User login
Vaping front and center at Hahn’s first FDA confirmation hearing
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
Stephen Hahn, MD, President Trump’s pick to head the Food and Drug Administration, faced questions from both sides of the aisle on youth vaping, but came up short when asked to commit to taking action, particularly on banning flavored vaping products.
Speaking at a Nov. 20 confirmation hearing before the Senate Health, Education, Labor, and Pensions Committee, Dr. Hahn said that youth vaping and e-cigarette use is “an important, urgent crisis in this country. I do not want to see another generation of Americans become addicted to tobacco and nicotine and I believe that we need to take aggressive to stop that.”
Sen. Patty Murray (D-Wash), the committee’s ranking member, asked Dr. Hahn whether he would work to finalize a ban flavored e-cigarette products, first proposed but then backed away from, by the president in September.
“I understand that the final compliance policy is under consideration by the administration, and I look forward to their decision,” Dr. Hahn said. “I am not privy to those decision-making processes, but I very much agree and support that aggressive action needs to be taken to protect our children.”
When pressed by Sen. Murray as to whether he told President Trump that he disagrees with the decision to back away the proposed ban, Dr. Hahn revealed that he has “not had a conversation with the president.”
Dr. Hahn, a radiation oncologist who currently serves as chief medical executive at MD Anderson Cancer Center, Houston, held firm to just coming up short of making that commitment when questioned by senators from both parties.
Sen. Mitt Romney (R-Utah) warned Dr. Hahn that the playing of politics would be unlike anything he has seen and is already being played out in the lobbying of the administration to change its stance on flavored e-cigarette products, which can run counter to the science about the harmful effects of these products.
“The question is how you will balance those things in which you put forward,” Sen. Romney asked. “How you will deal with this issue is a pretty good test case for how you would deal with this issue on an ongoing basis on matters not just related to vaping.”
He also brought up President Trump’s September announcement on a flavor ban and the administration’s signaling they are moving away from a flavor ban. “Is the FDA, under your leadership, able and willing to take action which will protect our kids, whether or not the White House wants you to take that action?”
Dr. Hahn cited his pledge as a doctor to always put the patient first and reiterated that “I take that pledge very seriously and I think if you ask anyone who has worked with me, they will tell you that I have upheld that pledge.”
But he fell short of saying that he would take actions that would oppose the White House, saying only that “patients need to come first and the decisions that we make need to be guided by science and data, congruent with the law.”
When asked by Sen. Romney if he saw any reason for holding off on a flavor ban, given the evidence that suggests flavored e-cigarette products are the gateway to youths nicotine addiction, Dr. Hahn said that he has seen the same evidence and that it requires “bold action,” but did not commit to a flavor ban. “I will use science and data to guide the decisions if I am fortunate enough to be confirmed, and I won’t back away from that.”
Sen. Doug Jones (D-Ala.) expressed concern about Dr. Hahn’s answers.
“I was less than happy with many of the answers you gave to members of this committee with regard to vaping and the potential ban on flavored e-cigarettes,” Sen. Jones said. “I think you can tell from the questions of so many senators that is one of the biggest issues that the United States Senate and Congress is facing right now. It is with this committee.”
Outside of vaping, much of the senators’ questioning was nonconfrontational, with questions spanning a gamut of issues facing the FDA.
Dr. Hahn offered his commitment to working with Congress to address drug shortages, noting nonspecifically that, “there are things that we can do to help.”
He also pledged to work with Congress on addressing patent reform to get more biosimilars to market in an effort to help drive down drug prices.
Regarding opioids, Dr. Hahn was asked about balancing the needs of those who legitimately need access to opioids against abuse and diversion.
“When I first went to medical school and started taking care of cancer patients, the teaching was that cancer patients should be treated liberally with opioids and that they don’t become addicted to pain medications,” he said. “We found out that wasn’t the case – and in some instances – with tragic consequences.”
He noted that pain therapy has evolved and that his institution now takes a multidisciplinary approach employing both opioid and nonopioid medications.
“I am very much a supporter of the multidisciplinary approach to treating pain,” he said. “I think it is something that we need to more of and if I am fortunate enough to be confirmed as commissioner of [FDA], I look forward to furthering the education efforts for providers and patients.”
Other areas he committed to included helping to improve clinical trial design for psychiatric medications and improving development of therapies for rare diseases.
Committee Chairman Lamar Alexander (R-Tenn.) said he plans to schedule a Dec. 3 vote to advance Dr. Hahn’s nomination to the full Senate for its consideration.
REPORTING FROM A SENATE SUBCOMMITTEE HEARING
Could the biosimilar market stall before it ever really started?
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett disclosed no relevant conflicts of interest.
To prepare for the entry of biosimilars to the market, AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett disclosed no relevant conflicts of interest.
To prepare for the entry of biosimilars to the market, AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett disclosed no relevant conflicts of interest.
To prepare for the entry of biosimilars to the market, AGA is taking the lead in educating health care providers and patients about biosimilars and how they can be used for IBD patient care. Learn more at www.gastro.org/biosimilars.
Feds propose new price transparency rules in health care
Three federal agencies have jointly issued a price transparency proposal that would require most employer-based health plans and health insurance issuers to disclose price and cost-sharing information up front.
The goal behind the proposal is to give consumers accurate estimates about the out-of-pocket costs they may incur for medical services, giving them the opportunity to shop around for medical treatment.
“Under the status quo, health care prices are about as clear as mud to patients,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services said in a statement, adding that “we are throwing open the shutters and bringing to light the price of care for American consumers. Kept secret, these prices are simply dollar amounts on a ledger; disclosed, they deliver fuel to the engines of competition among hospitals and insurers.”
