User login
Reducing Overuse of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Nonvariceal Gastrointestinal Bleeding in the Hospital: A Narrative Review and Implementation Guide
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).
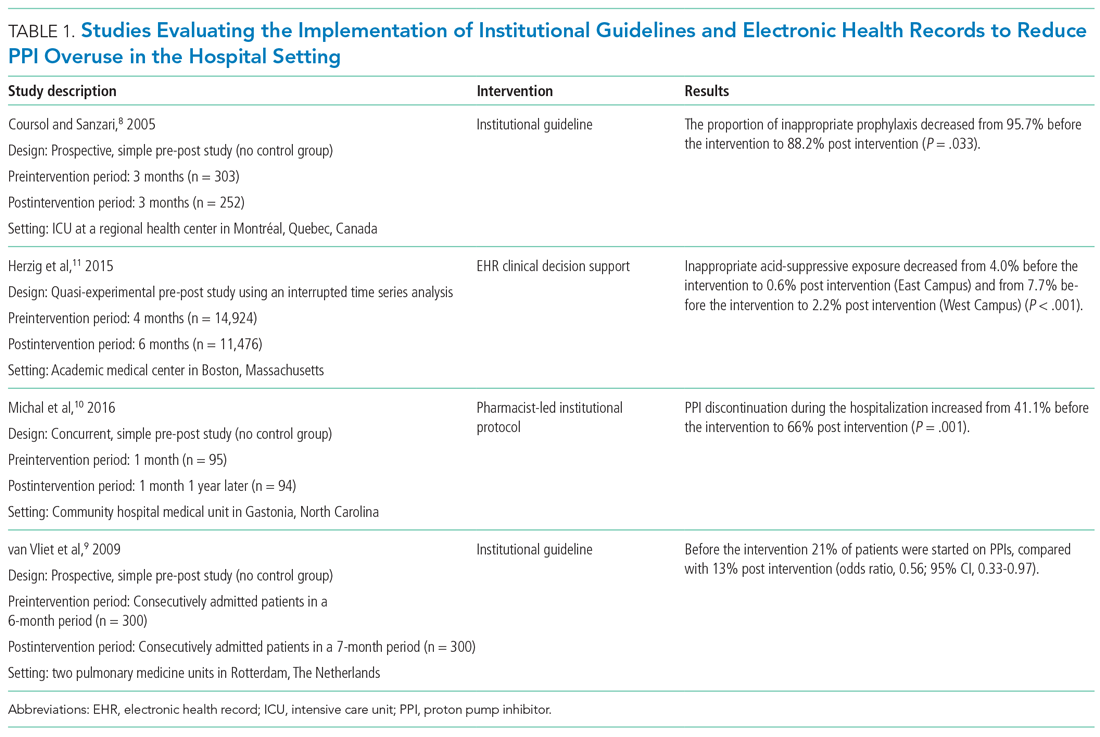
Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.
