User login
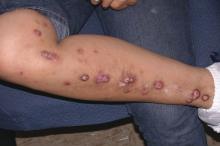
Tanning use disorder should be added to the DSM-5
Raghav Tripathi and his associates maintained in a letter to the editor of the Journal of the European Academy of Dermatology and Venereology.
“Strong evidence suggests that tanning use disorder should be included in the DSM-5,” they wrote, noting that individuals who show signs of tanning use disorder have problems quitting, and also are more likely to smoke cigarettes, drink alcohol excessively, and engage in other high-risk behaviors.
In the letter, Mr. Tripathi, a medical student at Case Western Reserve University, Cleveland, and his associates also noted that frequent tanners have been found to prefer tanning beds that used UV radiation (UVR) over beds that did not, even though they were blinded to which ones did and did not. Reports of pain relief and improved mood following UVR exposure, withdrawal symptoms upon discontinuation of UVR, and the successful use of opioid antagonists to reduce UVR dependence “underscore the importance of viewing tanning as a use disorder.”
The same “brain circuitry and neurotransmitters involved in the reward pathways of other use disorders” are associated with the addictive characteristics of UVR, they wrote.
In one study, people who were compulsive tanners were found to have an increase in “cerebral blood flow in the mesostriatal reward pathway when exposed to UVR.” In another study, opioid antagonism using naltrexone was found to reduce “UVR preference in frequent tanners.”
Understanding the biologic connections is crucial to advocating for formalization of the condition as a recognized disorder in the DSM-5; classification would not only increase awareness of the condition but also standardize approaches to diagnosis and treatment that are key to improving patient care, the authors wrote. Moreover, inclusion of the disorder in the DSM-5 could help to pave the way for inclusion in the ICD-10, which would have broader implications for limiting the overall harmful effects that tanning poses.
The authors had no relevant financial disclosures to report.
SOURCE: Tripathi R et al. J Eur Acad Dermatol Venereol. 2018 Oct 13. doi: 10.1111/jdv.15286.
Raghav Tripathi and his associates maintained in a letter to the editor of the Journal of the European Academy of Dermatology and Venereology.
“Strong evidence suggests that tanning use disorder should be included in the DSM-5,” they wrote, noting that individuals who show signs of tanning use disorder have problems quitting, and also are more likely to smoke cigarettes, drink alcohol excessively, and engage in other high-risk behaviors.
In the letter, Mr. Tripathi, a medical student at Case Western Reserve University, Cleveland, and his associates also noted that frequent tanners have been found to prefer tanning beds that used UV radiation (UVR) over beds that did not, even though they were blinded to which ones did and did not. Reports of pain relief and improved mood following UVR exposure, withdrawal symptoms upon discontinuation of UVR, and the successful use of opioid antagonists to reduce UVR dependence “underscore the importance of viewing tanning as a use disorder.”
The same “brain circuitry and neurotransmitters involved in the reward pathways of other use disorders” are associated with the addictive characteristics of UVR, they wrote.
In one study, people who were compulsive tanners were found to have an increase in “cerebral blood flow in the mesostriatal reward pathway when exposed to UVR.” In another study, opioid antagonism using naltrexone was found to reduce “UVR preference in frequent tanners.”
Understanding the biologic connections is crucial to advocating for formalization of the condition as a recognized disorder in the DSM-5; classification would not only increase awareness of the condition but also standardize approaches to diagnosis and treatment that are key to improving patient care, the authors wrote. Moreover, inclusion of the disorder in the DSM-5 could help to pave the way for inclusion in the ICD-10, which would have broader implications for limiting the overall harmful effects that tanning poses.
The authors had no relevant financial disclosures to report.
SOURCE: Tripathi R et al. J Eur Acad Dermatol Venereol. 2018 Oct 13. doi: 10.1111/jdv.15286.
Raghav Tripathi and his associates maintained in a letter to the editor of the Journal of the European Academy of Dermatology and Venereology.
“Strong evidence suggests that tanning use disorder should be included in the DSM-5,” they wrote, noting that individuals who show signs of tanning use disorder have problems quitting, and also are more likely to smoke cigarettes, drink alcohol excessively, and engage in other high-risk behaviors.
In the letter, Mr. Tripathi, a medical student at Case Western Reserve University, Cleveland, and his associates also noted that frequent tanners have been found to prefer tanning beds that used UV radiation (UVR) over beds that did not, even though they were blinded to which ones did and did not. Reports of pain relief and improved mood following UVR exposure, withdrawal symptoms upon discontinuation of UVR, and the successful use of opioid antagonists to reduce UVR dependence “underscore the importance of viewing tanning as a use disorder.”
The same “brain circuitry and neurotransmitters involved in the reward pathways of other use disorders” are associated with the addictive characteristics of UVR, they wrote.
In one study, people who were compulsive tanners were found to have an increase in “cerebral blood flow in the mesostriatal reward pathway when exposed to UVR.” In another study, opioid antagonism using naltrexone was found to reduce “UVR preference in frequent tanners.”
Understanding the biologic connections is crucial to advocating for formalization of the condition as a recognized disorder in the DSM-5; classification would not only increase awareness of the condition but also standardize approaches to diagnosis and treatment that are key to improving patient care, the authors wrote. Moreover, inclusion of the disorder in the DSM-5 could help to pave the way for inclusion in the ICD-10, which would have broader implications for limiting the overall harmful effects that tanning poses.
The authors had no relevant financial disclosures to report.
SOURCE: Tripathi R et al. J Eur Acad Dermatol Venereol. 2018 Oct 13. doi: 10.1111/jdv.15286.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Anthralin shows promise as second-line agent for pediatric alopecia areata
Given its limited systemic toxicity, , according to Sean Z. Wu, MD, of the department of dermatology, University of Cincinnati, and his associates.
In a retrospective study of 37 pediatric patients with AA, published in Pediatric Dermatology, Dr. Wu and his colleagues found that almost two-thirds experienced at least 50% regrowth of hair with topical anthralin treatment, but they described severe dermatitis and relapses as “potential drawbacks” of treatment.
The 37 patients were in the Cleveland Clinic AA areata database and began treatment with anthralin between 2004 and 2015, at aged 2-17 years (mean age 9). Over half (22) were females and most (31) were white. About 65% had patchy AA; the remainder had either alopecia totalis or alopecia universalis. Prior treatments included topical corticosteroids, minoxidil, and intralesional corticosteroids; four patients had not been treated previously. Patients were followed up from 51 days to more than 10 years, with a mean duration of 2.5 years. Treatment regimens, titrated up to achieve a mild to moderate dermatitis, included application of 0.5% cream for 5 minutes twice a week up to 1.0% cream for 30 minutes a day.
With topical anthralin, 12 (32%) of patients had complete scalp regrowth, 25 (68%) experienced at least 50% regrowth, and 5 (14%) had no response; in five patients, no follow-up information was available. Among those with at least 50% regrowth, the initial response was first noted at a mean of 3.4 months, and the mean time to maximal response was 15 months. This timeline suggests that treatment with anthralin should be continued beyond 1 year to ensure maximum beneficial results with hair regrowth, the authors wrote.
Factors associated with a positive response to treatment included less than 50% of scalp involvement. The two patients who used anthralin as monotherapy did not achieve a 50% scalp response, but the four treatment-naive patients were among those with at least 50% scalp regrowth, versus three of the five patients (60%) who had been treated previously with systemic steroids.
Two potential clinical limitations were noted during the study. Four patients had to stop treatment because of dermatitis, which suggests that patients and parents should be counseled about the potential for severe skin irritation with this treatment, the authors said. And among those who achieved at least 50% scalp regrowth, 16 of the 25 (64%) relapsed. The authors speculated that the effects of the drug could be temporary “or that the disease process may be able to overcome the anthralin effect over time.”
Dr. Wu and his coauthors cited the retrospective design and the small population size as major limitations of the study. Because some patients continued other treatments, it is “difficult to attribute scalp regrowth entirely to anthralin,” and variations in formulation and in treatment regimens are also factors to be considered, they cautioned. That AA has been found to spontaneously resolve, depending upon the severity of the disease, presents additional challenges for clinicians attempting to determine the extent to which anthralin offers therapeutic benefit, they pointed out.
In spite of the drug’s limitations, the authors concluded that the treatment was a safe and useful option as an “adjunct for those who fail first-line therapy with topical or intralesional corticosteroids.” They added that more work is needed to tailor treatment formulation, frequency, and duration to the specific needs of pediatric patients.
The authors had no relevant financial disclosures to report.
SOURCE: Wu SZ et al. Pediatr Dermatol. 2018;35:817-20.
Given its limited systemic toxicity, , according to Sean Z. Wu, MD, of the department of dermatology, University of Cincinnati, and his associates.
In a retrospective study of 37 pediatric patients with AA, published in Pediatric Dermatology, Dr. Wu and his colleagues found that almost two-thirds experienced at least 50% regrowth of hair with topical anthralin treatment, but they described severe dermatitis and relapses as “potential drawbacks” of treatment.
The 37 patients were in the Cleveland Clinic AA areata database and began treatment with anthralin between 2004 and 2015, at aged 2-17 years (mean age 9). Over half (22) were females and most (31) were white. About 65% had patchy AA; the remainder had either alopecia totalis or alopecia universalis. Prior treatments included topical corticosteroids, minoxidil, and intralesional corticosteroids; four patients had not been treated previously. Patients were followed up from 51 days to more than 10 years, with a mean duration of 2.5 years. Treatment regimens, titrated up to achieve a mild to moderate dermatitis, included application of 0.5% cream for 5 minutes twice a week up to 1.0% cream for 30 minutes a day.
