User login
Cosmeceutical ingredients to use before and after antiaging procedures
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
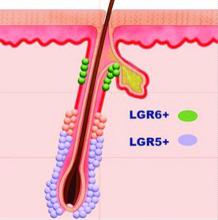
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Outcomes are improved when proper skincare is practiced before and after any type of dermatologic procedure. This column reviews These are ingredients commonly used before, during, and after procedures.
I will use the first person when I am expressing my personal opinion or experience versus data reported in published studies that I reference.
Ascorbic acid
Ascorbic acid (vitamin C) is an essential cofactor necessary for lysyl hydroxylase and prolyl hydroxylase to produce collagen. Many studies have demonstrated that the use of oral and topical ascorbic acid increases collagen production by fibroblasts.1-3 Several different ascorbic acid products, varying greatly in quality, are available on the market.
Ascorbic acid is very sensitive to light and air exposure and does not penetrate well if not at a pH of 2 or 2.5. There are aqueous and lipophilic formulations. Some are produced from L-ascorbic acid, while others are made from ascorbyl palmitate, or salts such as calcium ascorbate, magnesium ascorbate, magnesium ascorbyl phosphate, sodium ascorbate, and sodium ascorbyl phosphate. Consequently, one must closely evaluate any chosen ascorbic acid preparation and pay close attention to the form used in any studies. I am discussing ascorbic acid in general, but my statements only apply to properly formulated products. Most of the studies I quote used L-ascorbic acid, which is the form studied by the late Sheldon Pinnell, MD, who was an expert on ascorbic acid.
Properly formulated L-ascorbic acid products have a low pH. Unless formulated specifically to deter stinging, these low-pH preparations will sting wounded skin. For this reason, most ascorbic acid preparations should be avoided until the skin has completely re-epithelialized. I prefer using it preprocedure and after the procedure once the skin has re-epithelialized. Alster and West showed that use of ascorbic acid – in an aqueous solution formulated not to sting – after laser resurfacing resulted in a significant decrease in post‐CO2 laser resurfacing erythema by the eighth postoperative week when compared with laser‐irradiated skin that had not received topical vitamin C.4
I prefer using ascorbic acid in patients before and after procedures involving fillers, toxins, skin tightening, and nonablative lasers. In my experience, this improves collagen production. Also, I use ascorbic acid before microneedling, but not during or after. Several case reports have cited allergic granulomatous reactions when ascorbic acid is used during microneedling procedures,5 although these reports did not involve aqueous formulations.
Defensin
Defensins are peptides that play an important role in wound repair. Defensin has exhibited the capacity to activate the leucine-rich repeat-containing G-protein–coupled receptors 5 and 6 (also known as LGR5+ and LGR6+) stem cells.6 This accelerates wound healing by stimulating LGR stem cells to form new keratinocytes that populate the epidermis.7 Using defensins prior to procedures would theoretically speed wound healing, but no studies have been published in this area. Anecdotally, it has been used after microneedling without complication. I have not used defensin in this situation, but when I have asked the audience during lectures, many practitioners have reported using it and found that it accelerates healing.
Growth factors
Growth factors are essential in the skin because they are responsible for immunomodulation, regulation of cell division, wound healing, and tissue generation.1 There are several important growth factor families, including: transforming growth factor-beta (TGF-beta), epidermal growth factor (EGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).2 Because of the numerous different variables that play a role with growth factor function, it is difficult to know exactly which combinations are the most helpful to improve outcomes after procedures. There is some evidence to support the use of FGF, TGF-beta, and EGF, IGF, and PDGF to hasten skin healing.8,9 It is certain that growth factors play an important role in pre- and postprocedure skincare, but we do not yet know which growth factor combinations are the most effective.
Heparan sulfate
Heparan sulfate is a glycosaminoglycan found in the skin. Older cells are less responsive to growth factors than are younger cells; therefore, it is desirable to amplify the growth factor signal in older patients. Heparan sulfate has been shown to contribute to growth factors reaching the receptors on the cell surface and enhancing the cell’s ability to “hear” growth factor signals. Combining growth factors with enhancers such as heparan sulfate, defensins, ascorbic acid, and matrikines can improve outcomes of cosmetic procedures. There are not enough studies yet to substantiate which combinations are the most effective. However, I believe that if you are using a growth factor–containing product after a procedure, you should combine it with heparan sulfate to improve efficacy.
Heparan sulfate is not the same as the blood thinner heparin; however, it may affect clotting factors. It is prudent to stop heparan sulfate the day before a dermal filler procedure because of this theoretical risk. (I have not seen an increase in bruising in patients who use heparan sulfate prior to getting fillers.) I suggest using heparan sulfate–containing products with growth factors 24 hours after injecting fillers to try and enhance collagen synthesis that occurs after hyaluronic acid (HA) filler injections.10
Hyaluronic acid
Hyaluronic acid (HA) is known to increase penetration of drugs, as well as cosmeceutical ingredients.11 For this reason, it is often used before a procedure to increase efficacy of growth factors. Many practitioners report using it during microneedling to help the device glide across the skin. I have not observed or heard of any reports of adverse events from using it during microneedling.
