User login
The role of moisturizers and lubricants in genitourinary syndrome of menopause and beyond
FDA made the right call on flibanserin
An article published on Feb. 29 in JAMA Internal Medicine and the associated editorial questioning the safety, efficacy, and approval of flibanserin are both factually inaccurate and misrepresent the large body of sound scientific data that led to approval by the Food and Drug Administration in August 2015.
Female sexual dysfunction is prevalent. About 40% of women experience low desire and 6%-10% meet criteria for the diagnosis of hypoactive sexual desire disorder (HSDD). The FDA established female sexual dysfunction as an area of unmet medical need. HSDD has a clear, scientifically proven biologic basis. It causes women distress, and often affects a woman’s quality of life and her relationships. We have tools to diagnose HSDD in our office, and finally, we have the first-ever, FDA-approved treatment option for women. The FDA made the correct decision to approve flibanserin based on a careful review of the scientific data.
Flibanserin was approved based on the results of three pivotal trials in more than 11,000 patients documenting statistically significant improvement in sexually satisfying events, improvement in desire, and decrease in distress. As with all FDA-approved medications, flibanserin has side effects and drug interactions, but these are similar to, and in fact less severe than the side effects and drug interactions of other medications, including commonly prescribed antidepressants. The authors correctly conclude that the adverse events in the trials are mild and “reflects positively on the safety of flibanserin” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
It should be noted that flibanserin is similar in effectiveness and side effects to many other FDA-approved medications that work on brain neurotransmitters, such as antidepressants. As with other antidepressants, flibanserin is not effective in all women. The editorial’s claim that 90% of treated patients do not respond is factually inaccurate. The data in the pivotal trials demonstrate that 40%-50% of women respond with on average 2.5-4 additional sexually satisfying events over placebo. More significantly, 25% of responders had more than four additional sexually satisfying events per month. The author’s statement that the increase in sexually satisfying events was 0.5 per month is misleading because it includes nonresponders, and data with lower doses of flibanserin that are not effective.
Women are smart and are capable of understanding scientific data about medications including potential risks and potential benefits and of making informed treatment decisions.
I direct a menopause and sexual health clinic, and have prescribed flibanserin to approximately 15 premenopausal women with diagnosed acquired, generalized HSDD over the last 4 months after discussion of the data, the interaction with alcohol, drug interactions, and potential side effects. Similar to the reported data, approximately half of those women have had a robust response. None of the women have had significant side effects. All of the responders have continued the medication. Nonresponders all discontinued the medication after 8 weeks with no untoward effects.
I continue to discuss risk and benefit, and potential adverse effects with all women who remain on the drug in the same way I discuss risk and benefit with ongoing hormone therapy in postmenopausal women.
Prior to the FDA approval of flibanserin, women suffering with HSDD had only untested, unstudied compounded and off-label treatment options with unproven benefits and uncertain risks. Now with an FDA-approved product, patients can be confident about the dose and purity of medication, and are fully informed about the possible side effects and drug interactions as documented in a package insert. For this reason, I view flibanserin as a safer alternative for my patients with HSDD.
Finally, I take issue with the statement in the editorial that “the FDA approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2016.0073). That statement is a gross misrepresentation of the data and the FDA approval process. I was at the FDA when flibanserin was approved, and the FDA approval came after a careful and exhaustive review of the data. Give the FDA the credit it is due for doing the right thing for women based on science, not politics.
Dr. Larkin is director of the UC Health Women’s Center, Cincinnati; advocacy chair and a board member of the International Society for the Study of Women’s Sexual Health; and scientific chair of Even the Score. She reported one-time advisory work in 2016 for Valeant, the manufacturer of flibanserin.
An article published on Feb. 29 in JAMA Internal Medicine and the associated editorial questioning the safety, efficacy, and approval of flibanserin are both factually inaccurate and misrepresent the large body of sound scientific data that led to approval by the Food and Drug Administration in August 2015.