With topical anthralin, 12 (32%) of patients had complete scalp regrowth, 25 (68%) experienced at least 50% regrowth, and 5 (14%) had no response; in five patients, no follow-up information was available. Among those with at least 50% regrowth, the initial response was first noted at a mean of 3.4 months, and the mean time to maximal response was 15 months. This timeline suggests that treatment with anthralin should be continued beyond 1 year to ensure maximum beneficial results with hair regrowth, the authors wrote.
Factors associated with a positive response to treatment included less than 50% of scalp involvement. The two patients who used anthralin as monotherapy did not achieve a 50% scalp response, but the four treatment-naive patients were among those with at least 50% scalp regrowth, versus three of the five patients (60%) who had been treated previously with systemic steroids.
Two potential clinical limitations were noted during the study. Four patients had to stop treatment because of dermatitis, which suggests that patients and parents should be counseled about the potential for severe skin irritation with this treatment, the authors said. And among those who achieved at least 50% scalp regrowth, 16 of the 25 (64%) relapsed. The authors speculated that the effects of the drug could be temporary “or that the disease process may be able to overcome the anthralin effect over time.”
Dr. Wu and his coauthors cited the retrospective design and the small population size as major limitations of the study. Because some patients continued other treatments, it is “difficult to attribute scalp regrowth entirely to anthralin,” and variations in formulation and in treatment regimens are also factors to be considered, they cautioned. That AA has been found to spontaneously resolve, depending upon the severity of the disease, presents additional challenges for clinicians attempting to determine the extent to which anthralin offers therapeutic benefit, they pointed out.
In spite of the drug’s limitations, the authors concluded that the treatment was a safe and useful option as an “adjunct for those who fail first-line therapy with topical or intralesional corticosteroids.” They added that more work is needed to tailor treatment formulation, frequency, and duration to the specific needs of pediatric patients.
The authors had no relevant financial disclosures to report.
SOURCE: Wu SZ et al. Pediatr Dermatol. 2018;35:817-20.
Given its limited systemic toxicity, , according to Sean Z. Wu, MD, of the department of dermatology, University of Cincinnati, and his associates.
In a retrospective study of 37 pediatric patients with AA, published in Pediatric Dermatology, Dr. Wu and his colleagues found that almost two-thirds experienced at least 50% regrowth of hair with topical anthralin treatment, but they described severe dermatitis and relapses as “potential drawbacks” of treatment.
The 37 patients were in the Cleveland Clinic AA areata database and began treatment with anthralin between 2004 and 2015, at aged 2-17 years (mean age 9). Over half (22) were females and most (31) were white. About 65% had patchy AA; the remainder had either alopecia totalis or alopecia universalis. Prior treatments included topical corticosteroids, minoxidil, and intralesional corticosteroids; four patients had not been treated previously. Patients were followed up from 51 days to more than 10 years, with a mean duration of 2.5 years. Treatment regimens, titrated up to achieve a mild to moderate dermatitis, included application of 0.5% cream for 5 minutes twice a week up to 1.0% cream for 30 minutes a day.
With topical anthralin, 12 (32%) of patients had complete scalp regrowth, 25 (68%) experienced at least 50% regrowth, and 5 (14%) had no response; in five patients, no follow-up information was available. Among those with at least 50% regrowth, the initial response was first noted at a mean of 3.4 months, and the mean time to maximal response was 15 months. This timeline suggests that treatment with anthralin should be continued beyond 1 year to ensure maximum beneficial results with hair regrowth, the authors wrote.
Factors associated with a positive response to treatment included less than 50% of scalp involvement. The two patients who used anthralin as monotherapy did not achieve a 50% scalp response, but the four treatment-naive patients were among those with at least 50% scalp regrowth, versus three of the five patients (60%) who had been treated previously with systemic steroids.
Two potential clinical limitations were noted during the study. Four patients had to stop treatment because of dermatitis, which suggests that patients and parents should be counseled about the potential for severe skin irritation with this treatment, the authors said. And among those who achieved at least 50% scalp regrowth, 16 of the 25 (64%) relapsed. The authors speculated that the effects of the drug could be temporary “or that the disease process may be able to overcome the anthralin effect over time.”
Dr. Wu and his coauthors cited the retrospective design and the small population size as major limitations of the study. Because some patients continued other treatments, it is “difficult to attribute scalp regrowth entirely to anthralin,” and variations in formulation and in treatment regimens are also factors to be considered, they cautioned. That AA has been found to spontaneously resolve, depending upon the severity of the disease, presents additional challenges for clinicians attempting to determine the extent to which anthralin offers therapeutic benefit, they pointed out.
In spite of the drug’s limitations, the authors concluded that the treatment was a safe and useful option as an “adjunct for those who fail first-line therapy with topical or intralesional corticosteroids.” They added that more work is needed to tailor treatment formulation, frequency, and duration to the specific needs of pediatric patients.
The authors had no relevant financial disclosures to report.
SOURCE: Wu SZ et al. Pediatr Dermatol. 2018;35:817-20.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Additional research is needed to pinpoint protocols for optimal formulation, dosage, and duration of anthralin in pediatric patients.
Major finding: At least 50% regrowth of scalp hair was achieved in 68% of patients treated with topical anthralin.
Study details: A retrospective chart review of 37 patients with alopecia areata who started treatment at a mean age of 9 years.
Disclosures: The authors reported no relevant financial disclosures.
Source: Wu SZ et al. Pediatr Dermatol. 2018;35:817-20.
Despite declines in prenatal use of alcohol and cigarettes, cannabis use is on the rise
Despite general decreases in cigarette and alcohol use among pregnant women during 2002-2016, cannabis use in pregnancy persisted and has actually increased slightly over the past 14 years, reported Arpana Agrawal, PhD, and her associates, of the Washington University, St. Louis.
Dr. Agrawal and her associates analyzed data from the National Survey of Drug Use and Health from 2002-2016 to quantify changes in alcohol, cigarette, and cannabis use among pregnant women aged 18-44 years. Survey-adjusted prevalence estimates of alcohol, cigarette, and cannabis use in 2002 were 10%, 18%, and 3%, respectively, versus corresponding rates in 2016 of 8%, 10%, and 5%, according to their research letter in JAMA Pediatrics.
A sampling of 12,058 women aged 18-25 years (n = 8,170) or 26-44 years (n = 3,888) was taken from a population of more than 436,056 women included in the National Survey database. Fully 3,554 of the women included in the final study group were in their first trimester of pregnancy.
The findings in Dr. Agrawal’s study were similar to another study conducted by Brown et al., although the exact prevalence for cannabis use differed slightly.
In addition to observing a reduction in the use of alcohol and cigarettes, the authors also noted a significant decline in the combined use of alcohol and cigarettes together. For alcohol use specifically, the greatest decrease was seen among women aged 18-25 years. For cigarette smoking in pregnancy, the most significant decreases were seen in white women, those aged 18-25 years, and those who had achieved completion of high school or more advanced studies.
Overall, the effects were modest when sample sizes were stratified by trimester: decreases in cigarette smoking were observed in the first trimester, and more significantly, later in pregnancy. For alcohol, nominal reductions were observed in the second and third trimesters. Cannabis use, on the other hand, increased in the first trimester, but showed no significant increases during the second and third trimesters.
“Greater public awareness regarding the consequences of prenatal cannabis exposure in offspring health is necessary,” Dr. Agrawal and her colleagues wrote.
The authors had no relevant disclosures to report. Funding was provided by grants from the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the National Institute on Alcohol Abuse and Alcoholism.
SOURCE: Agrawal A et al. JAMA Pediatr. 2018 Nov 5. doi: 10.1001/jamapediatrics.2018.3096.
Despite general decreases in cigarette and alcohol use among pregnant women during 2002-2016, cannabis use in pregnancy persisted and has actually increased slightly over the past 14 years, reported Arpana Agrawal, PhD, and her associates, of the Washington University, St. Louis.
Dr. Agrawal and her associates analyzed data from the National Survey of Drug Use and Health from 2002-2016 to quantify changes in alcohol, cigarette, and cannabis use among pregnant women aged 18-44 years. Survey-adjusted prevalence estimates of alcohol, cigarette, and cannabis use in 2002 were 10%, 18%, and 3%, respectively, versus corresponding rates in 2016 of 8%, 10%, and 5%, according to their research letter in JAMA Pediatrics.
A sampling of 12,058 women aged 18-25 years (n = 8,170) or 26-44 years (n = 3,888) was taken from a population of more than 436,056 women included in the National Survey database. Fully 3,554 of the women included in the final study group were in their first trimester of pregnancy.
The findings in Dr. Agrawal’s study were similar to another study conducted by Brown et al., although the exact prevalence for cannabis use differed slightly.
In addition to observing a reduction in the use of alcohol and cigarettes, the authors also noted a significant decline in the combined use of alcohol and cigarettes together. For alcohol use specifically, the greatest decrease was seen among women aged 18-25 years. For cigarette smoking in pregnancy, the most significant decreases were seen in white women, those aged 18-25 years, and those who had achieved completion of high school or more advanced studies.