HA has been shown to accelerate wound healing in rats12 and dental procedures.13 For this reason, it is often used after laser resurfacing and microneedling procedures and on sutured and open wounds. HA can vary in chain link and molecular weight and whether or not it is cross linked. These differences affect efficacy and should be taken into consideration when choosing an HA product. Some formulations combine various forms of HA. Because HA may increase bruising because of its effects on fibrin formation,14 I prefer not to use it 2 days prior to or the day of filler injections.15
Hydroxy acids
Pretreating skin with hydroxy acids increases dermal matrix formation,16 promotes collagen synthesis,17 and hastens stratum corneum turnover.18 Although postprocedure healing times after pretreatment with hydroxy acids has not been studied, it is very likely that pretreatment with hydroxy acids speeds healing time by increasing collagen production and cell turnover. West and Alster showed that pretreating skin with hydroxy acids prior to CO2 resurfacing did not affect the incidence of postprocedure hyperpigmentation.19
Matrikines
Matrikines are peptides that occur when extracellular matrix (ECM) macromolecules are partially degraded. These peptides interact with cell surface receptors and activate intracellular signalling pathways to modulate ECM remodeling.20 Matrikines, such as tripeptides and hexapeptides, have been shown to remove damaged collagen and elastin from the ECM.21 It is thought that these matrikines help to prepare the skin for procedures by freeing up space to allow room for newly formed collagen. Using matrikines at least 2 weeks before procedures may precondition the skin to heal faster.22
The tripeptide glycyl-histidyl-lysine (GHK) is a good example of a matrikine. When it forms a complex with copper (II) ions (GHK–Cu) it can stimulate collagen and glycosaminoglycan synthesis23 and increase tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2, which play a role in wound remodeling.24
A serum that contains tripeptide-1, hexapeptide-12, lactoferrin, and phosphatidyl serine has been shown to speed resolution of bruises and inflammation when applied after procedures. It is believed that these ingredients activate macrophages to clear hemosiderin from the skin.
Retinoids
Derived from vitamin A, the retinoid family includes compounds such as adapalene, retinol, tazarotene, trifarotene, and tretinoin. Retinoids should be used for at least 2-4 weeks prior to procedures to improve outcomes. Multiple studies have cogently revealed that pretreatment with tretinoin accelerates wound healing.25-27 Kligman assessed healing after punch biopsy in the mid-1990s and found that the wounds on arms pretreated with tretinoin cream 0.05%-0.1% were significantly diminished by 35%-37% on days 1 and 4 and 47%-50% reduced on days 6, 8, and 11 as compared with the wounds on untreated arms.28 A tretinoin pretreatment regimen of 2-4 weeks is supported by the preponderance of studies29 because peak epidermal hypertrophy emerges after 7 days of tretinoin application and normalizes after 14 days of continued treatment.30 Such an approach gives the skin time to recover from any retinoid dermatitis before the procedure is performed. Pretreatment with adapalene requires an earlier initiation period and should be introduced 5-6 weeks before procedures because it exhibits a longer half-life.31
Topical retinoids should not be used after a procedure until re-epithelialization is complete. Hung et al. applied 0.05% tretinoin cream daily for 10 days prior to partial-thickness skin wounding in a porcine model, with results revealing that re-epithelialization was accelerated with preprocedure treatment while use after the procedure slowed wound healing.32
Skin care regimen design by procedure type
Procedures can be divided into six main types: nonablative, such as peels, intense pulsed light (IPL), and vascular or pigmented lasers; microneedling or other procedures that cause open channels into the dermis; injectables such as toxins and fillers; ablative, such as CO2, erbium, and fractionated lasers; sutured wounds; and unsutured wounds. Skincare regimens that are prescribed before and after each of these procedures should take into account the Baumann Skin Type, the procedure type, whether it is pre- or postprocedure, and lifestyle issues such as sun exposure. Once the pre- and postprocedure regimen has been designed, patients should be given specific instructions as to which brands, the exact products, and the order in which to apply them.
Conclusion
To ensure the best outcomes from surgical treatments, patient education is a key step. The more that patients know and understand about the ways in which they can prepare for their procedure and treat their skin after the procedure, the better the results. Providers should give this type of information in an easy-to-follow printed instruction sheet because studies show that patients cannot remember most of the oral instructions offered by practitioners. Patients should be encouraged to ask questions during their consultation and procedure and to express any concerns with the practitioner’s office should any arise after they have returned home. These steps help improve patient compliance, satisfaction, and outcomes. Please discuss your opinions and experience with me on LinkedIn. You can also see a lecture on this topic on my website, SkinGuru.com.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Murad S et al. Proc Natl Acad Sci U S A. 1981 May;78(5):2879-82.