Female sexual dysfunction is prevalent. About 40% of women experience low desire and 6%-10% meet criteria for the diagnosis of hypoactive sexual desire disorder (HSDD). The FDA established female sexual dysfunction as an area of unmet medical need. HSDD has a clear, scientifically proven biologic basis. It causes women distress, and often affects a woman’s quality of life and her relationships. We have tools to diagnose HSDD in our office, and finally, we have the first-ever, FDA-approved treatment option for women. The FDA made the correct decision to approve flibanserin based on a careful review of the scientific data.
Flibanserin was approved based on the results of three pivotal trials in more than 11,000 patients documenting statistically significant improvement in sexually satisfying events, improvement in desire, and decrease in distress. As with all FDA-approved medications, flibanserin has side effects and drug interactions, but these are similar to, and in fact less severe than the side effects and drug interactions of other medications, including commonly prescribed antidepressants. The authors correctly conclude that the adverse events in the trials are mild and “reflects positively on the safety of flibanserin” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
It should be noted that flibanserin is similar in effectiveness and side effects to many other FDA-approved medications that work on brain neurotransmitters, such as antidepressants. As with other antidepressants, flibanserin is not effective in all women. The editorial’s claim that 90% of treated patients do not respond is factually inaccurate. The data in the pivotal trials demonstrate that 40%-50% of women respond with on average 2.5-4 additional sexually satisfying events over placebo. More significantly, 25% of responders had more than four additional sexually satisfying events per month. The author’s statement that the increase in sexually satisfying events was 0.5 per month is misleading because it includes nonresponders, and data with lower doses of flibanserin that are not effective.
Women are smart and are capable of understanding scientific data about medications including potential risks and potential benefits and of making informed treatment decisions.
I direct a menopause and sexual health clinic, and have prescribed flibanserin to approximately 15 premenopausal women with diagnosed acquired, generalized HSDD over the last 4 months after discussion of the data, the interaction with alcohol, drug interactions, and potential side effects. Similar to the reported data, approximately half of those women have had a robust response. None of the women have had significant side effects. All of the responders have continued the medication. Nonresponders all discontinued the medication after 8 weeks with no untoward effects.
I continue to discuss risk and benefit, and potential adverse effects with all women who remain on the drug in the same way I discuss risk and benefit with ongoing hormone therapy in postmenopausal women.
Prior to the FDA approval of flibanserin, women suffering with HSDD had only untested, unstudied compounded and off-label treatment options with unproven benefits and uncertain risks. Now with an FDA-approved product, patients can be confident about the dose and purity of medication, and are fully informed about the possible side effects and drug interactions as documented in a package insert. For this reason, I view flibanserin as a safer alternative for my patients with HSDD.
Finally, I take issue with the statement in the editorial that “the FDA approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2016.0073). That statement is a gross misrepresentation of the data and the FDA approval process. I was at the FDA when flibanserin was approved, and the FDA approval came after a careful and exhaustive review of the data. Give the FDA the credit it is due for doing the right thing for women based on science, not politics.
Dr. Larkin is director of the UC Health Women’s Center, Cincinnati; advocacy chair and a board member of the International Society for the Study of Women’s Sexual Health; and scientific chair of Even the Score. She reported one-time advisory work in 2016 for Valeant, the manufacturer of flibanserin.
An article published on Feb. 29 in JAMA Internal Medicine and the associated editorial questioning the safety, efficacy, and approval of flibanserin are both factually inaccurate and misrepresent the large body of sound scientific data that led to approval by the Food and Drug Administration in August 2015.
Female sexual dysfunction is prevalent. About 40% of women experience low desire and 6%-10% meet criteria for the diagnosis of hypoactive sexual desire disorder (HSDD). The FDA established female sexual dysfunction as an area of unmet medical need. HSDD has a clear, scientifically proven biologic basis. It causes women distress, and often affects a woman’s quality of life and her relationships. We have tools to diagnose HSDD in our office, and finally, we have the first-ever, FDA-approved treatment option for women. The FDA made the correct decision to approve flibanserin based on a careful review of the scientific data.