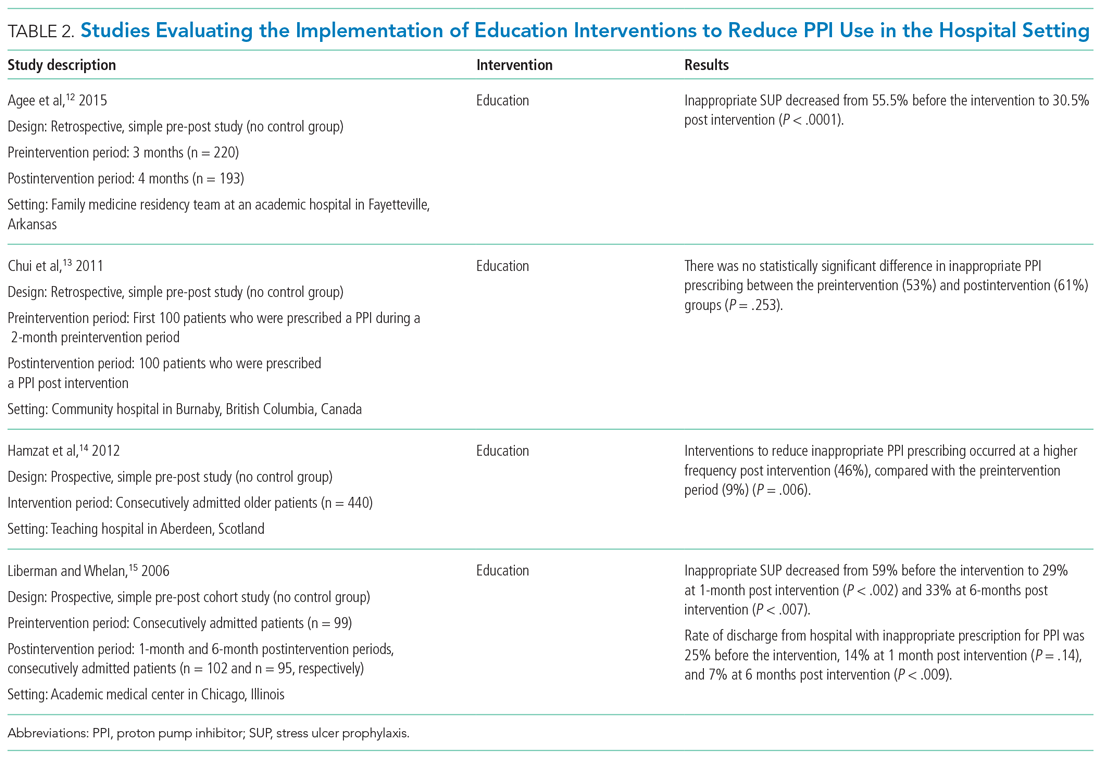
Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.
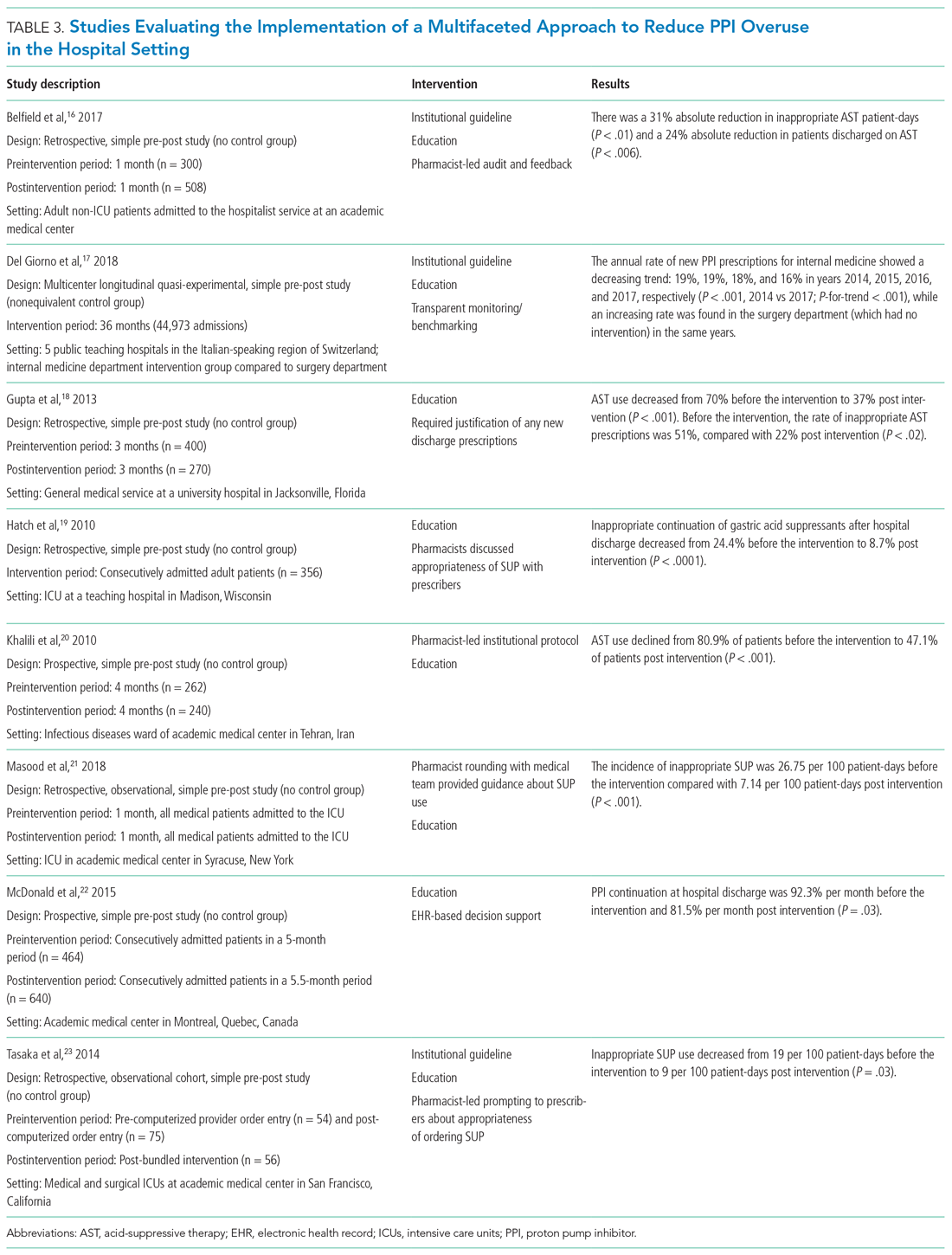
Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
Proton pump inhibitors (PPIs) are among the most commonly used drugs worldwide to treat dyspepsia and prevent gastrointestinal bleeding (GIB).1 Between 40% and 70% of hospitalized patients receive acid-suppressive therapy (AST; defined as PPIs or histamine-receptor antagonists), and nearly half of these are initiated during the inpatient stay.2,3 While up to 50% of inpatients who received a new AST were discharged on these medications,2 there were no evidence-based indications for a majority of the prescriptions.2,3
Growing evidence shows that PPIs are overutilized and may be associated with wide-ranging adverse events, such as acute and chronic kidney disease,4Clostridium difficile infection,5 hypomagnesemia,6 and fractures.7 Because of the widespread overuse and the potential harm associated with PPIs, a concerted effort to promote their appropriate use in the inpatient setting is necessary. It is important to note that reducing the use of PPIs does not increase the risks of GIB or worsening dyspepsia. Rather, reducing overuse of PPIs lowers the risk of harm to patients. The efforts to reduce overuse, however, are complex and difficult.
This article summarizes evidence regarding interventions to reduce overuse and offers an implementation guide based on this evidence. This guide promotes value-based quality improvement and provides a blueprint for implementing an institution-wide program to reduce PPI overuse in the inpatient setting. We begin with a discussion about quality initiatives to reduce PPI overuse, followed by a review of the safety outcomes associated with reduced use of PPIs.
METHODS
A focused search of the US National Library of Medicine’s PubMed database was performed to identify English-language articles published between 2000 and 2018 that addressed strategies to reduce PPI overuse for stress ulcer prophylaxis (SUP) and nonvariceal GIB. The following search terms were used: PPI and inappropriate use; acid-suppressive therapy and inappropriate use; PPI and discontinuation; acid-suppressive (or suppressant) therapy and discontinuation; SUP and cost; and histamine receptor antagonist and PPI. Inpatient or outpatient studies of patients aged 18 years or older were considered for inclusion in this narrative review, and all study types were included. The primary exclusion criterion was patients aged younger than 18 years. A manual review of the full text of the retrieved articles was performed and references were reviewed for missed citations.