2. Tajima S, Pinnell SR. J Dermatol Sci. 1996 Mar;11(3):250-3.
3. Geesin JC et al. J Invest Dermatol. 1988 Apr;90(4):420-4.
4. Alster TS, West TB. Dermatol Surg. 1998 Mar;24(3):331-4.
5. Soltani-Arabshahi R et al. JAMA Dermatol. 2014 Jan;150(1):68-72.
6. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
7. Hirsch T et al. J Gene Med. 2009 Mar;11(3):220-8.
8. Van Brunt J, Klausner A. Nat Biotechnol. 1988 Jan 1;6:25-30.
9. Lynch SE et al. J Clin Invest. 1989 Aug;84(2):640-6.
10. Wang F et al. Arch Dermatol. 2007 Feb;143(2):155-63.
11. Huang G, Huang H. Drug Deliv. 2018 Nov;25(1):766-72.
12. Celani LM. J Surg Clin Res. 2019 Oct. doi: 10.20398/jscr.v10i2.18825.
13. Yildirim S et al. J Periodontol. 2018 Jan;89(1):36-45.
14. Weigel PH et al. Ciba Found Symp. 1989;143:248-61; discussion 261-4, 281-5.
15. Basora JF et al. Am J Case Rep. 2014 May 9;15:199-202.
16. Okano Yet al. Exp Dermatol. 2003;12 Suppl 2:57-63.
17. Bernstein EF et al. Dermatol Surg. 2001 May;27(5):429-33.
18. Hood HL et al. Food Chem Toxicol. 1999 Nov;37(11):1105-11.
19. West TB, Alster TS. Dermatol Surg. 1999 Jan;25(1):15-7.
20. Maquart FX et al. M. Biochimie. 2005 Mar-Apr;87(3-4):353-60.
21. Pickart L et al. Biomed Res Int. 2015;2015:648108.
22. Widgerow AD et al. Aesthet Surg J. 2019 Apr 8;39 (Supplement 3):S103-11.
23. Maquart FX et al. FEBS Lett. 1988 Oct 10;238(2):343-6.
24. Siméon A et al. J Invest Dermatol. 1999 Jun;112(6):957-64.
25. Vagotis FL, Brundage SR. Aesthetic Plast Surg. 1995 May-Jun;19(3):243-6.
26. Stuzin JM. Plast Reconstr Surg. 2011 Mar;127(3):1343-5.
27. Elson ML. J Am Acad Dermatol. 1998 Aug;39:S79-81.
28. Popp C et al. Br J Dermatol. 1995 Jan;132(1):46-53.
29. Orringer JS et al. J Am Acad Dermatol. 2004 Dec;51(6):940-6.
30. Kim IH et al. J Korean Med Sci. 1996 Aug;11(4):335-41.
31. Basak PY et al. Eur J Dermatol. 2002 Mar-Apr;12(2):145-8.
32. Hung VC et al. Arch Dermatol. 1989 Jan;125(1):65-9.
Pyrrolidone carboxylic acid may be a key cutaneous biomarker
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Cynodon dactylon
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
medicine to treat cutaneous diseases, fevers, and rheumatism,as well as a variety of chronic inflammatory conditions.1,2 The Ayurvedic armamentarium is thought to be the most abundant source of botanically based drugs used to treat wounds.3 Unrelated to health concerns, with the possible exception of allergic reactions, C. dactylon – which originated in Africa, is widely dispersed in Europe, and became an invasive species in locations such as Bermuda – is also used on putting greens on golf courses in subtropical and tropical climates.4 This grass has been shown to be safe and effective for treating induced RA in rats.1,2 Recent findings are encouraging in the area of wound healing.
Chemical constituents
Among the numerous ingredients contained in C. dactylon are proteins, carbohydrates, minerals, terpenoids, vitamin C, palmitic acid, and alkaloids.3 Other key phytoconstituents known to impart beneficial health effects that are present in the plant include flavonoids (such as apigenin and luteolin), carotenoids (such as beta-carotene and neoxanthin), phenolics, phytosterols, glycosides, saponins, and volatile oils.3 Given such components, it should not be surprising that C. dactylon has demonstrated antioxidant activity by scavenging the 2,2-diphenyl-1-picrylhydrazyl radical.3
Wound healing
Given the reputation of C. dactylon as an effective compound used in traditional medicine for wound healing as a hemostatic agent, Biswas et al. set out in 2017 to determine if they could provide scientific validation of the botanical as a viable wound-healing option. The investigators first undertook to compare a 15% ointment of the extract with a placebo control and the standard framycetin on full-thickness punch wounds in Wistar rats. Across all parameters, results for the C. dactylon–treated group far exceeded the control group and were comparable with the framycetin group. Subsequently, in a pilot clinical study, the researchers assessed the botanical ointment in a small cohort (n = 12) of men and women aged 65-75 years (n = 12) with chronic and complicated wounds. Half were treated with a topical C. dactylon ointment and half were treated with a topical framycetin sulfate ointment. Comparable effects were seen across the groups, with significant contraction of wounds and wound area noted, along with significant development of granulation and epithelial tissues. Hematologic parameters indicating improvement were comparable between the groups. The investigators concluded that all patients treated with C. dactylon healed successfully. They added that the antioxidant activity of the constituent phenolic acids and flavonoids in C. dactylon likely play a key role in conferring potent wound-healing effects by promoting collagenesis.3
In 2018, Perumal et al. created a collagen-silica biocomposite enriched with C. dactylon extract and studied its wound-healing potential in vitro and in vivo in comparison with collagen as well as collagen-silica scaffold controls. The investigators found that the stability of the enriched product surpassed that of native collagen by virtue of the intermolecular interactions between the botanical ingredient and collagen. In a full-thickness excision wound model using Wistar rats, the biocomposite was associated with more rapid healing than wounds treated with collagen and the scaffold control.5
Arthritis
In 2009, Sindhu et al. orally administered C. dactylon to rats after intradermally inducing arthritis. The induction produced inflammation, and a marked rise in the levels of inflammatory mediators, C-reactive protein, myeloperoxidase, and nitrite. Resultant oxidative stress was noted with substantial declines in the activity of catalase, superoxide dismutase, and glutathione peroxidase, as well as levels of glutathione, vitamins C and E, and an increase in lipid peroxidation. Administration of C. dactylon yielded substantial changes, with mitigation of the inflammatory response and oxidative stress as well as diminution of the arthritic response nearly to the baseline condition. The investigators concluded that the botanical agent clearly demonstrates potential to protect against arthritis.2
A subsequent study in rats by Bhangale and Acharya supported the use of C. dactylon for RA, as its oral administration was found safe at all dose levels (100, 200 and 400 mg/kg), with 400 mg/kg as the most effective at ameliorating hemoglobin and red blood cell levels and C-reactive protein, as well as lowering tumor necrosis factor–alpha. The authors also noted that the ethanolic extract of C. dactylon contained alkaloids, flavonoids, and glycosides, all of which are known to confer health benefits.1
Allergy
In 2016, López-Matas et al. studied the profiles of sensitization to C. dactylon (as well as Phragmites communis) in subjects sensitized to grasses and evaluated cross-reactivity between these grasses as well as temperate ones. Patients received skin prick tests with a grass mixture, and 24 patients (80%) were found to have had positive results for C. dactylon (and 90% to P. communis). The researchers concluded that sensitization to these species appears to be engendered by allergens other than those present in sweet grasses.6
Mehta et al. reported in 2018 on their investigation of common allergens in Ambala, India, using intradermal tests in patients with asthma, allergic rhinitis, and eczema. The study included 100 patients over an 8-year period, with 197 allergens (50 types of pollen, 19 fungi, 17 insects, 14 types of dust, 6 kinds of animal dander, 7 varieties of fabric and feathers, 82 foods, dust mites, and parthenium) tested. Pollens (51%) were the major allergens, followed by foods (28.9%), insects (26.9%), fungi (12.6%), and dusts (6.7%). C. dactylon (5%) was among two other species ranking fourth among pollen allergens.7
Also that year, Sánchez et al. investigated whether growing conditions (rural vs. urban) might influence the nasal inflammatory response to C. dactylon among patients with allergic rhinitis. They observed that the urban extract provoked larger wheals, and more patients with rhinitis experienced a positive nasal challenge test than those administered the rural extract. The skin and nasal tests did not elicit reactions in healthy controls. The researchers reached the conclusion that growth of C. dactylon in an urban setting can produce alterations in the protein extract, with potential clinical ramifications for patients who experience allergic rhinitis.8
Conclusion
Regular readers of this column know of my interest in botanically sourced topical products. Such ingredients with an extensive history of traditional medical use are particularly compelling. Many of these compounds are found in the modern medical and dermatologic armamentaria. C. dactylon does boast a track record of use in Ayurvedic medicine. However, there is a paucity of modern research at the present time. While there are concerns about its allergenicity, some encouraging results have been seen in relation to RA and wound healing. Much more research is needed, though, before this botanical agent can be included feasibly for standard skin care.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Bhangale J, Acharya S. Pers. Indian J Exp Biol. 2014 Mar;52(3):215-22.
