User login
What is the diagnosis?
Syphilis
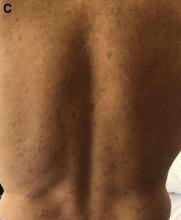
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
Syphilis
Although extremely rare, we checked a Venereal Disease Research Laboratory PCR, the results of which came back positive. A Treponema pallidum hemagglutination assay also returned positive with titers 1:5120 (ULN, < 1:80), confirming the diagnosis of syphilis. During his hospitalization, the patient developed a syphilitic skin rash on his back, chest, palms, feet, and soles (Figure C). The patient was started on penicillin G, 4 million units intravenously every 4 hours. The fever broke 36 hours after antibiotic initiation and 48 hours later, his bilirubin started to downtrend, followed by the alkaline phosphatase and GGT 3 days later. His rash completely disappeared 5 days after antibiotic initiation. He received a total of 2 weeks of penicillin G intravenously at 24 million units a day and his liver enzymes normalized 7 weeks later.
Syphilitic hepatitis is extremely rare and occurs in 0.2% of patients with secondary syphilis.1 There are few cases of syphilitic hepatitis in HIV carriers reported in the literature. Of the described cases, only two patients had an undetectable viral load.2,3 The clinical presentation of syphilitic hepatitis includes jaundice, pruritus, nausea, and vomiting, in addition to generalized symptoms of fatigue, malaise, and weight loss. Biochemically, alkaline phosphatase and GGT are predominantly elevated with mild elevation in the transaminases. Few cases describe an elevation in the bilirubin. Diagnosis is made based on treponemal testing and/or evaluation of tissue for spirochetes on liver biopsy. The majority of cases used penicillin G with excellent response. Doxycycline was also used in one case and ceftriaxone was used in another.
In our case, the patient had several other possible reasons for his liver enzyme elevation, including drug-induced liver injury, cocaine, and alcohol use, which could have contributed to his disturbed liver enzymes. The steady improvement in his cholestatic liver enzymes, fever, and rash, shortly after the initiation of penicillin G indicates that syphilis was the cause of his hepatitis. Given the improvement in his symptoms and biochemical markers, we refrained from obtaining a liver biopsy.
References
1. Lee M., Wang C., Dorer R. et al. A great masquerader: Acute syphilitic hepatitis. Dig Dis Sci. 2013;58:923-5.
2. Mullick CJ. Liappis A.P. Benator D.A. et al. Syphilitic hepatitis in HIV-infected patients: A report of 7 cases and review of the literature. Clin Infect Dis. 2004;39:e100-e105.
3. German MN. Matkowskyj K.A. Hoffman R.J. et al. A case of syphilitic hepatitis in an HIV-infected patient. Hum Pathol. 2018;79:184-7.
A 48-year-old man with HIV infection (PCR undetectable CD4 483) who used cocaine and was a heavy user of alcohol presented with jaundice, fever, and acute-onset left-upper quadrant abdominal pain. The pain was exacerbated by breathing. He had associated intermittent fevers and weight loss starting 3 weeks before presentation. He denied chest pain, nausea, vomiting, or a change in bowel habits. Home medications included dolutegravir, emtricitabine, tenofovir disoproxil fumarate, and recent intake of tamoxifen, clomiphene, and chorionic gonadotropin to counteract the effects of anabolic steroids that were used 4 months before presentation.
On examination, his temperature was 39°C, he was jaundiced, and he had icteric sclera. The abdomen was soft and nondistended with minimal left-upper quadrant tenderness. Blood work showed a white blood cell count of 7,800/mcL, hemoglobin of 12.2 g/dL, platelets of 378,000/mcL, alanine aminotransferase 236 IU/L (upper limit of normal [ULN], 65 IU/L), aspartate aminotransferase 166 IU/L (ULN, 50 IU/L), total bilirubin 3.4 mg/dL (ULN, 1.2 mg/dL), direct bilirubin 2.6 mg/dL (ULN, 0.3 mg/dL), alkaline phosphatase 1,064 IU/L (ULN, 120 IU/L), gamma-glutamyl transferase (GGT) of 655 (ULN, 50 IU/L), protein of 73 g/L, and albumin of 34 g/L. Lipase, lactate dehydrogenase, and international normalized ratio were normal. Blood smear was unrevealing. A contrasted computed tomography scan showed multiple subcentimetric mesenteric and multiple retroperitoneal lymph nodes, the largest of which was 1.3 cm in the aortocaval area. All medications were discontinued. Hepatitis A, B, and C serologies were negative, including hepatitis B and C PCR. Epstein-Barr virus IgM was negative and cytomegalovirus IgM was equivocal.