RESULTS
We identified a total of 1,497 unique citations through our initial search. After performing a manual review, we excluded 1,483 of the references and added an additional 2, resulting in 16 articles selected for inclusion. The selected articles addressed interventions falling into three main groupings: implementation of institutional guidelines with or without electronic health record (EHR)–based decision support, educational interventions alone, and multifaceted interventions. Each of these interventions is discussed in the sections that follow. Table 1, Table 2, and Table 3 summarize the results of the studies included in our narrative review.
QUALITY INITIATIVES TO REDUCE PPI OVERUSE
Institutional Guidelines With or Without EHR-Based Decision Support
Table 1 summarizes institutional guidelines, with or without EHR-based decision support, to reduce inappropriate PPI use. The implementation of institutional guidelines for the appropriate reduction of PPI use has had some success. Coursol and Sanzari evaluated the impact of a treatment algorithm on the appropriateness of prescriptions for SUP in the intensive care unit (ICU).8 Risk factors of patients in this study included mechanical ventilation for 48 hours, coagulopathy for 24 hours, postoperative transplant, severe burns, active gastrointestinal (GI) disease, multiple trauma, multiple organ failure, and septicemia. The three treatment options chosen for the algorithm were intravenous (IV) famotidine (if the oral route was unavailable or impractical), omeprazole tablets (if oral access was available), and omeprazole suspension (in cases of dysphagia and presence of nasogastric or orogastric tube). After implementation of the treatment algorithm, the proportion of inappropriate prophylaxis decreased from 95.7% to 88.2% (P = .033), and the cost per patient decreased from $11.11 to $8.49 Canadian dollars (P = .003).

Van Vliet et al implemented a clinical practice guideline listing specific criteria for prescribing a PPI.9 Their criteria included the presence of gastric or duodenal ulcer and use of a nonsteroidal anti-inflammatory drug (NSAID) or aspirin, plus at least one additional risk factor (eg, history of gastroduodenal hemorrhage or age >70 years). The proportion of patients started on PPIs during hospitalization decreased from 21% to 13% (odds ratio, 0.56; 95% CI, 0.33-0.97).
Michal et al utilized an institutional pharmacist-driven protocol that stipulated criteria for appropriate PPI use (eg, upper GIB, mechanical ventilation, peptic ulcer disease, gastroesophageal reflux disease, coagulopathy).10 Pharmacists in the study evaluated patients for PPI appropriateness and recommended changes in medication or discontinuation of use. This institutional intervention decreased PPI use in non-ICU hospitalized adults. Discontinuation of PPIs increased from 41% of patients in the preintervention group to 66% of patients in the postintervention group (P = .001).
In addition to implementing guidelines and intervention strategies, institutions have also adopted changes to the EHR to reduce inappropriate PPI use. Herzig et al utilized a computerized clinical decision support intervention to decrease SUP in non-ICU hospitalized patients.11 Of the available response options for acid-suppressive medication, when SUP was chosen as the only indication for PPI use a prompt alerted the clinician that “[SUP] is not recommended for patients outside the [ICU]”; the alert resulted in a significant reduction in AST for the sole purpose of SUP. With this intervention, the percentage of patients who had any inappropriate acid-suppressive exposure decreased from 4.0% to 0.6% (P < .001).
EDUCATION
Table 2 summarizes educational interventions to reduce inappropriate PPI use.

Agee et al employed a pharmacist-led educational seminar that described SUP indications, risks, and costs.12 Inappropriate SUP prescriptions decreased from 55.5% to 30.5% after the intervention (P < .0001). However, there was no reduction in the percentage of patients discharged on inappropriate AST.
Chui et al performed an intervention with academic detailing wherein a one-on-one visit with a physician took place, providing education to improve physician prescribing behavior.13 In this study, academic detailing focused on the most common instances for which PPIs were inappropriately utilized at that hospital (eg, surgical prophylaxis, anemia). Inappropriate use of double-dose PPIs was also targeted. Despite these efforts, no significant difference in inappropriate PPI prescribing was observed post intervention.
