User login
ACA marketplace sees late surge in new enrollees
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
Individuals new to the federal insurance marketplace made up nearly half of the last-minute enrollees, according to data released by the Centers for Medicare & Medicaid Services.
CMS officials said that of the 1.8 million people who purchased insurance coverage in the last 5 days of open enrollment, 48% were new customers. The open enrollment period was extended for 2 days past the original Dec. 15 deadline following a late surge in demand, including the single highest day of individuals enrolling for coverage – 600,000 on Dec. 15 – during the 3 years of the federal marketplace.
In total, 6 million people signed up for coverage through HealthCare.gov during this open enrollment season, which began Nov. 1, including 2.4 million newly enrolled people and 3.6 million returning to actively enroll in coverage. CMS did not have figures for how many people will be automatically reenrolled, and the figures released account for the federal marketplace only and do not include those who are enrolling through the 13 state-run marketplaces.
“It’s clear that people have been waiting for this open enrollment period to come to the marketplace and purchase coverage,” CMS Acting Administrator Andy Slavitt said during a Dec. 18 press briefing.
He cited a number of factors driving enrollment, including the increasing tax penalty for noncoverage, which can be $695 or more; a desire to have insurance; affordability, particularly when accounting for federal subsidies; and improvements to the shopping experience, including better cost estimates and decision support tools.
NIH, CDC get boost in House budget bill
The National Institutes of Health would see an almost 10% increase in funding under a budget deal announced Dec. 15 by House Speaker Paul Ryan (R-Wisc.).
Other provisions in the omnibus budget agreement include an increased budget for the Centers for Disease Control and Prevention, level budgets for the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology, and a reduced budget for the Agency for Healthcare Research and Quality.
The bill also prevents any new or additional spending on the Affordable Care Act.
The National Institutes of Health will see its budget increase to $32 billion, $2 billion more than the fiscal 2015 budget and $1 billion more than the White House had requested. The increases are targeted to a number of specific research areas, including Alzheimer’s disease, the BRAIN Initiative, antibiotics research, as well as for the Precision Medicine Initiative. The NIH increase also reflects increased budget levels for all individual institutes and centers.
CDC’s budget is increased by about $308 million – almost double the White House–requested increase – to $7.2 billion. The proposal prioritizes funding on disease prevention and biodefense research to prevent against infectious diseases as well as bioterrorism attacks, and helping the agency modernize laboratory facilities and work with states to address antibiotic resistance.
It also includes $70 million to target prescription drug abuse, $160 million for the Division for Heart Disease and Stroke Prevention, $170 million to help address preventable chronic diseases, $160 million in block grants for states to address their individual health needs, and $1.4 billion in budgeting for states to use for emergency preparedness for bioterror attacks or pandemic disease emergencies.
The budget proprosal also cuts more than $30 million from the Agency for Healthcare Research and Quality (AHRQ). The White House had asked for funding of the AHRQ to remain static.
Both the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology saw their budgets remain level; however, the bill makes no changes to the Meaningful Use program.
As part of Congress’ ongoing oversight into prescription drug pricing, the budget bill is calling for the U.S. Department of Health & Human Services to prepare a report examining the prices.
According to a committee report accompanying the bill language, HHS will prepare a report on prices, net of rebate, of the top 10 most commonly prescribed drugs and the top 10 highest-cost drugs paid under Medicare Part B and Part D, Medicaid, and by the Department of Veterans Affairs since 2003. The report, due 180 days after the enactment of the bill, would also evaluate access to drugs under the four programs as well as patient satisfaction with care.
The House is expected to vote on the budget proposal as early as Dec. 18.
The National Institutes of Health would see an almost 10% increase in funding under a budget deal announced Dec. 15 by House Speaker Paul Ryan (R-Wisc.).
Other provisions in the omnibus budget agreement include an increased budget for the Centers for Disease Control and Prevention, level budgets for the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology, and a reduced budget for the Agency for Healthcare Research and Quality.
The bill also prevents any new or additional spending on the Affordable Care Act.
The National Institutes of Health will see its budget increase to $32 billion, $2 billion more than the fiscal 2015 budget and $1 billion more than the White House had requested. The increases are targeted to a number of specific research areas, including Alzheimer’s disease, the BRAIN Initiative, antibiotics research, as well as for the Precision Medicine Initiative. The NIH increase also reflects increased budget levels for all individual institutes and centers.
CDC’s budget is increased by about $308 million – almost double the White House–requested increase – to $7.2 billion. The proposal prioritizes funding on disease prevention and biodefense research to prevent against infectious diseases as well as bioterrorism attacks, and helping the agency modernize laboratory facilities and work with states to address antibiotic resistance.
It also includes $70 million to target prescription drug abuse, $160 million for the Division for Heart Disease and Stroke Prevention, $170 million to help address preventable chronic diseases, $160 million in block grants for states to address their individual health needs, and $1.4 billion in budgeting for states to use for emergency preparedness for bioterror attacks or pandemic disease emergencies.
The budget proprosal also cuts more than $30 million from the Agency for Healthcare Research and Quality (AHRQ). The White House had asked for funding of the AHRQ to remain static.
Both the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology saw their budgets remain level; however, the bill makes no changes to the Meaningful Use program.
As part of Congress’ ongoing oversight into prescription drug pricing, the budget bill is calling for the U.S. Department of Health & Human Services to prepare a report examining the prices.
According to a committee report accompanying the bill language, HHS will prepare a report on prices, net of rebate, of the top 10 most commonly prescribed drugs and the top 10 highest-cost drugs paid under Medicare Part B and Part D, Medicaid, and by the Department of Veterans Affairs since 2003. The report, due 180 days after the enactment of the bill, would also evaluate access to drugs under the four programs as well as patient satisfaction with care.
The House is expected to vote on the budget proposal as early as Dec. 18.
The National Institutes of Health would see an almost 10% increase in funding under a budget deal announced Dec. 15 by House Speaker Paul Ryan (R-Wisc.).
Other provisions in the omnibus budget agreement include an increased budget for the Centers for Disease Control and Prevention, level budgets for the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology, and a reduced budget for the Agency for Healthcare Research and Quality.
The bill also prevents any new or additional spending on the Affordable Care Act.
The National Institutes of Health will see its budget increase to $32 billion, $2 billion more than the fiscal 2015 budget and $1 billion more than the White House had requested. The increases are targeted to a number of specific research areas, including Alzheimer’s disease, the BRAIN Initiative, antibiotics research, as well as for the Precision Medicine Initiative. The NIH increase also reflects increased budget levels for all individual institutes and centers.