2. Sindhu G et al. Immunopharmacol Immunotoxicol. 2009;31(4):647-53.
3. Biswas TK et al. J Ethnopharmacol. 2017 Feb 2;197:128-37.
4. Reasor EH et al. Planta. 2016 Oct;244(4):761-73.
5. Perumal RK et al. Mater Sci Eng C Mater Biol Appl. 2018 Nov 1;92:297-306.
6. López-Matas MA et al. J Investig Allergol Clin Immunol. 2016;26(5):295-303.
7. Mehta D et al. Indian J Dermatol. 2018 Jul-Aug;63(4):311-6.
8. Sánchez J et al. Allergy Rhinol (Providence). 2018 Dec 17;9:2152656718815870.
Piceatannol: The other potent antioxidant in grapes and wine
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Present in grape skins, passion fruit, and wine among several other plants and their derivatives, piceatannol is a natural stilbene, as well as an analogue of the much-studied antioxidant resveratrol. Similarly, piceatannol is thought to provide robust antioxidant and other salutary benefits.1,2
Two decades ago, the hydroxystilbenes piceatannol and transresveratrol were found, in a study of the antioxidant potential of natural products, to hinder carcinogen-induced preneoplastic lesion development in a murine mammary gland organ culture model.3 Piceatannol is naturally present in various plants and is a primary active ingredient in several. It is known to exhibit a wide range of biologic activities, including antioxidant, antibacterial, anti-inflammatory, and anticancer functions. Native to southern and southeastern Asia, Rhodomyrtus tomentosa (rose myrtle, which is a member of the Myrtaceae family), which has been utilized in traditional medicine in China, Malaysia, and Vietnam for myriad indications including wound healing, contains piceatannol as an active ingredient.4
The reported cutaneous benefits of piceatannol include promotion of collagen synthesis, suppression of melanin production, induction of the antioxidant glutathione, and the destruction of reactive oxygen species.5
Antimelanogenic activity
In 2007, Yokozawa and Kim looked into the capacity of piceatannol, given its antioxidant activities, to suppress melanogenesis. This ability was tested using the B16F10 melanoma culture system, and piceatannol was found to have a potent antityrosinase activity – stronger than kojic acid and resveratrol. Melanin content was also down-regulated by piceatannol. In addition, the researchers determined that piceatannol inhibited reactive oxygen species production, which improved the ratio of glutathione to oxidized glutathione. They concluded that the observed antimelanogenic activities of piceatannol could be attributed to its dynamic antioxidant qualities.6
Four years later, Matsui et al. ascertained that piceatannol (3,4,3’,5’-tetrahydroxy-trans-stilbene) is present in copious supply in the seeds of Passiflora edulis (passion fruit) and that this constituent of the fruit largely accounts for its antimelanogenic activities, as well as its promotion of collagen production.7
Anti-inflammatory activity
In 2014, Liu et al. used female HR-1 hairless mice in a study to shed light on the molecular mechanisms of the anti-inflammatory activity of topically applied piceatannol in vivo. Mice, either pretreated with piceatannol or not, were topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA), and pretreatment was found to yield diminished TPA-induced cyclooxygenase-2 (COX-2) expression and inducible nitric oxide synthase (iNOS). This occurred through the suppression of NF-kappa-B and AP-1 activation as a result of hindering IKK-beta activity and phosphorylation of mitogen-activated protein kinases.8
Photoprotection
Maruki-Uchida et al. studied the effects of the antioxidants piceatannol and its dimer scirpusin B, which is found in passion fruit, on human keratinocytes. In this 2013 study, they found that piceatannol dose-dependently up-regulated glutathione levels. In addition, piceatannol pretreatment blocked UVB-induced reactive oxygen species development. Pretreatment with piceatannol also reduced matrix metalloproteinase-1 activity in a nonirradiated medium of fibroblasts. The investigators concluded that piceatannol and piceatannol-rich passion fruit seed extract warrant attention as possible antiphotoaging cosmetic agents.9
With use of cultured normal human epidermal keratinocytes, Shiratake et al. in 2015 screened more than 50 plant extracts for ingredients that hinder UVB-induced damage. They identified the fruit R. tomentosa as the strongest inhibitor, with its primary component, piceatannol, demonstrating protective activities against UVB. Piceatannol decreased UVB-induced cyclobutane pyrimidine dimer synthesis, diminished prostaglandin E2 secretion, and promoted the cellular enzyme activity of DNA polymerases. The investigators concluded that rose myrtle extracts and piceatannol are potential photoprotective agents.10
Dry skin
In a 2018 randomized, placebo-controlled, double-blind trial Maruki-Uchida et al. assessed the effects of passion fruit seed extract on the skin of 32 healthy Japanese women (aged 35-54 years). Over an 8-week period, the subjects, all with dry skin, received either 5 mg of piceatannol (derived from passion fruit seed extract) or a dextrin placebo. Significant increases in cutaneous moisture content were noted in the subjects who consumed passion fruit after 4 and 8 weeks, compared with baseline and with the placebo group. Questionnaire results also indicated that perspiration and fatigue significantly decreased in the passion fruit group as compared with the placebo group. The researchers concluded that consumption of piceatannol-rich passion fruit seed extract can ameliorate dry skin and diminish fatigue.5
Conclusion
Although it gets much less attention than the related antioxidant resveratrol, piceatannol is hardly an insignificant bioactive compound. There is increasing evidence that suggests its potency as an antioxidant, as well as a potentially useful ingredient in skincare, particularly in addressing photoaging and dry skin. Much more research is necessary, of course, to determine how substantial a role this stilbene can play in providing skin protection and treatment.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Phytother Res. 2014 Nov;28(11):1581-8.