The “Transparency in Coverage” proposed rule, was released online on Nov. 15 jointly by the Department of Health & Human Services, the Department of Labor, and the Department of the Treasury. If finalized, it would give consumers “real-time, personalized access to cost-sharing information, including an estimate of their cost-sharing liability for all covered health care items and services through an online tool that most group health plans and health insurance issuers would be required to make available to all of their members, and in paper form, at the consumer’s request,” a fact sheet outlining the features of the proposed rule states.
Health plans would also “be required to disclose on a public website their negotiated rates for in-network providers and allowed amounts paid for out-of-network providers,” the fact sheet continues.
The proposal comes as the CMS finalized transparency-related rules in the 2020 update to the hospital outpatient prospective payment system (OPPS). The price transparency portion of the OPPS is scheduled to go into effect on Jan. 1, 2021.
A fact sheet on the OPPS states that each hospital in the United States will be required to “establish (and update) and make public a yearly list of the hospital’s standard charges for items and services provided by the hospital.”
That list must include all standard charges, including gross charges, discounted cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for all hospital items and services, as well as cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for 300 shoppable services (70 identified by CMS and 230 selected by the hospital). A shoppable service is a service that can be scheduled in advance.
“This final rule and the proposed rule will bring forward the transparency we need to finally begin reducing the overall health care costs,” Ms. Verma said. “Today’s rules usher in a new era that upends the status quo to empower patients and put them first.”
America’s Health Insurance Plans said in a statement that it is evaluating the proposal and the final OPPS rule through a lens of three core principles: that consumers deserve transparency about out-of-pocket costs to help them make informed decisions; that transparency should be achieved in a way that encourages, not undermines, competitive negotiations to lower costs; and that public programs and the free market work together to deliver on our commitments to affordable, quality, and value.
“Neither of these rules, together or separately, satisfies these principles,” AHIP stated.
The Federation of American Hospitals is already anticipating a legal challenge to the rules.
“Patients should have readily available and easy-to-understand cost-sharing information when they need to make health care decisions,” FAH President and CEO Chip Kahn said in a statement. “Health care pricing transparency ought to be defined by the right information at the right time. This final regulation on hospital transparency fails to meet the definition of price transparency useful for patients. Instead, it will only result in patient overload of useless information while distorting the competitive market for purchasing hospital care.”
Mr. Kahn said FAH plans “on joining with hospitals to file a legal challenge,” asserting that CMS has exceeded it’s authority with these rules.
Three federal agencies have jointly issued a price transparency proposal that would require most employer-based health plans and health insurance issuers to disclose price and cost-sharing information up front.
The goal behind the proposal is to give consumers accurate estimates about the out-of-pocket costs they may incur for medical services, giving them the opportunity to shop around for medical treatment.
“Under the status quo, health care prices are about as clear as mud to patients,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services said in a statement, adding that “we are throwing open the shutters and bringing to light the price of care for American consumers. Kept secret, these prices are simply dollar amounts on a ledger; disclosed, they deliver fuel to the engines of competition among hospitals and insurers.”
The “Transparency in Coverage” proposed rule, was released online on Nov. 15 jointly by the Department of Health & Human Services, the Department of Labor, and the Department of the Treasury. If finalized, it would give consumers “real-time, personalized access to cost-sharing information, including an estimate of their cost-sharing liability for all covered health care items and services through an online tool that most group health plans and health insurance issuers would be required to make available to all of their members, and in paper form, at the consumer’s request,” a fact sheet outlining the features of the proposed rule states.
Health plans would also “be required to disclose on a public website their negotiated rates for in-network providers and allowed amounts paid for out-of-network providers,” the fact sheet continues.
The proposal comes as the CMS finalized transparency-related rules in the 2020 update to the hospital outpatient prospective payment system (OPPS). The price transparency portion of the OPPS is scheduled to go into effect on Jan. 1, 2021.
A fact sheet on the OPPS states that each hospital in the United States will be required to “establish (and update) and make public a yearly list of the hospital’s standard charges for items and services provided by the hospital.”
That list must include all standard charges, including gross charges, discounted cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for all hospital items and services, as well as cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for 300 shoppable services (70 identified by CMS and 230 selected by the hospital). A shoppable service is a service that can be scheduled in advance.
“This final rule and the proposed rule will bring forward the transparency we need to finally begin reducing the overall health care costs,” Ms. Verma said. “Today’s rules usher in a new era that upends the status quo to empower patients and put them first.”
America’s Health Insurance Plans said in a statement that it is evaluating the proposal and the final OPPS rule through a lens of three core principles: that consumers deserve transparency about out-of-pocket costs to help them make informed decisions; that transparency should be achieved in a way that encourages, not undermines, competitive negotiations to lower costs; and that public programs and the free market work together to deliver on our commitments to affordable, quality, and value.
“Neither of these rules, together or separately, satisfies these principles,” AHIP stated.
The Federation of American Hospitals is already anticipating a legal challenge to the rules.
“Patients should have readily available and easy-to-understand cost-sharing information when they need to make health care decisions,” FAH President and CEO Chip Kahn said in a statement. “Health care pricing transparency ought to be defined by the right information at the right time. This final regulation on hospital transparency fails to meet the definition of price transparency useful for patients. Instead, it will only result in patient overload of useless information while distorting the competitive market for purchasing hospital care.”
Mr. Kahn said FAH plans “on joining with hospitals to file a legal challenge,” asserting that CMS has exceeded it’s authority with these rules.
Three federal agencies have jointly issued a price transparency proposal that would require most employer-based health plans and health insurance issuers to disclose price and cost-sharing information up front.
The goal behind the proposal is to give consumers accurate estimates about the out-of-pocket costs they may incur for medical services, giving them the opportunity to shop around for medical treatment.