CDC’s budget is increased by about $308 million – almost double the White House–requested increase – to $7.2 billion. The proposal prioritizes funding on disease prevention and biodefense research to prevent against infectious diseases as well as bioterrorism attacks, and helping the agency modernize laboratory facilities and work with states to address antibiotic resistance.
It also includes $70 million to target prescription drug abuse, $160 million for the Division for Heart Disease and Stroke Prevention, $170 million to help address preventable chronic diseases, $160 million in block grants for states to address their individual health needs, and $1.4 billion in budgeting for states to use for emergency preparedness for bioterror attacks or pandemic disease emergencies.
The budget proprosal also cuts more than $30 million from the Agency for Healthcare Research and Quality (AHRQ). The White House had asked for funding of the AHRQ to remain static.
Both the Centers for Medicare & Medicaid Services and the Office of the National Coordinator for Health Information Technology saw their budgets remain level; however, the bill makes no changes to the Meaningful Use program.
As part of Congress’ ongoing oversight into prescription drug pricing, the budget bill is calling for the U.S. Department of Health & Human Services to prepare a report examining the prices.
According to a committee report accompanying the bill language, HHS will prepare a report on prices, net of rebate, of the top 10 most commonly prescribed drugs and the top 10 highest-cost drugs paid under Medicare Part B and Part D, Medicaid, and by the Department of Veterans Affairs since 2003. The report, due 180 days after the enactment of the bill, would also evaluate access to drugs under the four programs as well as patient satisfaction with care.
The House is expected to vote on the budget proposal as early as Dec. 18.
Half of hospitals penalized in 2015 by CMS quality program will pay again in 2016
More than half of hospitals penalized under the Hospital-Acquired Condition Reduction Program in fiscal year 2015 will be penalized again in FY 2016, the Centers for Medicare & Medicaid Services reported.
The program, instituted as part of the Affordable Care Act, penalizes the lowest quartile of qualifying non-Maryland hospitals with the worst risk-adjusted HAC quality measures by reducing payments related to those discharges by 1%. Maryland hospitals are currently excluded from the program because of insufficient data.
The CMS reported that in FY2016, 758 out of the 3,308 hospitals subject to the program will face the payment reduction, up from 724 in FY 2015. “Out of the 758 hospitals in the worst performing quartile in FY 2016, approximately 53.7 percent were also in the worst performing quartile in FY 2015,” the agency said in a fact sheet.*
In general, the average performance improved on two of the three measures in both years of the program: the mean Patient Safety Indicator 90 Composite Index Value (tracking pressure ulcer, iatrogenic pneumothorax, central venous catheter-related bloodstream infections, postoperative hip fractures, perioperative pulmonary embolism or deep vein thrombosis, postoperative sepsis, postoperative wound dehiscence, and accident puncture or laceration) and the mean Central Line-Associated Blood Stream Infection Standardized Infection Ratio (SIR), the agency noted. The mean Catheter-Associated Urinary Tract Infection SIR increased slightly. A fourth measure, the mean Surgical Site Infection SIR, was added as measure for fiscal 2016.
gtwachtman@frontlinemedcom.com
*CORRECTION, 1/7/2016: An earlier version of this article did not clearly state the percentage of hospitals that were also in the worst performing quartile in FY 2015.
More than half of hospitals penalized under the Hospital-Acquired Condition Reduction Program in fiscal year 2015 will be penalized again in FY 2016, the Centers for Medicare & Medicaid Services reported.
The program, instituted as part of the Affordable Care Act, penalizes the lowest quartile of qualifying non-Maryland hospitals with the worst risk-adjusted HAC quality measures by reducing payments related to those discharges by 1%. Maryland hospitals are currently excluded from the program because of insufficient data.
The CMS reported that in FY2016, 758 out of the 3,308 hospitals subject to the program will face the payment reduction, up from 724 in FY 2015. “Out of the 758 hospitals in the worst performing quartile in FY 2016, approximately 53.7 percent were also in the worst performing quartile in FY 2015,” the agency said in a fact sheet.*
In general, the average performance improved on two of the three measures in both years of the program: the mean Patient Safety Indicator 90 Composite Index Value (tracking pressure ulcer, iatrogenic pneumothorax, central venous catheter-related bloodstream infections, postoperative hip fractures, perioperative pulmonary embolism or deep vein thrombosis, postoperative sepsis, postoperative wound dehiscence, and accident puncture or laceration) and the mean Central Line-Associated Blood Stream Infection Standardized Infection Ratio (SIR), the agency noted. The mean Catheter-Associated Urinary Tract Infection SIR increased slightly. A fourth measure, the mean Surgical Site Infection SIR, was added as measure for fiscal 2016.
gtwachtman@frontlinemedcom.com
*CORRECTION, 1/7/2016: An earlier version of this article did not clearly state the percentage of hospitals that were also in the worst performing quartile in FY 2015.
More than half of hospitals penalized under the Hospital-Acquired Condition Reduction Program in fiscal year 2015 will be penalized again in FY 2016, the Centers for Medicare & Medicaid Services reported.
The program, instituted as part of the Affordable Care Act, penalizes the lowest quartile of qualifying non-Maryland hospitals with the worst risk-adjusted HAC quality measures by reducing payments related to those discharges by 1%. Maryland hospitals are currently excluded from the program because of insufficient data.
The CMS reported that in FY2016, 758 out of the 3,308 hospitals subject to the program will face the payment reduction, up from 724 in FY 2015. “Out of the 758 hospitals in the worst performing quartile in FY 2016, approximately 53.7 percent were also in the worst performing quartile in FY 2015,” the agency said in a fact sheet.*
In general, the average performance improved on two of the three measures in both years of the program: the mean Patient Safety Indicator 90 Composite Index Value (tracking pressure ulcer, iatrogenic pneumothorax, central venous catheter-related bloodstream infections, postoperative hip fractures, perioperative pulmonary embolism or deep vein thrombosis, postoperative sepsis, postoperative wound dehiscence, and accident puncture or laceration) and the mean Central Line-Associated Blood Stream Infection Standardized Infection Ratio (SIR), the agency noted. The mean Catheter-Associated Urinary Tract Infection SIR increased slightly. A fourth measure, the mean Surgical Site Infection SIR, was added as measure for fiscal 2016.
gtwachtman@frontlinemedcom.com
*CORRECTION, 1/7/2016: An earlier version of this article did not clearly state the percentage of hospitals that were also in the worst performing quartile in FY 2015.
