User login
Docs to receive slightly better Medicare pay bump than originally proposed
Physicians will see a 0.41% increase to their payments under the Medicare physician fee schedule in 2018, a slight increase from the proposed 0.31% uptick but still short of the 0.5% increase promised under the Medicare Access and CHIP Reauthorization Act (MACRA).
Officials at the Centers for Medicare & Medicaid Services were unable to find adequate funding in so-called misvalued codes to back the larger increase, as required by law, according to the final version of the 2018 physician fee schedule, released Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“We finalized these changes based on stakeholder feedback and to better align with the MIPS data submission requirements for the quality performance category,” CMS said in a fact sheet detailing the provisions of the final rule.
CMS also is delaying the start of the appropriate use criteria (AUC) for imaging services, a program that would deny payments for imaging services unless the ordering physician consulted appropriate use criteria. The program will begin with an educational and operational testing year in 2020. Physicians will be required to start using AUCs and reporting this information on claims, but CMS will pay claims regardless of whether they correctly contain the required AUC data.
“This allows both clinicians and the agency to prepare for this new program,” the agency said in the fact sheet, CMS had proposed 2019 be the educational and operational testing year.
In response to comments submitted to the agency, CMS is changing its policy on billing codes for biosimilars administered under Medicare Part B.
“Effective January 1, 2018, newly approved biosimilar products with a common reference product will no longer be grouped in the same billing code,” the agency said in the fact sheet. “By encouraging innovation and greater manufacturer participation in the marketplace, we believe that this policy change will result in the licensing of more biosimilar products, thus creating a stable and robust market, driving market competition, and decreasing uncertainty about access and payment.”
The final rule implements proposed expansion of the Medicare Diabetes Prevention Program from a demonstration project to a nationwide program in 2018, however the implementation will be delayed for three months until April 1, 2018, rather than at the beginning of the year. The program provides payments to physicians based on performance goals being met by patients, including meeting certain numbers of service and maintenance sessions with the program and achieving specific weight loss goals.
CMS also finalized a number of new telemedicine payment codes.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Physicians will see a 0.41% increase to their payments under the Medicare physician fee schedule in 2018, a slight increase from the proposed 0.31% uptick but still short of the 0.5% increase promised under the Medicare Access and CHIP Reauthorization Act (MACRA).
Officials at the Centers for Medicare & Medicaid Services were unable to find adequate funding in so-called misvalued codes to back the larger increase, as required by law, according to the final version of the 2018 physician fee schedule, released Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“We finalized these changes based on stakeholder feedback and to better align with the MIPS data submission requirements for the quality performance category,” CMS said in a fact sheet detailing the provisions of the final rule.
CMS also is delaying the start of the appropriate use criteria (AUC) for imaging services, a program that would deny payments for imaging services unless the ordering physician consulted appropriate use criteria. The program will begin with an educational and operational testing year in 2020. Physicians will be required to start using AUCs and reporting this information on claims, but CMS will pay claims regardless of whether they correctly contain the required AUC data.
“This allows both clinicians and the agency to prepare for this new program,” the agency said in the fact sheet, CMS had proposed 2019 be the educational and operational testing year.
In response to comments submitted to the agency, CMS is changing its policy on billing codes for biosimilars administered under Medicare Part B.
“Effective January 1, 2018, newly approved biosimilar products with a common reference product will no longer be grouped in the same billing code,” the agency said in the fact sheet. “By encouraging innovation and greater manufacturer participation in the marketplace, we believe that this policy change will result in the licensing of more biosimilar products, thus creating a stable and robust market, driving market competition, and decreasing uncertainty about access and payment.”
The final rule implements proposed expansion of the Medicare Diabetes Prevention Program from a demonstration project to a nationwide program in 2018, however the implementation will be delayed for three months until April 1, 2018, rather than at the beginning of the year. The program provides payments to physicians based on performance goals being met by patients, including meeting certain numbers of service and maintenance sessions with the program and achieving specific weight loss goals.
CMS also finalized a number of new telemedicine payment codes.
Physicians will see a 0.41% increase to their payments under the Medicare physician fee schedule in 2018, a slight increase from the proposed 0.31% uptick but still short of the 0.5% increase promised under the Medicare Access and CHIP Reauthorization Act (MACRA).