“Under the status quo, health care prices are about as clear as mud to patients,” Seema Verma, administrator of the Centers for Medicare & Medicaid Services said in a statement, adding that “we are throwing open the shutters and bringing to light the price of care for American consumers. Kept secret, these prices are simply dollar amounts on a ledger; disclosed, they deliver fuel to the engines of competition among hospitals and insurers.”
The “Transparency in Coverage” proposed rule, was released online on Nov. 15 jointly by the Department of Health & Human Services, the Department of Labor, and the Department of the Treasury. If finalized, it would give consumers “real-time, personalized access to cost-sharing information, including an estimate of their cost-sharing liability for all covered health care items and services through an online tool that most group health plans and health insurance issuers would be required to make available to all of their members, and in paper form, at the consumer’s request,” a fact sheet outlining the features of the proposed rule states.
Health plans would also “be required to disclose on a public website their negotiated rates for in-network providers and allowed amounts paid for out-of-network providers,” the fact sheet continues.
The proposal comes as the CMS finalized transparency-related rules in the 2020 update to the hospital outpatient prospective payment system (OPPS). The price transparency portion of the OPPS is scheduled to go into effect on Jan. 1, 2021.
A fact sheet on the OPPS states that each hospital in the United States will be required to “establish (and update) and make public a yearly list of the hospital’s standard charges for items and services provided by the hospital.”
That list must include all standard charges, including gross charges, discounted cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for all hospital items and services, as well as cash prices, payer-specific negotiated charges, and deidentified minimum and maximum negotiated charges for 300 shoppable services (70 identified by CMS and 230 selected by the hospital). A shoppable service is a service that can be scheduled in advance.
“This final rule and the proposed rule will bring forward the transparency we need to finally begin reducing the overall health care costs,” Ms. Verma said. “Today’s rules usher in a new era that upends the status quo to empower patients and put them first.”
America’s Health Insurance Plans said in a statement that it is evaluating the proposal and the final OPPS rule through a lens of three core principles: that consumers deserve transparency about out-of-pocket costs to help them make informed decisions; that transparency should be achieved in a way that encourages, not undermines, competitive negotiations to lower costs; and that public programs and the free market work together to deliver on our commitments to affordable, quality, and value.
“Neither of these rules, together or separately, satisfies these principles,” AHIP stated.
The Federation of American Hospitals is already anticipating a legal challenge to the rules.
“Patients should have readily available and easy-to-understand cost-sharing information when they need to make health care decisions,” FAH President and CEO Chip Kahn said in a statement. “Health care pricing transparency ought to be defined by the right information at the right time. This final regulation on hospital transparency fails to meet the definition of price transparency useful for patients. Instead, it will only result in patient overload of useless information while distorting the competitive market for purchasing hospital care.”
Mr. Kahn said FAH plans “on joining with hospitals to file a legal challenge,” asserting that CMS has exceeded it’s authority with these rules.
FDA noncommittal on e-cigarette action
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
on when the agency would act and what actions it was planning on taking.
“I was actually shocked that, in a hearing that is focused in part on the youth vaping epidemic [that] your testimony, both written and oral here, made no mention of the administration’s Sept. 11 announcement that it intended to clear the market of all unauthorized non–tobacco-flavored vaping products,” said Patty Murray (D-Wash.), ranking member of the Senate Health, Education, Labor and Pensions Committee, during a Nov. 13 hearing to Mitchell Zeller, director of the FDA’s Center for Tobacco Products. “Why is that not included in your testimony?”
Director Zeller would only offer a vague response, testifying that the agency is “committed to doing everything that we can to prevent kids from using any tobacco product, including e-cigarettes, and that we are continuing to develop a policy approach that aligns with that concern.”
When Sen. Murray pressed further, Director Zeller deflected: “I think that any questions that the committee has about the announcement that the White House and anything related to what remains a deliberative process on policy is best referred to the White House itself.”
He would not even offer any perspective on when the FDA might take actual regulatory action when asked about it by Sen. Murray.
“I can’t give you a specific timeline, Senator, other than to say that the deliberative process continues,” Director Zeller responded, telling her that “I really would refer you and the committee to the White House to ask specific questions about where we are.”
The hearing, called to examine the response to lung illnesses and rising youth e-cigarette usage, shed no new light on the issue. And while Director Zeller outlined the numerous educational campaigns being aimed at convincing youth to not use e-cigarettes, Committee Chairman Lamar Alexander (R-Tenn.) questioned whether the FDA was doing an adequate job.
The FDA, from late 2017 to the end of 2020, “will wind up investing about $150 million in a massive, multimedia public education campaign to get the word out to kids” on the dangers of vaping, Director Zeller said, adding that the agency is “aggressively enforcing” youth access restrictions in targeting sellers of e-cigarette products to minors.
“Well, obviously we are not making much progress with youth use ... if one in four of American high schoolers, according to your statistics, are using e-cigarettes,” Sen. Alexander said.
While most on the committee were focused on the rising numbers of youth vaping and e-cigarette usage, Sen. Rand Paul (R-Ky.) cautioned that any regulatory action, particularly a ban on all flavored e-cigarette products, would adversely affect adults, particularly those who are turning to e-cigarettes as a smoking cessation tool.
His solution, noting that it is already illegal for kids to be purchasing vaping and e-cigarette products, was to increase the penalties for those found selling to minors, adding that “most adults are using the flavors as well” and it could lead them back to combustible tobacco products if they are prevented from accessing flavored e-cigarettes.
REPORTING FROM A SENATE HELP COMMITTEE HEARING
Oral anticancer spending in Part D tied in major part to price increases
Price increases are the main source of the increase in spending on oral anticancer drugs in the Medicare Part D prescription drug program, according to new research.