Senate calls for childproof packaging for ‘e-cig juice’
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
The Senate passed a bill that would require childproof packaging for liquid nicotine products.
The Child Nicotine Poisoning Prevention Act of 2015 (S. 142), also would codify Food and Drug Administration authority to regulate the packaging of liquid nicotine that is used to refill various electronic nicotine delivery systems.
S. 142 passed by unanimous consent in the Senate on Dec. 10. The House of Representatives has not taken action on the bill.
“Just a small amount of this stuff can injure or even kill a small child,” Sen. Bill Nelson (D-Fla.), the bill’s sponsor, said in a statement. “Making these bottles childproof is just common sense.”
In 2014, poison control centers received more than 3,000 calls related to e-cigarette nicotine exposure, including one toddler death, according to a statement from the American Academy of Pediatrics.
“With e-cigarettes becoming more and more common in households across the country, we cannot afford to wait another day to protect children from poisonous liquid nicotine,” AAP President Dr. Sandra Hassink said.
Ethicon recalls Gynecare Thermachoice III catheter
Ethicon has initiated a recall of its Gynecare Thermachoice III thermal balloon ablation silicone catheter.
The “voluntary recall involves only the catheter component of the Gynecare Thermachoice system (U.S. product codes TC003 and TC013). It does not involve other components of the Gynecare Thermachoice III Uterine Balloon Therapy System (controller, umbilical cable, etc.),” according to a Dec. 7 email from a company sales representative that was obtained by Ob.Gyn. News.
The thermal balloon ablation device is intended to ablate the endometrial lining of the uterus in premenopausal women who have completed childbearing and who experience menorrhagia from benign causes.
Ethicon said in the email that it is removing the product from the market “because stability data does not substantiate the labeled two-year shelf life of the product subject to the recall. There is no significant safety issue with the device.”
The company is advising physicians who have used the Thermachoice catheter to “continue to follow their patients in the usual manner.”
Ethicon officials did not respond to a request for comment on the product recall.
Ethicon has initiated a recall of its Gynecare Thermachoice III thermal balloon ablation silicone catheter.
The “voluntary recall involves only the catheter component of the Gynecare Thermachoice system (U.S. product codes TC003 and TC013). It does not involve other components of the Gynecare Thermachoice III Uterine Balloon Therapy System (controller, umbilical cable, etc.),” according to a Dec. 7 email from a company sales representative that was obtained by Ob.Gyn. News.
The thermal balloon ablation device is intended to ablate the endometrial lining of the uterus in premenopausal women who have completed childbearing and who experience menorrhagia from benign causes.
Ethicon said in the email that it is removing the product from the market “because stability data does not substantiate the labeled two-year shelf life of the product subject to the recall. There is no significant safety issue with the device.”
The company is advising physicians who have used the Thermachoice catheter to “continue to follow their patients in the usual manner.”
Ethicon officials did not respond to a request for comment on the product recall.
Ethicon has initiated a recall of its Gynecare Thermachoice III thermal balloon ablation silicone catheter.
The “voluntary recall involves only the catheter component of the Gynecare Thermachoice system (U.S. product codes TC003 and TC013). It does not involve other components of the Gynecare Thermachoice III Uterine Balloon Therapy System (controller, umbilical cable, etc.),” according to a Dec. 7 email from a company sales representative that was obtained by Ob.Gyn. News.
The thermal balloon ablation device is intended to ablate the endometrial lining of the uterus in premenopausal women who have completed childbearing and who experience menorrhagia from benign causes.
Ethicon said in the email that it is removing the product from the market “because stability data does not substantiate the labeled two-year shelf life of the product subject to the recall. There is no significant safety issue with the device.”
The company is advising physicians who have used the Thermachoice catheter to “continue to follow their patients in the usual manner.”
Ethicon officials did not respond to a request for comment on the product recall.
ACA: How will the demise of the primary care pay bump affect you?
The primary care “pay bump” is about to end.
Passed as part of the Affordable Care Act, the Primary Care Incentive Payment Program has added a 10% bonus to the Medicare physician fee schedule for doctors who self-designated a primary care specialty and have at least 60% of their Medicare billings designated for primary care services.
Depending on who was doing the math, physicians have been receiving between $3,900 and $5,000 per year, on average, paid quarterly, with some receiving as much as $9,000 per year. We asked a number of physicians how the end of the pay bump will affect them.
How will it affect you? Take the poll or leave a comment below.
Dr. Wanda Filer, president, American Academy of Family Physicians
“The concern, obviously, is that some [physicians] took these extra several thousand dollars and deployed them to get new resources for their practices and they often run on very thin margins with Medicare patients, so the concern is are they going to be able to continue some of that enhanced service? I have seen one story where those monies helped to support hiring a person. Obviously, it didn’t make the whole salary, but helped to support a person to try to do some more care coordination and the concern will be whatever these monies have been used for over these last few years, are we going to lose ground there?”
Dr. Filer noted that the expiration of the pay bump might be only a “short-term pain” in the transition to value-based payment models. “In a very short period of time, we are going to be doing more and more value-based payments and clearly primary care has a big opportunity to get a little bit bigger piece of the health care pie in terms of dollars because there is such a huge value in a coordinated system ... It really makes a difference right now if you continue that transition to medical home if you haven’t already done it because it will automatically position you into a value-based system.”
Dr. Wayne J. Riley, president, American College of Physicians
The expiration will not be a “catastrophic loss, but it is very disappointing in light of the fact that we know we have uneven distribution of primary care physician manpower and there are fewer and fewer medical students electing to choose primary care, principally because of the big pay differentials between primary care and some of the specialties. We think it sends the wrong message. It has the downstream effect of devaluing primary care … at a time when we know that our health care system really needs doctors who enjoy and take on the responsibilities of providing primary care services.
“The beauty of the primary care bonus in the Affordable Care Act, both the Medicaid primary care bonus which expired last year and Medicare’s which expires this year, is that it explicitly values primary care with the strong signal that primary care is needed, is valuable, and should be better compensated in order to make sure that our patients and our communities have access to good primary care physicians.”
Dr. Rajeev Kumar, medical director at Adventist Midwest Geriatrics Specialists of LaGrange, Ill.
The impact of bonus payment is “meaningful. My specialty is geriatrics and I am the lead physician for a group of 13 physicians. We all practice geriatrics; our practice is maybe 99.8% Medicare based. We definitely do benefit from this incentive. Traditionally there has always been a pretty substantial disparity between payments to primary care physicians and to specialty physicians and also procedure-based physicians and this has kind of negatively affected the number of physicians choosing primary care.”