Overall, the effects were modest when sample sizes were stratified by trimester: decreases in cigarette smoking were observed in the first trimester, and more significantly, later in pregnancy. For alcohol, nominal reductions were observed in the second and third trimesters. Cannabis use, on the other hand, increased in the first trimester, but showed no significant increases during the second and third trimesters.
“Greater public awareness regarding the consequences of prenatal cannabis exposure in offspring health is necessary,” Dr. Agrawal and her colleagues wrote.
The authors had no relevant disclosures to report. Funding was provided by grants from the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the National Institute on Alcohol Abuse and Alcoholism.
SOURCE: Agrawal A et al. JAMA Pediatr. 2018 Nov 5. doi: 10.1001/jamapediatrics.2018.3096.
Despite general decreases in cigarette and alcohol use among pregnant women during 2002-2016, cannabis use in pregnancy persisted and has actually increased slightly over the past 14 years, reported Arpana Agrawal, PhD, and her associates, of the Washington University, St. Louis.
Dr. Agrawal and her associates analyzed data from the National Survey of Drug Use and Health from 2002-2016 to quantify changes in alcohol, cigarette, and cannabis use among pregnant women aged 18-44 years. Survey-adjusted prevalence estimates of alcohol, cigarette, and cannabis use in 2002 were 10%, 18%, and 3%, respectively, versus corresponding rates in 2016 of 8%, 10%, and 5%, according to their research letter in JAMA Pediatrics.
A sampling of 12,058 women aged 18-25 years (n = 8,170) or 26-44 years (n = 3,888) was taken from a population of more than 436,056 women included in the National Survey database. Fully 3,554 of the women included in the final study group were in their first trimester of pregnancy.
The findings in Dr. Agrawal’s study were similar to another study conducted by Brown et al., although the exact prevalence for cannabis use differed slightly.
In addition to observing a reduction in the use of alcohol and cigarettes, the authors also noted a significant decline in the combined use of alcohol and cigarettes together. For alcohol use specifically, the greatest decrease was seen among women aged 18-25 years. For cigarette smoking in pregnancy, the most significant decreases were seen in white women, those aged 18-25 years, and those who had achieved completion of high school or more advanced studies.
Overall, the effects were modest when sample sizes were stratified by trimester: decreases in cigarette smoking were observed in the first trimester, and more significantly, later in pregnancy. For alcohol, nominal reductions were observed in the second and third trimesters. Cannabis use, on the other hand, increased in the first trimester, but showed no significant increases during the second and third trimesters.
“Greater public awareness regarding the consequences of prenatal cannabis exposure in offspring health is necessary,” Dr. Agrawal and her colleagues wrote.
The authors had no relevant disclosures to report. Funding was provided by grants from the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the National Institute on Alcohol Abuse and Alcoholism.
SOURCE: Agrawal A et al. JAMA Pediatr. 2018 Nov 5. doi: 10.1001/jamapediatrics.2018.3096.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Greatest increase in use of cannabis over the 14 years of the study was seen during the first trimester; the increase in use of cannabis in the second and third trimesters was not significant.
Study details: A study of 12,058 pregnant women from 2002-2016.
Disclosures: The authors had no relevant financial disclosures. Funding was provided by grants from the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the National Institute on Alcohol Abuse and Alcoholism.
Source: Agrawal A et al. JAMA Pediatr. 2018 Nov 5. doi: 10.1001/jamapediatrics.2018.3096.
Higher prenatal exposure to daylight tied to lower depression risk
Prenatal programming of the circadian and limbic systems might play a role in the odds of developing lifetime depression, a longitudinal study of almost 161,000 women shows.
“Our results could add support to an emerging hypothesis that perinatal photoperiod may influence depression risk,” wrote Elizabeth E. Devore of Brigham and Women’s Hospital, Boston, and her associates. “If replicated, ... these results could translate into safe and inexpensive light-related interventions for mothers and babies.”
In the study, which was published in the Journal of Psychiatric Research, Ms. Devore and her associates examined the influence of daylight exposure during maternal pregnancy and lifetime depression risk in the resulting offspring. They found that increased exposure to daylight during maternal pregnancy correlated with reduced lifetime risk of depression. wrote Ms. Devore, who also is affiliated with Harvard Medical School, Boston, and her associates.
The effects of daylight exposure were considered modest within the study population, but the authors emphasized that the finding would have much “larger effects at the population level,” given the occurrence of depression in the general population. They added that their findings reinforce a growing consensus that perinatal exposure to daylight could have the ability to influence the risk of developing a mood disorder.
The investigators accessed the Nurses’ Health Study (NHS) and the NHS II, established in 1976 and 1989, respectively, to assess risk factors for chronic conditions in female nurses. Both studies biennially surveyed demographic data on health, lifestyle, and medication use through mailed questionnaires. The first group was composed of 121,701 women aged 30-55 years; the second included 116,430 women aged 25-42 years. Altogether, 160,737 women born full-term were included in the study; 20,912 were excluded from the original survey group for not reporting depression status, as well as an additional 43,325 for not reporting their state of birth.
From data collected regarding participants’ day and state of birth, the researchers were able to estimate total length of daylight exposure during pregnancy using mathematical equations published by the National Oceanic and Atmospheric Administration.
Longitudinal coordinates pinpointing the center of population density for a participant’s birth state were used to identify the location of each participant during gestation. Using those assumptions, the authors were able to establish the two key data points evaluated for the study: total daylight exposure during pregnancy gestation, which was calculated by adding the lengths of all 280 days of the pregnancy, and extreme differences in daylight exposure that might have occurred throughout the pregnancy, which was measured by subtracting the longest and shortest day lengths during gestation.
The investigators paid particular attention to reported levels of depression, evidence of suicide, and personal characteristics and lifestyle factors, such as race, hair color, and early-life socioeconomic factors, including parents’ homeownership at the time of offspring birth; birth weight; history of having been breastfed; and parental occupation throughout the participant’s childhood.
Participants did not begin reporting antidepressant use for the first time until 1996; history of clinician-reported diagnoses of depression began in 2000, Ms. Devore and her associates reported.
Total daylight exposure during pregnancy was found to have “a borderline significant association with odds of lifetime depression,” but the trend was not convincing qualitatively, “and individual estimates across quintiles of exposure” were not considered to be statistically significant. In fact, the authors found that a larger difference between minimum and maximum daylight exposure throughout pregnancy significantly lowered lifetime risk of depression. Women with the largest differences in minimum/maximum daylight exposure during gestation had a 12% lower risk of depression in the NHS population. That reduced risk increased to 15% with the NHS II group. When both cohorts were combined, the reduced risk of depression was 13%.
When evaluating the role that daylight exposure plays with regard to trimester of pregnancy, the authors did note an association for the first trimester, but the association was much stronger for the second trimester; no association was found for the third trimester.
In terms of the effects of daylight exposure on incidence of suicide, no significant associations were found.
Because birth latitude and birth season were of key interest in this study, their relative contribution to total daylight exposure and extreme differences in exposure were considered. Citing observations from the National Health and Nutrition Examination Survey (NHANES), In this study, the authors found that women born in northern latitudes were found to have a 7% risk for lifetime depression, compared with women born in middle latitudes. Conversely, women born in southern latitudes had a 15% risk of depression. No association was found between birth season and incidence of depression, regardless of how season was defined.
The investigators cited several limitations. One is that they did not collect behavioral factors such as the time women spent outdoors. “Our method of exposure calculation relied on the assumption that participants’ mothers were exposed to sunlight from sunrise to sunset,” Ms. Devore and her associates wrote. This way of assessing exposure might have biased their results.
Nevertheless, they said, more studies are needed to examine the role that birth latitude and birth season might play with regard to depression.
The research for this study was supported by the National Institute of Mental Health and the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Additional infrastructure support for the Nurses’ Health Studies was provided by the National Cancer Institute.
The authors declared no conflicts of interest. Ms. Devore has reported receiving consulting fees from Epi Excellence and Bohn Epidemiology.
SOURCE: Devore EE et al. J. Psychiatric Res. 2018. 104(08):e20180225.
Prenatal programming of the circadian and limbic systems might play a role in the odds of developing lifetime depression, a longitudinal study of almost 161,000 women shows.
“Our results could add support to an emerging hypothesis that perinatal photoperiod may influence depression risk,” wrote Elizabeth E. Devore of Brigham and Women’s Hospital, Boston, and her associates. “If replicated, ... these results could translate into safe and inexpensive light-related interventions for mothers and babies.”
In the study, which was published in the Journal of Psychiatric Research, Ms. Devore and her associates examined the influence of daylight exposure during maternal pregnancy and lifetime depression risk in the resulting offspring. They found that increased exposure to daylight during maternal pregnancy correlated with reduced lifetime risk of depression. wrote Ms. Devore, who also is affiliated with Harvard Medical School, Boston, and her associates.
The effects of daylight exposure were considered modest within the study population, but the authors emphasized that the finding would have much “larger effects at the population level,” given the occurrence of depression in the general population. They added that their findings reinforce a growing consensus that perinatal exposure to daylight could have the ability to influence the risk of developing a mood disorder.
The investigators accessed the Nurses’ Health Study (NHS) and the NHS II, established in 1976 and 1989, respectively, to assess risk factors for chronic conditions in female nurses. Both studies biennially surveyed demographic data on health, lifestyle, and medication use through mailed questionnaires. The first group was composed of 121,701 women aged 30-55 years; the second included 116,430 women aged 25-42 years. Altogether, 160,737 women born full-term were included in the study; 20,912 were excluded from the original survey group for not reporting depression status, as well as an additional 43,325 for not reporting their state of birth.
