to immunomodulatory or proton pump inhibitor therapy: complications can include esophageal dysmotility, hypomotility, gastric paresis, reflux esophagitis, strictures, drug-related ulcer, malabsorption, bacterial overgrowth, and pseudo-obstruction.1
This patient had an underrecognized cause of dyspnea in the setting of systemic sclerosis. Vascular symptoms of limited cutaneous systemic sclerosis are typically attributed to Raynaud phenomenon; gastrointestinal symptoms are typically attributed to esophageal dysmotility; and associated dyspnea is often considered to represent pulmonary or cardiac involvement of the sclerosis. However, gastric antral vascular ectasia should be considered in any patient with scleroderma and evidence of anemia.
The prevalence of gastric antral vascular ectasia in patients with systemic sclerosis is estimated to be about 6%.2–4 It is a relatively rare cause of upper gastrointestinal blood loss that can be clinically silent until the patient develops severe iron deficiency anemia and symptoms of dyspnea, fatigue, or congestive heart failure.
Gastric antral vascular ectasia in scleroderma usually presents as iron deficiency anemia, and only presents overtly as hematemesis or melena 10% to 14% of the time.4 Because of the often occult nature of the bleeding, the condition may be clinically silent in the early phase. Symptoms of shortness of breath and fatigue may not develop until the anemia worsens rapidly or becomes severe. Anemia is present in almost all cases of gastric antral vascular ectasia (96% to 100%) and should be a strong clinical clue for early endoscopic evaluation in patients with scleroderma, especially if there is already suspicion of upper gastrointestinal bleeding.2–5
The distinctive endoscopic streaky pattern of ectasia along the stomach antrum seen in gastric antral vascular ectasia is called “watermelon stomach”4,5 because the striped pattern recalls the stripes of a watermelon. The endoscopic appearance can vary, however, from the watermelon pattern to a coalescence of angiodysplastic lesions termed “honeycomb stomach,” which can easily be mistaken for antral gastritis.4,5 Therefore, biopsy often serves to confirm the diagnosis, with histologic features including dilated mucosal capillaries with focal fibrin thrombosis and fibromuscular hyperplasia of the lamina propria.
Gastric antral vascular ectasia often requires multiple transfusions of red blood cells, as well as repeated treatments with endoscopic argon plasma coagulation, whereby ionized argon gas is used to conduct an electric current that coagulates the surface of the mucosa to a few millimeters depth.4–6
A knowledge of the association between scleroderma and gastric antral vascular ectasia can lead to earlier recognition and treatment and can avoid unnecessary testing and complications of severe anemia.
- Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009; 48(suppl 3):iii36–iii39.
- Ingraham KM, O’Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol 2010; 37:603–607.
- Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum 1996; 39:341–346.
- Marie I, Ducrotte P, Antonietti M, Herve S, Levesque H. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment Pharmacol Ther 2008; 28:412–421.
- Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion 2008; 77:131–137.
- Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol 2006; 43:191–195.
A 48-year-old man reports progressive exercise intolerance, shortness of breath, fatigue, and melena over the past month. He has a long history of Raynaud phenomenon, and 5 months ago he developed severe sclerodactyly in both hands, diagnosed as limited cutaneous systemic sclerosis (scleroderma).
He has no chest pain, swelling of the lower limbs, change in weight, cough, fever, chills, or sick contacts, and he has not traveled recently.
His symptoms began as fatigue and shortness of breath, which worsened until he began having episodes of abdominal pain with melena and dizzy spills, although he never passed out.
He is currently taking long-term low-dose prednisone and mycophenolate mofetil (Cell-Cept) for the systemic sclerosis, and omeprazole (Prilosec) for gastroesophageal reflux. His father had lupus, and his grandmother had colon cancer.
An outpatient workup for sclerosis-related lung and heart involvement is negative. The workup includes computed tomography of the chest, pulmonary function tests, and Doppler echocardiography.
He is afebrile, with a blood pressure of 105/60 mm Hg and a pulse of 98. His cardiopulmonary examination results are normal. He has mild epigastric tenderness without rebound or guarding. His hemoglobin concentration at the time of hospital admission is 7.8 g/dL, down from 14.5 g/dL recorded when limited cutaneous systemic sclerosis was diagnosed. Iron studies reveal iron deficiency.