2. Biogerontology. 2017 Aug;18(4):499-516.
3. Comb Chem High Throughput Screen. 1998 Apr;1(1):35-46.
4. Biomolecules. 2019 Feb 21. doi: 10.3390/biom9020076.
5. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):75-80.
6. Biol Pharm Bull. 2007 Nov;30(11):2007-11.
7. J Agric Food Chem. 2010 Oct 27;58(20):11112-8.
8. Inflamm Res. 2014 Dec;63(12):1013-21.
9. Biol Pharm Bull. 2013;36(5):845-9.
10. Mol Med Rep. 2015 Oct;12(4):5857-64.
Holy basil: A member of the Ocimum family
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
At least three particular species in the Ocimum family have been associated with a wide array of health benefits. This column will briefly discuss the as an “adaptogen” to counter life’s stresses. It is called “holy basil” because it is sacred to the Hindus who plant it around shrines.
O. sanctum (O. tenuiflorum)
Known popularly as holy basil in English and Tulsi in Sanskrit (in which the translation is “the incomparable one”), O. tenuiflorum is used for multiple indications in traditional medical practices in Southeast Asia, including Ayurveda, Siddha, and Unani.1,2
In Ayurvedic medicine, the leaves, stem, flower, root, seeds, and whole plant of O. sanctum have been used to treat various ailments, including skin diseases. Eugenol (1-hydroxy-2-methoxy-4-allylbenzene) is its primary constituent and the wide variety of biological activities associated with the plant (including antifertility, anticancer, antidiabetic, antifungal, antimicrobial, hepatoprotective, cardioprotective, antiemetic, antispasmodic, analgesic, adaptogenic, and diaphoretic) are ascribed to it.3
O. sanctum and its water-soluble flavonoids, orientin, and vicenin – as well as eugenol, its main nonpolar component – have been shown in animal studies and a few small clinical trials to act against various radiation-induced illnesses. Antioxidant, anti-inflammatory, and metal-chelating activity have been linked to these benefits.4 Indeed, multiple studies have demonstrated that O. sanctum exerts anti-inflammatory, analgesic, and immunomodulatory activities, among other beneficial functions, with phytochemical constituents such as eugenol, rosmarinic acid, apigenin, myrtenal, luteolin, beta-sitosterol, and carnosic acid playing critical roles.2
Several animal studies have also demonstrated that O. sanctum imparts wound-healing activity, such as increasing the rates of epithelialization and wound contraction and augmenting granulation tissue and hydroxyproline levels, with some evidence of benefits for also healing keloids and hypertrophic scars.1,5
Yamani et al. studied the antimicrobial activity of the flower spikes, leaves, and essential oil of O. sanctum grown in Australia in 2016. They found that, at concentrations of 4.5% and 2.25%, the oils prevented the growth of Staphylococcus aureus (including methicillin-resistant S. aureus) and Escherichia coli, and partly hindered the growth of Pseudomonas aeruginosa. Further, the investigators identified camphor, eucalyptol, and eugenol as the primary ingredients, among 54 observed, accountable for the antimicrobial activity. They concluded that O. sanctum essential oil has potential as a topical antimicrobial agent.6
A 2015 investigation into the antioxidant activities of 10 essential oils and 10 absolutes extracted from Thai aromatic plants revealed that O. sanctum was among four of the essential oils to display robust antioxidant activity in the 2,2-diphenyl-1-1-picrylhydrazyl and thiobarbituric acid reactive species tests. The study by Leelapornpisid et al. suggested that holy basil oil, along with ginger oil, Wan-sao-long leaf oil, and lemongrass oil, appear to have potential for use as natural antioxidants in cosmetic formulations aimed at preventing or treating cutaneous aging.7
O. gratissimum
O. gratissimum has been used in traditional medicine to treat a range of conditions, including skin and gastrointestinal infections and wounds.8
In 2007, Ajose reported on the results of history questionnaires filed by patients at a dermatology clinic in Lagos, Nigeria and oral interviews with vendors and prescribers of herbal formulations at busy markets in Lagos and Ijebu-Ode in southwest Nigeria, indicating that O. gratissimum was 1 of the 38 plants used for dermatologic purposes.9
In 2009, Nweze and Eze demonstrated that the ethanolic extract of the leaves of O. gratissimum displayed antibacterial activity, supporting its use in traditional medicine as well as a food spice that does not undermine conventional antibiotics, as is thought in some rural communities throughout the world.8O. gratissimum is a key ingredient of a topical cream formulation that is one component of a complete skin care line recently found to be effective in treating mild to moderate acne. The line includes an oral supplement for males, another for females, and the topical cream, which contains O. gratissimum and keratolytic ingredients (that is, salicylic acid, gluconolactone, and complex alpha-hydroxy acids). In the double-blind clinical trial, most patients were found to have exhibited satisfactory clinical responses according to the Global Acne Grading System.10
In 2015, Keziah et al. found that topical creams formulated with O. gratissimum and Lantana camara crude extracts and fractions were effective as mosquito repellents and might serve as natural alternatives to conventional products.11
O. basilicum
Also known as great basil or St. Joseph’s Wort, O. basilicum is native to tropical regions and is found abundantly from Southeast Asia to Africa. In a 2011 single-blind study, Rasul and Akhtar tested a formulation containing 3% basil in the inner aqueous phase and a base devoid of extract. The formulation exhibited significant effects in skin moisturization, and both creams conferred measurable benefits in stemming transepidermal water loss. Skin roughness, scaliness, smoothness, and wrinkles appeared to improve with the formulation as well. The researchers concluded that topically applied O. basilicum can deliver antiaging benefits.12
Antioxidant activity from myriad constituents, including quercetin, kaempferol, caffeic acid, rosmarinic acid, ferulic acid, rutin, and catechin, among others, has been cited for the potential of O. basilicum to confer an antiaging result.13,14
Conclusion
Various species in the Ocimum family have been used in traditional medicine for many years, with several reputed to impart dermatologic benefits. There are compelling reasons to continue to research these species in the continuing search to develop more effective topical formulations in the dermatologic armamentarium. As is often the case with botanical agents, we need to see much more evidence and clinical trials to establish if and how appropriate these Ocimum species are in the skin care realm. The word “adaptogen” is starting to be used frequently in the cosmeceutical world. Holy basil is an adaptogen.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. Rupani R, Chavez A. Clin Dermatol. 2018 May-Jun;36(3):306-9.