From data collected regarding participants’ day and state of birth, the researchers were able to estimate total length of daylight exposure during pregnancy using mathematical equations published by the National Oceanic and Atmospheric Administration.
Longitudinal coordinates pinpointing the center of population density for a participant’s birth state were used to identify the location of each participant during gestation. Using those assumptions, the authors were able to establish the two key data points evaluated for the study: total daylight exposure during pregnancy gestation, which was calculated by adding the lengths of all 280 days of the pregnancy, and extreme differences in daylight exposure that might have occurred throughout the pregnancy, which was measured by subtracting the longest and shortest day lengths during gestation.
The investigators paid particular attention to reported levels of depression, evidence of suicide, and personal characteristics and lifestyle factors, such as race, hair color, and early-life socioeconomic factors, including parents’ homeownership at the time of offspring birth; birth weight; history of having been breastfed; and parental occupation throughout the participant’s childhood.
Participants did not begin reporting antidepressant use for the first time until 1996; history of clinician-reported diagnoses of depression began in 2000, Ms. Devore and her associates reported.
Total daylight exposure during pregnancy was found to have “a borderline significant association with odds of lifetime depression,” but the trend was not convincing qualitatively, “and individual estimates across quintiles of exposure” were not considered to be statistically significant. In fact, the authors found that a larger difference between minimum and maximum daylight exposure throughout pregnancy significantly lowered lifetime risk of depression. Women with the largest differences in minimum/maximum daylight exposure during gestation had a 12% lower risk of depression in the NHS population. That reduced risk increased to 15% with the NHS II group. When both cohorts were combined, the reduced risk of depression was 13%.
When evaluating the role that daylight exposure plays with regard to trimester of pregnancy, the authors did note an association for the first trimester, but the association was much stronger for the second trimester; no association was found for the third trimester.
In terms of the effects of daylight exposure on incidence of suicide, no significant associations were found.
Because birth latitude and birth season were of key interest in this study, their relative contribution to total daylight exposure and extreme differences in exposure were considered. Citing observations from the National Health and Nutrition Examination Survey (NHANES), In this study, the authors found that women born in northern latitudes were found to have a 7% risk for lifetime depression, compared with women born in middle latitudes. Conversely, women born in southern latitudes had a 15% risk of depression. No association was found between birth season and incidence of depression, regardless of how season was defined.
The investigators cited several limitations. One is that they did not collect behavioral factors such as the time women spent outdoors. “Our method of exposure calculation relied on the assumption that participants’ mothers were exposed to sunlight from sunrise to sunset,” Ms. Devore and her associates wrote. This way of assessing exposure might have biased their results.
Nevertheless, they said, more studies are needed to examine the role that birth latitude and birth season might play with regard to depression.
The research for this study was supported by the National Institute of Mental Health and the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Additional infrastructure support for the Nurses’ Health Studies was provided by the National Cancer Institute.
The authors declared no conflicts of interest. Ms. Devore has reported receiving consulting fees from Epi Excellence and Bohn Epidemiology.
SOURCE: Devore EE et al. J. Psychiatric Res. 2018. 104(08):e20180225.
Prenatal programming of the circadian and limbic systems might play a role in the odds of developing lifetime depression, a longitudinal study of almost 161,000 women shows.
“Our results could add support to an emerging hypothesis that perinatal photoperiod may influence depression risk,” wrote Elizabeth E. Devore of Brigham and Women’s Hospital, Boston, and her associates. “If replicated, ... these results could translate into safe and inexpensive light-related interventions for mothers and babies.”
In the study, which was published in the Journal of Psychiatric Research, Ms. Devore and her associates examined the influence of daylight exposure during maternal pregnancy and lifetime depression risk in the resulting offspring. They found that increased exposure to daylight during maternal pregnancy correlated with reduced lifetime risk of depression. wrote Ms. Devore, who also is affiliated with Harvard Medical School, Boston, and her associates.
The effects of daylight exposure were considered modest within the study population, but the authors emphasized that the finding would have much “larger effects at the population level,” given the occurrence of depression in the general population. They added that their findings reinforce a growing consensus that perinatal exposure to daylight could have the ability to influence the risk of developing a mood disorder.
The investigators accessed the Nurses’ Health Study (NHS) and the NHS II, established in 1976 and 1989, respectively, to assess risk factors for chronic conditions in female nurses. Both studies biennially surveyed demographic data on health, lifestyle, and medication use through mailed questionnaires. The first group was composed of 121,701 women aged 30-55 years; the second included 116,430 women aged 25-42 years. Altogether, 160,737 women born full-term were included in the study; 20,912 were excluded from the original survey group for not reporting depression status, as well as an additional 43,325 for not reporting their state of birth.
From data collected regarding participants’ day and state of birth, the researchers were able to estimate total length of daylight exposure during pregnancy using mathematical equations published by the National Oceanic and Atmospheric Administration.
Longitudinal coordinates pinpointing the center of population density for a participant’s birth state were used to identify the location of each participant during gestation. Using those assumptions, the authors were able to establish the two key data points evaluated for the study: total daylight exposure during pregnancy gestation, which was calculated by adding the lengths of all 280 days of the pregnancy, and extreme differences in daylight exposure that might have occurred throughout the pregnancy, which was measured by subtracting the longest and shortest day lengths during gestation.
The investigators paid particular attention to reported levels of depression, evidence of suicide, and personal characteristics and lifestyle factors, such as race, hair color, and early-life socioeconomic factors, including parents’ homeownership at the time of offspring birth; birth weight; history of having been breastfed; and parental occupation throughout the participant’s childhood.
Participants did not begin reporting antidepressant use for the first time until 1996; history of clinician-reported diagnoses of depression began in 2000, Ms. Devore and her associates reported.
Total daylight exposure during pregnancy was found to have “a borderline significant association with odds of lifetime depression,” but the trend was not convincing qualitatively, “and individual estimates across quintiles of exposure” were not considered to be statistically significant. In fact, the authors found that a larger difference between minimum and maximum daylight exposure throughout pregnancy significantly lowered lifetime risk of depression. Women with the largest differences in minimum/maximum daylight exposure during gestation had a 12% lower risk of depression in the NHS population. That reduced risk increased to 15% with the NHS II group. When both cohorts were combined, the reduced risk of depression was 13%.
When evaluating the role that daylight exposure plays with regard to trimester of pregnancy, the authors did note an association for the first trimester, but the association was much stronger for the second trimester; no association was found for the third trimester.
In terms of the effects of daylight exposure on incidence of suicide, no significant associations were found.
Because birth latitude and birth season were of key interest in this study, their relative contribution to total daylight exposure and extreme differences in exposure were considered. Citing observations from the National Health and Nutrition Examination Survey (NHANES), In this study, the authors found that women born in northern latitudes were found to have a 7% risk for lifetime depression, compared with women born in middle latitudes. Conversely, women born in southern latitudes had a 15% risk of depression. No association was found between birth season and incidence of depression, regardless of how season was defined.
The investigators cited several limitations. One is that they did not collect behavioral factors such as the time women spent outdoors. “Our method of exposure calculation relied on the assumption that participants’ mothers were exposed to sunlight from sunrise to sunset,” Ms. Devore and her associates wrote. This way of assessing exposure might have biased their results.
Nevertheless, they said, more studies are needed to examine the role that birth latitude and birth season might play with regard to depression.
The research for this study was supported by the National Institute of Mental Health and the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health. Additional infrastructure support for the Nurses’ Health Studies was provided by the National Cancer Institute.
The authors declared no conflicts of interest. Ms. Devore has reported receiving consulting fees from Epi Excellence and Bohn Epidemiology.
SOURCE: Devore EE et al. J. Psychiatric Res. 2018. 104(08):e20180225.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Key clinical point: Future studies that are able to replicate findings have the potential to offer safe, inexpensive light-based treatments for both mothers and babies.
Major finding: Benefits of daytime light exposure are highest with second-trimester exposure.
Study details: Longitudinal cohort study of almost 161,000 women who were born full term.
Disclosures: The authors declared no conflicts of interest. Ms. Devore reported receiving consulting fees from Epi Excellence and Bohn Epidemiology.
Source: Devore EE et al. J Psychiatric Res. 2018.104(08):e20180225.
Initial screening not enough to catch all cases of preterm congenital hypothyroidism
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
reported Niamh McGrath, MD, of the department of pediatric endocrinology at Children’s University Hospital, Dublin, and her associates in the Journal of Pediatrics.
In a population-based prospective review of 898,424 records between 2004 and 2016, Dr. McGrath and her associates identified all preterm infants less than 33 weeks diagnosed with congenital hypothyroidism and receiving treatment with levothyroxine. Of the infants screened, just 53 were selected to participate in the study, including 26 who were diagnosed at the first thyroid-stimulating hormone (TSH) screening and 27 who had delayed TSH elevation.
Gestational age ranged from 23 to 33 weeks, median birth weight measured 1.2 kg, median serum TSH concentration at the time of diagnosis was 78.3 mU/L, and median free thyroxine concentration was 8.9 pmol/L.