“Annualized spending on the same oral anticancer drugs more than doubled in a 5-year period,” Kira Seiger from Harvard Medical School, Boston, and colleagues wrote in a research letter published online in JAMA Oncology.
“Use increased substantially, likely owing to expanded drug indications, increased Medicare Part D enrollment, and declining cancer mortality yielding longer treatment courses,” the authors continued. “However, increased spending was predominantly driven (56%) by rising drug costs, which is reflective of pharmaceutical pricing strategies.”
Increased use of these drugs accounted for 44% of the annualized spending on the 56 oral anticancer drugs in this study.
Researchers found that from 2007 through 2013, “anticancer drugs prices increased 5% above inflation annually, plus 10% per subsequent indication approved. Results of this study demonstrated a continued rise from 2013 through 2017 with a compound annual growth rate of 13% above inflation for drug cost per beneficiary.”
Ms. Seiger and colleagues noted that, from 2013 through 2017, more than $41.4 billion was spent on the 56 oral anticancer drugs examined for the study. These drugs carried an average out-of-pocket cost of $551, accounting for $2.1 billion of the amount spent on these drugs.
“High drug prices on market entry are often attributed to research and development expenses, though research and development may not explain the subsequent increases in drug costs demonstrated in this study,” the researchers stated.
They recommended policy makers consider capping price increases at a set percentage above inflation, “especially given that rising use reflects increased need for these therapies.”
The study was sponsored in part by the department of dermatology at Brigham and Women’s Hospital, Boston. Three of the five authors reported received fees from pharmaceutical manufacturers, and one reported serving as chair for the National Comprehensive Cancer Network.
SOURCE: Kira Seiger et al. JAMA Oncology. doi: 10.1001/jamaoncol.2019.4906.
Price increases are the main source of the increase in spending on oral anticancer drugs in the Medicare Part D prescription drug program, according to new research.
“Annualized spending on the same oral anticancer drugs more than doubled in a 5-year period,” Kira Seiger from Harvard Medical School, Boston, and colleagues wrote in a research letter published online in JAMA Oncology.
“Use increased substantially, likely owing to expanded drug indications, increased Medicare Part D enrollment, and declining cancer mortality yielding longer treatment courses,” the authors continued. “However, increased spending was predominantly driven (56%) by rising drug costs, which is reflective of pharmaceutical pricing strategies.”
Increased use of these drugs accounted for 44% of the annualized spending on the 56 oral anticancer drugs in this study.
Researchers found that from 2007 through 2013, “anticancer drugs prices increased 5% above inflation annually, plus 10% per subsequent indication approved. Results of this study demonstrated a continued rise from 2013 through 2017 with a compound annual growth rate of 13% above inflation for drug cost per beneficiary.”
Ms. Seiger and colleagues noted that, from 2013 through 2017, more than $41.4 billion was spent on the 56 oral anticancer drugs examined for the study. These drugs carried an average out-of-pocket cost of $551, accounting for $2.1 billion of the amount spent on these drugs.
“High drug prices on market entry are often attributed to research and development expenses, though research and development may not explain the subsequent increases in drug costs demonstrated in this study,” the researchers stated.
They recommended policy makers consider capping price increases at a set percentage above inflation, “especially given that rising use reflects increased need for these therapies.”
The study was sponsored in part by the department of dermatology at Brigham and Women’s Hospital, Boston. Three of the five authors reported received fees from pharmaceutical manufacturers, and one reported serving as chair for the National Comprehensive Cancer Network.
SOURCE: Kira Seiger et al. JAMA Oncology. doi: 10.1001/jamaoncol.2019.4906.
Price increases are the main source of the increase in spending on oral anticancer drugs in the Medicare Part D prescription drug program, according to new research.
“Annualized spending on the same oral anticancer drugs more than doubled in a 5-year period,” Kira Seiger from Harvard Medical School, Boston, and colleagues wrote in a research letter published online in JAMA Oncology.
“Use increased substantially, likely owing to expanded drug indications, increased Medicare Part D enrollment, and declining cancer mortality yielding longer treatment courses,” the authors continued. “However, increased spending was predominantly driven (56%) by rising drug costs, which is reflective of pharmaceutical pricing strategies.”
Increased use of these drugs accounted for 44% of the annualized spending on the 56 oral anticancer drugs in this study.
Researchers found that from 2007 through 2013, “anticancer drugs prices increased 5% above inflation annually, plus 10% per subsequent indication approved. Results of this study demonstrated a continued rise from 2013 through 2017 with a compound annual growth rate of 13% above inflation for drug cost per beneficiary.”
Ms. Seiger and colleagues noted that, from 2013 through 2017, more than $41.4 billion was spent on the 56 oral anticancer drugs examined for the study. These drugs carried an average out-of-pocket cost of $551, accounting for $2.1 billion of the amount spent on these drugs.
“High drug prices on market entry are often attributed to research and development expenses, though research and development may not explain the subsequent increases in drug costs demonstrated in this study,” the researchers stated.
They recommended policy makers consider capping price increases at a set percentage above inflation, “especially given that rising use reflects increased need for these therapies.”
The study was sponsored in part by the department of dermatology at Brigham and Women’s Hospital, Boston. Three of the five authors reported received fees from pharmaceutical manufacturers, and one reported serving as chair for the National Comprehensive Cancer Network.
SOURCE: Kira Seiger et al. JAMA Oncology. doi: 10.1001/jamaoncol.2019.4906.
FROM JAMA ONCOLOGY
Drug spending driving up Part B premiums and deductibles
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
Medicare beneficiaries charged the standard premium for Medicare Part B coverage will be paying $144.60 each month in 2020, up $9.10 from 2019.
Deductibles also will increase to $198 next year, up $13 from the current year.