Officials at the Centers for Medicare & Medicaid Services were unable to find adequate funding in so-called misvalued codes to back the larger increase, as required by law, according to the final version of the 2018 physician fee schedule, released Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“We finalized these changes based on stakeholder feedback and to better align with the MIPS data submission requirements for the quality performance category,” CMS said in a fact sheet detailing the provisions of the final rule.
CMS also is delaying the start of the appropriate use criteria (AUC) for imaging services, a program that would deny payments for imaging services unless the ordering physician consulted appropriate use criteria. The program will begin with an educational and operational testing year in 2020. Physicians will be required to start using AUCs and reporting this information on claims, but CMS will pay claims regardless of whether they correctly contain the required AUC data.
“This allows both clinicians and the agency to prepare for this new program,” the agency said in the fact sheet, CMS had proposed 2019 be the educational and operational testing year.
In response to comments submitted to the agency, CMS is changing its policy on billing codes for biosimilars administered under Medicare Part B.
“Effective January 1, 2018, newly approved biosimilar products with a common reference product will no longer be grouped in the same billing code,” the agency said in the fact sheet. “By encouraging innovation and greater manufacturer participation in the marketplace, we believe that this policy change will result in the licensing of more biosimilar products, thus creating a stable and robust market, driving market competition, and decreasing uncertainty about access and payment.”
The final rule implements proposed expansion of the Medicare Diabetes Prevention Program from a demonstration project to a nationwide program in 2018, however the implementation will be delayed for three months until April 1, 2018, rather than at the beginning of the year. The program provides payments to physicians based on performance goals being met by patients, including meeting certain numbers of service and maintenance sessions with the program and achieving specific weight loss goals.
CMS also finalized a number of new telemedicine payment codes.
More physicians excluded from MIPS under final CMS rule
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
More doctors will be exempt from participation in the Merit-Based Incentive Payment System in 2018, under a final rule issued by the Health & Human Service department.
The final rule excludes from MIPS participation any health care providers who are part of an advanced alternative payment model (APM), those who have $90,000 or less in Medicare Part B billings or who see 200 or fewer Medicare patients. For the 2017 reporting year, those levels were $30,000 and 100 patients.
In comments when the rule was a draft, many organizations suggested that CMS allow clinicians who are ready to participate in MIPS to opt in even if they fall into the MIPS low-volume threshold category. While the agency did not codify this suggestion, officials noted that they intend “revisit this policy in future rule making and are seeking comment on methods to implement this policy in a low-burden manner.”
Medical societies were generally in favor of the new higher threshold, but it was met with resistance from associations representing group practices.
“The transition to value is challenging and CMS understandably want to ease providers into value,” Jerry Penso, MD, president and CEO of the American Medical Group Association said in a statement. “But excluding providers isn’t the same as learning how to deliver care in a value-based world. Taking accountability for the quality and cost of care requires years of experience. Despite CMS’ intentions to ensure a smooth transition, AMGA is concerned that this rule actually hinders the prospects for value-based care.”
CMS is providing a number of enhancements for small practices participating in MIPS.
Small practices (15 or fewer providers) will get five bonus points under MIPS and will continue to earn points for partial data reporting of quality measures. They also will be able to join virtual groups to help aggregate their reporting and improve abilities to access payment bonuses. (Find a link to download a virtual group toolkit on page 4 of the CMS fact sheet for the final rule.)
CMS also is slowly phasing into the cost performance category, which will account for 10% of a MIPS score and will include Medicare spending per beneficiary and total per capita cost measures. These measures are carried over from the Value Modifier program and will require no action from providers to calculate. CMS will measure the performance in this category.
Finally, the agency included a hardship exemption for those affected by major hurricanes in the Gulf Coast and Puerto Rico in 2017. Currently, those who lost access to their EHRs because of the hurricanes, other natural disasters, or public health emergencies, they can file a hardship exemption to have their Advancing Care Information (formerly the meaningful use program) score reweighted to reflect the issues. Applications must be filed by Dec. 31, 2017. The final rule extends the reweighting policy to the other three categories (quality, cost, and improvement activities) through the 2018 performance year, with a deadline of Dec. 31, 2018, to file for a hardship exemption.
“Because our policies relating to reweighting the quality, cost, and improvement activities performance categories are not effective until next year, we are issuing an interim final rule for automatic extreme and uncontrollable circumstances where clinicians can be exempt form these categories in the transition year without submitting a hardship exception application,” CMS noted in the fact sheet. For 2017, that means clinicians in areas affected by the hurricanes who do not submit data will not receive any negative adjustment, Clinicians who do submit data will be scored as usual.