Flibanserin was approved based on the results of three pivotal trials in more than 11,000 patients documenting statistically significant improvement in sexually satisfying events, improvement in desire, and decrease in distress. As with all FDA-approved medications, flibanserin has side effects and drug interactions, but these are similar to, and in fact less severe than the side effects and drug interactions of other medications, including commonly prescribed antidepressants. The authors correctly conclude that the adverse events in the trials are mild and “reflects positively on the safety of flibanserin” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
It should be noted that flibanserin is similar in effectiveness and side effects to many other FDA-approved medications that work on brain neurotransmitters, such as antidepressants. As with other antidepressants, flibanserin is not effective in all women. The editorial’s claim that 90% of treated patients do not respond is factually inaccurate. The data in the pivotal trials demonstrate that 40%-50% of women respond with on average 2.5-4 additional sexually satisfying events over placebo. More significantly, 25% of responders had more than four additional sexually satisfying events per month. The author’s statement that the increase in sexually satisfying events was 0.5 per month is misleading because it includes nonresponders, and data with lower doses of flibanserin that are not effective.
Women are smart and are capable of understanding scientific data about medications including potential risks and potential benefits and of making informed treatment decisions.
I direct a menopause and sexual health clinic, and have prescribed flibanserin to approximately 15 premenopausal women with diagnosed acquired, generalized HSDD over the last 4 months after discussion of the data, the interaction with alcohol, drug interactions, and potential side effects. Similar to the reported data, approximately half of those women have had a robust response. None of the women have had significant side effects. All of the responders have continued the medication. Nonresponders all discontinued the medication after 8 weeks with no untoward effects.
I continue to discuss risk and benefit, and potential adverse effects with all women who remain on the drug in the same way I discuss risk and benefit with ongoing hormone therapy in postmenopausal women.
Prior to the FDA approval of flibanserin, women suffering with HSDD had only untested, unstudied compounded and off-label treatment options with unproven benefits and uncertain risks. Now with an FDA-approved product, patients can be confident about the dose and purity of medication, and are fully informed about the possible side effects and drug interactions as documented in a package insert. For this reason, I view flibanserin as a safer alternative for my patients with HSDD.
Finally, I take issue with the statement in the editorial that “the FDA approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers” (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2016.0073). That statement is a gross misrepresentation of the data and the FDA approval process. I was at the FDA when flibanserin was approved, and the FDA approval came after a careful and exhaustive review of the data. Give the FDA the credit it is due for doing the right thing for women based on science, not politics.
Dr. Larkin is director of the UC Health Women’s Center, Cincinnati; advocacy chair and a board member of the International Society for the Study of Women’s Sexual Health; and scientific chair of Even the Score. She reported one-time advisory work in 2016 for Valeant, the manufacturer of flibanserin.
STOP performing DXA scans in healthy, perimenopausal women
START counseling all women on lifestyle interventions to avoid fractures
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
STOP performing DXA scans in healthy, perimenopausal women
START counseling all women on lifestyle interventions to avoid fractures
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.
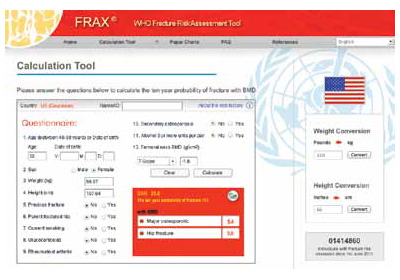
FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
CASE: Premature treatment for osteopenia
A 57-year-old woman presents to establish care and discuss concerns related to menopause and osteoporosis management. She is G2P2, healthy, 5 ft 6 in, and 130 lb. She underwent natural menopause at age 51; her vasomotor symptoms have been mild, and she has not used hormone therapy. Upon annual physical examination at age 52, her former physician referred her for a mammogram, bone-density assessment, and colonoscopy. Osteopenia (femoral neck T-score, –1.8) was noted on dual-energy x-ray absorptiometry (DXA), and alendronate 70 mg per week was started.
Today, she reports 6 months of worsening vaginal dryness. In addition, she mentions concerns about taking alendronate based on recent news reports describing serious potential adverse effects.