Hamzat et al implemented an educational strategy to reduce inappropriate PPI prescribing during hospital stays, which included dissemination of fliers, posters, emails, and presentations over a 4-week period.14 Educational efforts targeted clinical pharmacists, nurses, physicians, and patients. Appropriate indications for PPI use in this study included peptic ulcer disease (current or previous), H pylori infection, and treatment or prevention of an NSAID-induced ulcer. The primary outcome was a reduction in PPI dose or discontinuation of PPI during the hospital admission, which increased from 9% in the preintervention (pre-education) phase to 43% during the intervention (education) phase and to 46% in the postintervention (posteducation) phase (P = .006).
Liberman and Whelan also implemented an educational intervention among internal medicine residents to reduce inappropriate use of SUP; this intervention was based on practice-based learning and improvement methodology.15 They noted that the rate of inappropriate prophylaxis with AST decreased from 59% preintervention to 33% post intervention (P < .007).
MULTIFACETED APPROACHES
Table 3 summarizes several multifaceted approaches aimed at reducing inappropriate PPI use. Belfield et al utilized an intervention consisting of an institutional guideline review, education, and monitoring of AST by clinical pharmacists to reduce inappropriate use of PPI for SUP.16 With this intervention, the primary outcome of total inappropriate days of AST during hospitalization decreased from 279 to 116 (48% relative reduction in risk, P < .01, across 142 patients studied). Furthermore, inappropriate AST prescriptions at discharge decreased from 32% to 8% (P = .006). The one case of GIB noted in this study occurred in the control group.

Del Giorno et al combined audit and feedback with education to reduce new PPI prescriptions at the time of discharge from the hospital.17 The educational component of this intervention included guidance regarding potentially inappropriate PPI use and associated side effects and targeted multiple departments in the hospital. This intervention led to a sustained reduction in new PPI prescriptions at discharge during the 3-year study period. The annual rate of new PPI prescriptions was 19%, 19%, 18%, and 16% in years 2014, 2015, 2016, and 2017, respectively, in the internal medicine department (postintervention group), compared with rates of 30%, 29%, 36%, 36% (P < .001) for the same years in the surgery department (control group).
Education and the use of medication reconciliation forms on admission and discharge were utilized by Gupta et al to reduce inappropriate AST in hospitalized patients from 51% prior to intervention to 22% post intervention (P < .001).18 Furthermore, the proportion of patients discharged on inappropriate AST decreased from 69% to 20% (P < .001).
Hatch et al also used educational resources and pharmacist-led medication reconciliation to reduce use of SUP.19 Before the intervention, 24.4% of patients were continued on SUP after hospital discharge in the absence of a clear indication for use; post intervention, 11% of patients were continued on SUP after hospital discharge (of these patients, 8.7% had no clear indication for use). This represented a 64.4% decrease in inappropriately prescribed SUP after discharge (P < .0001).
Khalili et al combined an educational intervention with an institutional guideline in an infectious disease ward to reduce inappropriate use of SUP.20 This intervention reduced the inappropriate use of AST from 80.9% before the intervention to 47.1% post intervention (P < .001).
Masood et al implemented two interventions wherein pharmacists reviewed SUP indications for each patient during daily team rounds, and ICU residents and fellows received education about indications for SUP and the implemented initiative on a bimonthly basis.21 Inappropriate AST decreased from 26.75 to 7.14 prescriptions per 100 patient-days of care (P < .001).
McDonald et al combined education with a web-based quality improvement tool to reduce inappropriate exit prescriptions for PPIs.22 The proportion of PPIs discontinued at hospital discharge increased from 7.7% per month to 18.5% per month (P = .03).