The Centers for Medicare & Medicaid Services said in a statement announcing the hikes that the increases are “largely due to rising spending on physician administered drugs. These higher costs have a ripple effect and result in higher Part B premiums and deductibles.”
The formal details on the premium and deductible increases have been posted online and are scheduled for publication in the Federal Register on Nov. 13.
The CMS and Congress are looking into a number of options to help contain the spending on drugs, including the use of an international pricing index to put U.S. spending more in line with the lower prices offered in foreign countries, automatic rebates when drug prices rise faster than the rate of inflation, and a modern take on the failed competitive acquisition program.
The agency also announced increases in the inpatient hospital deductible that will be paid under Medicare Part A when beneficiaries are admitted into a hospital in 2020. The deductible increases to $1,408 next year, up from $1,364 this year. The daily coinsurance for the 61st-90th day increases to $352 from $341, while the daily coinsurance for lifetime reserve days increases to $704 from $682.
Skilled nursing facility coinsurance also rises during this same time period to $176 from $170.50.
More information on Part A deductibles can be found here, while information on Part A premiums can be found here.
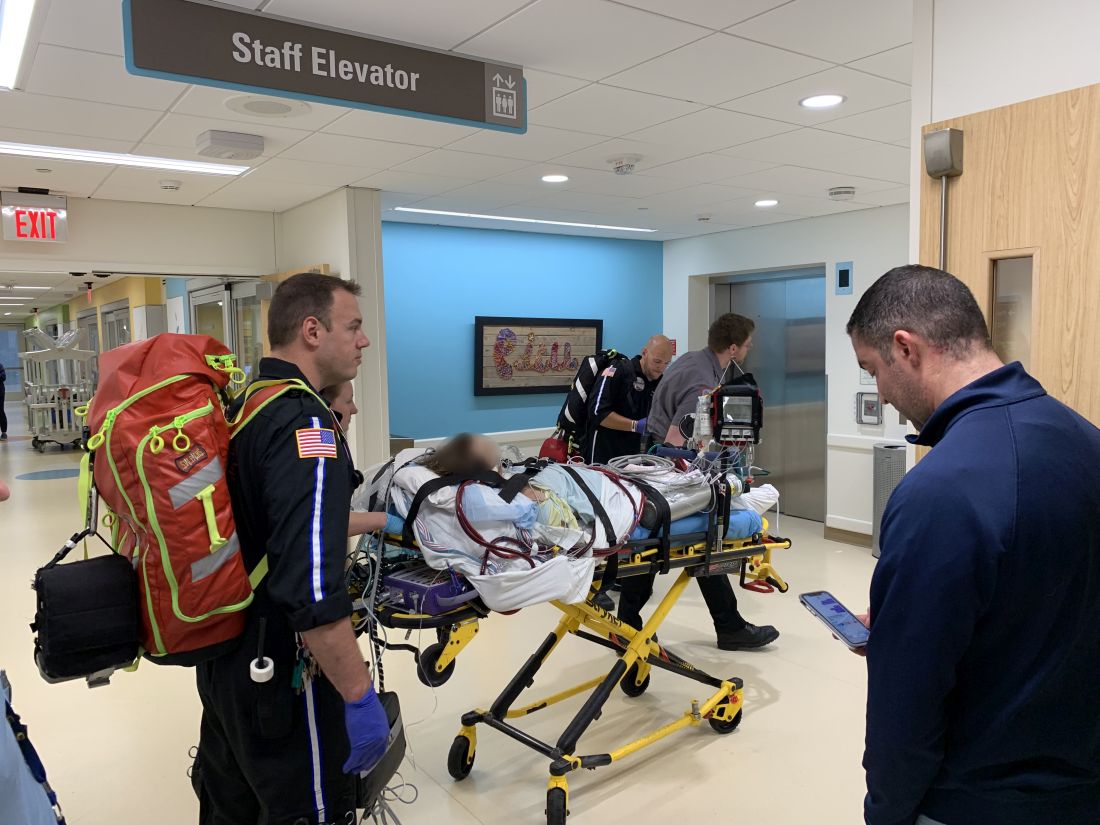
Teen survives double lung transplant after vaping injury
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
A Michigan teenager, described as an athlete and otherwise healthy, has survived a double lung transplant following lung damage attributed to vaping.
“On the 15th of October, the transplant team performed what we believe is the first double lung transplant done in the nation for a vaping-injury victim, who is a teenager,” Hassan Nemeh, MD, cardiothoracic surgeon with the Henry Ford Health System in Detroit, said during a Nov. 12, 2019, press conference to discuss the surgery.
“What I saw in his lungs is nothing that I have ever seen before and I have been doing lung transplants for 20 years,” Dr. Nemeh said. “There was an enormous amount of inflammation and scarring, in addition to multiple spots of dead tissue. The lung itself was so firm and scarred, we had to deliver it out of the chest. This is an evil that I haven’t faced before.”
He noted that the patient, now 17 years old but 16 when the surgical procedure occurred, is doing well in his recovery, and although the patient and the family are not yet ready to be identified, the health system made the decision to tell the story of the surgery as a cautionary tale.
“The reason we wanted to bring this case to public attention is because of the epidemic of e-cigarettes and vaping-induced lung injury that we are witnessing in the country,” including more than 2,000 cases of injury and 39 deaths that have been confirmed from lung failure related to e-cigarettes and vaping that have been reported to the Centers for Disease Control and Prevention, he said.
“Our teenage patient would have faced certain death if it weren’t for the lung transplant happening,” Dr. Nemeh said, adding that, while vaping and e-cigarettes are being presented as a benign habit, there are potentially very deadly consequences that Henry Ford Hospital System wanted to highlight. He described the patient’s lungs as essentially being nonfunctional with very little air being able to be passed into them, with the destruction to his native lung from pneumonia and dead tissue almost completely covering his lungs.