2. Baliga MS et al. Nutr Cancer. 2013;65 Suppl 1:26-35.
3. Prakash P, Gupta N. Indian J Physiol Pharmacol. 2005 Apr;49(2):125-31.
4. Baliga MS et al. J Cancer Res Ther. 2016 Jan-Mar;12(1):20-7.
5. Pazyar N et al. Skin Pharmacol Physiol. 2014;27(6):303-10.
6. Yamani HA et al. Front Microbiol. 2016 May 17;7:681.
7. Leelapornpisid P et al. J Cosmet Sci. 2015 Jul-Aug:66(4):219-31.
8. Nweze EI, Eze EE. BMC Complement Altern Med. 2009 Sep 28;9:37.
9. Ajose FOA. Int J Dermatol. 2007 Oct;46 Suppl 1:48-55.
10. Tolino E et al. G Ital Dermatol Venereol. 2018 Dec;153(6):866-871.
11. Keziah EA et al. J Insect Sci. 2015 Apr 15. doi: 10.1093/jisesa/iev025.
12. Rasul A, Akhtar N. Daru. 2011;19(5):344-50.
13. Jadoon S et al. Oxid Med Cell Longev. 2015;2015:709628.
14. Marwat SK et al. Asian J Chem. 2011;23(9):3773-82.
Morinda citrifolia (Noni) tree: Many names, even more applications
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
, which has been in use on the islands for two millennia.1-4 The plant, found abundantly in Southeast Asia, Australia, the Pacific Basin, and the Caribbean, is called Great Morinda or cheese fruit in Australia, Nono in Tahiti, Indian Mulberry in India, and Ba ji tian in China.4-6 It is also deployed for a wide range of health purposes in Brazil.7
Noni has been credited with conferring various salutary benefits against arthritis, diabetes, fever, gingivitis, headaches, infections, inflammation, respiratory illnesses, and tuberculosis.3,8 In alternative medicine, the fruit juice, which has been found to be safe, is used for multiple indications, with a slew of studies presenting evidence for anti-inflammatory, antioxidant, and apoptosis-inducing benefits against cancer.5,6 All parts of M. citrifolia – leaves, fruits, roots, bark, flowers, and seeds – have been used in traditional medical practices.8 This column will focus on recent research into the broad array of biologic activities attributed to the plant and possible dermatologic uses.
Diverse biologic properties
In 2007, Nayak et al. showed that the juice of M. citrifolia fruit significantly lowered sugar levels in diabetic rats and facilitated their wound healing.1
Three years later, Thani et al. determined that the leaves of M. citrifolia exert antiproliferative and antioxidative activities, with chemopreventive benefits seen against epidermoid and cervical cancers.9
In 2011, Serafini et al. confirmed the antibacterial, anti-inflammatory, antioxidant, and antinociceptive qualities of the aqueous extract from M. citrifolia leaves, with the extract shown to significantly lower leukocyte migration in doses of 200 and 400 mg/kg. Mild antibacterial properties were seen as was an antinociceptive effect at the higher dose in the acetic-acid-induced writhing test.3
A comprehensive literature review in 2017 by Torres et al. identified a varied and extensive list of biological activities of M. citrifolia, including immunostimulatory, antitumor, antidiabetic, antiobesity, antibacterial and antiseptic, antifungal, antiviral, anti-inflammatory, antinociceptive and analgesic, antioxidant, neuroprotective, wound healing, antiallergic, photoprotective, and antiwrinkle among several others. Despite its use in disease prevention and treatment around the world, the researchers call for more in vitro and in vivo models in addition to clinical trials to further examine the health benefits of Noni.7
Early in 2019, De La Cruz-Sánchez et al. determined that the methanolic extract of M. citrifolia displayed marked activity against methicillin-resistant Staphylococcus aureus (MRSA), thus supporting its continuing applications in traditional medical practice.2
Photoprotection and antiaging potential
Based on their prior work demonstrating that M. citrifolia fruit upregulates the production of type I collagen and glycosaminoglycans in primary cultures of normal human fibroblasts, Kim et al. isolated anthraquinone from the fruit and showed that it dose-dependently decreased the expression of collagenase matrix metalloproteinase-1 in human dermal fibroblasts. The investigators also found that an anthraquinone-containing nano-emulsion raised type I procollagen in nude mouse skin. They concluded, in this 2005 study, that Noni extract warrants consideration as an antiwrinkle agent given its proclivity to induce the production of collagen.10
In 2009, West et al. assessed a carbomer gel base containing the ethanol extract and juice pressed from Noni leaves for possible allergenic activity in a repeat-insult patch test in 49 volunteers. They also used a UVB-induced erythema model in 25 subjects to test the topical photoprotective potential of the ethanol extract and leaf juice. The investigators reported no allergic potential evinced by the patch tests, and in a histamine H-1 receptor antagonism assay, the leaves hindered receptor binding by 57%, suggesting anti-inflammatory activity. In the UVB test, the dose necessary to engender erythema was nearly 3.5 times higher than in untreated skin. The team concluded that M. citrifolia leaves are safe for topical application and show promise in lessening UVB-induced skin damage.11
A 2014 study on mice by Serafini et al. showed that the dorsal skin of mice treated for 7 days with topical M. citrifolia was protected from damage by exposure to UVA-UVB radiation as measured by skin thickness, transepidermal water loss, erythema, and histological changes.12
Conclusion
Morinda citrifolia has been used in traditional medicine for at least 2,000 years. Its reported list of uses covers an impressive gamut of indications.