Finally, the initiative implemented by Tasaka et al to reduce overutilization of SUP included an institutional guideline, a pharmacist-led intervention, and an institutional education and awareness campaign.23 Their initiative led to a reduction in inappropriate SUP both at the time of transfer out of the ICU (8% before intervention, 4% post intervention, P = .54) and at the time of discharge from the hospital (7% before intervention, 0% post intervention, P = .22).
REDUCING PPI USE AND SAFETY OUTCOMES
Proton pump inhibitors are often initiated in the hospital setting, with up to half of these new prescriptions continued at discharge.2,24,25 Inappropriate prescriptions for PPIs expose patients to excess risk of long-term adverse events.26 De-escalating PPIs, however, raises concern among clinicians and patients for potential recurrence of dyspepsia and GIB. There is limited evidence regarding long-term safety outcomes (including GIB) following the discontinuation of PPIs deemed to have been inappropriately initiated in the hospital. In view of this, clinicians should educate and monitor individual patients for symptom relapse to ensure timely and appropriate resumption of AST.
LIMITATIONS
Our literature search for this narrative review and implementation guide has limitations. First, the time frame we included (2000-2018) may have excluded relevant articles published before our starting year. We did not include articles published before 2000 based on concerns these might contain outdated information. Also, there may have been incomplete retrieval of relevant studies/articles due to the labor-intensive nature involved in determining whether PPI prescriptions are appropriate or inappropriate.
We noted that interventional studies aimed at reducing overuse of PPIs were often limited by a low number of participants; these studies were also more likely to be single-center interventions, which limits generalizability. In addition, the studies often had low methodological rigor and lacked randomization or controls. Moreover, to fully evaluate the sustainability of interventions, some of the studies had a limited postimplementation period. For multifaceted interventions, the efficacy of individual components of the interventions was not clearly evaluated. Moreover, there was a high risk of bias in many of the included studies. Some of the larger studies used overall AST prescriptions as a surrogate for more appropriate use. It would be advantageous for a site to perform a pilot study that provides well-defined parameters for appropriate prescribing, and then correlate with the total number of prescriptions (automated and much easier) thereafter. Further, although the evidence regarding appropriate PPI use for SUP and GIB has shifted rapidly in recent years, society guidelines have not been updated to reflect this change. As such, quality improvement interventions have predominantly focused on reducing PPI use for the indications reflected by these guidelines.
IMPLEMENTATION BLUEPRINT
The following are our recommendations for successfully implementing an evidence-based, institution-wide initiative to promote the appropriate use of PPIs during hospitalization. These recommendations are informed by the evidence review and reflect the consensus of the combined committees coauthoring this review.
For an initiative to succeed, participation from multiple disciplines is necessary to formulate local guidelines and design and implement interventions. Such an interdisciplinary approach requires advocates to closely monitor and evaluate the program; sustainability will be greatly facilitated by the active engagement of key stakeholders, including the hospital’s executive administration, supply chain, pharmacists, and gastroenterologists. Lack of adequate buy-in on the part of key stakeholders is a barrier to the success of any intervention. Accordingly, before selecting a particular intervention, it is important to understand local factors driving the overuse of PPI.
1. Develop evidence-based institutional guidelines for both SUP and nonvariceal upper GIB through an interdisciplinary workgroup.
- Establish an interdisciplinary group including, but not limited to, pharmacists, hospitalists, gastroenterologists, and intensivists so that changes in practice will be widely adopted as institutional policy.
- Incorporate the best evidence and clearly convey appropriate and inappropriate uses.
2. Integrate changes to the EHR.
- If possible, the EHR should be leveraged to implement changes in PPI ordering practices.
- While integrating changes to the EHR, it is important to consider informatics and implementation science, since the utility of hard stops and best practice alerts has been questioned in the setting of operational inefficiencies and alert fatigue.
- Options for integrating changes to the EHR include the following:
- Create an ordering pathway that provides clinical decision support for PPI use.
- Incorporate a best practice alert in the EMR to notify clinicians of institutional guidelines when they initiate an order for PPI outside of the pathway.