This story began with a morning call on Oct. 1 from the Children’s Hospital of Michigan alerting the Henry Ford Health System that they had a patient on life support because of complete lung failure who was not showing signs of healing and asking if the Henry Ford Health System could possibly handle a lung transplant for this patient.
Dr. Nemeh said that the patient was on a nontransportable extracorporeal membrane oxygenation (ECMO) machine at Children’s. Dr. Nemeh and the team at Henry Ford determined that the situation for the patient was so dire that they put a portable ECMO machine into the trunk of Dr. Nemeh’s car and delivered it to Children’s in order to facilitate the transfer of the patient for transplantation surgery.
Victor Coba, MD, a critical care specialist and medical director of the ECMO program at Henry Ford, said: “We evaluated the irreversible lung damage that had occurred associated with vaping. Working closely with the lung transplant team and noting that his lungs would not recover, we worked to get him on the lung transplant list.”
Lisa Allenspach, MD, pulmonologist and medical director of the lung transplant program at Henry Ford, reiterated the need for caution when it comes to vaping and e-cigarette use.
“Vaping-related injuries are all too common these days and, actually, our adolescents are faced with a crisis,” she said. “I believe we are just beginning to see the tip of the iceberg. Making sure that our teens understand the danger of vaping is of paramount importance.”
She did not disclose specific details about the teen’s use of vaping/e-cigarette products, so it is unknown whether the injury was caused by standard off-the-shelf products or if it was related to vaping cartridges containing tetrahydrocannabinol.
“We are here today to beg the public to pay special attention to the steps that were taken in this case,” said Nicholas Yeldo, MD, anesthesiology and critical care specialist with Henry Ford. “Without the heroic measures that were taken in this case, this young patient would have died. There is no doubt about it. ... This was not just an unlucky one. This is happening way, way too much.”
Dr. Allenspach was positive that the young patient could live a long life, noting that there are those who have received lung transplants have survived for 15-20 years and second transplants are possible.
Integrating lay navigation programs into cancer care is a challenge
The implementation of a lay navigation program as part of the delivery of cancer care presents a number of challenges, particularly because of the complexities of treating cancer, a new study has found.
Researchers looked at a lay navigator program, which uses nonclinical members to help provide information to cancer patients about their treatments, at the University of Alabama, and how the program was doing a year after its implementation.
“Integrating lay navigators into a complex clinical environment needs careful consideration at an organizational level, because this experience demonstrates that the integration process is not straightforward for clinical teams, patients, or navigators,” wrote Laura M. Holdsworth, PhD, Stanford (Calif.) University, and colleague. Their report is in the Journal of Oncology Practice.
A key difficulty discovered through the course of the research is that approximately two-thirds of concerns brought by patients to the lay navigators were clinical in nature, though only about a third (30%) required clinical follow-up and an additional 7% required social work follow-up.
“This seeming misalignment of nonclinical staff handling clinical issues is likely explained by the fact that clinical issues were often a request to repeat information previously delivered by a clinician,” the authors wrote. “The high proportion of clinical concerns brought to navigators is likely a consequence of navigators proactively contacting patients and thus being perceived as an accessible extension of the clinical team with whom to raise concern.”
Researchers found that clinical members did find navigators useful to the care team “specifically because they brought clinical issues to the attention of the clinical team that might otherwise have been missed.”
But on the other hand, some clinicians believed that “clinical concerns being raised with navigators were a source of concern and felt to be inappropriate, suggesting a lack of compatibility of a lay service layered onto complex clinical care,” Dr. Holdsworth and colleagues stated, adding that nurses “with a negative view of lay navigation seemed to lack knowledge and information about the navigator role, which created trust issues.”
Another potential issue is staff turnover within the navigator program and an ever-changing environment of patient education and support programs. Navigators can be very beneficial connecting patients to things such as support groups or helping to connect patients to insurers, but it requires a significant effort on the part of navigators to know, understand, and keep up to date with all the nonclinical opportunities that patients have, and high turnover can be an issue here.
Overall, though, the researchers note that the key finding “was that it was difficult to implement a lay navigation program outside of the clinical team for the purposes of cancer care coordination. The navigators were not integrated into the clinical teams and as such, the navigator role was treated with some suspicion by clinical team members; there was a sense of mistrust among some clinicians, and mismatched expectations around what navigators could or should be doing.”
SOURCE: Holdsworth L et al. J Oncol Pract, 2019 Nov. 6. doi: 10.1200/JOP.19.00339.
The implementation of a lay navigation program as part of the delivery of cancer care presents a number of challenges, particularly because of the complexities of treating cancer, a new study has found.
Researchers looked at a lay navigator program, which uses nonclinical members to help provide information to cancer patients about their treatments, at the University of Alabama, and how the program was doing a year after its implementation.
“Integrating lay navigators into a complex clinical environment needs careful consideration at an organizational level, because this experience demonstrates that the integration process is not straightforward for clinical teams, patients, or navigators,” wrote Laura M. Holdsworth, PhD, Stanford (Calif.) University, and colleague. Their report is in the Journal of Oncology Practice.
A key difficulty discovered through the course of the research is that approximately two-thirds of concerns brought by patients to the lay navigators were clinical in nature, though only about a third (30%) required clinical follow-up and an additional 7% required social work follow-up.
“This seeming misalignment of nonclinical staff handling clinical issues is likely explained by the fact that clinical issues were often a request to repeat information previously delivered by a clinician,” the authors wrote. “The high proportion of clinical concerns brought to navigators is likely a consequence of navigators proactively contacting patients and thus being perceived as an accessible extension of the clinical team with whom to raise concern.”
