User login
Traditional scoring tool limps as VAP screen
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more microbiologically confirmed cases of ventilator-associated pneumonia than did a traditional screening tool, in a study of patients in a surgical intensive care unit.
The findings were reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP.
The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, according to Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The investigators analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures.
VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said.
The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," Dr. Pieracci said.
However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, according to Dr. Pieracci.
"Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
emechcatie@frontlinemedcom.com
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more microbiologically confirmed cases of ventilator-associated pneumonia than did a traditional screening tool, in a study of patients in a surgical intensive care unit.
The findings were reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP.
The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, according to Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The investigators analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures.
VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said.
The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," Dr. Pieracci said.
However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, according to Dr. Pieracci.
"Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
emechcatie@frontlinemedcom.com
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more microbiologically confirmed cases of ventilator-associated pneumonia than did a traditional screening tool, in a study of patients in a surgical intensive care unit.
The findings were reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP.
The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, according to Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The investigators analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures.
VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said.
The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," Dr. Pieracci said.
However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, according to Dr. Pieracci.
"Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
emechcatie@frontlinemedcom.com
Key clinical point: The Clinical Pulmonary Infection Score (CPIS) was less useful than other variables, such as a new chest x-ray infiltrate or fever, in screening surgical ICU patients for VAP and determining the need for empiric antibiotic therapy.
Major finding: The sensitivity of the CPIS tool for VAP in surgical ICU patients was only 21%, compared with 91.1% for a new chest x-ray infiltrate and 89.0% for fever.
Data source: The study evaluated the value of the CPIS and other clinical and gram stain variables in screening for VAP, in 497 adult patients in a surgical ICU.
Disclosures: Dr. Pieracci had no relevant disclosures. The study was funded through institutional monies.
Postrecession increases in suicides not inevitable
Increases in suicides after a recession, such as those seen in Europe, Canada, and the United States after the most recent recession, are not inevitable, according to the authors of a report on "economic suicides" in Europe and North America.
"The evidence that some societies have successfully de-coupled economic shocks from adverse mental health outcomes reveals the hope that it will be possible to eliminate the association of economic shocks with a rise in suicidality," said Aaron Reeves, Ph.D., of the University of Oxford (England) and his coauthors, from the London School of Hygiene and Tropical Medicine. Their report, which reviewed suicide data in Europe, the United States, and Canada after the onset of recession in 2007, appears online in the British Journal of Psychiatry (2014 June 11 [doi: 10.1192/bjp.bp.114.144766]).
Using 2007 as the prerecession baseline, they said that after suicide rates had been falling in Europe, the rates started to increase, rising by 6.5% by 2009 and remaining elevated in 2011. The reversal in a prerecession drop in suicide rates also was documented in Canada, with a 4.5% increase in suicides from 2007 to 2009. And in the United States, where suicides had been increasing before the recession, an increase of 4.8% was found between 2007 and 2010.
These increases add up to at least 10,000 more so-called economic suicides than expected in Europe, the United States, and Canada since 2007, the authors pointed out.
But they questioned whether these suicides "are an inevitable accompaniment of economic hardship" and provided several examples of countries where the most recent or previous recessions were not associated with subsequent increases in suicides. For example, in Sweden, suicides declined between 1991 and 1992, during which time a marked rise in unemployment occurred. Also, the authors noted, the prevalence of suicides did not increase in Sweden during the most recent recession.
Factors that can help address the adverse effects of unemployment on suicide include access to secondary prevention, and programs that provide support for unemployed people and help them find work, they said. Noting that the medical community can be advocates of evidence-based prevention measures, they wrote, "recessions will continue to hurt, but need not cause self-harm."
The authors had no disclosures.
We have known since [Emile] Durkheim that suicide rates rise during times of economic hardship, but we now have evidence that at least three countries – Sweden, Finland, and Austria – experienced the recent Great Recession without additional suicides. Early case finding and antidepressant prescribing to clinically depressed, unemployed people inevitably help to prevent some deaths by suicide, but this is no panacea.
Too many people fall through the cracks or don’t respond to medication or are not adequately monitored. The authors mention the protective effectiveness of active labor market programs assisting the newly unemployed to find work and argue for their economic soundness. Associated with this notion is the beneficial effect of support, which can’t be underestimated as a major force in maintaining resilience.
I agree with the authors of this important paper that "voices of psychiatrists must not remain silent." In addition to the daily ministering to our patients in our clinical settings, we must continue to fight for their rights to state-of-the-art mental health care and our unwavering belief in their self-worth, so shattered with the scourge of un- or underemployment.
Dr. Michael F. Myers is professor of clinical psychiatry at SUNY Downstate Medical Center, Brooklyn, N.Y. He had no disclosures or conflicts of interest.
We have known since [Emile] Durkheim that suicide rates rise during times of economic hardship, but we now have evidence that at least three countries – Sweden, Finland, and Austria – experienced the recent Great Recession without additional suicides. Early case finding and antidepressant prescribing to clinically depressed, unemployed people inevitably help to prevent some deaths by suicide, but this is no panacea.
Too many people fall through the cracks or don’t respond to medication or are not adequately monitored. The authors mention the protective effectiveness of active labor market programs assisting the newly unemployed to find work and argue for their economic soundness. Associated with this notion is the beneficial effect of support, which can’t be underestimated as a major force in maintaining resilience.
I agree with the authors of this important paper that "voices of psychiatrists must not remain silent." In addition to the daily ministering to our patients in our clinical settings, we must continue to fight for their rights to state-of-the-art mental health care and our unwavering belief in their self-worth, so shattered with the scourge of un- or underemployment.
Dr. Michael F. Myers is professor of clinical psychiatry at SUNY Downstate Medical Center, Brooklyn, N.Y. He had no disclosures or conflicts of interest.
We have known since [Emile] Durkheim that suicide rates rise during times of economic hardship, but we now have evidence that at least three countries – Sweden, Finland, and Austria – experienced the recent Great Recession without additional suicides. Early case finding and antidepressant prescribing to clinically depressed, unemployed people inevitably help to prevent some deaths by suicide, but this is no panacea.
Too many people fall through the cracks or don’t respond to medication or are not adequately monitored. The authors mention the protective effectiveness of active labor market programs assisting the newly unemployed to find work and argue for their economic soundness. Associated with this notion is the beneficial effect of support, which can’t be underestimated as a major force in maintaining resilience.
I agree with the authors of this important paper that "voices of psychiatrists must not remain silent." In addition to the daily ministering to our patients in our clinical settings, we must continue to fight for their rights to state-of-the-art mental health care and our unwavering belief in their self-worth, so shattered with the scourge of un- or underemployment.
Dr. Michael F. Myers is professor of clinical psychiatry at SUNY Downstate Medical Center, Brooklyn, N.Y. He had no disclosures or conflicts of interest.
Increases in suicides after a recession, such as those seen in Europe, Canada, and the United States after the most recent recession, are not inevitable, according to the authors of a report on "economic suicides" in Europe and North America.
"The evidence that some societies have successfully de-coupled economic shocks from adverse mental health outcomes reveals the hope that it will be possible to eliminate the association of economic shocks with a rise in suicidality," said Aaron Reeves, Ph.D., of the University of Oxford (England) and his coauthors, from the London School of Hygiene and Tropical Medicine. Their report, which reviewed suicide data in Europe, the United States, and Canada after the onset of recession in 2007, appears online in the British Journal of Psychiatry (2014 June 11 [doi: 10.1192/bjp.bp.114.144766]).
Using 2007 as the prerecession baseline, they said that after suicide rates had been falling in Europe, the rates started to increase, rising by 6.5% by 2009 and remaining elevated in 2011. The reversal in a prerecession drop in suicide rates also was documented in Canada, with a 4.5% increase in suicides from 2007 to 2009. And in the United States, where suicides had been increasing before the recession, an increase of 4.8% was found between 2007 and 2010.
These increases add up to at least 10,000 more so-called economic suicides than expected in Europe, the United States, and Canada since 2007, the authors pointed out.
But they questioned whether these suicides "are an inevitable accompaniment of economic hardship" and provided several examples of countries where the most recent or previous recessions were not associated with subsequent increases in suicides. For example, in Sweden, suicides declined between 1991 and 1992, during which time a marked rise in unemployment occurred. Also, the authors noted, the prevalence of suicides did not increase in Sweden during the most recent recession.
Factors that can help address the adverse effects of unemployment on suicide include access to secondary prevention, and programs that provide support for unemployed people and help them find work, they said. Noting that the medical community can be advocates of evidence-based prevention measures, they wrote, "recessions will continue to hurt, but need not cause self-harm."
The authors had no disclosures.
Increases in suicides after a recession, such as those seen in Europe, Canada, and the United States after the most recent recession, are not inevitable, according to the authors of a report on "economic suicides" in Europe and North America.
"The evidence that some societies have successfully de-coupled economic shocks from adverse mental health outcomes reveals the hope that it will be possible to eliminate the association of economic shocks with a rise in suicidality," said Aaron Reeves, Ph.D., of the University of Oxford (England) and his coauthors, from the London School of Hygiene and Tropical Medicine. Their report, which reviewed suicide data in Europe, the United States, and Canada after the onset of recession in 2007, appears online in the British Journal of Psychiatry (2014 June 11 [doi: 10.1192/bjp.bp.114.144766]).