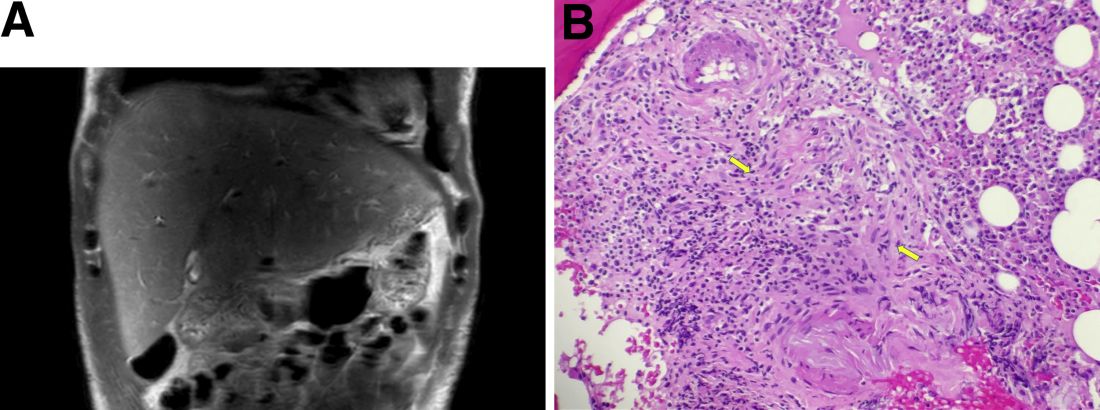
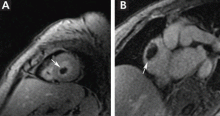
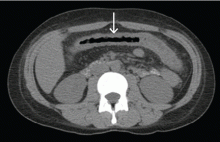
During this hospitalization, his cholestatic liver enzymes continued to rise, reaching a maximum value of total bilirubin of 7.8 mg/dL, direct bilirubin of 6.5 mg/dL, and 3 days later, alkaline phosphatase of 1,637 IU/L and GGT of 1,171 IU/L. Alanine aminotransferase and aspartate aminotransferase slowly downtrended during the hospitalization. Magnetic resonance cholangiopancreatography showed an edematous enlarged liver with minimal peripheral intrahepatic dilatation without an obstructing mass or extrahepatic biliary ductal dilatation (Figure A). Comprehensive autoimmune hepatic serology, iron studies, ceruloplasmin, and alpha-1 antitrypsin labs were negative. The patient remained febrile, so a positron emission tomography computed tomography scan was done and it showed active and enlarged (2.8-cm) portocaval and porta hepatis lymph nodes. Bone marrow biopsy showed no lymphoproliferative disorder, but there was a small poorly formed granuloma (Figure B, between the arrows).
What other testing would you obtain to evaluate this patient's fever and abnormal liver enzymes?
Previously published in Gastroenterology
Multiple intracardiac thrombi
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
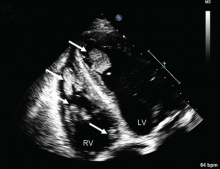
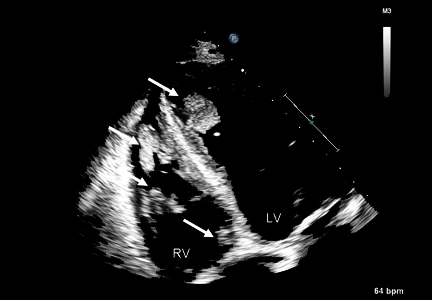
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
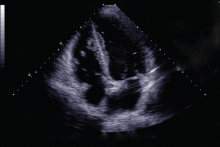
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
A 60-year-old woman presented with sudden swelling and pain in her right arm. She reported progressive lower-extremity edema and abdominal girth over the past month, associated with shortness of breath and orthopnea. She had a remote history of two spontaneous abortions.
Duplex ultrasonography revealed massive venous thrombosis extending from the antecubital fossa to the right atrium. Transthoracic echocardiography revealed severe left ventricular (LV) dysfunction and multiple echo-dense masses in the LV apex, the right ventricle, and the left atrium, as well as at the base of the tricuspid valve (Figure 1). There was no evidence of a structural heart defect, eg, patent foramen ovale, atrial septal defect, or ventricular septal defect. Cardiovascular magnetic resonance imaging (MRI) confirmed the densities as thrombi (Figure 2). Her ejection fraction was 35%.
Blood testing on admission showed a prolonged partial thromboplastin time of 55.0 sec (reference range 22.7–35.6) and a prothrombin time of 13.4 sec (reference range 11.3–14.5). Tissue thromboplastin inhibition at a dilution of 1:50 was elevated at 1.5 sec (reference range 0.7–1.3), as was the tissue thromboplastin inhibition at a dilution of 1:500—ie, 1.6 sec (0.7–1.3). Dilute Russell viper venom testing and anticardiolipin antibody immunoglobulin G and M testing were negative. The lupus antiphospholipid antibody test and the hexagonal lipid neutralization test were positive.