Modern medicine is beginning to catch up with new research conducted on this copious and beloved plant. That said, much more data, particularly from human clinical trials, are necessary to elucidate the most appropriate dermatologic roles for M. citrifolia. I just started growing a Noni tree in my yard because some patients have reported using it on their skin. I will report back and let you know how it goes. It is flowering now!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nayak BS et al. J Wound Care. 2007 Feb;16(2):83-6.
2. De La Cruz-Sánchez NG et al. Microb Pathog. 2019 Mar;128:347-53.
3. Serafini MR et al. J Med Food. 2011 Oct;14(10):1159-66.
4. Wang MY, Su C. Ann N Y Acad Sci. 2001 Dec;952:161-8.
5. Gupta RK, Patel AK. Asian Pac J Cancer Prev. 2013;14(8):4495-9.
6. Brown AC. Phytother Res. 2012 Oct;26(10):1427-40.
7. Torres MAO et al. Phytother Res. 2017 Jul;31(7):971-9.
8. Potterat O, Hamburger M. Planta Med. 2007 Mar;73(3):191-9.
9. Thani W et al. Southeast Asian J Trop Med Public Health. 2010 Mar;41(2):482-9.
10. Kim SW et al. J Med Food. 2005 Winter;8(4):552-5.
11. West BJ et al. J Nat Med. 2009 Jul;63(3):351-4.
12. Serafini MR et al. Biomed Res Int. 2014;2014:587819. doi: 10.1155/2014/587819.
Beware of natural fruit and nut ingredients in latex-allergic patients
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
It has been 40 years since the first reported case of IgE-mediated natural rubber latex allergy, which was soon followed by a global epidemic of allergic and anaphylactic reactions.1,2 Resolution came through insightful work in the 1990s that led to the removal of cornstarch powder and a switch to nonpowdered latex and synthetic examination gloves.2 Also discovered during this period was the cross-reactivity of many patients to latex and various fruits.
Research substantiates reports
Blanco et al. conducted a prospective study in their outpatient clinic in 25 patients diagnosed with latex allergy, published in 1994.They used a clinical questionnaire, skin-prick tests, skin test with a latex extract, and identification of total and specific IgE to help ascertain clinical characteristics and cross-reactivity. Of the 23 women and 2 men in the study (mean age 33, plus or minus 9 years), 9 (36%) experienced latex-induced reactions characterized by systemic anaphylaxis. In 13 patients (52%), 42 food allergies were identified, and 23 included systemic anaphylaxis. Avocado (9), chestnut (9), banana (7), kiwi (5), and papaya (3) were the most common foods to cause hypersensitivities. The researchers concluded that their small study supported the reality of a “latex-fruit syndrome.”3
Another study aimed to characterize the cross-reactivity of latex and foods and evaluate clinical significance. Beezhold et al. examined 47 patients allergic to latex and 46 nonallergic controls. The investigators found immunologic reactivity to foods to be prevalent (33 latex-allergic patients and seven controls), with 27% of food skin-prick tests positive in the latex-allergic group. In addition, clinical symptoms were linked to 27% of positive skin-prick tests. Among the 17 patients who displayed clinical allergies to at least one food, 14 showed local sensitivity reactions, with anaphylaxis noted in 11. Avocado (53%), potato (40%), banana (38%), tomato (28%), chestnut (28%), and kiwi (17%) were the foods most frequently cited for provoking a skin test reaction. The authors observed extensive cross-reactivity between latex sensitivity and particular foods, with potatoes and tomatoes reported for the first time.4
In 1997, Brehler et al. studied serum samples from 136 patients whose immediate hypersensitivity to latex proteins was clinically observable and documented. The samples were assessed for IgE antibodies against several fruits, with fruit-specific IgE antibodies recorded in 69.1%. Radioallergosorbent (RAST) -inhibition tests yielded the recognition of cross-reacting IgE antibodies in latex and multiple fruit allergens: avocado, banana, chestnut, fig, kiwi, mango, melon, papaya, passion fruit, peach, pineapple, and tomato. The investigators recorded 112 intolerance reactions and noted that 42.5% of their patients reported allergic symptoms after consuming these fruits. Fruit-specific IgE antibodies were detected in only 32.1% of these patients, suggesting to the researchers that serologic tests were suboptimal in forecasting food hypersensitivities in patients who are allergic to latex.5
Cross-reactivity with banana
Mäkinen-Kiljunen studied 47 patients to investigate banana allergy in patients with latex allergy in 1994, measuring latex-, banana-, and pollen-specific (birch, timothy, and mugwort) IgE. Thirty-one patients were also given skin-prick tests with banana and were queried about reactions after consuming bananas. Of the 47 sera samples, latex RAST results were positive in 31 and banana RAST results in 26. RAST results from latex and banana were correlated (25 of the 31 latex RAST-positive samples were also banana RAST-positive), but not with pollen. Sixteen of the 31 patients who ate banana reported symptoms, and 11 of the 31 patients given the banana skin-prick test showed positive results. The author confirmed the cross-reactivity of IgE antibodies for latex and banana, identifying for the first time a structurally similar antigen/allergen as at least one antigen from banana fused with an antigen from latex in crossed-line immunoelectrophoresis.6