Using 2007 as the prerecession baseline, they said that after suicide rates had been falling in Europe, the rates started to increase, rising by 6.5% by 2009 and remaining elevated in 2011. The reversal in a prerecession drop in suicide rates also was documented in Canada, with a 4.5% increase in suicides from 2007 to 2009. And in the United States, where suicides had been increasing before the recession, an increase of 4.8% was found between 2007 and 2010.
These increases add up to at least 10,000 more so-called economic suicides than expected in Europe, the United States, and Canada since 2007, the authors pointed out.
But they questioned whether these suicides "are an inevitable accompaniment of economic hardship" and provided several examples of countries where the most recent or previous recessions were not associated with subsequent increases in suicides. For example, in Sweden, suicides declined between 1991 and 1992, during which time a marked rise in unemployment occurred. Also, the authors noted, the prevalence of suicides did not increase in Sweden during the most recent recession.
Factors that can help address the adverse effects of unemployment on suicide include access to secondary prevention, and programs that provide support for unemployed people and help them find work, they said. Noting that the medical community can be advocates of evidence-based prevention measures, they wrote, "recessions will continue to hurt, but need not cause self-harm."
The authors had no disclosures.
FROM THE BRITISH JOURNAL OF PSYCHIATRY
Key clinical point: Interventions aimed at helping patients whose mental health is adversely affected by recessions might have an effect on reducing the suicide risk.
Major finding: The suicide rates increased by almost 5% in the United States and Canada, and 6.5% in Europe, since the onset of recession in 2007, but no increases in several countries affected by the same recession indicates that suicides can be prevented.
Data source: The report compared suicide trends after the 2007 recession in Europe and North America.
Disclosures: The authors had no disclosures.
Viral reactivation common in septic patients, study finds
Critically ill patients with sepsis have a markedly higher prevalence of different viruses than do nonseptic critically ill patients and healthy controls, judging from the findings of a study of more than 800 patients.
These findings provide evidence that the reactivation of latent viruses "is extremely common in patients with prolonged sepsis and is consistent with development of immunosuppression," the authors concluded.
For some of the viruses, the levels detected in septic patients were comparable to the levels in organ transplant recipients, "who are pharmacologically immunosuppressed, providing further support that our findings are indicative of clinically relevant immunosuppression," Dr. Anthony Walton, of the department of anesthesiology, Washington University, St. Louis, and his coauthors wrote. The study was published online June 6 in PLoS One (2014;9:e98819 [doi: 10.1371/journal.pone.0098819]).
In what they said is the first study to evaluate the effect of sepsis on "multiple families of viruses," the investigators addressed whether sepsis progresses from a hyperinflammatory phase early in the course of sepsis to an immunosuppressive state, a "controversial hypothesis" for explaining the course of sepsis, they wrote.
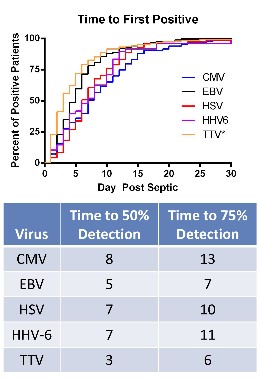
The researchers compared levels of viruses that included cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), human herpesvirus 6 (HHV-6), and the anellovirus TTV in whole blood and plasma of 560 critically ill patients with sepsis and 161 critically ill patients who did not have sepsis, who were not immunocompromised at baseline; and 164 healthy, age-matched controls, who were ambulatory and whose blood sample was obtained before elective surgery. The median age of the patients was 63-64 years; the median APACHE II score was 18 in the septic group and 5 in the critically ill, nonseptic group; and the median length of stay in the ICU was 11 days in the septic group and 2 days in the critically ill, nonseptic group. Mortality was 26% among those with sepsis and 6% among the critically ill, nonseptic group.
Among the investigators’ key findings were these:
• CMV seropositivity was detected in about 70% of the patients in the three groups, indicating they had been infected previously. Among these patients, CMV levels were markedly elevated in 24.2% of the septic patients, compared with 1.1% of the critically ill, nonseptic patients and none of the healthy controls.
• EBV was detected in 53.2% of those who were septic, compared with 12.1% of the critically ill, nonseptic patients and 3.6% of the healthy controls.
• HSV was detected in 14.1% of the septic patients, compared with 1.5% of the critically ill, nonseptic patients and none of the healthy controls.
• HHV-6 was detected in 10.4% of those who were septic, compared with less than 1% of the critically ill, nonseptic patients and 3.3% of the healthy controls.
• TTV was detected in almost 78% of the septic patients, close to 64% if the critically ill, nonseptic patients, and 60.1% of the healthy controls, but levels were lower among the latter two groups.
The authors said that it is "likely that viral detection in the setting of sepsis is not due to primary infection but rather to viral reactivation." Almost 43% of those with sepsis had evidence of at least two viruses, which, combined with the "magnitude of viral loads ... provides strong evidence that host immunity is impaired in sepsis," they added.
Among their other findings was that in the septic patients, the detection rate of the viruses increased for all the viruses with increasing number of days spent in the ICU, and septic patients who had CMV detected in the plasma had significantly higher 90-day mortality than did septic patients with no CMV detected.
Limitations of the study include the fact that it does not address whether the increased prevalence of viral reactivation among the septic patients "is merely a marker of impaired immunity or contributes to sepsis morbidity/mortality," they noted. But the implications of their results include the possibility that tracking the viral load of different viruses in septic patients "may be useful as a biomarker of host immunity in sepsis."
The study was funded by the National Institutes of Health. One of the 13 authors is an employee of Biomérieux, a company that is working on a method to monitor levels of different viruses in the blood as an indicator of immune status. No other author disclosures were listed.
The investigators have demonstrated that reactivation of latent viral infections may well contribute to the death of critically ill septic patients. Some of the viral reactivations were associated with secondary fungal infection as well.
Although viral DNA was detected as early as 1 day into sepsis, the bulk of the manifested reactivations occurred over the subsequent 2 weeks. Viral reactivation is a clear marker that the "late" immune suppression of sepsis is a real phenomenon and leads to real sequelae.
Nevertheless, it is not yet clear exactly how this information will become useful in practice, as the cost of daily DNA screening for multiple viruses would be prohibitive, unless high-volume demand drives pricing down. One can see, under that scenario, how viral reactivation could be the signal that immune augmentation therapy is required, and that it might be beneficial. This work is not quite ready for prime time, but it is getting ever closer.
Dr. Steven Q. Simpson is professor of medicine University of Kansas, Kansas City. He is also founder of the Kansas Sepsis Project. He had no disclosures.
The investigators have demonstrated that reactivation of latent viral infections may well contribute to the death of critically ill septic patients. Some of the viral reactivations were associated with secondary fungal infection as well.
Although viral DNA was detected as early as 1 day into sepsis, the bulk of the manifested reactivations occurred over the subsequent 2 weeks. Viral reactivation is a clear marker that the "late" immune suppression of sepsis is a real phenomenon and leads to real sequelae.
Nevertheless, it is not yet clear exactly how this information will become useful in practice, as the cost of daily DNA screening for multiple viruses would be prohibitive, unless high-volume demand drives pricing down. One can see, under that scenario, how viral reactivation could be the signal that immune augmentation therapy is required, and that it might be beneficial. This work is not quite ready for prime time, but it is getting ever closer.
Dr. Steven Q. Simpson is professor of medicine University of Kansas, Kansas City. He is also founder of the Kansas Sepsis Project. He had no disclosures.
The investigators have demonstrated that reactivation of latent viral infections may well contribute to the death of critically ill septic patients. Some of the viral reactivations were associated with secondary fungal infection as well.
Although viral DNA was detected as early as 1 day into sepsis, the bulk of the manifested reactivations occurred over the subsequent 2 weeks. Viral reactivation is a clear marker that the "late" immune suppression of sepsis is a real phenomenon and leads to real sequelae.
Nevertheless, it is not yet clear exactly how this information will become useful in practice, as the cost of daily DNA screening for multiple viruses would be prohibitive, unless high-volume demand drives pricing down. One can see, under that scenario, how viral reactivation could be the signal that immune augmentation therapy is required, and that it might be beneficial. This work is not quite ready for prime time, but it is getting ever closer.
Dr. Steven Q. Simpson is professor of medicine University of Kansas, Kansas City. He is also founder of the Kansas Sepsis Project. He had no disclosures.
Critically ill patients with sepsis have a markedly higher prevalence of different viruses than do nonseptic critically ill patients and healthy controls, judging from the findings of a study of more than 800 patients.
These findings provide evidence that the reactivation of latent viruses "is extremely common in patients with prolonged sepsis and is consistent with development of immunosuppression," the authors concluded.
For some of the viruses, the levels detected in septic patients were comparable to the levels in organ transplant recipients, "who are pharmacologically immunosuppressed, providing further support that our findings are indicative of clinically relevant immunosuppression," Dr. Anthony Walton, of the department of anesthesiology, Washington University, St. Louis, and his coauthors wrote. The study was published online June 6 in PLoS One (2014;9:e98819 [doi: 10.1371/journal.pone.0098819]).
In what they said is the first study to evaluate the effect of sepsis on "multiple families of viruses," the investigators addressed whether sepsis progresses from a hyperinflammatory phase early in the course of sepsis to an immunosuppressive state, a "controversial hypothesis" for explaining the course of sepsis, they wrote.