Researchers found that clinical members did find navigators useful to the care team “specifically because they brought clinical issues to the attention of the clinical team that might otherwise have been missed.”
But on the other hand, some clinicians believed that “clinical concerns being raised with navigators were a source of concern and felt to be inappropriate, suggesting a lack of compatibility of a lay service layered onto complex clinical care,” Dr. Holdsworth and colleagues stated, adding that nurses “with a negative view of lay navigation seemed to lack knowledge and information about the navigator role, which created trust issues.”
Another potential issue is staff turnover within the navigator program and an ever-changing environment of patient education and support programs. Navigators can be very beneficial connecting patients to things such as support groups or helping to connect patients to insurers, but it requires a significant effort on the part of navigators to know, understand, and keep up to date with all the nonclinical opportunities that patients have, and high turnover can be an issue here.
Overall, though, the researchers note that the key finding “was that it was difficult to implement a lay navigation program outside of the clinical team for the purposes of cancer care coordination. The navigators were not integrated into the clinical teams and as such, the navigator role was treated with some suspicion by clinical team members; there was a sense of mistrust among some clinicians, and mismatched expectations around what navigators could or should be doing.”
SOURCE: Holdsworth L et al. J Oncol Pract, 2019 Nov. 6. doi: 10.1200/JOP.19.00339.
The implementation of a lay navigation program as part of the delivery of cancer care presents a number of challenges, particularly because of the complexities of treating cancer, a new study has found.
Researchers looked at a lay navigator program, which uses nonclinical members to help provide information to cancer patients about their treatments, at the University of Alabama, and how the program was doing a year after its implementation.
“Integrating lay navigators into a complex clinical environment needs careful consideration at an organizational level, because this experience demonstrates that the integration process is not straightforward for clinical teams, patients, or navigators,” wrote Laura M. Holdsworth, PhD, Stanford (Calif.) University, and colleague. Their report is in the Journal of Oncology Practice.
A key difficulty discovered through the course of the research is that approximately two-thirds of concerns brought by patients to the lay navigators were clinical in nature, though only about a third (30%) required clinical follow-up and an additional 7% required social work follow-up.
“This seeming misalignment of nonclinical staff handling clinical issues is likely explained by the fact that clinical issues were often a request to repeat information previously delivered by a clinician,” the authors wrote. “The high proportion of clinical concerns brought to navigators is likely a consequence of navigators proactively contacting patients and thus being perceived as an accessible extension of the clinical team with whom to raise concern.”
Researchers found that clinical members did find navigators useful to the care team “specifically because they brought clinical issues to the attention of the clinical team that might otherwise have been missed.”
But on the other hand, some clinicians believed that “clinical concerns being raised with navigators were a source of concern and felt to be inappropriate, suggesting a lack of compatibility of a lay service layered onto complex clinical care,” Dr. Holdsworth and colleagues stated, adding that nurses “with a negative view of lay navigation seemed to lack knowledge and information about the navigator role, which created trust issues.”
Another potential issue is staff turnover within the navigator program and an ever-changing environment of patient education and support programs. Navigators can be very beneficial connecting patients to things such as support groups or helping to connect patients to insurers, but it requires a significant effort on the part of navigators to know, understand, and keep up to date with all the nonclinical opportunities that patients have, and high turnover can be an issue here.
Overall, though, the researchers note that the key finding “was that it was difficult to implement a lay navigation program outside of the clinical team for the purposes of cancer care coordination. The navigators were not integrated into the clinical teams and as such, the navigator role was treated with some suspicion by clinical team members; there was a sense of mistrust among some clinicians, and mismatched expectations around what navigators could or should be doing.”
SOURCE: Holdsworth L et al. J Oncol Pract, 2019 Nov. 6. doi: 10.1200/JOP.19.00339.
FROM JOURNAL OF ONCOLOGY PRACTICE
MIPS, E/M changes highlight 2020 Medicare fee schedule
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
A Merit-based Incentive Payment System (MIPS) overhaul and evaluation and management changes to support the care of complex patients highlight the final Medicare physician fee schedule for 2020.
The new MIPS Value Pathways (MVPs) framework “aims to align and connect measures and activities across the quality, cost, promoting interoperability, and improvement activities performance categories of MIPS for different specialties or conditions,” the Centers for Medicare & Medicaid Services said in a fact sheet outlining the updates to the Quality Payment Program.
CMS noted that the framework will have measures aimed at population health and public health priorities, as well as reducing the reporting burden of the MIPS program and providing enhanced data and feedback to clinicians.
“We also intend to analyze existing Medicare information so that we can provide clinicians and patients with more information to improve health outcomes,” the agency wrote. “We believe the MVPs framework will help simplify MIPS, create a more cohesive and meaningful participation experience, improve value, reduce clinician burden, and better align with APMs [advanced alternative payment models] to help ease transition between the two tracks.
While the specifics of how the pathways will work are yet to be determined, the goal is to reduce the reporting burden while increasing its clinical applicability
Under the current MIPS structure, clinicians report on a specific number of measures chosen from a menu that may or may not be relevant to the care of patients with a specific disease, such as diabetes.
The MVPs framework will have some “foundation” measures in the first 2 years linked to promoting interoperability and population health that all clinicians will use. These, however, will be coupled with additional measures across the other MIPS categories (quality, cost, and improvement) that are specifically related to diabetes treatment. The expectation is that disease-specific measures plus foundation measures will add up to fewer measures than clinicians currently report, according to CMS.
Over the next 3-5 years, disease-specific measures will be refined and foundation measures expanded to include enhanced performance feedback and patient-reported outcomes.