On the advanced APM track, under which physicians take on more risk in exchange for a potential for greater bonus payments, CMS said it is making it easier for clinicians to participate, including extending certain revenue and expenditure provisions for an additional 2 years that are used to determine nominal risk, changing the medical home models to slower the increase of the minimal amount of financial risk taken on, and making it easier for clinicians to earn bonus payments for APMs that begin or end mid-year.
The final rule is scheduled for publication in the Federal Register on Nov. 16.
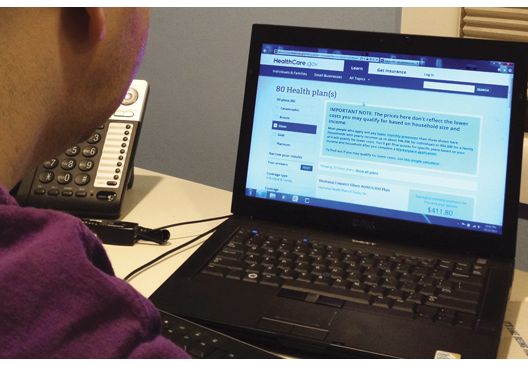
Government uncertainty drives jump in ACA silver plan insurance premiums
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements. However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Mike Nelson, MD, FCCP, comments: One need not “google” too long to find that the United States performs quite poorly in overall health care when compared with other nations, despite spending more than any of the comparators…we’re 37th this year. This information from Avalere Health portends a further drop in our ranking next year. The privilege of good health is a responsibility of the individual, but the right to affordable health care is a responsibility of the government. It is time for our legislators to stop playing partisan politics and start communicating to propose a workable and affordable solution.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements. However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements. However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Government uncertainty drives jump in ACA silver plan insurance premiums
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Silver plans on the Affordable Care Act insurance exchanges in 2018 will see an average premium increase of 34% nationwide, according to new research from Avalere Health.
“Plans are raising premiums in 2018 to account for market uncertainty and the federal government’s failure to pay for cost-sharing reductions,” Caroline Pearson, senior vice president at Avalere, said in a statement. “These premium increases may allow insurers to remain in the market and enrollees in all regions to have access to coverage.”
The expected premium changes are highly variable by state. Iowa has the highest change in its silver plans, with an average premium increase of 69% for its silver plans, while at the other end of the spectrum, Alaska is actually seeing a 22% decrease.
“These rates may change prior to open enrollment depending on how states respond to the elimination of CSR [cost-sharing reduction] funding for the 2018 plan year,” Avalere notes in its new analysis, adding that states may allow plans to refile for rate hikes now that CSR funding is likely dead. “In states where this occurs, it is expected that the newly updated rates will be substantially higher for the 2018 plan year.”
There was a glimmer of hope that the CSR payments would resume after a compromise was reached in the Senate Health, Education, Labor & Pensions Committee by Chairman Lamar Alexander (R-Tenn.) and Ranking Member Patty Murray (D-Wash.) that would offer 2 years of funding along with flexibility in the waiver program to allow states to tweak Affordable Care Act requirements, which is supported by AGA (http://www.gastro.org/news_items/aga-supports-alexander-murray-agreement-to-stabilize-individual-market). However, Speaker Paul Ryan (R-Wisc.) said the House would not be taking on any more health care action for the remainder of the year.
A spokeswoman from America’s Health Insurance Plans said in an interview that, although the CSR payments are no more, premium tax credits still exist to help lower-income individuals obtain insurance coverage.
Biosimilars poised to save $54 billion over the next decade
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
Biosimilars could reduce overall spending on biologic products by $54 billion from 2017 to 2026, according to new research from the Rand Corp.
Given the level of uncertainty surrounding the biosimilars market, however, the range of savings could be as low at $24 billion or as high as $150 billion.
“Because of limited U.S. experience with biosimilars, the key assumptions on market share and biosimilar prices are ‘best guesses’ based on anecdotes or professional opinion,” Andrew Mulcahy, PhD, a health policy researcher at Rand, and his colleagues, wrote in a perspective report.
“Whether actual cost savings end up above or below our baseline estimate hinges in large part on whether manufacturers continue to have a business case to invest in developing and marketing biosimilars,” the authors noted, citing a number of areas, including intellectual property litigation, payment, price competition, nonprice competition from reference biologic manufacturers, naming convention, and interchangeability.