The antral ectasia is treated with argon plasma coagulation during the endoscopic examination.
Afterward, the patient's hemoglobin stabilizes, and the melena resolves. He is discharged on an oral proton pump inhibitor, with instructions to follow up for another endoscopic session in 1 month.
GASTROINTESTINAL FEATURES OF SYSTEMIC SCLEROSIS
Sclerodermal disorders have diverse manifestations that always include characteristic cutaneous signs. While there are several well-recognized symptomatic conditions commonly associated with scleroderma, attention must also be paid to the less common causes of these symptoms. Scleroderma has gastrointestinal complications that can easily be missed and may not respond to immunomodulatory or proton pump inhibitor therapy: complications can include esophageal dysmotility, hypomotility, gastric paresis, reflux esophagitis, strictures, drug-related ulcer, malabsorption, bacterial overgrowth, and pseudo-obstruction.1
This patient had an underrecognized cause of dyspnea in the setting of systemic sclerosis. Vascular symptoms of limited cutaneous systemic sclerosis are typically attributed to Raynaud phenomenon; gastrointestinal symptoms are typically attributed to esophageal dysmotility; and associated dyspnea is often considered to represent pulmonary or cardiac involvement of the sclerosis. However, gastric antral vascular ectasia should be considered in any patient with scleroderma and evidence of anemia.
The prevalence of gastric antral vascular ectasia in patients with systemic sclerosis is estimated to be about 6%.2–4 It is a relatively rare cause of upper gastrointestinal blood loss that can be clinically silent until the patient develops severe iron deficiency anemia and symptoms of dyspnea, fatigue, or congestive heart failure.
Gastric antral vascular ectasia in scleroderma usually presents as iron deficiency anemia, and only presents overtly as hematemesis or melena 10% to 14% of the time.4 Because of the often occult nature of the bleeding, the condition may be clinically silent in the early phase. Symptoms of shortness of breath and fatigue may not develop until the anemia worsens rapidly or becomes severe. Anemia is present in almost all cases of gastric antral vascular ectasia (96% to 100%) and should be a strong clinical clue for early endoscopic evaluation in patients with scleroderma, especially if there is already suspicion of upper gastrointestinal bleeding.2–5
The distinctive endoscopic streaky pattern of ectasia along the stomach antrum seen in gastric antral vascular ectasia is called “watermelon stomach”4,5 because the striped pattern recalls the stripes of a watermelon. The endoscopic appearance can vary, however, from the watermelon pattern to a coalescence of angiodysplastic lesions termed “honeycomb stomach,” which can easily be mistaken for antral gastritis.4,5 Therefore, biopsy often serves to confirm the diagnosis, with histologic features including dilated mucosal capillaries with focal fibrin thrombosis and fibromuscular hyperplasia of the lamina propria.
Gastric antral vascular ectasia often requires multiple transfusions of red blood cells, as well as repeated treatments with endoscopic argon plasma coagulation, whereby ionized argon gas is used to conduct an electric current that coagulates the surface of the mucosa to a few millimeters depth.4–6
A knowledge of the association between scleroderma and gastric antral vascular ectasia can lead to earlier recognition and treatment and can avoid unnecessary testing and complications of severe anemia.
A 48-year-old man reports progressive exercise intolerance, shortness of breath, fatigue, and melena over the past month. He has a long history of Raynaud phenomenon, and 5 months ago he developed severe sclerodactyly in both hands, diagnosed as limited cutaneous systemic sclerosis (scleroderma).
He has no chest pain, swelling of the lower limbs, change in weight, cough, fever, chills, or sick contacts, and he has not traveled recently.
His symptoms began as fatigue and shortness of breath, which worsened until he began having episodes of abdominal pain with melena and dizzy spills, although he never passed out.
He is currently taking long-term low-dose prednisone and mycophenolate mofetil (Cell-Cept) for the systemic sclerosis, and omeprazole (Prilosec) for gastroesophageal reflux. His father had lupus, and his grandmother had colon cancer.