The researchers compared levels of viruses that included cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), human herpesvirus 6 (HHV-6), and the anellovirus TTV in whole blood and plasma of 560 critically ill patients with sepsis and 161 critically ill patients who did not have sepsis, who were not immunocompromised at baseline; and 164 healthy, age-matched controls, who were ambulatory and whose blood sample was obtained before elective surgery. The median age of the patients was 63-64 years; the median APACHE II score was 18 in the septic group and 5 in the critically ill, nonseptic group; and the median length of stay in the ICU was 11 days in the septic group and 2 days in the critically ill, nonseptic group. Mortality was 26% among those with sepsis and 6% among the critically ill, nonseptic group.
Among the investigators’ key findings were these:
• CMV seropositivity was detected in about 70% of the patients in the three groups, indicating they had been infected previously. Among these patients, CMV levels were markedly elevated in 24.2% of the septic patients, compared with 1.1% of the critically ill, nonseptic patients and none of the healthy controls.
• EBV was detected in 53.2% of those who were septic, compared with 12.1% of the critically ill, nonseptic patients and 3.6% of the healthy controls.
• HSV was detected in 14.1% of the septic patients, compared with 1.5% of the critically ill, nonseptic patients and none of the healthy controls.
• HHV-6 was detected in 10.4% of those who were septic, compared with less than 1% of the critically ill, nonseptic patients and 3.3% of the healthy controls.
• TTV was detected in almost 78% of the septic patients, close to 64% if the critically ill, nonseptic patients, and 60.1% of the healthy controls, but levels were lower among the latter two groups.
The authors said that it is "likely that viral detection in the setting of sepsis is not due to primary infection but rather to viral reactivation." Almost 43% of those with sepsis had evidence of at least two viruses, which, combined with the "magnitude of viral loads ... provides strong evidence that host immunity is impaired in sepsis," they added.
Among their other findings was that in the septic patients, the detection rate of the viruses increased for all the viruses with increasing number of days spent in the ICU, and septic patients who had CMV detected in the plasma had significantly higher 90-day mortality than did septic patients with no CMV detected.
Limitations of the study include the fact that it does not address whether the increased prevalence of viral reactivation among the septic patients "is merely a marker of impaired immunity or contributes to sepsis morbidity/mortality," they noted. But the implications of their results include the possibility that tracking the viral load of different viruses in septic patients "may be useful as a biomarker of host immunity in sepsis."
The study was funded by the National Institutes of Health. One of the 13 authors is an employee of Biomérieux, a company that is working on a method to monitor levels of different viruses in the blood as an indicator of immune status. No other author disclosures were listed.
Critically ill patients with sepsis have a markedly higher prevalence of different viruses than do nonseptic critically ill patients and healthy controls, judging from the findings of a study of more than 800 patients.
These findings provide evidence that the reactivation of latent viruses "is extremely common in patients with prolonged sepsis and is consistent with development of immunosuppression," the authors concluded.
For some of the viruses, the levels detected in septic patients were comparable to the levels in organ transplant recipients, "who are pharmacologically immunosuppressed, providing further support that our findings are indicative of clinically relevant immunosuppression," Dr. Anthony Walton, of the department of anesthesiology, Washington University, St. Louis, and his coauthors wrote. The study was published online June 6 in PLoS One (2014;9:e98819 [doi: 10.1371/journal.pone.0098819]).
In what they said is the first study to evaluate the effect of sepsis on "multiple families of viruses," the investigators addressed whether sepsis progresses from a hyperinflammatory phase early in the course of sepsis to an immunosuppressive state, a "controversial hypothesis" for explaining the course of sepsis, they wrote.
The researchers compared levels of viruses that included cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV), human herpesvirus 6 (HHV-6), and the anellovirus TTV in whole blood and plasma of 560 critically ill patients with sepsis and 161 critically ill patients who did not have sepsis, who were not immunocompromised at baseline; and 164 healthy, age-matched controls, who were ambulatory and whose blood sample was obtained before elective surgery. The median age of the patients was 63-64 years; the median APACHE II score was 18 in the septic group and 5 in the critically ill, nonseptic group; and the median length of stay in the ICU was 11 days in the septic group and 2 days in the critically ill, nonseptic group. Mortality was 26% among those with sepsis and 6% among the critically ill, nonseptic group.
Among the investigators’ key findings were these:
• CMV seropositivity was detected in about 70% of the patients in the three groups, indicating they had been infected previously. Among these patients, CMV levels were markedly elevated in 24.2% of the septic patients, compared with 1.1% of the critically ill, nonseptic patients and none of the healthy controls.
• EBV was detected in 53.2% of those who were septic, compared with 12.1% of the critically ill, nonseptic patients and 3.6% of the healthy controls.
• HSV was detected in 14.1% of the septic patients, compared with 1.5% of the critically ill, nonseptic patients and none of the healthy controls.
• HHV-6 was detected in 10.4% of those who were septic, compared with less than 1% of the critically ill, nonseptic patients and 3.3% of the healthy controls.
• TTV was detected in almost 78% of the septic patients, close to 64% if the critically ill, nonseptic patients, and 60.1% of the healthy controls, but levels were lower among the latter two groups.
The authors said that it is "likely that viral detection in the setting of sepsis is not due to primary infection but rather to viral reactivation." Almost 43% of those with sepsis had evidence of at least two viruses, which, combined with the "magnitude of viral loads ... provides strong evidence that host immunity is impaired in sepsis," they added.
Among their other findings was that in the septic patients, the detection rate of the viruses increased for all the viruses with increasing number of days spent in the ICU, and septic patients who had CMV detected in the plasma had significantly higher 90-day mortality than did septic patients with no CMV detected.
Limitations of the study include the fact that it does not address whether the increased prevalence of viral reactivation among the septic patients "is merely a marker of impaired immunity or contributes to sepsis morbidity/mortality," they noted. But the implications of their results include the possibility that tracking the viral load of different viruses in septic patients "may be useful as a biomarker of host immunity in sepsis."
The study was funded by the National Institutes of Health. One of the 13 authors is an employee of Biomérieux, a company that is working on a method to monitor levels of different viruses in the blood as an indicator of immune status. No other author disclosures were listed.
FROM PLOS ONE
Key clinical point: Reactivation of latent viruses may underlie the development of sepsis in critically ill patients and contribute to their death.
Major finding: Evidence of reactivation with multiple viruses in septic patients – which included almost 43% who were positive for at least two viruses – and the magnitude of viral loads in septic patients indicate that patients with sepsis are immunosuppressed.
Data source: The study compared levels of cytomegalovirus, herpes simplex, and other viruses in 560 critically ill septic patients and 161 critically ill nonseptic patients in intensive care units, and 164 healthy, age-matched controls.
Disclosures: The study was funded by the National Institutes of Health. One of the 13 authors is an employee of Biomérieux, a company that is working on a method to monitor levels of different viruses in the blood as an indicator of immune status. No other author disclosures were listed.
One in 10 heart attack patients has unrecognized diabetes
Ten percent of patients presenting with an acute myocardial infarction had undiagnosed diabetes at the time of their heart attack, underlining the importance of evaluating such patients for diabetes while they are hospitalized, investigators have reported.
The study found that 287 (10.1%) of the 2,854 patients enrolled in a 24-site U.S. acute MI registry, who were not known to have type 2 diabetes on admission, actually had diabetes, reported Dr. Suzanne V. Arnold, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo. The data were presented at the American Heart Association’s Quality of Care and Outcomes Research conference.
The diabetes diagnosis was based on hemoglobin A1c levels of 6.5% or higher. If no HbA1c result was available, the diagnosis was based on at least two fasting glucose levels of 126 mg/dL or higher, or at least one fasting glucose level of 126 mg/dL or higher plus a glucose level at presentation of at least 200 mg/dL.
Of the 287 patients who were identified as having unrecognized diabetes in the study, almost 70% (198) had not been diagnosed by the physician who treated them during their hospitalization. This lack of a diagnosis was indicated by patients not having received education about diabetes while hospitalized or not being discharged with a diabetes medication.
If a physician checked the HbA1c for a patient with an acute MI as part of routine clinical care, however, the likelihood that the patient would be diagnosed with diabetes was increased 18-fold, a highly statistically significant finding, Dr. Arnold said.
Dr. Arnold had no disclosures.
The registry study is the TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) study and is sponsored by the National Institutes of Health/the National Heart Lung and Blood Institute. Genentech funded this analysis of the TRIUMPH registry data.
That 10% of patients with acute myocardial infarction (MI) were found to have undiagnosed diabetes is perhaps unsurprising, considering the known association. However, the lack of response to the diagnosis in 70% of MI patients brings to light the potential benefit of adding diabetes diagnostics and initiation of appropriate treatments to the inpatient checklist. Doing so would prompt evidenced-based care that could [affect] both the acute treatment of the primary event (via selection of interventions and medications known to benefit the diabetic patient), as well as important secondary prevention.
Dr. Claudia K. Geyer is the hospital medicine fellowship director at Central Maine Medical Center in Lewiston.
That 10% of patients with acute myocardial infarction (MI) were found to have undiagnosed diabetes is perhaps unsurprising, considering the known association. However, the lack of response to the diagnosis in 70% of MI patients brings to light the potential benefit of adding diabetes diagnostics and initiation of appropriate treatments to the inpatient checklist. Doing so would prompt evidenced-based care that could [affect] both the acute treatment of the primary event (via selection of interventions and medications known to benefit the diabetic patient), as well as important secondary prevention.
Dr. Claudia K. Geyer is the hospital medicine fellowship director at Central Maine Medical Center in Lewiston.