Dr. Kumar said the cut will likely affect his personal income. “I will probably take home less money. My specialty is such that I am not going to be able shut my doors and say I won’t take Medicare patients. My entire practice is based on Medicare patients. It is a loss that we have to absorb. I can’t pay my secretaries, my billers any less because they would leave and find somebody else to work for. It eventually affects my bottom line as a primary care doctor.”
Dr. Victor Roberts of Endocrine Associates of (Lake May) Florida
“The amount of money which the American College of Physicians predicted a general internist [who qualifies for the incentive payment] would receive is approximately $8,000 extra per year. It’s unlikely that this additional payment will make or break a practice, particularly if it is paid out quarterly. That would be $2,000 every 90 days. Based on current primary care reimbursements, and considering the complex work the primary care medical professionals do for our patients, the Medicare Bonus Payment Plan is, in my opinion, inadequate.”
Although Dr. Roberts’ practice focuses on endocrinology, diabetes, and metabolism, many of the ICD treatment codes it uses qualify it to participate in PCIP. “We have not seen a dramatic increase in revenue for ambulatory office-based work,” he said.
How the program affects physicians could also be a function of the size of the practice and whether the physician is employed by a system, he added.
Dr. Charles Crecelius, geriatrician at Barnes Jewish Medical Group of St. Louis
I have “just changed from a private practice small physician group to a larger hospital-based group practice, so my perspective is changing somewhat. In our smaller private practice, [the end of the incentive payments] was going to be a serious blow to our fiscal stability as we see predominantly older patients and depended on the income to support our ability to modernize our practice. Under value-based medicine, we might be able to make up some of the difference by performing well, but our ability to make up all the income is very doubtful. We were seriously contemplating curtailing new Medicare patients and increasing our younger population practice.
“Now that we have just joined a larger group, we will be insulated from any immediate impact of this additional income as our payment structure has no bearing on this additional source of revenue. The decreased total revenue to the larger group practice will be substantially diluted by the total much larger non-affected incomes – surgery, younger patients, ancillary income, etc. The payment structure to the individual physician never did take into account this additional payment – and in fact in the past this was a source of contention to some of the members of this larger group who saw a lot of Medicare patients, I am told. Curiously now it will not impact them.
“This loss of additional income will have a greater impact on the smaller groups and solo practices, and will be one more nail in the coffin of traditional small primary care practices. While not a major influence in our decision to join a larger practice – EHRs, meeting quality metrics, controlling costs were bigger more longstanding issues – it did play a role.”
The primary care “pay bump” is about to end.
Passed as part of the Affordable Care Act, the Primary Care Incentive Payment Program has added a 10% bonus to the Medicare physician fee schedule for doctors who self-designated a primary care specialty and have at least 60% of their Medicare billings designated for primary care services.
Depending on who was doing the math, physicians have been receiving between $3,900 and $5,000 per year, on average, paid quarterly, with some receiving as much as $9,000 per year. We asked a number of physicians how the end of the pay bump will affect them.
How will it affect you? Take the poll or leave a comment below.
Dr. Wanda Filer, president, American Academy of Family Physicians
“The concern, obviously, is that some [physicians] took these extra several thousand dollars and deployed them to get new resources for their practices and they often run on very thin margins with Medicare patients, so the concern is are they going to be able to continue some of that enhanced service? I have seen one story where those monies helped to support hiring a person. Obviously, it didn’t make the whole salary, but helped to support a person to try to do some more care coordination and the concern will be whatever these monies have been used for over these last few years, are we going to lose ground there?”
Dr. Filer noted that the expiration of the pay bump might be only a “short-term pain” in the transition to value-based payment models. “In a very short period of time, we are going to be doing more and more value-based payments and clearly primary care has a big opportunity to get a little bit bigger piece of the health care pie in terms of dollars because there is such a huge value in a coordinated system ... It really makes a difference right now if you continue that transition to medical home if you haven’t already done it because it will automatically position you into a value-based system.”
Dr. Wayne J. Riley, president, American College of Physicians
The expiration will not be a “catastrophic loss, but it is very disappointing in light of the fact that we know we have uneven distribution of primary care physician manpower and there are fewer and fewer medical students electing to choose primary care, principally because of the big pay differentials between primary care and some of the specialties. We think it sends the wrong message. It has the downstream effect of devaluing primary care … at a time when we know that our health care system really needs doctors who enjoy and take on the responsibilities of providing primary care services.
“The beauty of the primary care bonus in the Affordable Care Act, both the Medicaid primary care bonus which expired last year and Medicare’s which expires this year, is that it explicitly values primary care with the strong signal that primary care is needed, is valuable, and should be better compensated in order to make sure that our patients and our communities have access to good primary care physicians.”
Dr. Rajeev Kumar, medical director at Adventist Midwest Geriatrics Specialists of LaGrange, Ill.
The impact of bonus payment is “meaningful. My specialty is geriatrics and I am the lead physician for a group of 13 physicians. We all practice geriatrics; our practice is maybe 99.8% Medicare based. We definitely do benefit from this incentive. Traditionally there has always been a pretty substantial disparity between payments to primary care physicians and to specialty physicians and also procedure-based physicians and this has kind of negatively affected the number of physicians choosing primary care.”
Dr. Kumar said the cut will likely affect his personal income. “I will probably take home less money. My specialty is such that I am not going to be able shut my doors and say I won’t take Medicare patients. My entire practice is based on Medicare patients. It is a loss that we have to absorb. I can’t pay my secretaries, my billers any less because they would leave and find somebody else to work for. It eventually affects my bottom line as a primary care doctor.”
Dr. Victor Roberts of Endocrine Associates of (Lake May) Florida
“The amount of money which the American College of Physicians predicted a general internist [who qualifies for the incentive payment] would receive is approximately $8,000 extra per year. It’s unlikely that this additional payment will make or break a practice, particularly if it is paid out quarterly. That would be $2,000 every 90 days. Based on current primary care reimbursements, and considering the complex work the primary care medical professionals do for our patients, the Medicare Bonus Payment Plan is, in my opinion, inadequate.”
Although Dr. Roberts’ practice focuses on endocrinology, diabetes, and metabolism, many of the ICD treatment codes it uses qualify it to participate in PCIP. “We have not seen a dramatic increase in revenue for ambulatory office-based work,” he said.
How the program affects physicians could also be a function of the size of the practice and whether the physician is employed by a system, he added.