For half of the infants ultimately diagnosed, congenital hypothyroidism was not detected during the initial newborn screening. The authors also noted that 25% of patients with delayed TSH elevation had been exposed to iodine while undergoing surgery for necrotizing enterocolitis; after age 28 days, four of these infants were found to have elevated TSH. They cautioned that while this finding emphasizes the need for close monitoring and repeat screening of infants who have been exposed to iodine for up to 1 month following exposure, they could not be certain that the iodine exposure was responsible for the transient hypothyroidism in these patients.
Dr. McGrath and her associates emphasized the importance of repeat TSH screening for all preterm infants, noting that standard protocol screenings conducted just once at age 2 weeks or 4 weeks are not sufficient to effectively identify all cases of congenital hypothyroidism in which TSH elevation is delayed. Instead, they recommended measuring TSH on days 3-5 and at 1 week, 2 weeks, 4 weeks, and term-corrected gestational age.
“Our data are consistent with studies showing a high incidence of delayed TSH rise, particularly in very-low-birth-weight infants,” the authors wrote. They speculated that delays in detecting primary congenital hypothyroidism could be caused by the suppression of TSH secretion as a result of “hypothalamic-pituitary immaturity, medication administration, and effects of serious neonatal illness.” In fact, had standard, recommended 2-week-only screening protocols been followed with their patient population, fully 48% of infants with delayed TSH elevation would have been overlooked; half of these patients were later found to have decompensated hypothyroidism.
Neurodevelopmental disability caused by congenital hypothyroidism, which affects roughly 1 in every 2,000-4,000 births, is increasingly being prevented with newborn screenings that identify the condition early, but the incidence of congenital hypothyroidism has increased considerably in the past 20 years. The authors attribute this increase to the gradual change in screening cutoff levels and the rise in number of preterm infants who are surviving.
The need for a second screening, as well as appropriate timing and optimal TSH cutoff, are “subjects of active debate,” the authors wrote. The latest European screening guidelines recommend second screenings for preterm and low-birth-weight infants at either 2 weeks of age or 2 weeks following preliminary screening. Although they make no recommendations regarding additional screenings, American Academy of Pediatrics guidelines, published in 2006, cite a “disproportionate incidence” of delayed increase in TSH and congenital hypothyroidism in infants with very low birth weight. Dr. McGrath and her associates speculated that not all screening programs have adopted repeat screening of preterm infants, perhaps because of the low yield results, “the transient nature of most cases” detected, as well as conflicting long-term data on neurodevelopmental outcomes.
Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
SOURCE: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Periodic screenings are key to preventing permanent, decompensated hypothyroidism.
Major finding: High incidence of delayed TSH rise is common, especially in very-low-birth-weight infants.
Study details: A population-based prospective review of 898,424 records.
Disclosures: Dr. McGrath receives funding from the Children’s Fund for Health. The authors declared no conflicts of interest.
Source: McGrath N et al. J Pediatr. 2018 Oct 24. doi: 10.1016/j.jpeds.2018.09.044.
Review finds some therapies show promise in managing prurigo nodularis
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Recent success in the use of (PN) suggests that safer and more effective systemic therapies that provide relief and management of this debilitating chronic skin condition are possible, reported Azam A. Qureshi, BA, of the department of dermatology, George Washington University, Washington, and his coauthors.
Their report was published in the Journal of the American Academy of Dermatology.
They conducted a systematic review of 35 clinical studies of different therapies for PN, published between Jan. 1, 1990, and March 22, 2018, using PubMed and Scopus databases. Their goal was twofold: to provide a summary of current evidence-based therapies and their corresponding level of evidence ratings, and to help researchers “identify gaps in PN treatment development and study.”
From 706 studies, the authors selected 35 clinical studies of treatment strategies for PN, for which the pathogenesis is virtually unknown, they noted. The studies included 15 prospective cohort studies, 11 retrospective reviews, 8 randomized controlled trials (with 10-127 patients), and 1 case series. Studies that failed to report treatment outcomes and those with fewer than five patients were excluded from the review.
Treatment modalities included topical agents, phototherapy and photochemotherapy, thalidomide, systemic immunomodulatory drugs, antiepileptics and antidepressants, and emerging treatment approaches. Many of the treatments evaluated in the review were found to offer limited promise for clinical application as a result of low efficacy or frequent side effects. The authors attributed the overall lack of success with treatments to the “heterogeneous nature of the etiology of chronic pruritus.”
Thalidomide was found to have limited use given its poor safety profile; lenalidomide achieved better outcomes, but treatment in one case was stopped because of possible drug-induced neuropathy or myopathy, they wrote. The systemic immunomodulatory agents methotrexate and cyclosporine exhibited successful treatment but also were found to have poor safety profiles. Greater promise was seen with antiepileptics and antidepressants, which were associated with fewer side effects. “Antidepressants, such as mirtazapine and amitriptyline, and antiepileptics, such as gabapentin and pregabalin, can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” senior author Adam Friedman, MD, professor of dermatology at George Washington University, said in an interview.
Among the most positive outcomes achieved were those in which promising newer treatments, including targeting IL-31 signaling and opioid receptor modulation, were used. In particular, the authors cited nemolizumab, an IL-31 receptor A antagonist, which is currently in a phase 2 trial of PN. Beneficial effects of extended release nalbuphine, an opioid k-receptor agonist and mu-receptor antagonist, were found in an unpublished multicenter, double-blind randomized controlled trial, with no serious adverse effects attributed to treatment.
Among the emerging treatments are the neurokinin-1 receptor antagonists, which include serlopitant, which showed beneficial effects compared with placebo in an 8-week randomized controlled study, they wrote. The neurokinin-1 receptor “is a target of substance P, a mediator of itch and a probable pathogenic agent in PN,” they wrote. “Binding by these agents likely disrupts substance P signaling, thereby halting PN pathogenesis,” they added.
“Antidepressants such as mirtazapine and amitriptyline and antiepileptics such as gabapentin and pregabalin can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” Dr. Friedman said in the interview.
Mr. Qureshi had no relevant financial disclosures. Dr. Friedman has received honoraria for participation in advisory boards for Menlo Therapeutics, and serves as an investigator for Kiniksa Pharmaceuticals. A third author received honoraria for participation in advisory boards for Menlo, and several other companies, and received grant/research support as an investigator from Menlo, Kiniksa, and several other companies.
SOURCE: Qureshi AA et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.020.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Recent success in the use of (PN) suggests that safer and more effective systemic therapies that provide relief and management of this debilitating chronic skin condition are possible, reported Azam A. Qureshi, BA, of the department of dermatology, George Washington University, Washington, and his coauthors.
Their report was published in the Journal of the American Academy of Dermatology.
They conducted a systematic review of 35 clinical studies of different therapies for PN, published between Jan. 1, 1990, and March 22, 2018, using PubMed and Scopus databases. Their goal was twofold: to provide a summary of current evidence-based therapies and their corresponding level of evidence ratings, and to help researchers “identify gaps in PN treatment development and study.”
From 706 studies, the authors selected 35 clinical studies of treatment strategies for PN, for which the pathogenesis is virtually unknown, they noted. The studies included 15 prospective cohort studies, 11 retrospective reviews, 8 randomized controlled trials (with 10-127 patients), and 1 case series. Studies that failed to report treatment outcomes and those with fewer than five patients were excluded from the review.
Treatment modalities included topical agents, phototherapy and photochemotherapy, thalidomide, systemic immunomodulatory drugs, antiepileptics and antidepressants, and emerging treatment approaches. Many of the treatments evaluated in the review were found to offer limited promise for clinical application as a result of low efficacy or frequent side effects. The authors attributed the overall lack of success with treatments to the “heterogeneous nature of the etiology of chronic pruritus.”
Thalidomide was found to have limited use given its poor safety profile; lenalidomide achieved better outcomes, but treatment in one case was stopped because of possible drug-induced neuropathy or myopathy, they wrote. The systemic immunomodulatory agents methotrexate and cyclosporine exhibited successful treatment but also were found to have poor safety profiles. Greater promise was seen with antiepileptics and antidepressants, which were associated with fewer side effects. “Antidepressants, such as mirtazapine and amitriptyline, and antiepileptics, such as gabapentin and pregabalin, can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” senior author Adam Friedman, MD, professor of dermatology at George Washington University, said in an interview.
Among the most positive outcomes achieved were those in which promising newer treatments, including targeting IL-31 signaling and opioid receptor modulation, were used. In particular, the authors cited nemolizumab, an IL-31 receptor A antagonist, which is currently in a phase 2 trial of PN. Beneficial effects of extended release nalbuphine, an opioid k-receptor agonist and mu-receptor antagonist, were found in an unpublished multicenter, double-blind randomized controlled trial, with no serious adverse effects attributed to treatment.
Among the emerging treatments are the neurokinin-1 receptor antagonists, which include serlopitant, which showed beneficial effects compared with placebo in an 8-week randomized controlled study, they wrote. The neurokinin-1 receptor “is a target of substance P, a mediator of itch and a probable pathogenic agent in PN,” they wrote. “Binding by these agents likely disrupts substance P signaling, thereby halting PN pathogenesis,” they added.
“Antidepressants such as mirtazapine and amitriptyline and antiepileptics such as gabapentin and pregabalin can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” Dr. Friedman said in the interview.
Mr. Qureshi had no relevant financial disclosures. Dr. Friedman has received honoraria for participation in advisory boards for Menlo Therapeutics, and serves as an investigator for Kiniksa Pharmaceuticals. A third author received honoraria for participation in advisory boards for Menlo, and several other companies, and received grant/research support as an investigator from Menlo, Kiniksa, and several other companies.