Getting over these hurdles could require legislative or regulatory solutions.
“The pervasive uncertainty in the U.S. biosimilar market – including questions as to whether the market will be sustainable and lead to cost savings, as intended – presents two choices for policymakers,” Dr. Mulcahy and his colleagues wrote. “One strategy is to let the market continue to develop under current policies,” with stability coming from experience.
The alternative could be policy levers to “help steer the U.S. biosimilar market more quickly to a sustainable, competitive state,” they continued. “For example, regulators at the FDA could experiment with new approaches to provide stronger, earlier signals through guidance documents or other mechanisms on expectations surrounding interchangeability and other topics.”
The FDA appears to be moving on the latter. In an Oct. 23 blog post, FDA Commissioner Scott Gottlieb, MD, and Leah Christl, PhD, associate director for therapeutic biologics in the office of new drugs at the FDA’s Center for Drug Evaluation and Research, outlined a number of recent tools to help biosimilar adoption. The resources provide basics such as the basic definition associated with biosimilars (i.e., what is a biosimilar and a reference product, and what it means to be interchangeable), the standards of approval that biosimilars must go through, and easily accessible information on what the FDA is using to review biosimilarity.
“Next, FDA plans to embark on additional research with health care professionals to learn more about the types of information prescribers need to properly communicate with their patients about biosimilars,” Commissioner Gottlieb and Dr. Christl wrote. “An increase in market competition, offered by a growing complement of biosimilars, may lead to meaningfully reduced costs for both patients and our health care system.”
The Centers for Medicare & Medicaid Services also plays a role in developing policy to spur biosimilar adoption, Dr. Mulcahy and his colleagues wrote. They note work being done by the Medicare Payment Advisory Commission on recommendations that could address payment for physician-administered biosimilars under Part B, as well as incentives in the Part D prescription drug program to steer patients and providers toward lower cost biosimilars when appropriate. CMS changed current payment policy for biosimilars for 2018, which may have an effect.
“Beyond FDA regulation, payment, and coverage, both government and industry could play a role in educating patients and providers about the potential cost savings from biosimilars, much like both groups have done for generic drugs,” they stated. “While our study does not address whether policy action is needed now, it is likely that the answer will become clearer over the next 1 to 3 years as the market continues to develop.”
ACR: Use comparative effectiveness research for clinical decisions, not payers’ cost decisions
The goal of comparative effectiveness research should be to find the best products to meet a clinical situation instead of a tool to be used by payers to make cost-based coverage decisions, the American College of Rheumatology recently said.
The ACR’s Committee on Rheumatologic Care recently updated its position statement on CER, noting that high-quality comparative effectiveness research and cost-effectiveness analysis “can and should inform individual provider and patient decisions about the relative value of diagnostic and therapeutic options.”
One concern that Dr. Fahey noted, specific to the rheumatology space, is that there currently are few CER data to draw upon, which as a result, makes it harder to have meaningful conversations about clinical value.
“Quite frankly right now, a lot of times those decisions are already heavily influenced by the payer’s formulary structure and what is on their preferred tier and what is on their second, third, or fourth tier,” he said. “We just don’t have enough comparative effectiveness data yet to be a huge influence. The formulary and step edits for each individual payer are still based extremely heavily on their cost and rebates. This is why, circling back to the whole question to begin with ... when we updated the position statement, we sort of reinforced our concern that this should be made to guide clinical decisions and not payer-type coverage decisions.”
He noted that the ACR does have registries that are building real-world data about the various products, but there is still a ways to go to have an effective database that would be usable for quality CER information.
“We are hopeful that, over time, especially with things like registries, the ACR has a registry that compiles real-world data from our members about our patients, that we will be able to come up with a more real-world comparison between different medications,” Dr. Fahey said. “The concern is that the payers will use the results of comparative effectiveness research to make very black-and-white decisions about their formulary and about treatment options and things like that.”
The position statement says that the ACR is doing its part to help collect information. In 2014, it launched the Rheumatology Informatics System for Effectiveness (RISE), which “allows rheumatologists throughout the country to seamlessly and effortlessly transfer anonymous patient outcome data to a national registry.” According to the position statement, “thus far, RISE has collected more than one million patient encounters, positioning RISE to become the premier source for real-world CER data in rheumatology.”
“It would be fantastic if we already had the data about which medication provided the best value,” Dr. Fahey said. “Our drugs are extremely expensive, at least our biologic drugs, and we understand that the goal is to provide both good quality care but cost efficient care.”