Dr. Charles Crecelius, geriatrician at Barnes Jewish Medical Group of St. Louis
I have “just changed from a private practice small physician group to a larger hospital-based group practice, so my perspective is changing somewhat. In our smaller private practice, [the end of the incentive payments] was going to be a serious blow to our fiscal stability as we see predominantly older patients and depended on the income to support our ability to modernize our practice. Under value-based medicine, we might be able to make up some of the difference by performing well, but our ability to make up all the income is very doubtful. We were seriously contemplating curtailing new Medicare patients and increasing our younger population practice.
“Now that we have just joined a larger group, we will be insulated from any immediate impact of this additional income as our payment structure has no bearing on this additional source of revenue. The decreased total revenue to the larger group practice will be substantially diluted by the total much larger non-affected incomes – surgery, younger patients, ancillary income, etc. The payment structure to the individual physician never did take into account this additional payment – and in fact in the past this was a source of contention to some of the members of this larger group who saw a lot of Medicare patients, I am told. Curiously now it will not impact them.
“This loss of additional income will have a greater impact on the smaller groups and solo practices, and will be one more nail in the coffin of traditional small primary care practices. While not a major influence in our decision to join a larger practice – EHRs, meeting quality metrics, controlling costs were bigger more longstanding issues – it did play a role.”
The primary care “pay bump” is about to end.
Passed as part of the Affordable Care Act, the Primary Care Incentive Payment Program has added a 10% bonus to the Medicare physician fee schedule for doctors who self-designated a primary care specialty and have at least 60% of their Medicare billings designated for primary care services.
Depending on who was doing the math, physicians have been receiving between $3,900 and $5,000 per year, on average, paid quarterly, with some receiving as much as $9,000 per year. We asked a number of physicians how the end of the pay bump will affect them.
How will it affect you? Take the poll or leave a comment below.
Dr. Wanda Filer, president, American Academy of Family Physicians
“The concern, obviously, is that some [physicians] took these extra several thousand dollars and deployed them to get new resources for their practices and they often run on very thin margins with Medicare patients, so the concern is are they going to be able to continue some of that enhanced service? I have seen one story where those monies helped to support hiring a person. Obviously, it didn’t make the whole salary, but helped to support a person to try to do some more care coordination and the concern will be whatever these monies have been used for over these last few years, are we going to lose ground there?”
Dr. Filer noted that the expiration of the pay bump might be only a “short-term pain” in the transition to value-based payment models. “In a very short period of time, we are going to be doing more and more value-based payments and clearly primary care has a big opportunity to get a little bit bigger piece of the health care pie in terms of dollars because there is such a huge value in a coordinated system ... It really makes a difference right now if you continue that transition to medical home if you haven’t already done it because it will automatically position you into a value-based system.”
Dr. Wayne J. Riley, president, American College of Physicians
The expiration will not be a “catastrophic loss, but it is very disappointing in light of the fact that we know we have uneven distribution of primary care physician manpower and there are fewer and fewer medical students electing to choose primary care, principally because of the big pay differentials between primary care and some of the specialties. We think it sends the wrong message. It has the downstream effect of devaluing primary care … at a time when we know that our health care system really needs doctors who enjoy and take on the responsibilities of providing primary care services.
“The beauty of the primary care bonus in the Affordable Care Act, both the Medicaid primary care bonus which expired last year and Medicare’s which expires this year, is that it explicitly values primary care with the strong signal that primary care is needed, is valuable, and should be better compensated in order to make sure that our patients and our communities have access to good primary care physicians.”
Dr. Rajeev Kumar, medical director at Adventist Midwest Geriatrics Specialists of LaGrange, Ill.
The impact of bonus payment is “meaningful. My specialty is geriatrics and I am the lead physician for a group of 13 physicians. We all practice geriatrics; our practice is maybe 99.8% Medicare based. We definitely do benefit from this incentive. Traditionally there has always been a pretty substantial disparity between payments to primary care physicians and to specialty physicians and also procedure-based physicians and this has kind of negatively affected the number of physicians choosing primary care.”
Dr. Kumar said the cut will likely affect his personal income. “I will probably take home less money. My specialty is such that I am not going to be able shut my doors and say I won’t take Medicare patients. My entire practice is based on Medicare patients. It is a loss that we have to absorb. I can’t pay my secretaries, my billers any less because they would leave and find somebody else to work for. It eventually affects my bottom line as a primary care doctor.”
Dr. Victor Roberts of Endocrine Associates of (Lake May) Florida
“The amount of money which the American College of Physicians predicted a general internist [who qualifies for the incentive payment] would receive is approximately $8,000 extra per year. It’s unlikely that this additional payment will make or break a practice, particularly if it is paid out quarterly. That would be $2,000 every 90 days. Based on current primary care reimbursements, and considering the complex work the primary care medical professionals do for our patients, the Medicare Bonus Payment Plan is, in my opinion, inadequate.”
Although Dr. Roberts’ practice focuses on endocrinology, diabetes, and metabolism, many of the ICD treatment codes it uses qualify it to participate in PCIP. “We have not seen a dramatic increase in revenue for ambulatory office-based work,” he said.
How the program affects physicians could also be a function of the size of the practice and whether the physician is employed by a system, he added.
Dr. Charles Crecelius, geriatrician at Barnes Jewish Medical Group of St. Louis
I have “just changed from a private practice small physician group to a larger hospital-based group practice, so my perspective is changing somewhat. In our smaller private practice, [the end of the incentive payments] was going to be a serious blow to our fiscal stability as we see predominantly older patients and depended on the income to support our ability to modernize our practice. Under value-based medicine, we might be able to make up some of the difference by performing well, but our ability to make up all the income is very doubtful. We were seriously contemplating curtailing new Medicare patients and increasing our younger population practice.
“Now that we have just joined a larger group, we will be insulated from any immediate impact of this additional income as our payment structure has no bearing on this additional source of revenue. The decreased total revenue to the larger group practice will be substantially diluted by the total much larger non-affected incomes – surgery, younger patients, ancillary income, etc. The payment structure to the individual physician never did take into account this additional payment – and in fact in the past this was a source of contention to some of the members of this larger group who saw a lot of Medicare patients, I am told. Curiously now it will not impact them.
“This loss of additional income will have a greater impact on the smaller groups and solo practices, and will be one more nail in the coffin of traditional small primary care practices. While not a major influence in our decision to join a larger practice – EHRs, meeting quality metrics, controlling costs were bigger more longstanding issues – it did play a role.”