That 10% of patients with acute myocardial infarction (MI) were found to have undiagnosed diabetes is perhaps unsurprising, considering the known association. However, the lack of response to the diagnosis in 70% of MI patients brings to light the potential benefit of adding diabetes diagnostics and initiation of appropriate treatments to the inpatient checklist. Doing so would prompt evidenced-based care that could [affect] both the acute treatment of the primary event (via selection of interventions and medications known to benefit the diabetic patient), as well as important secondary prevention.
Dr. Claudia K. Geyer is the hospital medicine fellowship director at Central Maine Medical Center in Lewiston.
Ten percent of patients presenting with an acute myocardial infarction had undiagnosed diabetes at the time of their heart attack, underlining the importance of evaluating such patients for diabetes while they are hospitalized, investigators have reported.
The study found that 287 (10.1%) of the 2,854 patients enrolled in a 24-site U.S. acute MI registry, who were not known to have type 2 diabetes on admission, actually had diabetes, reported Dr. Suzanne V. Arnold, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo. The data were presented at the American Heart Association’s Quality of Care and Outcomes Research conference.
The diabetes diagnosis was based on hemoglobin A1c levels of 6.5% or higher. If no HbA1c result was available, the diagnosis was based on at least two fasting glucose levels of 126 mg/dL or higher, or at least one fasting glucose level of 126 mg/dL or higher plus a glucose level at presentation of at least 200 mg/dL.
Of the 287 patients who were identified as having unrecognized diabetes in the study, almost 70% (198) had not been diagnosed by the physician who treated them during their hospitalization. This lack of a diagnosis was indicated by patients not having received education about diabetes while hospitalized or not being discharged with a diabetes medication.
If a physician checked the HbA1c for a patient with an acute MI as part of routine clinical care, however, the likelihood that the patient would be diagnosed with diabetes was increased 18-fold, a highly statistically significant finding, Dr. Arnold said.
Dr. Arnold had no disclosures.
The registry study is the TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) study and is sponsored by the National Institutes of Health/the National Heart Lung and Blood Institute. Genentech funded this analysis of the TRIUMPH registry data.
Ten percent of patients presenting with an acute myocardial infarction had undiagnosed diabetes at the time of their heart attack, underlining the importance of evaluating such patients for diabetes while they are hospitalized, investigators have reported.
The study found that 287 (10.1%) of the 2,854 patients enrolled in a 24-site U.S. acute MI registry, who were not known to have type 2 diabetes on admission, actually had diabetes, reported Dr. Suzanne V. Arnold, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo. The data were presented at the American Heart Association’s Quality of Care and Outcomes Research conference.
The diabetes diagnosis was based on hemoglobin A1c levels of 6.5% or higher. If no HbA1c result was available, the diagnosis was based on at least two fasting glucose levels of 126 mg/dL or higher, or at least one fasting glucose level of 126 mg/dL or higher plus a glucose level at presentation of at least 200 mg/dL.
Of the 287 patients who were identified as having unrecognized diabetes in the study, almost 70% (198) had not been diagnosed by the physician who treated them during their hospitalization. This lack of a diagnosis was indicated by patients not having received education about diabetes while hospitalized or not being discharged with a diabetes medication.
If a physician checked the HbA1c for a patient with an acute MI as part of routine clinical care, however, the likelihood that the patient would be diagnosed with diabetes was increased 18-fold, a highly statistically significant finding, Dr. Arnold said.
Dr. Arnold had no disclosures.
The registry study is the TRIUMPH (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status) study and is sponsored by the National Institutes of Health/the National Heart Lung and Blood Institute. Genentech funded this analysis of the TRIUMPH registry data.
FROM THE AHA/QCOR ANNUAL CONFERENCE
Key clinical point: Obtaining a HbA1c level in patients presenting with an acute MI who are not known to have diabetes will help identify those who actually have the disease.
Major finding: The lab criteria for a diabetes diagnosis were met by about 10% of patients admitted with an acute MI who were not known to have diabetes – a diagnosis missed by their physicians while they were hospitalized in almost 70% of cases.
Data source: The study looked for a diagnosis of diabetes, based on HbA1c levels or fasting glucose levels, in 2,854 patients hospitalized with an acute MI who did not have a diabetes diagnosis.
Disclosures: Dr. Arnold had no disclosures. The TRIUMPH registry is sponsored by the NIH/NHLBI. Genentech funded this analysis of the TRIUMPH registry data.
Less-frequent antibiotics as effective as daily vancomycin for skin infections
Treatment with once-weekly dalbavancin and treatment with a one-time dose of oritavancin for acute bacterial skin and skin-structure infections had similar outcomes to conventional treatment with vancomycin, in studies reported in the June 5 issue of the New England Journal of Medicine.
In the noninferiority studies, the effects of treatment with the two long-acting intravenously administered antibiotics on methicillin-resistant Staphylococcus aureus (MRSA) infections were comparable to treatment with vancomycin, administered intravenously twice a day (followed by oral linezolid in the dalbavancin study).
Both dalbavancin, which was recently approved by the Food and Drug Administration, and oritavancin, which is being reviewed by the FDA, are lipoglycopeptide antibiotics with activity against gram-positive bacteria. Because of their prolonged half-lives, oritavancin can be administered as a single dose and dalbavancin can be administered once a week. The authors of both studies referred to the substantial medical costs associated with the inpatient treatment of acute bacterial skin and skin structure infections.
In the randomized double-blind, international study of oritavancin, the SOLO I trial, patients with acute bacterial skin and skin structure infections (cellulitis, wound infections, or an abscess), thought to be caused by a gram-positive pathogen were treated with a single intravenous dose of 1,200 mg of oritavancin (475 patients) or intravenous vancomycin twice a day for 7-10 days (479), at a dose of 1g or 15 mg/kg of body weight. Their mean age was 45 years, almost 9% were aged 65 years or older, more than half were white men, and about 34% were obese. Almost 20% had diabetes, and of the approximately 60% who had a pathogen isolated, almost all had a gram-positive pathogen known to cause acute bacterial skin and skin-structure infections, with S. aureus being the most common.
The primary endpoint, evaluated early in treatment (48-72 hours after the drug was administered) was a composite of the following: cessation of spreading or a reduction in the baseline lesion size, absence of fever, and no need for rescue antibiotic to be administered. This endpoint was met by 82.3% of those on oritavancin and 78.9% of those on vancomycin, which met the prespecified non-inferiority margin of 10%, reported the authors, led by Dr. G. Ralph Corey, professor of medicine, Duke University, Durham, NC. (N. Engl. J. Med. 2014;370:2180-90 [doi:10.1056/NEJMoa1310422]).
These results were similar when analyzed by body mass index, whether the patient had diabetes or MRSA infection, and by race, sex, or type of lesion. The investigator-assessed clinical cure rates 7-14 days after treatment ended were about 80% in both groups, and the proportion of patients with at least a 20% reduction in the area of the lesion 48-72 hours after treatment started was 86.9% among those on oritavancin vs. 82.9% of those on vancomycin. More patients on oritavancin experienced nausea; otherwise, the proportion of adverse events and serious adverse events were similar in the two treatment groups.
While the study had limitations, a single-dose treatment for acute bacterial skin and skin structure infections "that results in an early and sustained clinical response could have the potential to reduce the complications associated with multiple intravenous administrations in patients with these infections, improve adherence to treatment, improve quality of life, and reduce the utilization of health care resources," the authors concluded.
The dalbavancin studies, known as the Discover I and II studies, enrolled about 1,300 patients with cellulitis, a major abscess, or a wound infection; the median size of the infected area was 351cm2 in one study and 336 cm2 in the other. Patients were treated with dalbavancin (1g IV, followed by a 500 mg intravenous dose on day 8) or vancomycin (1 g or 15 mg/kg of body weight) every 12 hours for at least 3 days, with the option of changing to linezolid (600 mg) every 12 hours for a total of 10-14 days of treatment. Overall, 13% of the patients had diabetes, and more than 85% had a temperature over 38 degrees° C at baseline. The mean age was 49-50 years, almost 60% were male, almost 90% were white, and about half had systemic inflammatory response syndrome).
In a pooled analysis of the studies, 79.7% of those treated with dalbavancin and 79.8% of those treated with vancomycin-linezolid met the primary endpoint of an early clinical response (cessation of spread of infection-related erythema and absence of fever 48-72 hours after starting treatment).
The results were consistent at early and later time points, and results "were robust in patients with major abscess, cellulitis, or wound infection; in those with S. aureus, including MRSA, or Streptococcus pyogenes infection; and in those treated as an outpatient," reported the authors, led by Dr. Helen Boucher of Tufts University, Boston. (N. Engl. J. Med. 2014;370:2169-79 [doi:10.1056/NEJMoa1310480]).
Fewer patients on dalbavancin had adverse events (almost 33% vs. almost 38% of those on vancomycin-linezolid). The most common adverse events associated with treatment in both groups were nausea (almost 3% in both groups), diarrhea (0.8% among those on dalbavancin, vs. 2.5% among those on vancomycin-linezolid) and pruritus (0.6% vs. 2.3%).
The oritavancin study was funded by the Medicines Company, the drug’s manufacturer, and several authors are employees of the company. Dr. Corey’s disclosures include having received personal fees from the Medicines Company during the study. Other disclosures include fees paid to one author’s institution to conduct the study; other authors had no disclosures.