The goal of comparative effectiveness research should be to find the best products to meet a clinical situation instead of a tool to be used by payers to make cost-based coverage decisions, the American College of Rheumatology recently said.
The ACR’s Committee on Rheumatologic Care recently updated its position statement on CER, noting that high-quality comparative effectiveness research and cost-effectiveness analysis “can and should inform individual provider and patient decisions about the relative value of diagnostic and therapeutic options.”
One concern that Dr. Fahey noted, specific to the rheumatology space, is that there currently are few CER data to draw upon, which as a result, makes it harder to have meaningful conversations about clinical value.
“Quite frankly right now, a lot of times those decisions are already heavily influenced by the payer’s formulary structure and what is on their preferred tier and what is on their second, third, or fourth tier,” he said. “We just don’t have enough comparative effectiveness data yet to be a huge influence. The formulary and step edits for each individual payer are still based extremely heavily on their cost and rebates. This is why, circling back to the whole question to begin with ... when we updated the position statement, we sort of reinforced our concern that this should be made to guide clinical decisions and not payer-type coverage decisions.”
He noted that the ACR does have registries that are building real-world data about the various products, but there is still a ways to go to have an effective database that would be usable for quality CER information.
“We are hopeful that, over time, especially with things like registries, the ACR has a registry that compiles real-world data from our members about our patients, that we will be able to come up with a more real-world comparison between different medications,” Dr. Fahey said. “The concern is that the payers will use the results of comparative effectiveness research to make very black-and-white decisions about their formulary and about treatment options and things like that.”
The position statement says that the ACR is doing its part to help collect information. In 2014, it launched the Rheumatology Informatics System for Effectiveness (RISE), which “allows rheumatologists throughout the country to seamlessly and effortlessly transfer anonymous patient outcome data to a national registry.” According to the position statement, “thus far, RISE has collected more than one million patient encounters, positioning RISE to become the premier source for real-world CER data in rheumatology.”
“It would be fantastic if we already had the data about which medication provided the best value,” Dr. Fahey said. “Our drugs are extremely expensive, at least our biologic drugs, and we understand that the goal is to provide both good quality care but cost efficient care.”
The goal of comparative effectiveness research should be to find the best products to meet a clinical situation instead of a tool to be used by payers to make cost-based coverage decisions, the American College of Rheumatology recently said.
The ACR’s Committee on Rheumatologic Care recently updated its position statement on CER, noting that high-quality comparative effectiveness research and cost-effectiveness analysis “can and should inform individual provider and patient decisions about the relative value of diagnostic and therapeutic options.”
One concern that Dr. Fahey noted, specific to the rheumatology space, is that there currently are few CER data to draw upon, which as a result, makes it harder to have meaningful conversations about clinical value.
“Quite frankly right now, a lot of times those decisions are already heavily influenced by the payer’s formulary structure and what is on their preferred tier and what is on their second, third, or fourth tier,” he said. “We just don’t have enough comparative effectiveness data yet to be a huge influence. The formulary and step edits for each individual payer are still based extremely heavily on their cost and rebates. This is why, circling back to the whole question to begin with ... when we updated the position statement, we sort of reinforced our concern that this should be made to guide clinical decisions and not payer-type coverage decisions.”
He noted that the ACR does have registries that are building real-world data about the various products, but there is still a ways to go to have an effective database that would be usable for quality CER information.
“We are hopeful that, over time, especially with things like registries, the ACR has a registry that compiles real-world data from our members about our patients, that we will be able to come up with a more real-world comparison between different medications,” Dr. Fahey said. “The concern is that the payers will use the results of comparative effectiveness research to make very black-and-white decisions about their formulary and about treatment options and things like that.”
The position statement says that the ACR is doing its part to help collect information. In 2014, it launched the Rheumatology Informatics System for Effectiveness (RISE), which “allows rheumatologists throughout the country to seamlessly and effortlessly transfer anonymous patient outcome data to a national registry.” According to the position statement, “thus far, RISE has collected more than one million patient encounters, positioning RISE to become the premier source for real-world CER data in rheumatology.”
“It would be fantastic if we already had the data about which medication provided the best value,” Dr. Fahey said. “Our drugs are extremely expensive, at least our biologic drugs, and we understand that the goal is to provide both good quality care but cost efficient care.”