“We recognize that this will be a significant shift in the way clinicians may potentially participate in MIPS, therefore we want to work closely with clinicians, patients, specialty societies, third parties, and others to establish the MVPs,” CMS officials said.
In the meantime, there are changes to the current MIPS program. Category weighting remains unchanged for the 2020 performance year (payable in 2022), with the performance threshold being 45 points and the exceptional performance threshold being 85 points.
In the quality performance category, the data completeness threshold is increased to 70%, while the agency continues to remove low-bar, standard-of-care process measures and adding new specialty sets, such as audiology, chiropractic medicine, pulmonology, and endocrinology.
In the cost category, 10 new episode-based measures were added to help expand access to this category. In the improvement activities category, CMS reduced barriers to obtaining a patient-centered medical home designation and increased the participation threshold for a practice from a single clinician to 50% of the clinicians in the practice. In the promoting interoperability category, the agency included queries to a prescription drug–monitoring program as an option measure, removed the verify opioid treatment–agreement measure, and reduced the threshold for a group to be considered hospital based from 100% to 75% being hospital based in order for a group to be excluded from reporting measures in this category.
One change not made in the MIPS update is threshold for exclusion from participating in the MIPS program, which has generated continued criticism over the years from the American Medical Group Association, which represents multispecialty practices.
“Overall, CMS expects Part B payment adjustments of 1.4% for those providers who participate in the program,” AMGA officials said in a statement. “However, Congress authorized up to a 9% payment adjustment for the 2020 performance year. While not every provider will achieve the highest possible adjustment, CMS’ continued policy of excluding otherwise eligible providers from participating in MIPS makes it impossible to achieve sustainable payments to cover the cost of participation. Thus, AMGA members have expressed that the program is no longer a viable tool for transitioning to value-based care.”
The physician fee schedule also finalized a number of provisions aimed at reducing administrative burden and increasing the time physicians have with patients. The changes will save clinicians 2.3 million hours per year in burden reduction, according to CMS.
New evaluation and management services (E/M) codes will allow clinicians to choose the appropriate level of coding based on either the medical decision making or time spent with the patient. In 2021, an add-on code will be implemented for prolonged service times for when clinicians spend more time treating complex patients, according to a CMS fact sheet.
Beginning in 2020, clinicians will be paid for care management services for patients with one serious and high-risk condition. Previously, a patient would need at least two serious and high-risk conditions for clinicians to get paid for care management services. For those with multiple chronic conditions, a Medicare-specific code has been added that covers patient visits that last beyond 20 minutes allowed in the current coding for chronic care management services.
The E/M changes are “a significant step in reducing administrative burden that gets in the way of patient care. Now it’s time for vendors and payors to take the necessary steps to align their systems with the E/M office visit code changes by the time the revisions are deployed on Jan. 1, 2021,” Patrice Harris, MD, president of the American Medical Association, said in a statement.
The American College of Physicians also applauded the change.
“Medicare has long undervalued E/M codes by internal medicine physicians, family physicians, and other cognitive and primary care physicians,” ACP said in a statement, adding that it is “extremely pleased that CMS’s final payment rules will strengthen primary and cognitive care by improving E/M codes and payment levels and reducing administrative burdens.”
The changes also will help address physician shortages, according to ACP officials.
“Fewer physicians are going into office-based internal medicine and other primary care disciplines in large part because Medicare and other payers have long undervalued their services and imposed unreasonable documentation requirements,” they wrote. “CMS’s new rule can help reverse this trend at a time when an aging population will need more primary care physicians, especially internal medicine specialists, to care for them.”
Opioid use disorder treatment programs will be covered by Medicare beginning in 2020. Enrolled opioid treatment programs will receive a bundled payment based on weekly episodes of care that cover Food and Drug Administration–approved medications that treat opioid use disorder, the dispensing and administering those medications, counseling, individual and group therapy, and toxicology testing.
The physician fee schedule also includes codes for telehealth services related to the opioid treatment bundle.
CMS also is finalizing updates on physician supervision of physician assistants to give physician assistants “greater flexibility to practice more broadly in the current health care system in accordance with state law and state scope of practice,” the fact sheet notes.
SOURCE: CMS Medicare Physician Fee Schedule for calendar year 2020.
Could the biosimilar market stall before it ever really started?
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
NATIONAL HARBOR, MD. – If the United States does not step up and create a thriving biosimilars market soon, it risks destroying the market not only domestically but internationally as well.
This was the warning Gillian Woollett, senior vice president at Avalere, provided to attendees at the annual meeting of the Academy of Managed Care Pharmacy.
She prefaced her warning by quoting Alex Azar, secretary of Health & Human Services, who said that those “trying to hold back biosimilars are simply on the wrong side of history,” though Ms. Woollett said they “may be on the right side of the current economic model in the United States.”
And despite the probusiness, procompetition philosophy of current HHS leadership, there has been very little movement on creating a competitive market for biosimilars in the United States, evidenced by the very expensive regulatory requirements that biosimilar manufacturers need to meet in order to get products to market.
“It’s not that we won’t have competition in the U.S.,” she said. “I think we will. We do have that innovation. ... It’s just that biosimilars may not ultimately be part of that competition. And for that, we will pay a price, and I actually think the whole world will pay a price because if we are not providing the [return on investment], I am not sure the other markets can sustain it.”
One issue biosimilars have is the lack of recognition of the value that they bring.
“That biosimilars offer the same clinical outcomes at a lower price is yet to be a recognized value,” she said. “To me that’s a really surprising situation in the United States.”
Ms. Woollett continued: “It’s not even acknowledged as a basic truth in the United States, which again suggests the business model may not be there for biosimilars.”
Ms. Woollett disclosed no conflicts of interest relevant to her presentation.
REPORTING FROM AMCP NEXUS 2019