The dalbavancin study was funded by Durata Therapeutics, the drug’s manufacturer, and two of the six authors are Durata employees. Dr. Boucher disclosed having received fees for serving on advisory boards for Durata and other pharmaceutical companies. Disclosures of the remaining three authors included having received consulting and lecture fees and advisory board fees from various companies, including Durata. One author (not a Durata employee) also disclosed owning stock or stock options in several companies, including Durata.
In May, the FDA approved dalbavancin for the treatment of acute bacterial skin and skin structure infections; it is being marketed as Dalvance. In February, the Medicines Company announced that the application for approval of oritavancin for the treatment of skin and skin structure infections had been accepted by the FDA.
While neither oritavancin nor dalbavancin is more efficacious than vancomycin is, they are easier to administer and "make it possible to treat patients with complicated skin and skin-structure infections who might otherwise require hospitalization" as outpatients, "without compromising efficacy and without the need for laboratory monitoring or an indwelling intravenous catheter," Dr. Henry Chambers wrote in an accompanying editorial. By reducing or eliminating hospitalization-related expenses and risks, "this approach could profoundly affect how these infections are managed," he added. However, he cautioned that broader clinical use is needed to determine the safety of these two agents, and it is unclear how effective they will be for other types of infections, noting that more clinical trials are needed to "define the safety and efficacy profile." (N. Engl. J. Med. 2014;370:2238-39 [doi:10.1056/NEJMe1405078]).
Dr. Chambers is professor of medicine, University of California, San Francisco, and is chief of the division of infectious diseases, San Francisco General Hospital. He disclosed having received personal fees from Cubist Pharmaceuticals, Pfizer, AstraZeneca, and Theravance, and personal fees and other support from Trius outside of this submitted work.
While neither oritavancin nor dalbavancin is more efficacious than vancomycin is, they are easier to administer and "make it possible to treat patients with complicated skin and skin-structure infections who might otherwise require hospitalization" as outpatients, "without compromising efficacy and without the need for laboratory monitoring or an indwelling intravenous catheter," Dr. Henry Chambers wrote in an accompanying editorial. By reducing or eliminating hospitalization-related expenses and risks, "this approach could profoundly affect how these infections are managed," he added. However, he cautioned that broader clinical use is needed to determine the safety of these two agents, and it is unclear how effective they will be for other types of infections, noting that more clinical trials are needed to "define the safety and efficacy profile." (N. Engl. J. Med. 2014;370:2238-39 [doi:10.1056/NEJMe1405078]).
Dr. Chambers is professor of medicine, University of California, San Francisco, and is chief of the division of infectious diseases, San Francisco General Hospital. He disclosed having received personal fees from Cubist Pharmaceuticals, Pfizer, AstraZeneca, and Theravance, and personal fees and other support from Trius outside of this submitted work.
While neither oritavancin nor dalbavancin is more efficacious than vancomycin is, they are easier to administer and "make it possible to treat patients with complicated skin and skin-structure infections who might otherwise require hospitalization" as outpatients, "without compromising efficacy and without the need for laboratory monitoring or an indwelling intravenous catheter," Dr. Henry Chambers wrote in an accompanying editorial. By reducing or eliminating hospitalization-related expenses and risks, "this approach could profoundly affect how these infections are managed," he added. However, he cautioned that broader clinical use is needed to determine the safety of these two agents, and it is unclear how effective they will be for other types of infections, noting that more clinical trials are needed to "define the safety and efficacy profile." (N. Engl. J. Med. 2014;370:2238-39 [doi:10.1056/NEJMe1405078]).
Dr. Chambers is professor of medicine, University of California, San Francisco, and is chief of the division of infectious diseases, San Francisco General Hospital. He disclosed having received personal fees from Cubist Pharmaceuticals, Pfizer, AstraZeneca, and Theravance, and personal fees and other support from Trius outside of this submitted work.
Treatment with once-weekly dalbavancin and treatment with a one-time dose of oritavancin for acute bacterial skin and skin-structure infections had similar outcomes to conventional treatment with vancomycin, in studies reported in the June 5 issue of the New England Journal of Medicine.
In the noninferiority studies, the effects of treatment with the two long-acting intravenously administered antibiotics on methicillin-resistant Staphylococcus aureus (MRSA) infections were comparable to treatment with vancomycin, administered intravenously twice a day (followed by oral linezolid in the dalbavancin study).
Both dalbavancin, which was recently approved by the Food and Drug Administration, and oritavancin, which is being reviewed by the FDA, are lipoglycopeptide antibiotics with activity against gram-positive bacteria. Because of their prolonged half-lives, oritavancin can be administered as a single dose and dalbavancin can be administered once a week. The authors of both studies referred to the substantial medical costs associated with the inpatient treatment of acute bacterial skin and skin structure infections.
In the randomized double-blind, international study of oritavancin, the SOLO I trial, patients with acute bacterial skin and skin structure infections (cellulitis, wound infections, or an abscess), thought to be caused by a gram-positive pathogen were treated with a single intravenous dose of 1,200 mg of oritavancin (475 patients) or intravenous vancomycin twice a day for 7-10 days (479), at a dose of 1g or 15 mg/kg of body weight. Their mean age was 45 years, almost 9% were aged 65 years or older, more than half were white men, and about 34% were obese. Almost 20% had diabetes, and of the approximately 60% who had a pathogen isolated, almost all had a gram-positive pathogen known to cause acute bacterial skin and skin-structure infections, with S. aureus being the most common.
The primary endpoint, evaluated early in treatment (48-72 hours after the drug was administered) was a composite of the following: cessation of spreading or a reduction in the baseline lesion size, absence of fever, and no need for rescue antibiotic to be administered. This endpoint was met by 82.3% of those on oritavancin and 78.9% of those on vancomycin, which met the prespecified non-inferiority margin of 10%, reported the authors, led by Dr. G. Ralph Corey, professor of medicine, Duke University, Durham, NC. (N. Engl. J. Med. 2014;370:2180-90 [doi:10.1056/NEJMoa1310422]).
These results were similar when analyzed by body mass index, whether the patient had diabetes or MRSA infection, and by race, sex, or type of lesion. The investigator-assessed clinical cure rates 7-14 days after treatment ended were about 80% in both groups, and the proportion of patients with at least a 20% reduction in the area of the lesion 48-72 hours after treatment started was 86.9% among those on oritavancin vs. 82.9% of those on vancomycin. More patients on oritavancin experienced nausea; otherwise, the proportion of adverse events and serious adverse events were similar in the two treatment groups.
While the study had limitations, a single-dose treatment for acute bacterial skin and skin structure infections "that results in an early and sustained clinical response could have the potential to reduce the complications associated with multiple intravenous administrations in patients with these infections, improve adherence to treatment, improve quality of life, and reduce the utilization of health care resources," the authors concluded.
The dalbavancin studies, known as the Discover I and II studies, enrolled about 1,300 patients with cellulitis, a major abscess, or a wound infection; the median size of the infected area was 351cm2 in one study and 336 cm2 in the other. Patients were treated with dalbavancin (1g IV, followed by a 500 mg intravenous dose on day 8) or vancomycin (1 g or 15 mg/kg of body weight) every 12 hours for at least 3 days, with the option of changing to linezolid (600 mg) every 12 hours for a total of 10-14 days of treatment. Overall, 13% of the patients had diabetes, and more than 85% had a temperature over 38 degrees° C at baseline. The mean age was 49-50 years, almost 60% were male, almost 90% were white, and about half had systemic inflammatory response syndrome).
In a pooled analysis of the studies, 79.7% of those treated with dalbavancin and 79.8% of those treated with vancomycin-linezolid met the primary endpoint of an early clinical response (cessation of spread of infection-related erythema and absence of fever 48-72 hours after starting treatment).
The results were consistent at early and later time points, and results "were robust in patients with major abscess, cellulitis, or wound infection; in those with S. aureus, including MRSA, or Streptococcus pyogenes infection; and in those treated as an outpatient," reported the authors, led by Dr. Helen Boucher of Tufts University, Boston. (N. Engl. J. Med. 2014;370:2169-79 [doi:10.1056/NEJMoa1310480]).
Fewer patients on dalbavancin had adverse events (almost 33% vs. almost 38% of those on vancomycin-linezolid). The most common adverse events associated with treatment in both groups were nausea (almost 3% in both groups), diarrhea (0.8% among those on dalbavancin, vs. 2.5% among those on vancomycin-linezolid) and pruritus (0.6% vs. 2.3%).
The oritavancin study was funded by the Medicines Company, the drug’s manufacturer, and several authors are employees of the company. Dr. Corey’s disclosures include having received personal fees from the Medicines Company during the study. Other disclosures include fees paid to one author’s institution to conduct the study; other authors had no disclosures.
The dalbavancin study was funded by Durata Therapeutics, the drug’s manufacturer, and two of the six authors are Durata employees. Dr. Boucher disclosed having received fees for serving on advisory boards for Durata and other pharmaceutical companies. Disclosures of the remaining three authors included having received consulting and lecture fees and advisory board fees from various companies, including Durata. One author (not a Durata employee) also disclosed owning stock or stock options in several companies, including Durata.
In May, the FDA approved dalbavancin for the treatment of acute bacterial skin and skin structure infections; it is being marketed as Dalvance. In February, the Medicines Company announced that the application for approval of oritavancin for the treatment of skin and skin structure infections had been accepted by the FDA.