Docs should engage employers directly if they want better payment models
WASHINGTON – If doctors want to improve their reimbursement and at the same time be a catalyst for reducing costs to the overall health care delivery system, they need to be stronger advocates for themselves.
This was the message of Harold D. Miller, president and CEO of the Center for Healthcare Quality and Payment Reform, in his presentation to the American Gastroenterological Association Partners in Value meeting on Oct. 6.
He called specifically on doctors to exercise initiative and accept the accountability that comes with leading the charge for payment reform.
“I think there is a much better way, which is bottom up, which is to say ask the physicians and the hospitals to say what are the ways you can improve care and reduce costs,” he continued. “Then get payers to provide adequate support for that but have physicians take accountability for actually achieving those results they think are possible. And then you have patients who get good care and you keep physicians and hospitals financially sustainable, which nobody in Washington is talking about how to actually do.”
Getting to that point is going to require physicians to be much more proactive in who they communicate with to get the information that is necessary to build payment models from the bottom up.
“I think there are lots of potential solutions, but I think it needs to be talked about,” Mr. Miller said. “If I were to leave you with one message, the problem is that employers, Congress, etc., are not hearing from physicians that you want to do something different.”
He noted that part of the issue is the adversarial relationship doctors have with the payer community, noting that most “health plans demonize you all.”
Mr. Miller added: “They go in to employers and they tell employers that the only thing standing between the employers and certain health insurance bankruptcy is the health plan because all the doctors want to do is spend more money.”
To change that, it is going to require doctors to be much more proactive in reaching out past the payer middleman to start engaging directly with employers.
“Employers do not see doctors as their partners,” Mr. Miller noted. “The people who pay have to start seeing you as wanting to solve the same problem they are trying to solve.”
And working with employers could help physicians insofar as getting access to data that would be crucial in developing the kinds of payment models that would benefit all of the financial aspects of health care delivery while at the same time improving care.
Mr. Miller recounted how various state and local governments in Maine were trying to extract clinical information that might not be ascertained from claims data from Anthem, the largest or second largest health insurance company in America. Initially, Anthem balked, prompting the state and local entities to issue requests for information and seek to replace Anthem as the main provider for its health insurance coverage.
“Anthem completely changed its attitude,” he said. “All of a sudden, Anthem was back in. ... Anthem felt that impact all the way in Indianapolis. Sam Nussbaum, MD [former chief medical officer at Anthem], said to me, ‘We were punished in Maine.’ ”
He noted that some big employers are seeking out direct contracts with health systems because they are not getting support from the health plans.
“But they need to hear from you and what it is you need and what you are going to do with it,” he said. “It is not just give us data, see ya. It is here’s what we are trying to do. Here is the information we need and why we need it. If you say to an employer ‘I want to know how many patients are being hospitalized so that I can help you reduce hospitalizations,’ do you think they are going to say nah, we are too busy for that?”
WASHINGTON – If doctors want to improve their reimbursement and at the same time be a catalyst for reducing costs to the overall health care delivery system, they need to be stronger advocates for themselves.
This was the message of Harold D. Miller, president and CEO of the Center for Healthcare Quality and Payment Reform, in his presentation to the American Gastroenterological Association Partners in Value meeting on Oct. 6.
He called specifically on doctors to exercise initiative and accept the accountability that comes with leading the charge for payment reform.
“I think there is a much better way, which is bottom up, which is to say ask the physicians and the hospitals to say what are the ways you can improve care and reduce costs,” he continued. “Then get payers to provide adequate support for that but have physicians take accountability for actually achieving those results they think are possible. And then you have patients who get good care and you keep physicians and hospitals financially sustainable, which nobody in Washington is talking about how to actually do.”
Getting to that point is going to require physicians to be much more proactive in who they communicate with to get the information that is necessary to build payment models from the bottom up.
“I think there are lots of potential solutions, but I think it needs to be talked about,” Mr. Miller said. “If I were to leave you with one message, the problem is that employers, Congress, etc., are not hearing from physicians that you want to do something different.”
He noted that part of the issue is the adversarial relationship doctors have with the payer community, noting that most “health plans demonize you all.”
Mr. Miller added: “They go in to employers and they tell employers that the only thing standing between the employers and certain health insurance bankruptcy is the health plan because all the doctors want to do is spend more money.”
To change that, it is going to require doctors to be much more proactive in reaching out past the payer middleman to start engaging directly with employers.