- Consider restricting the authority to order IV PPIs by requiring a code or password or implement another means of using the EHR to limit the supply of PPI.
- Limit the duration of IV PPI by requiring daily renewal of IV PPI dosing or by altering the period of time that use of IV PPI is permitted (eg, 48 to 72 hours).
- PPIs should be removed from any current order sets that include medications for SUP.
3. Foster pharmacy-driven interventions.
- Consider requiring pharmacist approval for IV PPIs.
- Pharmacist-led review and feedback to clinicians for discontinuation of inappropriate PPIs can be effective in decreasing inappropriate utilization.
4. Provide education, audit data, and obtain feedback.
- Data auditing is needed to measure the efficacy of interventions. Outcome measures may include the number of non-ICU and ICU patients who are started on a PPI during an admission; the audit should be continued through discharge. A process measure may be the number of pharmacist calls for inappropriate PPIs. A balancing measure would be ulcer-specific upper GIB in patients who do not receive SUP during their admission. (Upper GIB from other etiologies, such as varices, portal hypertensive gastropathy, and Mallory-Weiss tear would not be affected by PPI SUP.)
- Run or control charts should be utilized, and data should be shared with project champions and ordering clinicians—in real time if possible.
- Project champions should provide feedback to colleagues; they should also work with hospital leadership to develop new strategies to improve adherence.
- Provide ongoing education about appropriate indications for PPIs and potential adverse effects associated with their use. Whenever possible, point-of-care or just-in-time teaching is the preferred format.
CONCLUSION
Excessive use of PPIs during hospitalization is prevalent; however, quality improvement interventions can be effective in achieving sustainable reductions in overuse. There is a need for the American College of Gastroenterology to revisit and update their guidelines for management of patients with ulcer bleeding to include stronger evidence-based recommendations on the proper use of PPIs.27 These updated guidelines could be used to update the implementation blueprint.
Quality improvement teams have an opportunity to use the principles of value-based healthcare to reduce inappropriate PPI use. By following the blueprint outlined in this article, institutions can safely and effectively tailor the use of PPIs to suitable patients in the appropriate settings. Reduction of PPI overuse can be employed as an institutional catalyst to promote implementation of further value-based measures to improve efficiency and quality of patient care.
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
1. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Exp Rev Clin Pharmacol. 2018;11(11):1123-1134. https://doi.org/10.1080/17512433.2018.1531703
2. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122. https://doi.org/10.1111/j.1572-0241.2000.03259.x
3. Ahrens D, Behrens G, Himmel W, Kochen MM, Chenot JF. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. https://doi.org/10.1111/j.1742-1241.2012.02973.x
4. Moledina DG, Perazella MA. PPIs and kidney disease: from AIN to CKD. J Nephrol. 2016;29(5):611-616. https://doi.org/10.1007/s40620-016-0309-2
5. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. https://doi.org/10.1038/ajg.2012.108
6. Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37(7):1237-1241. https://doi.org/10.3109/0886022x.2015.1057800
7. Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947-2953. https://doi.org/10.1001/jama.296.24.2947
8. Coursol CJ, Sanzari SE. Impact of stress ulcer prophylaxis algorithm study. Ann Pharmacother. 2005;39(5):810-816. https://doi.org/10.1345/aph.1d129
9. van Vliet EPM, Steyerberg EW, Otten HJ, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29(2):213-221. https://doi.org/10.1111/j.1365-2036.2008.03875.x