Treatment with once-weekly dalbavancin and treatment with a one-time dose of oritavancin for acute bacterial skin and skin-structure infections had similar outcomes to conventional treatment with vancomycin, in studies reported in the June 5 issue of the New England Journal of Medicine.
In the noninferiority studies, the effects of treatment with the two long-acting intravenously administered antibiotics on methicillin-resistant Staphylococcus aureus (MRSA) infections were comparable to treatment with vancomycin, administered intravenously twice a day (followed by oral linezolid in the dalbavancin study).
Both dalbavancin, which was recently approved by the Food and Drug Administration, and oritavancin, which is being reviewed by the FDA, are lipoglycopeptide antibiotics with activity against gram-positive bacteria. Because of their prolonged half-lives, oritavancin can be administered as a single dose and dalbavancin can be administered once a week. The authors of both studies referred to the substantial medical costs associated with the inpatient treatment of acute bacterial skin and skin structure infections.
In the randomized double-blind, international study of oritavancin, the SOLO I trial, patients with acute bacterial skin and skin structure infections (cellulitis, wound infections, or an abscess), thought to be caused by a gram-positive pathogen were treated with a single intravenous dose of 1,200 mg of oritavancin (475 patients) or intravenous vancomycin twice a day for 7-10 days (479), at a dose of 1g or 15 mg/kg of body weight. Their mean age was 45 years, almost 9% were aged 65 years or older, more than half were white men, and about 34% were obese. Almost 20% had diabetes, and of the approximately 60% who had a pathogen isolated, almost all had a gram-positive pathogen known to cause acute bacterial skin and skin-structure infections, with S. aureus being the most common.
The primary endpoint, evaluated early in treatment (48-72 hours after the drug was administered) was a composite of the following: cessation of spreading or a reduction in the baseline lesion size, absence of fever, and no need for rescue antibiotic to be administered. This endpoint was met by 82.3% of those on oritavancin and 78.9% of those on vancomycin, which met the prespecified non-inferiority margin of 10%, reported the authors, led by Dr. G. Ralph Corey, professor of medicine, Duke University, Durham, NC. (N. Engl. J. Med. 2014;370:2180-90 [doi:10.1056/NEJMoa1310422]).
These results were similar when analyzed by body mass index, whether the patient had diabetes or MRSA infection, and by race, sex, or type of lesion. The investigator-assessed clinical cure rates 7-14 days after treatment ended were about 80% in both groups, and the proportion of patients with at least a 20% reduction in the area of the lesion 48-72 hours after treatment started was 86.9% among those on oritavancin vs. 82.9% of those on vancomycin. More patients on oritavancin experienced nausea; otherwise, the proportion of adverse events and serious adverse events were similar in the two treatment groups.
While the study had limitations, a single-dose treatment for acute bacterial skin and skin structure infections "that results in an early and sustained clinical response could have the potential to reduce the complications associated with multiple intravenous administrations in patients with these infections, improve adherence to treatment, improve quality of life, and reduce the utilization of health care resources," the authors concluded.
The dalbavancin studies, known as the Discover I and II studies, enrolled about 1,300 patients with cellulitis, a major abscess, or a wound infection; the median size of the infected area was 351cm2 in one study and 336 cm2 in the other. Patients were treated with dalbavancin (1g IV, followed by a 500 mg intravenous dose on day 8) or vancomycin (1 g or 15 mg/kg of body weight) every 12 hours for at least 3 days, with the option of changing to linezolid (600 mg) every 12 hours for a total of 10-14 days of treatment. Overall, 13% of the patients had diabetes, and more than 85% had a temperature over 38 degrees° C at baseline. The mean age was 49-50 years, almost 60% were male, almost 90% were white, and about half had systemic inflammatory response syndrome).
In a pooled analysis of the studies, 79.7% of those treated with dalbavancin and 79.8% of those treated with vancomycin-linezolid met the primary endpoint of an early clinical response (cessation of spread of infection-related erythema and absence of fever 48-72 hours after starting treatment).
The results were consistent at early and later time points, and results "were robust in patients with major abscess, cellulitis, or wound infection; in those with S. aureus, including MRSA, or Streptococcus pyogenes infection; and in those treated as an outpatient," reported the authors, led by Dr. Helen Boucher of Tufts University, Boston. (N. Engl. J. Med. 2014;370:2169-79 [doi:10.1056/NEJMoa1310480]).
Fewer patients on dalbavancin had adverse events (almost 33% vs. almost 38% of those on vancomycin-linezolid). The most common adverse events associated with treatment in both groups were nausea (almost 3% in both groups), diarrhea (0.8% among those on dalbavancin, vs. 2.5% among those on vancomycin-linezolid) and pruritus (0.6% vs. 2.3%).
The oritavancin study was funded by the Medicines Company, the drug’s manufacturer, and several authors are employees of the company. Dr. Corey’s disclosures include having received personal fees from the Medicines Company during the study. Other disclosures include fees paid to one author’s institution to conduct the study; other authors had no disclosures.
The dalbavancin study was funded by Durata Therapeutics, the drug’s manufacturer, and two of the six authors are Durata employees. Dr. Boucher disclosed having received fees for serving on advisory boards for Durata and other pharmaceutical companies. Disclosures of the remaining three authors included having received consulting and lecture fees and advisory board fees from various companies, including Durata. One author (not a Durata employee) also disclosed owning stock or stock options in several companies, including Durata.
In May, the FDA approved dalbavancin for the treatment of acute bacterial skin and skin structure infections; it is being marketed as Dalvance. In February, the Medicines Company announced that the application for approval of oritavancin for the treatment of skin and skin structure infections had been accepted by the FDA.
Key clinical point: Treatment with one dose of intravenous oritavancin and treatment with once weekly doses of intravenous dalbavancin were noninferior to conventional treatment with intravenous vancomycin administered twice a day for at least 7 days in studies of patients with acute skin infections.
Major finding: About 80% of patients in all the treatment groups meeting the primary composite endpoint that included a cessation to the spread of erythema and no fever 48-72 hours after starting treatment.
Data source: The oritavancin study was a randomized double-blind, noninferiority study of 954 adults with acute bacterial skin and skin structure infections. Dalbavancin was evaluated in two studies of more seriously ill patients with similar infections.
Disclosures: The oritavancin study was funded by the Medicines Company, the drug’s manufacturer, and several authors are employees of the company. Disclosures of the lead author, Dr. G. Ralph Corey, include having received personal fees from the Medicines Company during the study.
Traditional scoring tool limps as VAP screen in surgical ICU study
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more cases of microbiologically confirmed cases of ventilator-associated pneumonia than a traditional screening tool, in a study of patients in a surgical intensive care unit reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP. The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, said Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The study analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures. VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said. The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," he said. However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, Dr. Pieracci said. "Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
The study was funded through institutional monies. Dr. Pieracci had no relevant disclosures.
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more cases of microbiologically confirmed cases of ventilator-associated pneumonia than a traditional screening tool, in a study of patients in a surgical intensive care unit reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP. The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, said Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The study analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures. VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said. The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," he said. However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, Dr. Pieracci said. "Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
The study was funded through institutional monies. Dr. Pieracci had no relevant disclosures.
BALTIMORE – Screening based on a new chest x-ray infiltrate or fever correctly identified more cases of microbiologically confirmed cases of ventilator-associated pneumonia than a traditional screening tool, in a study of patients in a surgical intensive care unit reported at the annual meeting of the Surgical Infection Society.
In the study, the Clinical Pulmonary Infection Score (CPIS) clinical score (using the threshold of 6) would have missed almost 80% of the cases of microbiologically confirmed VAP. The finding of a new chest x-ray infiltrate was highly sensitive for diagnosing VAP, identifying most cases of VAP, followed by fever as the next most sensitive variable. Each had a sensitivity of about 90%, said Dr. Fredric Pieracci of the department of surgery, Denver Health Medical Center, University of Colorado.
Another notable finding of the study was that the presence of organisms on gram stain in the early VAP window (within 5 days of intubation) was highly sensitive for diagnosing VAP, he added.
VAP is the most common nosocomial infection in intubated, critically ill surgical patients and is the most common reason antibiotics are prescribed in the surgical intensive care unit (SICU), he said. Screening criteria for VAP vary widely, but every algorithm includes some variation of the CPIS, with a score that ranges from 0 to 12. Although the CPIS screening tool, which uses variables that include tracheal secretions and chest x-ray results, has come under scrutiny, it is commonly used, with a result over 6 used as the threshold for both obtaining a lower respiratory tract culture and initiating empiric treatment.
The study analyzed the results of 1,013 bronchoalveolar lavage cultures from 497 SICU patients aged 18-88 years, over a 3-year period (2009-2012). Most of the patients (81%) were males and 71% were trauma patients; cultures were obtained a median of 8 days after intubation (range, 1-109 days), and patients had a median of two cultures. VAP was defined microbiologically as at least 105 CFU/mL if no antibiotics had been given within the previous 72 hours; or at least 104 CFU/mL if antibiotics had been given within the previous 72 hours. CPIS scores were calculated retrospectively.
Of the 1,013 cultures, 438 (43%) met the VAP criteria, and 310 of the 497 patients (62%) had at least one episode of VAP.
Most of the CPIS clinical scores were 4, 5, or 6. When the likelihood of VAP was analyzed, CPIS clinical scores from 1 to 9 all correlated with about a 40% chance of VAP, Dr. Pieracci said. The median CPIS clinical score was 5 for those diagnosed with VAP as well as those not diagnosed with VAP, based on the microbiologic criteria.