This patient is taking osteoporosis medication unnecessarily
BMD screening was not indicated in this patient at age 52. Schnatz and colleagues found that 41% of 612 women who had been referred for and underwent DXA screening did not meet the criteria for such screening, according to the 2006 Osteoporosis Position Statement of The North American Menopause Society.1 In addition, nearly 18% of those women who underwent DXA screening without the proper indications for it were being treated for osteopenia or osteoporosis with a bisphosphonate, raloxifene, or calcitonin therapy.
These groups should be screened for BMD2–4:
- women aged 65 and older
- women younger than age 65 who are at high risk for fracture
- adults with fracture after age 50
- adults with a medical condition or who are taking a medication that is associated with low bone mass.
Bone-health treatment was not indicated in this patient at age 52. The World Health Organization’s fracture risk assessment tool (FRAX) indicated that the patient’s risk of having a major osteoporotic fracture in the next 10 years was 5.4%, and her calculated risk of hip fracture in the next 10 years was 0.5% (FIGURE). Relying on both the patient’s T-score and FRAX results, alendronate therapy should not have been initiated.
These groups meet the criteria for treatment with a prescription medication according to FRAX results4:
- >20% risk of major osteoporotic fracture in next 10 years
- >3% risk of hip fracture in the next 10 years.

FRAX calculation tool
What now?
This patient is at low risk for osteoporosis. With an estimated rate of bone loss of 0.5% per year, over the next 8 years (from age 57 to age 65), she is unlikely to have osteoporosis at age 65. Therefore, her next BMD screening should be when she reaches age 65, according to current screening guidelines.4
Treatment holiday. Although bisphosphonate side effects, including esophageal irritation and osteonecrosis of the jaw and atypical femur fracture, are rare, it makes no sense for this patient to continue her bisphosphonate since its use was not indicated in the first place. Since bisphosphonates have been shown to accumulate in bone, their treatment effect can last up to 2 years after discontinuing therapy. Ceasing treatment for 1 to 2 years, after 3 to 5 years of continuous treatment, is a good option to minimize drug exposure and the risk of complications while preserving bone density and fracture risk reduction in patients at low risk for fracture. In patients at high risk for fracture, the 1- to 2-year drug holiday should occur after 10 years of bisphosphonate treatment.5,6
Your patient’s lifestyle can help her avoid fracture. Tell her.
Counsel your patients to exercise (30 to 45 minutes per day of weight-bearing exercise), limit their alcohol intake, and ensure adequate calcium and vitamin D intake. Food sources, rather than supplement sources, of calcium are better for bone health. The total daily calcium and vitamin D intake for women should be7-10:
- Calcium: Age older than 50 years = 1200 mg/d
- Vitamin D: Until age 70 = 600 IU/d; after age 70 = 800 IU/d.
We want to hear from you! Tell us what you think.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.
1. Schnatz PF, Marakovitz KA, Dubois M, O’Sullivan DM. Osteoporosis screening and treatment guidelines: are they being followed? Menopause. 2011;18(10):1072-1078.
2. North American Menopause Society. Management of osteoporosis in postmenopausal women: 2010 position statement. Menopause. 2010;17(1):25-54.
3. US Preventive Services Task Force. Screening for osteoporosis: recommendation statement. http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteors.htm. Published January 18 2011. Accessed December 6, 2012.
4. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. http://www.nof.org/files/nof/public/content/file/344/upload/159.pdf. Revised January 2010. Accessed December 6 2012.
5. Watts NB, Diab D. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565.
6. Black DM, Bauer DC, Schwartz AV, Cummings ST, Rosen CJ. Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med. 2012;366(22):2051-2053.
7. Manson JE, Bassuk SS. NAMS Practice Pearl: Calcium supplements: Do they help or harm? North American Menopause Society. http://www.menopause.org/publications/clinical-practice-materials/practice-pearls. Published September 6 2012. Accessed December 6, 2012.
8. Jackson RD, LaCrois AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683.
9. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58.
10. Ross AC, Taylor CL, Yaktine AL, Del Valle HB. eds; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011.