SOURCE: Qureshi AA et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.020.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Recent success in the use of (PN) suggests that safer and more effective systemic therapies that provide relief and management of this debilitating chronic skin condition are possible, reported Azam A. Qureshi, BA, of the department of dermatology, George Washington University, Washington, and his coauthors.
Their report was published in the Journal of the American Academy of Dermatology.
They conducted a systematic review of 35 clinical studies of different therapies for PN, published between Jan. 1, 1990, and March 22, 2018, using PubMed and Scopus databases. Their goal was twofold: to provide a summary of current evidence-based therapies and their corresponding level of evidence ratings, and to help researchers “identify gaps in PN treatment development and study.”
From 706 studies, the authors selected 35 clinical studies of treatment strategies for PN, for which the pathogenesis is virtually unknown, they noted. The studies included 15 prospective cohort studies, 11 retrospective reviews, 8 randomized controlled trials (with 10-127 patients), and 1 case series. Studies that failed to report treatment outcomes and those with fewer than five patients were excluded from the review.
Treatment modalities included topical agents, phototherapy and photochemotherapy, thalidomide, systemic immunomodulatory drugs, antiepileptics and antidepressants, and emerging treatment approaches. Many of the treatments evaluated in the review were found to offer limited promise for clinical application as a result of low efficacy or frequent side effects. The authors attributed the overall lack of success with treatments to the “heterogeneous nature of the etiology of chronic pruritus.”
Thalidomide was found to have limited use given its poor safety profile; lenalidomide achieved better outcomes, but treatment in one case was stopped because of possible drug-induced neuropathy or myopathy, they wrote. The systemic immunomodulatory agents methotrexate and cyclosporine exhibited successful treatment but also were found to have poor safety profiles. Greater promise was seen with antiepileptics and antidepressants, which were associated with fewer side effects. “Antidepressants, such as mirtazapine and amitriptyline, and antiepileptics, such as gabapentin and pregabalin, can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” senior author Adam Friedman, MD, professor of dermatology at George Washington University, said in an interview.
Among the most positive outcomes achieved were those in which promising newer treatments, including targeting IL-31 signaling and opioid receptor modulation, were used. In particular, the authors cited nemolizumab, an IL-31 receptor A antagonist, which is currently in a phase 2 trial of PN. Beneficial effects of extended release nalbuphine, an opioid k-receptor agonist and mu-receptor antagonist, were found in an unpublished multicenter, double-blind randomized controlled trial, with no serious adverse effects attributed to treatment.
Among the emerging treatments are the neurokinin-1 receptor antagonists, which include serlopitant, which showed beneficial effects compared with placebo in an 8-week randomized controlled study, they wrote. The neurokinin-1 receptor “is a target of substance P, a mediator of itch and a probable pathogenic agent in PN,” they wrote. “Binding by these agents likely disrupts substance P signaling, thereby halting PN pathogenesis,” they added.
“Antidepressants such as mirtazapine and amitriptyline and antiepileptics such as gabapentin and pregabalin can offer significant relief to a good number of patients, though success is variable and as we found in our study, the evidence available is limited,” Dr. Friedman said in the interview.
Mr. Qureshi had no relevant financial disclosures. Dr. Friedman has received honoraria for participation in advisory boards for Menlo Therapeutics, and serves as an investigator for Kiniksa Pharmaceuticals. A third author received honoraria for participation in advisory boards for Menlo, and several other companies, and received grant/research support as an investigator from Menlo, Kiniksa, and several other companies.
SOURCE: Qureshi AA et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.020.
Key clinical point: Additional research is needed to develop more individualized treatment strategies, but data for some treatments are promising.
Major finding: Treatments identified as having beneficial effects in the review included nemolizumab, an IL-31 receptor A antagonist; nalbuphine, an opioid k-receptor agonist and mu-receptor antagonist, and serlopitant, a neurokinin-1 receptor antagonist.
Study details: A systematic review of 35 studies evaluating treatments for PN, published between Jan. 1, 1990, and March 22, 2018.
Disclosures: Mr. Qureshi had no relevant financial disclosures. Dr. Friedman has received honoraria for participation in advisory boards for Menlo Therapeutics, and serves as an investigator for Kiniksa Pharmaceuticals. A third author received honoraria for participation in advisory boards for Menlo, and several other companies, and received grant/research support as an investigator from Menlo, Kiniksa, and several other companies.
Source: Qureshi AA et al. J Am Acad Dermatol. 2018. doi: 10.1016/j.jaad.2018.09.020.
Platelet-rich plasma injections yield substantial improvement in androgenetic alopecia
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
Autologous treatment with injected (AGA) after three monthly treatments, in a study that compared two treatment regimens.
PRP is gaining popularity because of its efficacy in stimulating fibroblast proliferation, triggering the production of collagen and elastin, and boosting the quantity and quality of the extracellular matrix, noted the investigators, Amelia K. Hausauer, MD, in private practice in Campbell, Calif., and Derek H. Jones, MD, in private practice in Los Angeles. Both are also with the department of dermatology at the University of California, Los Angeles.
They undertook this study to determine the optimal number and timing of treatments in patients with AGA, comparing two different injection protocols over a 6-month period. The study evaluated 40 healthy men (30) and women (10), whose mean age was 44 years, with AGA stages Norwood-Hamilton II-V (in men) and Ludwig I2-II1 (in women), recruited from a private practice in Los Angeles between November 2016 and January 2017. They were randomly assigned to one of two treatment groups: three monthly sessions followed by a fourth injection 3 months later (group 1), or two treatments, one at baseline and the second 3 months later (group 2). One of the men dropped out for reasons unrelated to the treatment.
Those with clinically stable effects of Food and Drug Administration–approved AGA treatments for 12 months were permitted to participate while continuing those treatments (topical minoxidil and/or oral finasteride), since PRP is often coadministered with other therapies. But additional products, devices, or medications used for hair regrowth were not allowed. The washout period for antiandrogen therapies was 90 days.
At 3 months, the mean increase in hair counts was significant in the first group only, but at 6 months, both groups experienced significant increases in hair count (P less than .001). However, those in the first group had superior results at 6 months, with a mean 30% increase in hair counts from baseline, compared with a 7% increase in the second group (P less than .001).
Both groups had significant increases in the mean hair shaft caliber at 3 and 6 months.
Overall, 82% of participants who completed treatment reported being satisfied or highly satisfied, and 72% expressed interest in continuing treatment after the study period; almost two-thirds considered the procedure “tolerable.”
While the authors stipulated that they did not undertake the study primarily to predict treatment response to PRP, they uncovered some significant trends that they said warranted further evaluation, including the finding that those who had experienced hair loss for less than 5-6 years were more likely to have rapid and pronounced treatment response.
Their overall findings correlated with those of previous studies supporting the increase in density of hair or hair numbers, but the existing literature draws from studies that have been open label or unblinded, which makes it difficult to evaluate them head to head. The novel, subdermal injection technique used in the study “allows for fewer, more widely spaced injection points than the traditional nappage procedure ... because PRP can diffuse further once in the deeper, subgaleal space,” they wrote. The investigators noted similar response between men and women, which is important given sparse data on the efficacy of PRP in women.
Weaknesses of the study included its small sample size and short follow-up period, the authors noted. Longer-duration studies have reported relapse between 3 and 12 months.
This study is the first of its kind to directly compare efficacy rates of two injection protocols, the authors wrote, cautioning that future studies are necessary to “fine-tune preparation methods, determine optimal maintenance schedule(s), and parse out clinical predictors of efficacy.”
Eclipse Aesthetics (the manufacturer of the PRP preparation kits) provided funding for this study, but the authors acknowledged no significant interest with commercial supporters.
SOURCE: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
FROM DERMATOLOGIC SURGERY
Key clinical point: Starting off with monthly PRP injections may yield more hair growth than a protocol that uses less frequently administered injections.
Major finding: Of the patients who completed treatment, 82% were satisfied with the results.
Study details: A prospective, randomized trial comparing two early-phase treatment protocols in 40 patients.
Disclosures: Eclipse Aesthetics provided funding for this study; the authors said they had no significant interest with commercial supporters.
Source: Hausauer A et al. Dermatol Surg. 2018 Sep;44(9):1191-200.
RSV-related risk of hospitalization higher in Down syndrome patients
reported Andrea Beckhaus, MD, and Jose Castro-Rodriguez, MD, PhD, at the Pontificia Universidad Católica de Chile in Santiago.
Because DS is the most common chromosomal disorder, affecting 1 in every 800 children born worldwide, and respiratory infections are the leading cause of hospitalization in children with DS, especially during the first year of life, the results of this study are important and have considerable consequences for public health, the authors observed.
The economic burden on families of children with DS and the health care systems that treat them is, not surprisingly, significantly higher given their sevenfold higher need for supplemental oxygen therapy, threefold likely increased risk of ICU admission, fivefold higher likely need for ventilator support, and average increased length of hospital stay by nearly 5 days.
In the 15 years from 1997 to 2012, RSV-related hospital charges for infants with DS increased by nearly 80% from $10,141 (US dollars) to $18,217, compared with charges for infants not at high risk, which increased by more than 60% from $6,983 to $11,273 during the same period, according to a study (PLoS One. 2016;11[4]:e0152208).