In 1998, Mikkola et al. investigated whether proteins similar to hevein, a major natural rubber latex allergen, are present in banana and account for cross-reactivity between these botanicals. Immunoblotting revealed that 9 of 15 sera from latex-allergic patients with IgE to hevein also bound to 32- and 33-kd banana proteins. Studies using ELISA [enzyme-linked immunosorbent assay] showed that the common presentation of hypersensitivity to banana among patients allergic to latex could be attributed to cross-reacting IgE antibodies binding to epitopes in hevein and in the then-newly identified hevein-like endochitinase found in banana.7
Cross-reactivity with avocado
In response to reports of an association between allergy to natural rubber latex and avocado, Ahlroth et al. investigated cross-reactive proteins between natural rubber latex and avocado in 1995 by using skin-prick tests with fresh avocado on 11 patients and the sera of 18 patients with known latex allergy for IgE antibodies. Fourteen of the 18 sera were found to have IgE antibodies binding to 17 distinct avocado proteins, with multiple immunoblot experiments and skin-prick test results (positive in 7 of 11 patients) revealing marked immunologic cross-reactivity between latex and avocado.8
In 1998, Chen et al. set out to identify the cross-sensitizing allergen between latex and avocado, with hevein suspected. The researchers looked at sera samples from 118 health care workers allergic to latex and 78 patients with spina bifida who were allergic to latex. They noted a robust correlation between the prevalence of seropositive IgE antibodies to avocado in the presence of hevein-specific IgE antibodies in both groups. All members in the spina bifida group and 91 (73%) of the health care workers had positive IgE antibodies to hevein and high IgE values to avocado. Additional results supported the conclusion that sensitization to avocado in the majority of people allergic to latex is engendered by IgE-binding epitopes found in hevein.9
A year later, Diaz-Perales et al. considered the potential relevance of chitinases and complex glycans as factors in the then newly described latex/food syndrome, particularly in avocado, banana, and chestnuts. The investigators culled extracts from 20 various plant foods as well as latex. In immunoblot inhibition assays, the primary allergen and class I chitinase in avocado, Prs a 1, and the latex extract potently or completely blocked IgE binding by these constituents. Polyclonal antibodies to chitinases and sera from patients with latex/fruit allergy responded to reactive proteins of about 30-45 kd (putative class I chitinases) in chestnut, cherimoya, kiwi, mango, papaya, passion fruit, tomato, and wheat flour extracts. The glycans complex was deemed to be irrelevant in latex/fruit cross-reactivity, but the researchers found the putative class I chitinases to be notable players in the latex/fruit syndrome.10
According to Wagner and Breitender, anywhere from 30%-50% of people with known latex allergy also evince a related hypersensitivity or allergy to various plant-derived foods, with avocado, banana, chestnut, kiwi, peach, tomato, potato, and bell pepper among the foods most frequently linked to latex/fruit syndrome. They summarize that several plant defense proteins have been shown to be involved in the syndrome, with the most prominent, class I chitinases with an N-terminal hevein-like domain, having been found to cross-react with hevein (Hev b 6.02), a major IgE-binding allergen for individuals allergic to latex. A beta-1,3-glucanase, a key latex allergen, has also shown cross-reactivity with proteins of bell pepper, and another significant latex allergen, Hev b 7, a patatin-like protein, cross-reacts with its analogous protein in potato.11
Conclusion
It is unknown whether latex allergy precedes or follows food allergy.11 The latex/food syndrome itself merits attention as a significant source of hypersensitivity to natural cosmeceutical ingredients. Dermatologists should be aware of the lengthy list of cross-reacting plant-derived products, particularly when it comes to reviewing topical product ingredients with susceptible or allergic patients. Latex-allergic patients may react to these natural ingredients in food or when topically applied to the skin.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Nutter AF. Br J Dermatol 1979 Nov;101(5):597-8.
2. Kelly KJ et al. J Allergy Clin Immunol Pract. 2017 Sep-Oct;5(5):1212-16.
3. Blanco C et al. Ann Allergy. 1994 Oct;73(4):309-14.
4. Beezhold DH et al. Clin Exp Allergy. 1996 Apr;26(4):416-22.
5. Brehler R et al. Allergy. 1997 Apr;52(4):404-10.
6. Mäkinen-Kiljunen S. J Allergy Clin Immunol. 1994 Jun;93(6):990-6.
7. Mikkola JH et al. J Allergy Clin Immunol. 1998 Dec;102(6 Pt 1):1005-12.
8. Ahlroth M et al. J Allergy Clin Immunol. 1995 Aug;96(2):167-73.
9. Chen Z et al. J Allergy Clin Immunol. 1998 Sep;102(3):476-81.
10. Diaz-Perales A et al. J Allergy Clin Immunol. 1999 Sep;104(3 Pt 1):681-7.
11. Wagner S et al. Biochem Soc Trans. 2002 Nov;30(Pt 6):935-40.
Parabens – friend or foe?
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Bakuchiol
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at dermnews@mdedge.com.
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
A primer on cannabis for cosmeceuticals: Research and treatments for particular skin conditions
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at dermnews@mdedge.com.
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at dermnews@mdedge.com.
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at dermnews@mdedge.com.
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.