The patient’s clinical presentation of extensive unprovoked venous thrombosis and her laboratory profile together suggested the antiphospholipid antibody syndrome.
SURGICAL TREATMENT NOT AN OPTION
Given her extensive clot burden, surgical thrombectomy was not an option. Instead, warfarin therapy was started and resulted in a progressive diminution of the thrombi. At 4-month follow-up, the thrombi had nearly resolved (Figure 3), and her LV ejection fraction had increased to 45% to 50%. Eighteen months later, she was diagnosed with cholangiocarcinoma. In retrospect, we believe the cancer predisposed the patient to the hypercoagulable state and, subsequently, to thrombosis.
DIAGNOSING AND TREATING LEFT VENTRICULAR THROMBOSIS
Ventricular thrombosis is a serious problem, most commonly associated with extensive myocardial infarction. It is a relatively common complication of myocardial infarction and of ischemic and nonischemic cardiomy-opathies.1 In this population, the incidence of LV thrombosis is reported to be in the range of 10% to 25%, and it increases with increasing LV end-diastolic diameter, lower ejection fraction, and anterior-wall-motion akinesia, and with the presence of apical aneurysms.2 It is an important cause of morbidity and death, whether the thrombus is sessile or mobile.
How diagnostic imaging tests compare
The diagnosis of LV thrombosis requires a certain level of suspicion and has traditionally relied on echocardiography. However, several studies have raised doubt about the sensitivity of echocardiography for the detection of left or right ventricular thrombi.3 In a 2006 report, the sensitivity of transthoracic echocardiography in detecting LV thrombi was 23% and the sensitivity of transesophageal echocardiography was 40%.4 In contrast, delayed-enhancement cardiovascular MRI had a sensitivity near 90%. Similarly, in another study,5 contrast-enhanced echocardiography had a low but higher sensitivity of nearly 60%.5 Therefore, cardiovascular MRI is emerging as the new gold standard test for the detection of this important complication of ventricular dysfunction and myocardial infarction.
Treatment and screening
The optimal management of intraventricular thrombi is poorly defined. It has been suggested from case series that large, mobile, or protruding LV thrombi have more potential for embolization and, therefore, that patients with these findings may benefit from surgical thrombectomy.6 Oral anticoagulation has been reported to dissolve intraventricular thrombi, with success rates from 13% to 59%.7 A prospective study of enoxaparin in 26 patients with LV thrombi reported resolution rates close to 73% at 3-week follow-up.8
There are no guidelines at present on which to base recommendations for screening patients for intracavitary thrombi or for starting empiric anticoagulation in those at risk.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
- Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008; 52:148–157.
- Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002; 106:2873–2876.
- Tsang BK, Platts DG, Javorsky G, Brown MR. Right ventricular thrombus detection and multimodality imaging using contrast echocardiography and cardiac magnetic resonance imaging. Heart Lung Circ 2012; 21:185–188.
- Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006; 152:75–84.
- Weinsaft JW, Kim RJ, Ross M, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009; 2:969–979.
- Nili M, Deviri E, Jortner R, Strasberg B, Levy MJ. Surgical removal of a mobile, pedunculated left ventricular thrombus: report of 4 cases. Ann Thorac Surg 1988; 46:396–400.
- Heik SC, Kupper W, Hamm C, et al. Efficacy of high dose intravenous heparin for treatment of left ventricular thrombi with high embolic risk. J Am Coll Cardiol 1994; 24:1305–1309.
- Meurin P, Tabet JY, Renaud N, et al. Treatment of left ventricular thrombi with a low molecular weight heparin. Int J Cardiol 2005; 98:319–323.
Cytomegalovirus colitis
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
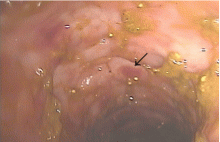
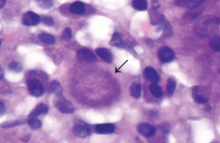
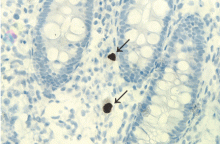
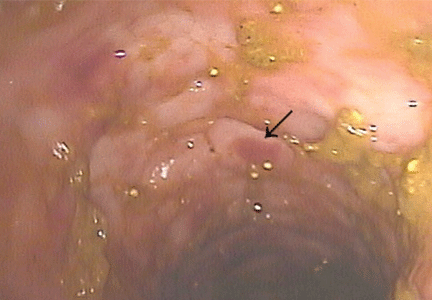
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.