“Employers do not see doctors as their partners,” Mr. Miller noted. “The people who pay have to start seeing you as wanting to solve the same problem they are trying to solve.”
And working with employers could help physicians insofar as getting access to data that would be crucial in developing the kinds of payment models that would benefit all of the financial aspects of health care delivery while at the same time improving care.
Mr. Miller recounted how various state and local governments in Maine were trying to extract clinical information that might not be ascertained from claims data from Anthem, the largest or second largest health insurance company in America. Initially, Anthem balked, prompting the state and local entities to issue requests for information and seek to replace Anthem as the main provider for its health insurance coverage.
“Anthem completely changed its attitude,” he said. “All of a sudden, Anthem was back in. ... Anthem felt that impact all the way in Indianapolis. Sam Nussbaum, MD [former chief medical officer at Anthem], said to me, ‘We were punished in Maine.’ ”
He noted that some big employers are seeking out direct contracts with health systems because they are not getting support from the health plans.
“But they need to hear from you and what it is you need and what you are going to do with it,” he said. “It is not just give us data, see ya. It is here’s what we are trying to do. Here is the information we need and why we need it. If you say to an employer ‘I want to know how many patients are being hospitalized so that I can help you reduce hospitalizations,’ do you think they are going to say nah, we are too busy for that?”
WASHINGTON – If doctors want to improve their reimbursement and at the same time be a catalyst for reducing costs to the overall health care delivery system, they need to be stronger advocates for themselves.
This was the message of Harold D. Miller, president and CEO of the Center for Healthcare Quality and Payment Reform, in his presentation to the American Gastroenterological Association Partners in Value meeting on Oct. 6.
He called specifically on doctors to exercise initiative and accept the accountability that comes with leading the charge for payment reform.
“I think there is a much better way, which is bottom up, which is to say ask the physicians and the hospitals to say what are the ways you can improve care and reduce costs,” he continued. “Then get payers to provide adequate support for that but have physicians take accountability for actually achieving those results they think are possible. And then you have patients who get good care and you keep physicians and hospitals financially sustainable, which nobody in Washington is talking about how to actually do.”
Getting to that point is going to require physicians to be much more proactive in who they communicate with to get the information that is necessary to build payment models from the bottom up.
“I think there are lots of potential solutions, but I think it needs to be talked about,” Mr. Miller said. “If I were to leave you with one message, the problem is that employers, Congress, etc., are not hearing from physicians that you want to do something different.”
He noted that part of the issue is the adversarial relationship doctors have with the payer community, noting that most “health plans demonize you all.”
Mr. Miller added: “They go in to employers and they tell employers that the only thing standing between the employers and certain health insurance bankruptcy is the health plan because all the doctors want to do is spend more money.”
To change that, it is going to require doctors to be much more proactive in reaching out past the payer middleman to start engaging directly with employers.
“Employers do not see doctors as their partners,” Mr. Miller noted. “The people who pay have to start seeing you as wanting to solve the same problem they are trying to solve.”
And working with employers could help physicians insofar as getting access to data that would be crucial in developing the kinds of payment models that would benefit all of the financial aspects of health care delivery while at the same time improving care.
Mr. Miller recounted how various state and local governments in Maine were trying to extract clinical information that might not be ascertained from claims data from Anthem, the largest or second largest health insurance company in America. Initially, Anthem balked, prompting the state and local entities to issue requests for information and seek to replace Anthem as the main provider for its health insurance coverage.
“Anthem completely changed its attitude,” he said. “All of a sudden, Anthem was back in. ... Anthem felt that impact all the way in Indianapolis. Sam Nussbaum, MD [former chief medical officer at Anthem], said to me, ‘We were punished in Maine.’ ”
He noted that some big employers are seeking out direct contracts with health systems because they are not getting support from the health plans.
“But they need to hear from you and what it is you need and what you are going to do with it,” he said. “It is not just give us data, see ya. It is here’s what we are trying to do. Here is the information we need and why we need it. If you say to an employer ‘I want to know how many patients are being hospitalized so that I can help you reduce hospitalizations,’ do you think they are going to say nah, we are too busy for that?”
Societies unite to develop cardiology subspecialty MOC products
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Four medical societies are banding together to help cardiology subspecialists get through the maintenance of certification process.
The American College of Cardiology, Heart Failure Society of America, Heart Rhythm Society, and Society for Cardiovascular Angiography and Interventions are working together to develop new modules to help subspecialists meet the American Board of Internal Medicine’s current 10-year maintenance of certification examination.