Gap between public, private hospital payment rates widening
Payments from private insurers were about 75% higher than those from Medicare for a standardized hospital inpatient stay in 2012, according to a study from the Agency for Healthcare Research and Quality.
In analyzing data from the Medicare Expenditure Panel Survey from 1996-2012, researchers found that “payment rates for privately insured patients exceeded those for Medicare and Medicaid beneficiaries throughout the study period, but the difference widened rapidly in the later half of the period,” Thomas Selden, Ph. D., director of division of research and modeling at the AHRQ Center for Financing, Access, and Cost Trends, and his colleagues, said in a report in December issue of Health Affairs (2015 Dec 7. doi: 10.1377/hlthaff.2015.0706).
Conversely, for the period of 1996-2001, private payors paid approximately 10% more than did public payors for a standardized inpatient stay, Dr. Selden and his colleagues noted.
While the authors found no clear cause for the divergent public and private payment rates, they suggested that it makes sense to determine how much of it is a result of cost-shifting from public to private payers, “given the growing body of evidence that challenges the existence of such cost shifting.”
They also questioned whether hospitals are “exploiting market concentration or engaging in a technological ‘arm’s race,’ in which they invest heavily in expensive diagnostic and surgical equipment,” as well as the effect of declining private inpatient hospital stays from 13.6 million in 2008 to 11.2 million in 2012.
“Understanding how these provisions will interact with preexisting trends in private and public payment is yet another question that takes on greater urgency in light of the payment rate differentials we observed,” the authors said.
The authors reported no conflicts of interest.
Payments from private insurers were about 75% higher than those from Medicare for a standardized hospital inpatient stay in 2012, according to a study from the Agency for Healthcare Research and Quality.
In analyzing data from the Medicare Expenditure Panel Survey from 1996-2012, researchers found that “payment rates for privately insured patients exceeded those for Medicare and Medicaid beneficiaries throughout the study period, but the difference widened rapidly in the later half of the period,” Thomas Selden, Ph. D., director of division of research and modeling at the AHRQ Center for Financing, Access, and Cost Trends, and his colleagues, said in a report in December issue of Health Affairs (2015 Dec 7. doi: 10.1377/hlthaff.2015.0706).
Conversely, for the period of 1996-2001, private payors paid approximately 10% more than did public payors for a standardized inpatient stay, Dr. Selden and his colleagues noted.
While the authors found no clear cause for the divergent public and private payment rates, they suggested that it makes sense to determine how much of it is a result of cost-shifting from public to private payers, “given the growing body of evidence that challenges the existence of such cost shifting.”
They also questioned whether hospitals are “exploiting market concentration or engaging in a technological ‘arm’s race,’ in which they invest heavily in expensive diagnostic and surgical equipment,” as well as the effect of declining private inpatient hospital stays from 13.6 million in 2008 to 11.2 million in 2012.
“Understanding how these provisions will interact with preexisting trends in private and public payment is yet another question that takes on greater urgency in light of the payment rate differentials we observed,” the authors said.
The authors reported no conflicts of interest.
Payments from private insurers were about 75% higher than those from Medicare for a standardized hospital inpatient stay in 2012, according to a study from the Agency for Healthcare Research and Quality.
In analyzing data from the Medicare Expenditure Panel Survey from 1996-2012, researchers found that “payment rates for privately insured patients exceeded those for Medicare and Medicaid beneficiaries throughout the study period, but the difference widened rapidly in the later half of the period,” Thomas Selden, Ph. D., director of division of research and modeling at the AHRQ Center for Financing, Access, and Cost Trends, and his colleagues, said in a report in December issue of Health Affairs (2015 Dec 7. doi: 10.1377/hlthaff.2015.0706).
Conversely, for the period of 1996-2001, private payors paid approximately 10% more than did public payors for a standardized inpatient stay, Dr. Selden and his colleagues noted.
While the authors found no clear cause for the divergent public and private payment rates, they suggested that it makes sense to determine how much of it is a result of cost-shifting from public to private payers, “given the growing body of evidence that challenges the existence of such cost shifting.”
They also questioned whether hospitals are “exploiting market concentration or engaging in a technological ‘arm’s race,’ in which they invest heavily in expensive diagnostic and surgical equipment,” as well as the effect of declining private inpatient hospital stays from 13.6 million in 2008 to 11.2 million in 2012.
“Understanding how these provisions will interact with preexisting trends in private and public payment is yet another question that takes on greater urgency in light of the payment rate differentials we observed,” the authors said.
The authors reported no conflicts of interest.
FROM HEALTH AFFAIRS
ACA: Larger nonprofits seek permission to opt out of contraceptive coverage
One in 10 nonprofit organizations with at least 1,000 workers sought an accommodation to not provide Affordable Care Act–required contraceptive coverage to their employees, but instead allow workers to access the copay-free coverage separately, according to new research from the Kaiser Family Foundation.
The findings “indicate that a minority of nonprofits have elected an accommodation to the contraceptive coverage requirement,” according to survey results published Dec. 1.
Certain religious-affiliated organizations object to providing health care coverage for contraceptive services, and the Supreme Court ruled in the Hobby Lobby v. Burwell case that certain closely held companies could be excepted from providing coverage if doing so violated their religious beliefs. In November 2015, the Supreme Court agreed to hear another case – this one brought by nonprofit, religiously affiliated groups seeking to be exempt from the mandate.
“For workers and their dependents, the distinction between an accommodation and an exemption is the difference between guaranteed no-cost contraceptive coverage and having to pay out of pocket for services that could potentially exceed hundreds of dollars a year,” Laurie Sobel, a KFF senior policy analyst, and her colleagues said in the survey report.
The report notes that the government does not collect uniform information on whether a nonprofit has a religious affiliation, but adds that religion does not need to be a primary focus of the organization to be eligible for the accommodation.
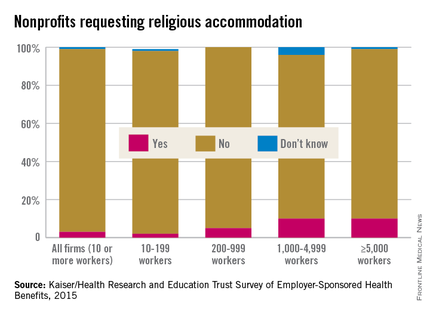
According to the survey results, 3% of all nonprofits with at least 10 workers sought some kind of accommodation. For the smallest of organizations (10-199 workers), the accommodation request rate was 2%, while 5% of organizations with 200-999 workers requested an accommodation.