An outpatient workup for sclerosis-related lung and heart involvement is negative. The workup includes computed tomography of the chest, pulmonary function tests, and Doppler echocardiography.
He is afebrile, with a blood pressure of 105/60 mm Hg and a pulse of 98. His cardiopulmonary examination results are normal. He has mild epigastric tenderness without rebound or guarding. His hemoglobin concentration at the time of hospital admission is 7.8 g/dL, down from 14.5 g/dL recorded when limited cutaneous systemic sclerosis was diagnosed. Iron studies reveal iron deficiency.
The antral ectasia is treated with argon plasma coagulation during the endoscopic examination.
Afterward, the patient's hemoglobin stabilizes, and the melena resolves. He is discharged on an oral proton pump inhibitor, with instructions to follow up for another endoscopic session in 1 month.
GASTROINTESTINAL FEATURES OF SYSTEMIC SCLEROSIS
Sclerodermal disorders have diverse manifestations that always include characteristic cutaneous signs. While there are several well-recognized symptomatic conditions commonly associated with scleroderma, attention must also be paid to the less common causes of these symptoms. Scleroderma has gastrointestinal complications that can easily be missed and may not respond to immunomodulatory or proton pump inhibitor therapy: complications can include esophageal dysmotility, hypomotility, gastric paresis, reflux esophagitis, strictures, drug-related ulcer, malabsorption, bacterial overgrowth, and pseudo-obstruction.1
This patient had an underrecognized cause of dyspnea in the setting of systemic sclerosis. Vascular symptoms of limited cutaneous systemic sclerosis are typically attributed to Raynaud phenomenon; gastrointestinal symptoms are typically attributed to esophageal dysmotility; and associated dyspnea is often considered to represent pulmonary or cardiac involvement of the sclerosis. However, gastric antral vascular ectasia should be considered in any patient with scleroderma and evidence of anemia.
The prevalence of gastric antral vascular ectasia in patients with systemic sclerosis is estimated to be about 6%.2–4 It is a relatively rare cause of upper gastrointestinal blood loss that can be clinically silent until the patient develops severe iron deficiency anemia and symptoms of dyspnea, fatigue, or congestive heart failure.
Gastric antral vascular ectasia in scleroderma usually presents as iron deficiency anemia, and only presents overtly as hematemesis or melena 10% to 14% of the time.4 Because of the often occult nature of the bleeding, the condition may be clinically silent in the early phase. Symptoms of shortness of breath and fatigue may not develop until the anemia worsens rapidly or becomes severe. Anemia is present in almost all cases of gastric antral vascular ectasia (96% to 100%) and should be a strong clinical clue for early endoscopic evaluation in patients with scleroderma, especially if there is already suspicion of upper gastrointestinal bleeding.2–5
The distinctive endoscopic streaky pattern of ectasia along the stomach antrum seen in gastric antral vascular ectasia is called “watermelon stomach”4,5 because the striped pattern recalls the stripes of a watermelon. The endoscopic appearance can vary, however, from the watermelon pattern to a coalescence of angiodysplastic lesions termed “honeycomb stomach,” which can easily be mistaken for antral gastritis.4,5 Therefore, biopsy often serves to confirm the diagnosis, with histologic features including dilated mucosal capillaries with focal fibrin thrombosis and fibromuscular hyperplasia of the lamina propria.
Gastric antral vascular ectasia often requires multiple transfusions of red blood cells, as well as repeated treatments with endoscopic argon plasma coagulation, whereby ionized argon gas is used to conduct an electric current that coagulates the surface of the mucosa to a few millimeters depth.4–6
A knowledge of the association between scleroderma and gastric antral vascular ectasia can lead to earlier recognition and treatment and can avoid unnecessary testing and complications of severe anemia.
- Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009; 48(suppl 3):iii36–iii39.
- Ingraham KM, O’Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol 2010; 37:603–607.
- Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum 1996; 39:341–346.
- Marie I, Ducrotte P, Antonietti M, Herve S, Levesque H. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment Pharmacol Ther 2008; 28:412–421.
- Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion 2008; 77:131–137.
- Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol 2006; 43:191–195.
- Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009; 48(suppl 3):iii36–iii39.
- Ingraham KM, O’Brien MS, Shenin M, Derk CT, Steen VD. Gastric antral vascular ectasia in systemic sclerosis: demographics and disease predictors. J Rheumatol 2010; 37:603–607.
- Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum 1996; 39:341–346.
- Marie I, Ducrotte P, Antonietti M, Herve S, Levesque H. Watermelon stomach in systemic sclerosis: its incidence and management. Aliment Pharmacol Ther 2008; 28:412–421.
- Selinger CP, Ang YS. Gastric antral vascular ectasia (GAVE): an update on clinical presentation, pathophysiology and treatment. Digestion 2008; 77:131–137.
- Chaves DM, Sakai P, Oliveira CV, Cheng S, Ishioka S. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol 2006; 43:191–195.
Unmasking gastric cancer
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
A 50-year-old male Japanese immigrant with a history of smoking and occasional untreated heartburn presented with the recent onset of flank pain, weight loss, headache, syncope, and blurred vision.
Previously healthy, he began feeling moderate pain in his left flank 1 month ago; it was diagnosed as kidney stones and was treated conservatively. Two weeks later he had an episode of syncope and soon after developed blurred vision, mainly in his left eye, along with severe bifrontal headache. An eye examination and magnetic resonance imaging of the brain indicated optic neuritis, for which he was given glucocorticoids intravenously for 3 days, with moderate improvement.
As his symptoms continued over the next 2 weeks, he lost 20 lb (9.1 kg) due to the pain, loss of appetite, nausea, and occasional vomiting.
Positron emission tomography showed the retroperitoneal infiltrative process and the thickened gastric cardia to be hypermetabolic (Figure 1C).
The area of retroperitoneal infiltration was biopsied under CT guidance, and pathologic study showed poorly differentiated carcinoma with signet-ring cells, a feature of gastric cancer.
The patient underwent lumbar puncture. His cerebrospinal fluid had 206 white blood cells/μL (reference range 0–5) and large numbers of poorly differentiated malignant cells, most consistent with adenocarcinoma on cytologic study.
duodenoscopy (EGD) revealed a large, ulcerated, submucosal, nodular mass in the cardia of the stomach extending to the gastroesophageal junction (Figure 2A). Biopsy of the mass again revealed poorly differentiated adenocarcinoma with scattered signet-ring cells undermining the gastric mucosa, favoring a gastric origin (Figure 2B).
THREE SUBTYPES OF GASTRIC CANCER
Worldwide, gastric cancer is the third most common type of cancer and the second most common cause of cancer-related deaths.1 In the United States, blacks and people of Asian ancestry have almost twice the risk of death, with the highest incidence and mortality rates.2,3
Most cases of gastric adenocarcinoma can be categorized as either intestinal or diffuse, but a new proximal subtype is emerging.4
Intestinal-type gastric adenocarcinoma is the most common subtype and accounts for almost all the ethnic and geographic variation in incidence.2 The lesions are often ulcerative and distal; the pathogenesis is stepwise and is initiated by chronic inflammation. Risk factors include old age, Helicobacter pylori infection, tobacco smoking, family history, and high salt intake, with an observed risk-reduction with the use of nonsteroidal anti-inflammatory drugs and with a high intake of fruits and vegetables.3
Diffuse gastric adenocarcinoma, on the other hand, has a uniform distribution worldwide, and its incidence is increasing. It typically carries a poor prognosis. Evidence thus far has shown its pathogenesis to be independent of chronic inflammation, but it has a strong tendency to be hereditary.3
Proximal gastric adenocarcinoma is observed in the gastric cardia and near the gastroesophageal junction. It is often grouped with the distal esophageal adenocarcinomas and has similar risk factors, including reflux disease, obesity, alcohol abuse, and tobacco smoking. Interestingly, however, H pylori infection does not contribute to the pathogenesis of this type, and it may even have a protective role.3
DIFFICULT TO DETECT EARLY
Gastric cancer is difficult to detect early enough in its course to be cured. Understanding its risk factors, recognizing its common symptoms, and regarding its uncommon symptoms with suspicion may lead to earlier diagnosis and more effective treatment.