The sensitivity of the CPIS clinical score, when the threshold of greater than 6 was used, was only 21%, so by using the CPIS, "we would have missed almost 80% of the VAP cases in this group of patients," he pointed out.
Every case of VAP had at least one of the following: fever, a new chest x-ray infiltrate, or the presence of organisms on gram stain.
Of the individual components of the CPIS, the most sensitive for diagnosing VAP were the new finding on chest x-ray (a sensitivity of 91.1%), and fever (a sensitivity of 89.0%).
When the gram stain results were added to the CPIS clinical score, there was a marginal improvement in sensitivity, "but it was still a very poor screening tool," he said. However, the presence of organisms on the gram stain "was highly sensitive for diagnosing VAP, with a reasonably high negative predictive value" in the early VAP window, when cultures were sent within 5 days of intubation, he said.
The results indicate that the CPIS clinical score in the study "had poor discriminative ability for diagnosing VAP in all the clinical scenarios we tested," and it had a sensitivity that was acceptable only at a threshold lower than 6, Dr. Pieracci said. "Based on these data, we recommend abandoning the CPIS clinical score as a screening tool for VAP," and instead, adopting the three criteria and withholding antibiotic therapy in patients with no organisms on gram stain in the early VAP window.
"What we’ve adopted and are now studying is a screening algorithm that differentiates between the early and late period, and is based on either fever or new chest x-ray finding," Dr. Pieracci said. If the gram stain is negative in the early VAP window, then withholding empiric antibiotics is recommended; this is the only scenario identified in which empiric antibiotics could be safely withheld, he added.
The study was funded through institutional monies. Dr. Pieracci had no relevant disclosures.
AT THE SIS ANNUAL MEETING
Key clinical point: The Clinical Pulmonary Infection Score (CPIS) was less useful than other variables, such as a new chest x-ray infiltrate or fever, in screening surgical ICU patients for VAP and determining the need for empiric antibiotic therapy.
Major finding: The sensitivity of the CPIS tool for VAP in surgical ICU patients was only 21%, compared with 91.1% for a new chest x-ray infiltrate and 89.0% for fever.
Data source: The study evaluated the value of the CPIS and other clinical and gram stain variables in screening for VAP, in 497 adult patients in a surgical ICU.
Disclosures: Dr. Pieracci had no relevant disclosures. The study was funded through institutional monies.
CDC: U.S. measles cases at 20-year high
As of May 23, 288 measles cases were reported to the Centers for Disease Control and Prevention, the highest number reported by that date in the United States since 1994.
The cases have been reported in people aged from 2 weeks to 65 years, most (69%) in unvaccinated patients or those whose vaccination status is not known (20%). Nearly all cases (97%) have been associated with importation from at least 18 countries, particularly the Philippines, which is in the midst of a large outbreak that began in October 2013.
To date, there have been no deaths or cases of encephalitis, but 43 people (15%) have been hospitalized. Complications have included five cases of pneumonia, one case of hepatitis, one case of pancytopenia, and one case of thrombocytopenia.
In 1994, a total of 764 cases had been reported in the United States by May 23.
The large and growing number of cases reported this year "is a wake up call for parents and clinicians," Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases, said during a press briefing May 29. "If you’re a parent or clinician, remember that measles may be forgotten but it isn’t gone," she added.
The large number of reports "emphasizes the need for health care providers to have a heightened awareness of the potential for measles in their communities and the importance of vaccination to prevent measles," according to the CDC report (MMWR 2014; 63:1-4).
Dr. Schuchat advised health care providers to review a patient’s vaccination history before they travel and to not take for granted that adult patients are vaccinated. "As people are caring for middle-aged and younger adults, they shouldn’t take for granted that they got all the recommended vaccines when they were a child," she said. If a patient’s records are not available, or patients do not know if they have been vaccinated, a dose of the measles, mumps, and rubella (MMR) vaccine can be administered, as recommended by the CDC in this situation, she pointed out.
More than half the cases (151) reported through May 23 have been in people aged 20 years and older.
The mean size of the reported outbreaks is 5 cases, with a range of 3-138 cases. The outbreak of 138 cases is in Ohio, affecting mostly unvaccinated people in Amish communities, and is ongoing, Dr. Schuchat said. This outbreak has been linked to importation from the Philippines, she said, noting that individuals in Ohio traveled to that country to do service work.
Of the 195 U.S. residents with measles who were not vaccinated, 85% were not vaccinated for religious, philosophical, or personal reasons, not because of their age or a medical reason, according to the CDC report.
Other details of the 288 cases include:
• This year, measles cases have been reported in New York City and in 18 states, including multiple communities in California.
• Eighteen cases (6%) have been in children under age 12 months (who are too young for routine vaccination), 48 (17%) were aged 1-4 years, and 71 (25%) were aged 5-19 years.
• A total of 45 cases were directly imported to the United States – 40 in U.S. residents traveling abroad and 5 in residents of other countries visiting the United States; 22 of those cases were from the Philippines.
• From Jan. 1 through April 20, 2014, over 32,000 measles cases and 41 measles-related deaths have been reported in the Philippines.
Measles was considered eliminated in the United States in 2000, which means there was no spread within the country for more than 12 months. But Dr. Schuchat described this as "a very fragile state," pointing out that indigenous spread occurs if the chains of transmission cannot be broken, referring to an outbreak of over 20,000 cases in France a few years ago.
Two doses of MMR vaccine are recommended, one at age 12-15 months and the second between 4 and 6 years. The CDC recommends a dose for infants as young as 6 months traveling internationally (who will still need to receive two doses later if they receive one dose between 6 and 11 months).
As of May 23, 288 measles cases were reported to the Centers for Disease Control and Prevention, the highest number reported by that date in the United States since 1994.
The cases have been reported in people aged from 2 weeks to 65 years, most (69%) in unvaccinated patients or those whose vaccination status is not known (20%). Nearly all cases (97%) have been associated with importation from at least 18 countries, particularly the Philippines, which is in the midst of a large outbreak that began in October 2013.
To date, there have been no deaths or cases of encephalitis, but 43 people (15%) have been hospitalized. Complications have included five cases of pneumonia, one case of hepatitis, one case of pancytopenia, and one case of thrombocytopenia.
In 1994, a total of 764 cases had been reported in the United States by May 23.
The large and growing number of cases reported this year "is a wake up call for parents and clinicians," Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases, said during a press briefing May 29. "If you’re a parent or clinician, remember that measles may be forgotten but it isn’t gone," she added.
The large number of reports "emphasizes the need for health care providers to have a heightened awareness of the potential for measles in their communities and the importance of vaccination to prevent measles," according to the CDC report (MMWR 2014; 63:1-4).
Dr. Schuchat advised health care providers to review a patient’s vaccination history before they travel and to not take for granted that adult patients are vaccinated. "As people are caring for middle-aged and younger adults, they shouldn’t take for granted that they got all the recommended vaccines when they were a child," she said. If a patient’s records are not available, or patients do not know if they have been vaccinated, a dose of the measles, mumps, and rubella (MMR) vaccine can be administered, as recommended by the CDC in this situation, she pointed out.
More than half the cases (151) reported through May 23 have been in people aged 20 years and older.
The mean size of the reported outbreaks is 5 cases, with a range of 3-138 cases. The outbreak of 138 cases is in Ohio, affecting mostly unvaccinated people in Amish communities, and is ongoing, Dr. Schuchat said. This outbreak has been linked to importation from the Philippines, she said, noting that individuals in Ohio traveled to that country to do service work.
Of the 195 U.S. residents with measles who were not vaccinated, 85% were not vaccinated for religious, philosophical, or personal reasons, not because of their age or a medical reason, according to the CDC report.
Other details of the 288 cases include:
• This year, measles cases have been reported in New York City and in 18 states, including multiple communities in California.
• Eighteen cases (6%) have been in children under age 12 months (who are too young for routine vaccination), 48 (17%) were aged 1-4 years, and 71 (25%) were aged 5-19 years.
• A total of 45 cases were directly imported to the United States – 40 in U.S. residents traveling abroad and 5 in residents of other countries visiting the United States; 22 of those cases were from the Philippines.
• From Jan. 1 through April 20, 2014, over 32,000 measles cases and 41 measles-related deaths have been reported in the Philippines.
Measles was considered eliminated in the United States in 2000, which means there was no spread within the country for more than 12 months. But Dr. Schuchat described this as "a very fragile state," pointing out that indigenous spread occurs if the chains of transmission cannot be broken, referring to an outbreak of over 20,000 cases in France a few years ago.
Two doses of MMR vaccine are recommended, one at age 12-15 months and the second between 4 and 6 years. The CDC recommends a dose for infants as young as 6 months traveling internationally (who will still need to receive two doses later if they receive one dose between 6 and 11 months).
As of May 23, 288 measles cases were reported to the Centers for Disease Control and Prevention, the highest number reported by that date in the United States since 1994.
The cases have been reported in people aged from 2 weeks to 65 years, most (69%) in unvaccinated patients or those whose vaccination status is not known (20%). Nearly all cases (97%) have been associated with importation from at least 18 countries, particularly the Philippines, which is in the midst of a large outbreak that began in October 2013.
To date, there have been no deaths or cases of encephalitis, but 43 people (15%) have been hospitalized. Complications have included five cases of pneumonia, one case of hepatitis, one case of pancytopenia, and one case of thrombocytopenia.
