User login
What is the best empiric therapy for community-acquired cellulitis?
Case
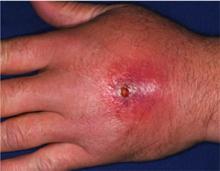
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue, and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but can affect other locations resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy, specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization, and those that develop following contact with healthcare facilities (e.g., hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Since CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful from 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g., clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for skin and SSTI caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethox-azole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized, prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism. TH
Dr. Clarke is a hospitalist and clinical instructor at Emory University School of Medicine, Atlanta. Dr. Dressler is associate professor and director of education, section of hospital medicine, and associate program director of the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit is an instructor in clinical medicine at Emory University School of Medicine.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297.
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.