Most nonprofits affiliated with the Catholic Church are likely among those seeking the accommodation due to the church’s objection to birth control, according to the report, which noted that in 2011, 10% of all nonprofit hospitals were Catholic hospitals and 10 of the 25 largest health systems in the United States were nonprofit Catholic-sponsored systems. Additionally, 260 of the approximately 1,700 private nonprofit colleges and universities have Catholic affiliations.
One in 10 nonprofit organizations with at least 1,000 workers sought an accommodation to not provide Affordable Care Act–required contraceptive coverage to their employees, but instead allow workers to access the copay-free coverage separately, according to new research from the Kaiser Family Foundation.
The findings “indicate that a minority of nonprofits have elected an accommodation to the contraceptive coverage requirement,” according to survey results published Dec. 1.
Certain religious-affiliated organizations object to providing health care coverage for contraceptive services, and the Supreme Court ruled in the Hobby Lobby v. Burwell case that certain closely held companies could be excepted from providing coverage if doing so violated their religious beliefs. In November 2015, the Supreme Court agreed to hear another case – this one brought by nonprofit, religiously affiliated groups seeking to be exempt from the mandate.
“For workers and their dependents, the distinction between an accommodation and an exemption is the difference between guaranteed no-cost contraceptive coverage and having to pay out of pocket for services that could potentially exceed hundreds of dollars a year,” Laurie Sobel, a KFF senior policy analyst, and her colleagues said in the survey report.
The report notes that the government does not collect uniform information on whether a nonprofit has a religious affiliation, but adds that religion does not need to be a primary focus of the organization to be eligible for the accommodation.

According to the survey results, 3% of all nonprofits with at least 10 workers sought some kind of accommodation. For the smallest of organizations (10-199 workers), the accommodation request rate was 2%, while 5% of organizations with 200-999 workers requested an accommodation.
Most nonprofits affiliated with the Catholic Church are likely among those seeking the accommodation due to the church’s objection to birth control, according to the report, which noted that in 2011, 10% of all nonprofit hospitals were Catholic hospitals and 10 of the 25 largest health systems in the United States were nonprofit Catholic-sponsored systems. Additionally, 260 of the approximately 1,700 private nonprofit colleges and universities have Catholic affiliations.
One in 10 nonprofit organizations with at least 1,000 workers sought an accommodation to not provide Affordable Care Act–required contraceptive coverage to their employees, but instead allow workers to access the copay-free coverage separately, according to new research from the Kaiser Family Foundation.
The findings “indicate that a minority of nonprofits have elected an accommodation to the contraceptive coverage requirement,” according to survey results published Dec. 1.
Certain religious-affiliated organizations object to providing health care coverage for contraceptive services, and the Supreme Court ruled in the Hobby Lobby v. Burwell case that certain closely held companies could be excepted from providing coverage if doing so violated their religious beliefs. In November 2015, the Supreme Court agreed to hear another case – this one brought by nonprofit, religiously affiliated groups seeking to be exempt from the mandate.
“For workers and their dependents, the distinction between an accommodation and an exemption is the difference between guaranteed no-cost contraceptive coverage and having to pay out of pocket for services that could potentially exceed hundreds of dollars a year,” Laurie Sobel, a KFF senior policy analyst, and her colleagues said in the survey report.
The report notes that the government does not collect uniform information on whether a nonprofit has a religious affiliation, but adds that religion does not need to be a primary focus of the organization to be eligible for the accommodation.

According to the survey results, 3% of all nonprofits with at least 10 workers sought some kind of accommodation. For the smallest of organizations (10-199 workers), the accommodation request rate was 2%, while 5% of organizations with 200-999 workers requested an accommodation.
Most nonprofits affiliated with the Catholic Church are likely among those seeking the accommodation due to the church’s objection to birth control, according to the report, which noted that in 2011, 10% of all nonprofit hospitals were Catholic hospitals and 10 of the 25 largest health systems in the United States were nonprofit Catholic-sponsored systems. Additionally, 260 of the approximately 1,700 private nonprofit colleges and universities have Catholic affiliations.
Sleep medicine specialists issue statement on drowsy driving
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.
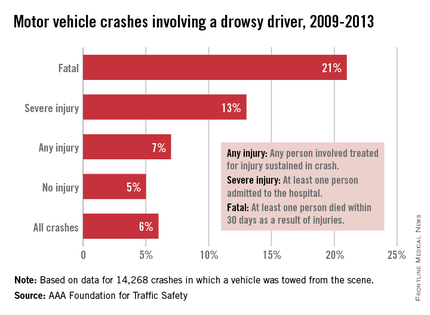
AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.

AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
In an effort to combat drowsy driving, the American Academy of Sleep Medicine is calling for better education on the symptoms.
The organization also is calling for more research to understand the thresholds for when sleepiness while driving becomes dangerous.
“Driving while drowsy can have the same consequences as driving while under the influence of drugs and alcohol: drowsiness is similar to alcohol in how it compromises driving ability by reducing alertness and attentiveness, delaying reaction times, and hindering decision-making skills,” the American Academy of Sleep Medicine (AASM) said in a policy statement published Nov. 11, 2015, in the Journal of Clinical Sleep Medicine (doi: 10.5664/jcsm.5200).
AASM is incorporating drowsy driving education online as part of its broader National Healthy Sleep Awareness Project.
The group identified a number of symptoms of drowsy driving, including frequent yawning or difficulty keeping eyes open, “nodding off” or difficulty keeping your head up, inability to remember driving the last few miles, missing road signs or turns, difficulty maintaining speed, and drifting out of your driving lane.

AASM is calling for collaboration among sleep physicians, state departments of motor vehicles and licensing, highway patrol, and the insurance industry to develop policies and procedures that reduce drowsy driving, educational material to be used in driver’s education and licensing examination, drowsy driving educational insurance discount programs, and manufacturing and infrastructure technologies that mitigate drowsy driving.
In addition, AASM “encourages more research that better defines indicators of drowsy driving, identifies the threshold at which sleepiness while driving becomes dangerous, and provides the public with simple methods to determine when they might be too tired to drive safely.”
It also warned that consumption of caffeine can temporarily increase alertness but is not a substitute for healthy sleep, and things like turning on the radio, opening the window, or turning on the air conditioner “are not effective techniques for staying awake while driving.”
FROM JOURNAL OF CLINICAL SLEEP MEDICINE
Senate panel targets drug prices during FDA commissioner nomination hearing
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
WASHINGTON – Drug pricing was front and center as a Senate panel convened to review the nomination of Dr. Robert M. Califf as commissioner of the Food and Drug Administration.