Our patient’s proximal gastric cancer was diagnosed late even though he had several risk factors for it (he was Japanese, he was a smoker, and he had gastroesophageal reflux disease) because of a late and atypical presentation with misleading paraneoplastic symptoms.
Early diagnosis is difficult because most patients have no symptoms in the early stage; weight loss and abdominal pain are often late signs of tumor progression.
Screening may be justified in high-risk groups in the United States, although the issue is debatable. Diagnostic imaging is the only effective method for screening,5 with EGD considered the first-line targeted evaluation should there be suspicion of gastric cancer either from the clinical presentation or from barium swallow.6 Candidates for screening may include elderly patients with atrophic gastritis or pernicious anemia, immigrants from countries with high rates of gastric carcinoma, and people with a family history of gastrointestinal cancer.7
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55:74–108.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12:354–362.
- Shah MA, Kelsen DP. Gastric cancer: a primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J Natl Compr Canc Netw 2010; 8:437–447.
- Fine G, Chan K. Alimentary tract. In:Kissane JM, editor. Anderson’s Pathology. 8th ed. Saint Louis, MO: Mosby; 1985:1055–1095.
- Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol 2006; 13:221–228.
- Cappell MS, Friedel D. The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders. Med Clin North Am 2002; 86:1165–1216.
- Hisamuchi S, Fukao P, Sugawara N, et al. Evaluation of mass screening programme for stomach cancer in Japan. In:Miller AB, Chamberlain J, Day NE, et al, editors. Cancer Screening. Cambridge, UK: Cambridge University Press; 1991:357–372.
A 40-year-old woman with excoriated skin lesions
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
She denies any history of itching, insect bites, exposure to new medications or jewelry, allergies, recent change in medications, travel, or intravenous drug abuse.
Q: Which is the most likely diagnosis?
- Allergic contact dermatitis
- Xerosis
- Dermatotillomania
- Folliculitis
- Infestation (scabies)
A: Dermatotillomania, ie, pathologic skin picking, is the correct diagnosis. On further questioning, the patient reveals that the wounds have been self-inflicted over many years, starting in her adolescence. The wounds are located only in areas she can reach. She admits that social and emotional stressors had made the condition significantly worse and that lately she had lost control of her skin-picking. She denies nail-biting, trichotillomania, or obsessive-compulsive behavior.
As for the other possible diagnoses:
Allergic contact dermatitis occurs when contact with a particular substance elicits a hypersensitivity reaction. This reaction is of the delayed type (type IV). The affected individual can develop skin erythema and swelling with vesicles that are intensely pruritic at the contact site. The erythema may become evident hours after exposure, or not until weeks later, which can make the diagnosis difficult at times.
Our patient’s lesions were not pruritic, and she denied recent exposure to allergens.
Xerosis. Xerotic (dry) skin is usually rough, with fine scales and fissures. Xerosis can affect people of all ages and is often more intense during the winter. It affects mainly the arms, legs, and hands. Patients note pruritus, which can be treated with liberal use of emollients and tepid water baths.
Our patient’s lesions were scarred, hyperpigmented, and nonpruritic.
Folliculitis is a superficial infection of the hair follicle that presents as an erythematous pustule on the extremities, buttocks, or scalp. The pustule can be tender to palpation and can progress to an abscess that requires incision and drainage and intravenous antibiotics. A moist environment and poor hygiene are predisposing factors. Staphylococcus aureus is the culprit in most cases.
Our patient’s lesions were on the chest and upper back, where hair follicles were sparse or absent, and there was no erythema or tenderness.
Scabies is a skin infestation with Sarcoptes scabiei mites, which burrow in the skin and cause intense pruritus, especially at night. Scabies usually affects the sides and webs of the fingers and skin folds. Sexual contact is a common way of transmission; however, transmission can also occur by sharing beds and towels.
Patients with dermatotillomania lack intense pruritus, and skin-picking occurs during the day, while the patient is awake.