10. Michal J, Henry T, Street C. Impact of a pharmacist-driven protocol to decrease proton pump inhibitor use in non-intensive care hospitalized adults. Am J Health Syst Pharm. 2016;73(17 Suppl 4):S126-S132. https://doi.org/10.2146/ajhp150519
11. Herzig SJ, Guess JR, Feinbloom DB, et al. Improving appropriateness of acid-suppressive medication use via computerized clinical decision support. J Hosp Med. 2015;10(1):41-45. https://doi.org/10.1002/jhm.2260
12. Agee C, Coulter L, Hudson J. Effects of pharmacy resident led education on resident physician prescribing habits associated with stress ulcer prophylaxis in non-intensive care unit patients. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S48-S52. https://doi.org/10.2146/sp150013
13. Chui D, Young F, Tejani AM, Dillon EC. Impact of academic detailing on proton pump inhibitor prescribing behaviour in a community hospital. Can Pharm J (Ott). 2011;144(2):66-71. https://doi.org/10.3821/1913-701X-144.2.66
14. Hamzat H, Sun H, Ford JC, Macleod J, Soiza RL, Mangoni AA. Inappropriate prescribing of proton pump inhibitors in older patients: effects of an educational strategy. Drugs Aging. 2012;29(8):681-690. https://doi.org/10.1007/bf03262283
15. Liberman JD, Whelan CT. Brief report: Reducing inappropriate usage of stress ulcer prophylaxis among internal medicine residents. A practice-based educational intervention. J Gen Intern Med. 2006;21(5):498-500. https://doi.org/10.1111/j.1525-1497.2006.00435.x
16. Belfield KD, Kuyumjian AG, Teran R, Amadi M, Blatt M, Bicking K. Impact of a collaborative strategy to reduce the inappropriate use of acid suppressive therapy in non-intensive care unit patients. Ann Pharmacother. 2017;51(7):577-583. https://doi.org/10.1177/1060028017698797
17. Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52-59. https://doi.org/10.1016/j.ejim.2017.11.002
18. Gupta R, Marshall J, Munoz JC, Kottoor R, Jamal MM, Vega KJ. Decreased acid suppression therapy overuse after education and medication reconciliation. Int J Clin Pract. 2013;67(1):60-65. https://doi.org/10.1111/ijcp.12046
19. Hatch JB, Schulz L, Fish JT. Stress ulcer prophylaxis: reducing non-indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565-1571. https://doi.org/10.1345/aph.1p167
20. Khalili H, Dashti-Khavidaki S, Hossein Talasaz AH, Tabeefar H, Hendoiee N. Descriptive analysis of a clinical pharmacy intervention to improve the appropriate use of stress ulcer prophylaxis in a hospital infectious disease ward. J Manag Care Pharm. 2010;16(2):114-121. https://doi.org/10.18553/jmcp.2010.16.2.114
21. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist-based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. https://doi.org/10.1177/0046958018759116
22. McDonald EG, Jones J, Green L, Jayaraman D, Lee TC. Reduction of inappropriate exit prescriptions for proton pump inhibitors: a before-after study using education paired with a web-based quality-improvement tool. J Hosp Med. 2015;10(5):281-286. https://doi.org/10.1002/jhm.2330
23. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462-469. https://doi.org/10.1177/1060028013517088
24. Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21(10):1203-1209. https://doi.org/10.1111/j.1365-2036.2005.02454.x
25. Pham CQ, Regal RE, Bostwick TR, Knauf KS. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacother. 2006;40(7-8):1261-1266. https://doi.org/10.1345/aph.1g703
26. Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors [editorial]. JAMA Intern Med. 2016;176(2):172-174. https://doi.org/10.1001/jamainternmed.2015.7927
27. Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345-360; quiz 361. https://doi.org/10.1038/ajg.2011.480
© 2021 Society of Hospital Medicine
What Is the Best Empiric Therapy for Community-Acquired Cellulitis?
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
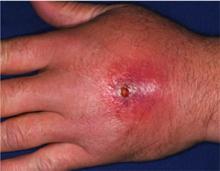
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
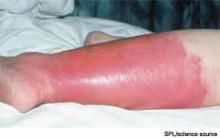
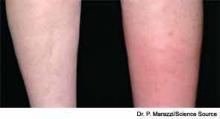
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
What is the best empiric therapy for community-acquired cellulitis?
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.