During a Nov. 17 hearing of the Senate Health, Education, Labor, and Pensions (HELP) Committee, many members keyed in on the role of the FDA in trying to bring down drug prices.
When asked about the price of prescription drugs, Dr. Califf was clear in stating that the agency’s role was not to set prices, but suggested that the FDA could have an impact on pricing by making the approval process for both brand and generic products more efficient, including revamping the clinical trials needed for approval to make them cheaper and more inclusive, while improving the quality of the data produced.
“We think that we can do trials that are actually bigger and include more patients that are more representative for a much lower cost,” Dr. Califf said, responding to Sen. Orrin Hatch’s (R-Utah) concerns about length of time of data exclusivity and fears that calls for shortening it might lead to higher drug prices as companies try to recoup development costs.
He also pointed to the use of electronic health records as a way to broaden clinical trials at a lower cost.
“The key here is using electronic health records that we already have,” Dr. Califf said, adding that once interoperability issues are addressed, EHRs will enable manufacturers to develop therapies “at a much lower cost with better information about safety and efficacy.”
In looking at rising generic prices, Committee Chairman Lamar Alexander (R-Tenn) focused on generic approval times, noting that generic manufacturers estimated that it took on average 48 months for a generic to get approval in 2014, up from 30 months in 2011.
A more rapid approval, “if safe and effective, might have some effect on lowering drug prices,” Sen. Alexander said.
Dr. Califf responded that FDA is already ahead of the generic user fee goals that have been set and the agency has been clearing the more simple applications from its backlog.
“But we have this backlog of applications that are requiring a back-and-forth because we want generic drugs to be just as safe and effective as the innovative drugs and when the applications [are not complete and there are questions] those get held up,” he responded, adding that he was “confident” that once the backlog is cleared over the next several years, generic applications will be processed quickly.
However, his answers were not convincing to at least one member. Presidential hopeful Sen. Bernie Sanders (I-Vt.) was not convinced Dr. Califf will do enough in this area.
“At a time when millions of Americans cannot afford to purchase the prescription drugs they need, we need a new leader at the FDA who is prepared to stand up to the pharmaceutical companies and work to substantially lower drug prices. Unfortunately, I have come to the conclusion that Dr. Califf is not that person,” Sanders said in an Oct. 9 statement.
Sen. Sanders reaffirmed his objection, saying that nothing he heard during the hearing has changed his opinions.
And while he openly objected to the nomination, both the chairman and ranking member of the committee both openly endorsed the White House nominee.
In his prepared remarks, Mr. Alexander noted that his staff “has spent 2 months carefully reviewing everything you submitted and has not found anything that would call into doubt your ability to lead the FDA fairly and impartially.”
Likewise, Ranking Member Patty Murray (D-Wash.) said during the hearing that after “careful consideration and review, I am confident that Dr. Califf would contribute leadership and expertise as we work to find new ways to advance medical innovation for patients and families, and improve the health and well-being across this country. He is a strong nominee for the role of FDA commissioner.”
In his opening statement, Dr. Califf outlined his priorities if confirmed as commissioner, including strengthening and better supporting FDA’s workforce, finishing work in an number of areas – including improving the nation’s food supply, tobacco regulation, better combating antibiotic resistant bacteria, medical countermeasure development, and the White House’s Precision Medicine Initiative – and “further develop the science base that informs FDA’s decision making.”
During his testimony, he also said there is “a need to work on postmarket surveillance of devices,” suggesting that a similar program, the FDA’s Sentinel Initiative drug surveillance program, be developed. Dr. Califf also said a new pathway is needed for approving drug/device combination products.
In the area of medical apps, Dr. Califf said there needs to be more clarity as to where the line for regulation should be. As an example, he said that an app that monitors heart rate does not need much oversight, but if that app were connected to an implantable defibrillator, that would require more regulatory oversight.
Sen. Sanders cited Dr. Califf’s ties to the pharmaceutical industry as the prime reason for opposing the nomination.
Dr. Califf noted that for any of the research he had conducted, even when primarily funded from industry, he was clear that manufacturers, while being able to make suggestions for analysis and publication of results, did not have final say, and he said that numerous studies were rejected because companies would not agree to full access to databases or independence with the final publication of results.
One area that was not raised during the hearing, but was raised by the Project on Government Oversight regarding the ROCKET AF trial of rivaroxaban (Xarelto), for which he was co-primary investigator (N Engl J Med. 2011 Sep 8;365:883-91).
“Dr. Califf’s handling of the Xarelto trial raises concerns about his judgment in overseeing the pharmaceutical industry.” POGO Executive Director Danielle Brian said in a statement. “As senators consider Dr. Califf’s confirmation to run the FDA, they should ask tough questions about the Xarelto clinical trial.”
FDA primary reviewers recommended against approval based on a number of issues with the trial, but FDA ultimately decided in favor of bringing the drug to market.
A cardiologist by training, Dr. Califf has received support from the American College of Cardiology.
In an Oct. 21, 2015, letter to the Senate HELP Committee, ACC called Dr. Califf “the right person to lead the FDA as commissioner based on his impressive medical knowledge, clinical research experience, and visionary leadership abilities.”
Dr. Califf joined the FDA in February as deputy commissioner of medical products and currently provides executive leadership to FDA’s Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, the Center for Devices and Radiological Health, and the Center for Tobacco Products.
He also oversees FDA’s Office of Special Medical Programs and plays a critical role in providing high-level advice and policy direction on the agency’s medical product and tobacco priorities.
Prior to coming to FDA, he was vice chancellor of clinical and translational research and professor of medicine in the division of cardiology at Duke University in Durham, N.C. He has held multiple positions at the university, including director of the Duke Translational Medicine Institute and founding director of the Duke Clinical Research Institute.
He also served as a member of FDA’s Cardiovascular and Renal Drugs Advisory Committee and the agency’s Science Board Subcommittee on Science and Technology.
“We believe that with Dr. Califf’s diverse background, and his exemplary knowledge of clinical and translational medicine, he will continue to improve the FDA’s drug approval process while ensuring that patients are receiving the safest and most effective treatments as quickly as possible. We urge his immediate confirmation,” a coalitions of 50 medical and advocacy groups, including the American Academy of Pediatrics, American Society of Clinical Oncology, American Association of Cancer Research, National Organization for Rare Disorders, and the Personalized Medicine Coalition, said in an Oct. 29, 2015, letter to the Senate HELP Committee.
AT THE SENATE HELP COMMITTEE HEARING


