SELF-INFLICTED WOUNDS
Pathologic skin-picking, neurotic excoriation, excoriated acne, or dermatotillomania results from scratching, picking, gouging, or squeezing of one’s skin via teeth, fingernails, tweezers, or other objects.1–3 Lesions are usually found on skin that the patient can easily reach, such as the face, chest, upper and lower extremities, and upper back.4
The prevalence of pathologic skin-picking is estimated at 2% in dermatology patients.5 The overall prevalence of psychiatric disorders in all dermatology outpatients is estimated at 30% to 40%. Women outnumber men with this disorder.6
Dermatotillomania is thought to be on the spectrum of obsessive-compulsive disorder, in which patients exhibit impulses and compulsions.5 It starts in childhood or early adulthood, with an average lifetime duration of 21 years.7 It is usually associated with anxiety, depression, obsessive-compulsive traits, eating disorders, body dysmorphic disorders, or hypochondriasis. Psychosocial stress is the main trigger. Patients report feelings of tension and stress before picking and relief while picking; there is no suicidal ideation.8
Treatments are both pharmacologic and behavioral.9 Cognitive behavioral therapy and habit reversal therapy have each been successful when used alone.8 In addition, several case reports10 and double-blind studies11,12 have shown that treatment with a selective serotonin-reuptake inhibitor (SSRI) can reduce pathologic skin-picking.13,14 However, SSRIs have also been reported to induce or aggravate this behavior in patients with underlying mild skin-picking and a family history of skin-picking.15 Thus, it is pertinent to extract a detailed history from the patient before prescribing an SSRI.
We referred our patient for behavioral therapy and prescribed fluoxetine (Prozac) 20 mg daily. She showed improvement in symptoms in 4 weeks and has since stopped skin-picking completely.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.
- Arnold LM. Phenomenology and therapeutic options for dermatotillomania. Expert Rev Neurother 2002; 2:725–730.
- Bohne A, Keuthen N, Wilhelm S. Pathologic hairpulling, skin picking, and nail biting. Ann Clin Psychiatry 2005; 17:227–232.
- Gattu S, Rashid RM, Khachemoune A. Self-induced skin lesions: a review of dermatitis artefacta. Cutis 2009; 84:247–251.
- Keuthen NJ, Deckersbach T, Wilhelm S, et al. Repetitive skin-picking in a student population and comparison with a sample of self-injurious skin-pickers. Psychosomatics 2000; 41:210–215.
- Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology and approaches to treatment. CNS Drugs 2001; 15:351–359.
- Wilhelm S, Keuthen NJ, Deckersbach T, et al. Self-injurious skin picking: clinical characteristics and comorbidity. J Clin Psychiatry 1999; 60:454–459.
- Gupta MA, Gupta AK, Haberman HF. Neurotic excoriations: a review and some new perspectives. Compr Psychiatry 1986; 27:381–386.
- Rosenbaum MS, Ayllon T. The behavioral treatment of neurodermatitis through habit-reversal. Behav Res Ther 1981; 19:313–318.
- Deckersbach T, Wilhelm S, Keuthen N. Self-injurious skin picking: clinical characteristics, assessment methods, and treatment modalities. Brief Treatment and Crisis Intervention 2003; 3:249–260.
- Sharma H. Psychogenic excoriation responding to fluoxetine: a case report. J Indian Med Assoc 2008; 106:245,262.
- Bloch MR, Elliott M, Thompson H, Koran LM. Fluoxetine in pathologic skin-picking: open-label and double-blind results. Psychosomatics 2001; 42:314–319.
- Simeon D, Stein DJ, Gross S, Islam N, Schmeidler J, Hollander E. A double-blind trial of fluoxetine in pathologic skin picking. J Clin Psychiatry 1997; 58:341–347.
- Gupta MA, Gupta AK. The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 2001; 15:512–518.
- Keuthen NJ, Jameson M, Loh R, Deckersbach T, Wilhelm S, Dougherty DD. Open-label escitalopram treatment for pathological skin picking. Int Clin Psychopharmacol 2007; 22:268–274.
- Denys D, van Megen HJ, Westenberg HG. Emerging skin-picking behaviour after serotonin reuptake inhibitor-treatment in patients with obsessive-compulsive disorder: possible mechanisms and implications for clinical care. J Psychopharmacol 2003; 17:127–129.