In 1994, a total of 764 cases had been reported in the United States by May 23.
The large and growing number of cases reported this year "is a wake up call for parents and clinicians," Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases, said during a press briefing May 29. "If you’re a parent or clinician, remember that measles may be forgotten but it isn’t gone," she added.
The large number of reports "emphasizes the need for health care providers to have a heightened awareness of the potential for measles in their communities and the importance of vaccination to prevent measles," according to the CDC report (MMWR 2014; 63:1-4).
Dr. Schuchat advised health care providers to review a patient’s vaccination history before they travel and to not take for granted that adult patients are vaccinated. "As people are caring for middle-aged and younger adults, they shouldn’t take for granted that they got all the recommended vaccines when they were a child," she said. If a patient’s records are not available, or patients do not know if they have been vaccinated, a dose of the measles, mumps, and rubella (MMR) vaccine can be administered, as recommended by the CDC in this situation, she pointed out.
More than half the cases (151) reported through May 23 have been in people aged 20 years and older.
The mean size of the reported outbreaks is 5 cases, with a range of 3-138 cases. The outbreak of 138 cases is in Ohio, affecting mostly unvaccinated people in Amish communities, and is ongoing, Dr. Schuchat said. This outbreak has been linked to importation from the Philippines, she said, noting that individuals in Ohio traveled to that country to do service work.
Of the 195 U.S. residents with measles who were not vaccinated, 85% were not vaccinated for religious, philosophical, or personal reasons, not because of their age or a medical reason, according to the CDC report.
Other details of the 288 cases include:
• This year, measles cases have been reported in New York City and in 18 states, including multiple communities in California.
• Eighteen cases (6%) have been in children under age 12 months (who are too young for routine vaccination), 48 (17%) were aged 1-4 years, and 71 (25%) were aged 5-19 years.
• A total of 45 cases were directly imported to the United States – 40 in U.S. residents traveling abroad and 5 in residents of other countries visiting the United States; 22 of those cases were from the Philippines.
• From Jan. 1 through April 20, 2014, over 32,000 measles cases and 41 measles-related deaths have been reported in the Philippines.
Measles was considered eliminated in the United States in 2000, which means there was no spread within the country for more than 12 months. But Dr. Schuchat described this as "a very fragile state," pointing out that indigenous spread occurs if the chains of transmission cannot be broken, referring to an outbreak of over 20,000 cases in France a few years ago.
Two doses of MMR vaccine are recommended, one at age 12-15 months and the second between 4 and 6 years. The CDC recommends a dose for infants as young as 6 months traveling internationally (who will still need to receive two doses later if they receive one dose between 6 and 11 months).
Key clinical point: Be aware of the potential for measles to appear in the community and emphasize the importance of vaccination to patients.
Major finding: From Jan. 1 through May 23 of this year, 288 cases of measles were reported in people age 2 weeks-65 years; most patients (89%) were not vaccinated or their vaccination status was not known.
Data source: Reports of confirmed measles cases from state and local health departments through May 23.
Disclosures: The report was provided by the Centers for Disease Control and Prevention.
Prevention bundle led to drop in postoperative pressure ulcers
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
BALTIMORE – Implementing a bundle of preventive interventions led to a significant drop in pressure ulcers acquired during hospitalization among high-risk surgical patients, according to a prospective study.
Moreover, the beneficial effect of the program was sustained for more than a year after the program was implemented, reported Dr. Sylvia Martinez of Baylor College of Medicine and the Michael E. DeBakey VA Medical Center in Houston.
Dr. Martinez pointed out that strategies used to prevent hospital-acquired pressure ulcers are not standardized, and that in an effort to improve quality of care, save money, and reduce mortality, the Centers for Medicare & Medicaid Services no longer reimburses medical facilities for stage III and IV hospital-acquired pressure ulcers.
More than 7,000 high-risk patients were included in the study. During the 6 months before the plan was implemented at the medical center, the mean pressure ulcer rate overall was 3.37, dropping to 1.45 during the 6 months after implementation, a significant difference (P = .004). At 18 months, the rate was 0.89. During this time, the mean rates of hospital-acquired pressure ulcers in the entire VA system did not significantly change, ranging from 1.68 to 1.85, Dr. Martinez reported at the annual meeting of the Surgical Infection Society.
The study was done to address the relatively high rate of pressure ulcers in the medical center’s surgical service. Dr. Martinez and her associates evaluated the impact of an evidence-based hospital-acquired pressure ulcer prevention bundle, developed by an interdisciplinary team.
Patients were classified as high risk if they met at least two of four criteria (age greater than 62 years, albumin less than 3.5 g/dL, ASA score greater than 3, and surgery lasting for more than 3 hours).
The components of the program included:
• Comprehensive skin assessment and documentation and identification of any ulcers on admission, transfer, and discharge.
• Use of operating room beds with pressure redistributing mattresses, with careful positioning and padding of pressure points.
• Turning of patients every 2 hours with pressure point relief via wedges, heel pads, and pillows, as well as massages of bony prominences.
• Assessment of patients for pressure ulcers on daily rounds (led by the unit nurse manager) and use of daily checklists in high-risk patients.
The pressure ulcer rate was calculated by the number of discharged acute-care patients with a stage II or greater hospital-acquired pressure ulcer, divided by number patient days in the hospital (the discharge date minus the admission date for all discharged acute-care patients who were hospitalized for at least 48 hours).
During the period studied, 21,377 operations were performed. Almost 5,000 (4,692) were during the 6-month period prior to implementation of the program, from January to June 2012, and 16,685 were done in the 18 months after implementation (including 4,461 during the first 6 months). Overall, 34% of the patients were determined to be at high risk.
The rates dropped significantly in different parts of the surgical service: In the surgical intensive care unit, the rate dropped from 2.93 before implementation to 1.25 at 6 months and 0.86 at 18 months post implementation. In the surgical wards, the rate dropped from 2.93 to 0.83 at 6 months, and was 0.86 at 18 months.
Implementation of this bundle "may decrease hospital-acquired pressure ulcer rates in high risk surgical patients," with sustained lower rates, which "can lead to decreased costs, complications, and length of stay, and improved quality of life for our patients," Dr. Martinez concluded.
Among the study’s limitations were that it was conducted at one hospital and it did not evaluate which elements of the prevention bundle were most important, she said.
Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
AT THE SIS ANNUAL MEETING
Key clinical point: Use preventive measures to reduce pressure ulcers in high-risk surgical patients.
Major finding: The rate of pressure ulcers acquired during hospitalization dropped from 3.37 during the 6 months before a prevention bundle was implemented to 0.89 18 months afterward.
Data source: A prospective study of surgical patients at high risk for developing bedsores, comparing rates of hospital-acquired pressure ulcers 6 and 18 months after preventive interventions with the 6-month period beforehand.
Disclosures: Dr. Martinez had no disclosures. The study was funded by the U.S. Department of Veterans Affairs.
Implantable device approved for remotely monitoring PA in heart failure patients
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.
An implantable device that provides measurements of pulmonary arterial pressure in patients with class III heart failure has been approved by the Food and Drug Administration, based on a study that showed the use of the device to remotely monitor patients reduced heart failure hospitalization rates.
The CardioMEMS HF System "is the first permanently implantable wireless system intended to provide PA pressure measurements, including systolic, diastolic, and mean PA pressures," according to the FDA statement announcing the approval on May 28. This information is remotely reviewed by the patient’s physician, who "can make decisions regarding the status of the patient and, if necessary, initiate changes in medical therapy, with the goal of reducing hospitalization due to heart failure," the statement said.
It is specifically approved for patients with New York Heart Association (NYHA) class III heart failure (HF) who have been hospitalized for heart failure in the previous year.
The three components of the system are the battery-free sensor/monitor that is permanently implanted in the pulmonary artery, a transvenous catheter that deploys the sensor in the distal PA, and an electronic system that receives and processes the signals from the sensor/monitor and transfers the PA pressure measurements to a secure database, the statement said. Patients can be monitored from their home or another remote location.
Approval was based on a study of 550 patients with NYHA class III HF and a recent hospitalization for HF, who had the device implanted. Physicians had access to daily PA measurements only for the patients randomized to the treatment group, and adjusted HF medications based on the values provided. At 6 months, the HF hospitalization rate was significantly lower among those in the treatment group. The FDA statement noted that at 6 months, almost 99% of the patients who had the device implanted or in whom implantation was attempted had no complications related to the device or system, and all of the devices that were implanted were operating normally.
However, concerns about the study held up approval of the device for several years, and at a meeting in December 2011, the majority of the FDA’s Circulatory System Devices Panel agreed that the risks of the device outweighed the benefits. The manufacturer, CardioMEMS, provided follow-up data and further analyses of the data that were provided at another meeting of the panel, in October 2013. At that meeting, the majority of the panel agreed that the benefits of the device outweighed its risks for monitoring patients who met the criteria specified in the indication that has been approved, patients with NYHA class III heart failure who have been hospitalized for HF in the previous year.
In the May 28 statement, the FDA said that the company is required to conduct a postmarketing study to evaluate the performance of the device when used outside of a clinical trial. One concern of the panelists who supported approval at the 2013 meeting was that the benefit in terms of HF hospitalizations was not evident in women in the study, which they said could have been due to the low number of women enrolled in the trial, and they recommended that the device be studied in more women.
A CPAP alternative, implantable tongue stimulator is ahead for apnea
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.