Dr. Beckhaus and Dr. Castro-Rodriguez conducted their systematic review and meta-analysis to evaluate RSV-associated morbidity in children with DS. Following a search of four electronic data bases, a total of 12 studies published between 2004 and 2017 across 10 different countries were identified, including six in Europe, three in Asia, two in the United States, and one in Latin America. Altogether, 3,662 children with DS and 1,145,509 without DS were included in the review.
“Any potential strategy to reduce RSV infection (e.g., prophylaxis with monoclonal antibodies or new vaccines) in children with DS could decrease their morbidity and mortality,” the authors noted. Specifically, they cited the humanized monoclonal antibody palivizumab, which is used for prophylaxis against RSV. Palivizumab is known to reduce hospitalizations in children at high risk from comorbid conditions, including chronic lung disease, hemodynamically significant congenital heart disease (CHD), neuromuscular disease, immunodeficiency, and prematurity. At present, palivizumab is not recommended by the American Academy of Pediatrics for routine use to prevent RSV infection in patients with DS who are not already qualified for other reasons because there are insufficient data to recommend routine prophylactic use of the drug in children with Down syndrome.
The authors cited recent studies in Canada and Japan that may warrant further reconsideration of the AAP’s recommendations, however. A fourfold lowering of RSV-related hospitalizations was observed during the first 2 years of life among 532 children with DS receiving palivizumab who were included in a recent prospective Canadian study. The drug also was found to be safe and effective for preventing lower respiratory tract infections caused by RSV in a recent Japanese multicenter postmarketing surveillance study that evaluated palivizumab prophylaxis for RSV infection in 138 children with DS who did not have hemodynamically significant CHD; only 2 of the children treated required hospitalization.
“More cost-utility studies used to determine the efficacy of RSV immunoprophylaxis in this specific high-risk patient population need to be done,” Dr. Beckhaus and Dr. Castro-Rodriguez recommended.
The review was not without limitations. Not all of the studies included subgroups of participants with DS with CHD and without CHD or other additional risk factors. However, when only those studies were considered that had data for participants with DS without other risk factors, almost all results were similar.
One key strength of the study concerned the study methodology. Using the Newcastle–Ottawa scale, the risk of bias among the 12 studies was generally low, the total number of participants was considerably high, and the outcomes selected had importance for the patients and also public health implications. “It is important to remark that the vast majority of the outcomes that were analyzed had no or unimportant bias,” they said.
Dr. Beckhaus and Dr. Castro-Rodriguez had no relevant disclosures to disclose.
SOURCE: Beckhaus A et al. J Pediatrics. 2018;142(3):e20180225.
reported Andrea Beckhaus, MD, and Jose Castro-Rodriguez, MD, PhD, at the Pontificia Universidad Católica de Chile in Santiago.
Because DS is the most common chromosomal disorder, affecting 1 in every 800 children born worldwide, and respiratory infections are the leading cause of hospitalization in children with DS, especially during the first year of life, the results of this study are important and have considerable consequences for public health, the authors observed.
The economic burden on families of children with DS and the health care systems that treat them is, not surprisingly, significantly higher given their sevenfold higher need for supplemental oxygen therapy, threefold likely increased risk of ICU admission, fivefold higher likely need for ventilator support, and average increased length of hospital stay by nearly 5 days.
In the 15 years from 1997 to 2012, RSV-related hospital charges for infants with DS increased by nearly 80% from $10,141 (US dollars) to $18,217, compared with charges for infants not at high risk, which increased by more than 60% from $6,983 to $11,273 during the same period, according to a study (PLoS One. 2016;11[4]:e0152208).
Dr. Beckhaus and Dr. Castro-Rodriguez conducted their systematic review and meta-analysis to evaluate RSV-associated morbidity in children with DS. Following a search of four electronic data bases, a total of 12 studies published between 2004 and 2017 across 10 different countries were identified, including six in Europe, three in Asia, two in the United States, and one in Latin America. Altogether, 3,662 children with DS and 1,145,509 without DS were included in the review.
“Any potential strategy to reduce RSV infection (e.g., prophylaxis with monoclonal antibodies or new vaccines) in children with DS could decrease their morbidity and mortality,” the authors noted. Specifically, they cited the humanized monoclonal antibody palivizumab, which is used for prophylaxis against RSV. Palivizumab is known to reduce hospitalizations in children at high risk from comorbid conditions, including chronic lung disease, hemodynamically significant congenital heart disease (CHD), neuromuscular disease, immunodeficiency, and prematurity. At present, palivizumab is not recommended by the American Academy of Pediatrics for routine use to prevent RSV infection in patients with DS who are not already qualified for other reasons because there are insufficient data to recommend routine prophylactic use of the drug in children with Down syndrome.
The authors cited recent studies in Canada and Japan that may warrant further reconsideration of the AAP’s recommendations, however. A fourfold lowering of RSV-related hospitalizations was observed during the first 2 years of life among 532 children with DS receiving palivizumab who were included in a recent prospective Canadian study. The drug also was found to be safe and effective for preventing lower respiratory tract infections caused by RSV in a recent Japanese multicenter postmarketing surveillance study that evaluated palivizumab prophylaxis for RSV infection in 138 children with DS who did not have hemodynamically significant CHD; only 2 of the children treated required hospitalization.
“More cost-utility studies used to determine the efficacy of RSV immunoprophylaxis in this specific high-risk patient population need to be done,” Dr. Beckhaus and Dr. Castro-Rodriguez recommended.
The review was not without limitations. Not all of the studies included subgroups of participants with DS with CHD and without CHD or other additional risk factors. However, when only those studies were considered that had data for participants with DS without other risk factors, almost all results were similar.
One key strength of the study concerned the study methodology. Using the Newcastle–Ottawa scale, the risk of bias among the 12 studies was generally low, the total number of participants was considerably high, and the outcomes selected had importance for the patients and also public health implications. “It is important to remark that the vast majority of the outcomes that were analyzed had no or unimportant bias,” they said.
Dr. Beckhaus and Dr. Castro-Rodriguez had no relevant disclosures to disclose.
SOURCE: Beckhaus A et al. J Pediatrics. 2018;142(3):e20180225.
reported Andrea Beckhaus, MD, and Jose Castro-Rodriguez, MD, PhD, at the Pontificia Universidad Católica de Chile in Santiago.
Because DS is the most common chromosomal disorder, affecting 1 in every 800 children born worldwide, and respiratory infections are the leading cause of hospitalization in children with DS, especially during the first year of life, the results of this study are important and have considerable consequences for public health, the authors observed.
The economic burden on families of children with DS and the health care systems that treat them is, not surprisingly, significantly higher given their sevenfold higher need for supplemental oxygen therapy, threefold likely increased risk of ICU admission, fivefold higher likely need for ventilator support, and average increased length of hospital stay by nearly 5 days.
In the 15 years from 1997 to 2012, RSV-related hospital charges for infants with DS increased by nearly 80% from $10,141 (US dollars) to $18,217, compared with charges for infants not at high risk, which increased by more than 60% from $6,983 to $11,273 during the same period, according to a study (PLoS One. 2016;11[4]:e0152208).
Dr. Beckhaus and Dr. Castro-Rodriguez conducted their systematic review and meta-analysis to evaluate RSV-associated morbidity in children with DS. Following a search of four electronic data bases, a total of 12 studies published between 2004 and 2017 across 10 different countries were identified, including six in Europe, three in Asia, two in the United States, and one in Latin America. Altogether, 3,662 children with DS and 1,145,509 without DS were included in the review.
“Any potential strategy to reduce RSV infection (e.g., prophylaxis with monoclonal antibodies or new vaccines) in children with DS could decrease their morbidity and mortality,” the authors noted. Specifically, they cited the humanized monoclonal antibody palivizumab, which is used for prophylaxis against RSV. Palivizumab is known to reduce hospitalizations in children at high risk from comorbid conditions, including chronic lung disease, hemodynamically significant congenital heart disease (CHD), neuromuscular disease, immunodeficiency, and prematurity. At present, palivizumab is not recommended by the American Academy of Pediatrics for routine use to prevent RSV infection in patients with DS who are not already qualified for other reasons because there are insufficient data to recommend routine prophylactic use of the drug in children with Down syndrome.
The authors cited recent studies in Canada and Japan that may warrant further reconsideration of the AAP’s recommendations, however. A fourfold lowering of RSV-related hospitalizations was observed during the first 2 years of life among 532 children with DS receiving palivizumab who were included in a recent prospective Canadian study. The drug also was found to be safe and effective for preventing lower respiratory tract infections caused by RSV in a recent Japanese multicenter postmarketing surveillance study that evaluated palivizumab prophylaxis for RSV infection in 138 children with DS who did not have hemodynamically significant CHD; only 2 of the children treated required hospitalization.
“More cost-utility studies used to determine the efficacy of RSV immunoprophylaxis in this specific high-risk patient population need to be done,” Dr. Beckhaus and Dr. Castro-Rodriguez recommended.
The review was not without limitations. Not all of the studies included subgroups of participants with DS with CHD and without CHD or other additional risk factors. However, when only those studies were considered that had data for participants with DS without other risk factors, almost all results were similar.
One key strength of the study concerned the study methodology. Using the Newcastle–Ottawa scale, the risk of bias among the 12 studies was generally low, the total number of participants was considerably high, and the outcomes selected had importance for the patients and also public health implications. “It is important to remark that the vast majority of the outcomes that were analyzed had no or unimportant bias,” they said.