The groups first must reach an agreement with ABIM; they can then collaborate to enhance the existing ACC self-assessment program (SAP) line with CathSAP, EPSAP, and Heart Failure SAP products to help fulfill the MOC needs of interventionalists, electrophysiologists, and heart failure specialists, respectively.
The societies hope to make the SAPs available in time for the ABIM rollout of the 2-year Knowledge Check-in assessment option. The current plan is for the rollout of a general cardiology product in 2019; electrophysiologists, heart failure, and interventionalists in 2020; and adult congenital in 2023.
“It is the shared goal of ACC, HFSA, HRS, and SCAI to help our collective members ensure their patients are receiving the highest-quality, evidence-based care,” ACC President Mary Norine Walsh, MD, said in a statement. “In offering additional pathways for cardiologists who wish to maintain their professional certification, we can more effectively and efficiently help busy clinicians keep up with current knowledge in their specific areas of practice.”
Trump halts ACA cost-sharing reduction subsidy payments
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.
“The Democrats ObamaCare [sic] is imploding. Massive subsidy payments to their pet insurance companies has stopped. Dems should call me to fix!” President Trump tweeted Oct. 13.
The action prompted criticism from medical organizations and health insurers alike.
In a statement, American Medical Association President David Barbe said he was “deeply discouraged” by the cuts. “This most recent action by the administration creates still more uncertainty in the ACA marketplace just as the abbreviated open enrollment period is about to begin, further undermining the law and threatening access to meaningful health insurance coverage for millions of Americans,” Dr. Barbe said.
“We need constructive solutions that increase consumer choice, lower consumer costs, and stabilize local markets,” America’s Health Insurance Plans and the Blue Cross Blue Shield Association said in a joint statement. “Terminating this critical program will do just the opposite. This action will make it harder for patients to access the care they need. Costs will go up and choice will be restricted.”
The U.S. Senate Committee on Health, Education, Labor & Pensions was working on a bipartisan, narrowly focused bill that would have, among other things, codified cost-sharing reduction payments in legislation for at least a year, after witnesses from across the political spectrum testified during four hearings that maintenance of the payments would bring stability to the individual market. That effort was derailed when Senate Majority Leader Mitch McConnell (R-Ky.) instead pushed a broad repeal-and-replace effort, which never reached the Senate floor for consideration when it was clear the action did not have enough votes to pass.
The president’s move follows an executive order that could further destabilize the individual market through broader use of association health plans and an expansion of short-term health insurance plans, neither of which would have to meet the coverage and benefits requirements of the Affordable Care Act.
SHM pushes to protect patients from ‘surprise’ out-of-network expenses
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.
Patients entering a hospital should not be on the hook for costs related to out-of-network insurance coverage when that hospital is in-network, according to the Society of Hospital Medicine and other major medical societies, especially if it is an emergency situation and the patient is unable to make an informed choice regarding who is administering care to them.
“We want to see it come to a resolution that does not put patients in jeopardy for paying these extra costs when they are going a hospital that is in-network, and they assume that the physicians are in-network,” Ron Greeno, MD, FCCP, MHM, president of the Society of Hospital Medicine, said in an interview.
Other groups signing onto the resolution include the American College of Emergency Physicians, the American Academy of Orthopedic Surgeons, the American College of Radiology, the American Society of Anesthesiologists, the College of American Pathologists, the American Association of Neurological Surgeons, and the Congress of Neurological Surgeons.
“States are tackling this on a state-by-state basis and creating laws that are meant to protect patients from being placed in legal jeopardy,” Dr. Greeno said. “But you still want to maintain the rights of the health plan and the physicians to negotiate in good faith. That is basically the stance we take.”
According to Dr. Greeno, the joint resolution passed at the AMA meeting was “designed to make recommendations to states who are considering such laws.” The medical societies want to provide guidance on what to include in those laws that will make the process fair. “If you have a law that says ‘out of network doctors cannot balance bill at a hospital that is in-network,’ then the health plans have no reason to negotiate in good faith,” he said. “They will just pay those doctors whatever they feel like paying them.”
Ultimately, though, the resolution was about medical societies affirming their desire to protect patients from burdensome, unexpected bills.
“We want to make sure whatever laws are passed that they actually protect the patients while maintaining the ability of physicians and health plans to negotiate in good faith to a mutual resolution,” Dr. Greeno said.