Dr. Beckhaus and Dr. Castro-Rodriguez had no relevant disclosures to disclose.
SOURCE: Beckhaus A et al. J Pediatrics. 2018;142(3):e20180225.
FROM PEDIATRICS
Key clinical point: Strategies to reduce RSV are needed to decrease morbidity and mortality in children with Down syndrome.
Major finding: Down syndrome children with RSV-related hospitalization had sevenfold higher need for supplemental oxygen therapy, threefold likely increased risk of ICU admission, fivefold higher likely need for ventilator support, and average increased length of hospital stay by nearly 5 days.
Study details: Systematic review and 12-study meta-analysis of 3,662 children with DS and 1,145,509 without DS.
Disclosures: The authors had no relevant financial disclosures.
Source: Beckhaus A et al. J Pediatrics. 2018;142(3):e20180225.
Consider potty seats when you see contact dermatitis on toddler bottoms
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
In such cases, be on the alert for contact dermatitis, reported Claire O. Dorfman, DO, of Lehigh Valley Health Network, Allentown, Pa., and her associates at Hershey (Pa.) Medical Center.
A 3-year-old white boy with a 6-month history of a pruritic rash on his buttocks and bilateral posterior thighs was treated without improvement at the pediatric dermatology clinic with low-potency topical corticosteroids, as well as topical antibiotic and antifungal agents.
Only mild improvement was seen once disposable paper toilet seat covers were added to treatment regimen. Following the purchase of a new potty seat through an online retailer, the child’s mother discovered a number of consumer product reviews also detailing similar complaints about the manufacturer, Prince Lionheart WeePOD Basix, by more than 30 other consumers. Photos highlighting identical rash presentation in other toddlers confirmed that the toilet seat was responsible for the allergic reaction. A warning had been posted by the manufacturer but this warning was not provided by the online retailer.
Use of the seat was immediately discontinued, and complete resolution of lesions was achieved within 1 month; subsequently, a report to the Consumer Product Safety Commission was made.
Allergic contact dermatitis to toilet seats is becoming increasingly common, the authors noted. Although the source of allergies is varied, wood historically has been identified as the most common material associated with the condition. Polypropylene and polyurethane foam also have been found to cause irritation. However, in the case reported by Dr. Dorfman and her associates, the precise irritant could not be identified because of the atypical pattern of the lesions and their irregular presentation on the buttocks and thighs. They speculated that this irregularity could be attributed to “the small, round shape of the seat and the squirmy behavior of a toddler,” because the typical arciform distribution was not present. Relief was not achieved with the paper liners because they did not completely cover the seat.
Because the rash resolved when the seat was replaced, parents declined patch testing. As a result, it was not possible to identify the specific allergenic component of the polyurethane. The polyurethanes used to make the seats are synthetic polymers that contain isocyanates, and frequently diaminodiphenylmethane, a curing agent. Possible allergy to the dyes used during manufacture also was considered but the presenting rash was reported in all four of the available colors made.
Although it was speculated that exposure to cleansers could be to blame for possible irritant dermatitis given reports of cracking of the potty seat, the mother and several online reviews indicated only soap and water were used, not harsh cleaning agents.
The clinicians had no relevant financial disclosures.
SOURCE: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
FROM THE JOURNAL OF PEDIATRIC DERMATOLOGY
Key clinical point: Suspect contact dermatitis in cases of unexplained pruritic rash.
Major finding: Allergic contact dermatitis to toilet seats is becoming increasingly common.
Study details: A case study.
Disclosures: The authors had no relevant financial disclosures.
Source: Dorfman CO et al. Pediatr Dermatol. 2018 May 29. doi: 10.1111/pde.13534.
Pediatric asthma patients should be considered priority for flu vaccine
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
Children with asthma who present to emergency departments for treatment are significantly more likely to test positive for one or more respiratory pathogens, reported Dr. Joanna Merckx of the Montreal Children’s Hospital at the McGill University Health Centre, and her associates.
Nearly two-thirds of patients tested positive for one or more respiratory viruses in a study conducted by Dr. Merckx and her associates. “Given the documented safety of influenza immunization in children with asthma and its expected protective effect,” such cases should be among those prioritized to receive influenza immunization.
In reviewing the findings of the study, Dr. Merckx and her colleagues sought to determine whether closely evaluating the effects of specific respiratory pathogens could be useful in further developing appropriate preventive treatments for children with asthma; improving efforts to diagnose pathogens at the time of ED treatment; and identifying patients at higher risk of treatment failure who could be candidates for more intensive treatment protocols.
Children aged 1-17 years presenting to one of five EDs in the Pediatric Emergency Research Canada network during 2011-2013 with moderate or severe asthma flares were considered for the study. All eligible DOORWAY study participants with a valid respiratory specimen were included in the study and received a standardized dose of oral and bronchodilator treatment with salbutamol; those with severe exacerbations also received ipratropium bromide (Atrovent).
Within 1 hour of study inclusion, patients were tested by way of nasopharyngeal aspirate or swab. Patients identified with coinfection presented with two or more pathogens. Failure of ED management was defined as patients admitted to the hospital for asthma; ED treatment lasting 8 or more hours after corticosteroid treatment; or returns to the ED within 72 hours after discharge that led to hospital admission or prolonged ED stay.
Of 1,012 children enrolled in the study, 958 were assessed for worsening of asthma symptoms. Of the 958 respiratory specimens tested, 62% tested positive for one or more pathogens, 8.5% were found to have coinfection, of which respiratory syncytial virus (RSV) and coronavirus were the most frequent copathogens. Rhinovirus was the most prevalent pathogen, occurring in 29%, and of these, rhinovirus C was the most frequent species (18.2%), followed by RSV (17.9%); only two patients tested positive for Mycoplasma pneumoniae.
Children with a laboratory-confirmed pathogen were younger, had higher tobacco exposure, and were slightly more likely to present with fever (29% vs. 24%), compared with children without a laboratory-confirmed pathogen. Children with rhinovirus were less often febrile (16% vs 41%) and less frequently diagnosed with pneumonia (5% vs. 16%.) than those without a rhinovirus infection The proportion of children presenting with a severe exacerbation of asthma was 33%.
Overall, 17% of patients experienced treatment failure. Those with current respiratory infection were at increased risk of treatment failure, for a risk difference of 8% (95% confidence interval, 3.3%-13.1%). RSV, influenza, and parainfluenza virus (PIV) were associated with 21%, 38%, and 47% higher risks of treatment failure, respectively, noted Dr. Merckx and her associates. These resulted in absolute risks of 9%, 25%, and 34%, respectively, the authors reported in Pediatrics.
Coronavirus, adenovirus, enterovirus D68, and the presence of a coinfection, however, were not found to increase the risk of treatment failure, they noted.
Although rhinovirus may play a role in triggering reactions that require medical attention, such cases still appear to respond favorably to treatment, they said.
A separate study cited by Dr. Merckx and her associates observed the same outcome for rhinovirus patients but more patients diagnosed with nonrhinovirus pathogens, especially human metapneumovirus (hMPV) and PIV, had moderate, rather than severe, symptoms and were much more likely to experience higher treatment failure, particularly those infected with RSV, influenza, and PIV.
“It appears reasonable to pursue strategies to improve immunization coverage for influenza and invest in efforts for the development of vaccines for RSV and rhinovirus,” they said.
In cases in which respiratory pathogens were present (especially nonrhinovirus pathogens), greater treatment failure occurred, despite use of inhaler and corticosteroids. The researchers noted that severity of condition at time of treatment and patient response to treatment should be considered as two separate, distinct dimensions of viral infection impact in children with acute asthma. “The high prevalence of rhinovirus C in children presenting with asthma exacerbation, its presumed association with asthma-related hospitalization, and its peak in the fall,” also should be considered as a leading cause for more potential severe disease.
Dr. Merckx and her associates did point to several possibly significant implications with their findings. Intensifying treatment using inhaled anticholinergics or magnesium sulfate could block the vagally mediated reflex bronchoconstriction typically seen in cases of asthma exacerbation worsened by viral infection. Although these therapies currently only are used only in severe reactions, it may be useful to examine their efficacy in any cases triggered by RSV, influenza, and PIV because these have been associated with a poor treatment response.
While it still is necessary to clarify its mechanism of action, azithromycin’s demonstrated benefit in preschoolers with severe reactions suggests it could be a possible alternative pathogen–nonspecific therapy to address antineutrophilic inflammation, they said.
Any treatment intensification would first require clear identification of responsible pathogens using on-site diagnostic measures in the ED. Until such testing is possible, preventive measures need to be prioritized, they advised.
Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
SOURCE: Merckx J et al. Pediatrics. 2018 Jun;142(1):e20174105.
FROM PEDIATRICS
Key clinical point: Nearly two-thirds of emergency asthma cases test positive for multiple pathogens.
Major finding: Risk of treatment failure is higher with respiratory syncytial virus, influenza, and parainfluenza virus.
Study details: Ancillary multicenter, prospective, ethics-approved cohort study of 958 children with asthma.
Disclosures: Dr. Merckx had no relevant disclosures; two of her associates reported receiving grants, salary rewards, and/or unrestricted donations from various pharmaceutical companies or foundations.
Source: Merckx J et al. Pediatrics. 2018;142(1):e20174105.