User login
Need-to-know information for the 2016-2017 flu season
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
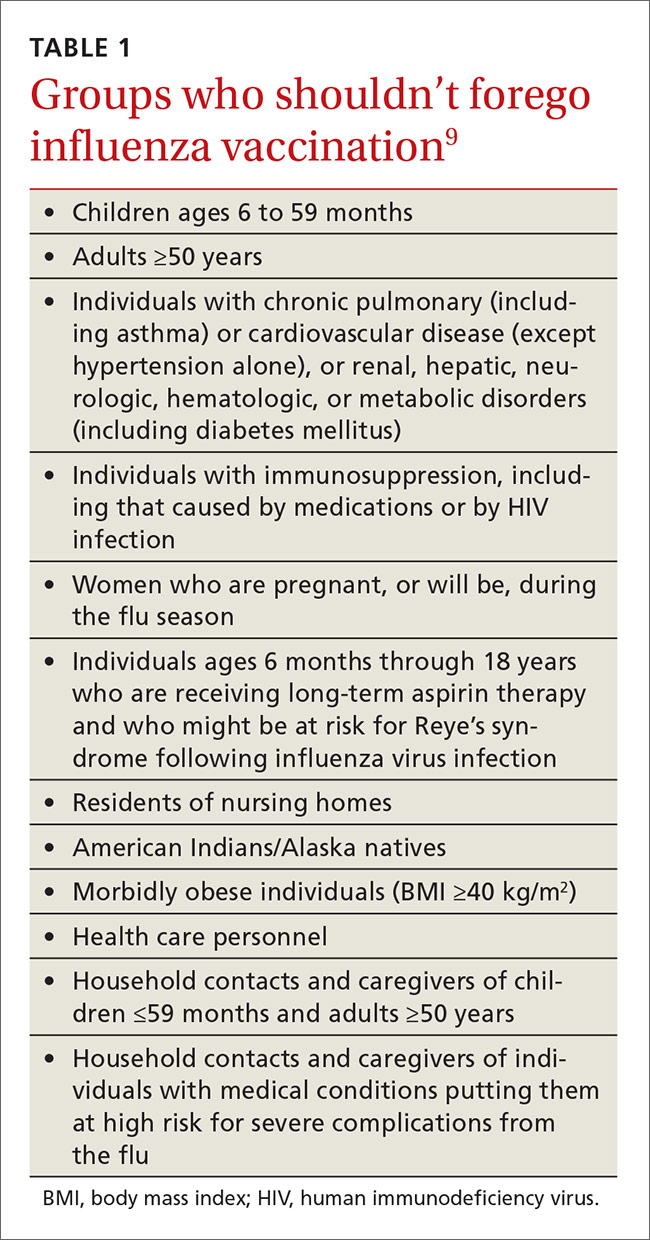
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
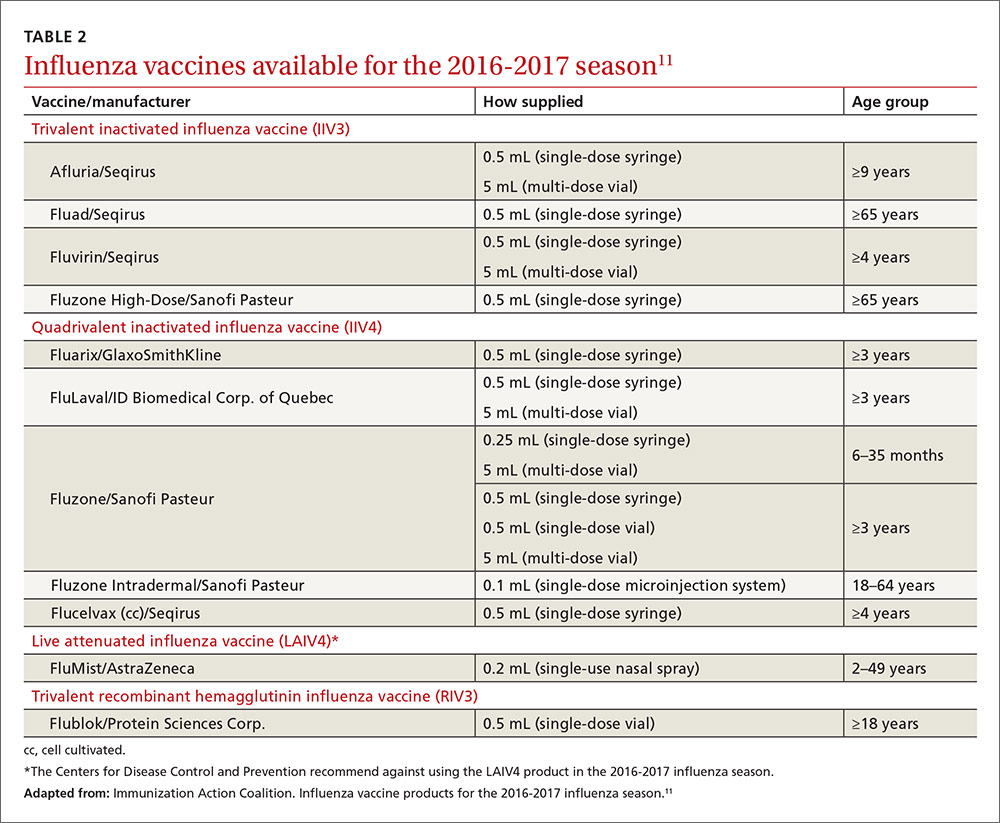
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
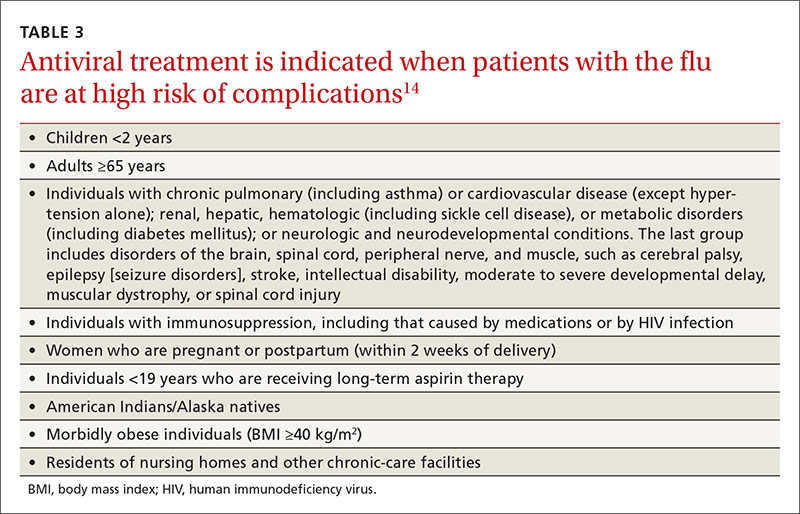
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
Colorectal cancer screening: The USPSTF’s final word
USPSTF update: Screening for abnormal blood glucose, diabetes
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
The resurgence of syphilis: New USPSTF screening recommendations
8 USPSTF recommendations FPs need to know about
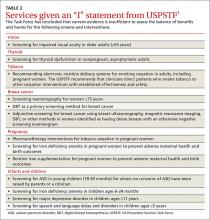
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
From The Journal of Family Practice | 2016;65(5):338-341.
Who benefits most from aspirin to prevent CVD and colorectal cancer?
What every FP needs to know about Zika virus
Immunization update: This year’s changes
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
2 new USPSTF draft recommendations—what you need to know
USPSTF urges extra step before treating hypertension
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
Screening for and treating high blood pressure (HBP) to prevent cardiovascular and renal disease is a tried-and-true preventive intervention that is supported by strong evidence. And not surprisingly, when the US Preventive Services Task Force (USPSTF) recently updated its 2007 recommendation for blood pressure screening for adults, it once again gave an A recommendation for those ages 18 years and older. What is noteworthy, however, is that this update concentrates on the accuracy of blood pressure measurement methods and optimal frequency of screening.1
The most significant modification of past recommendations is that HBP found with office measurement of blood pressure (OMBP) should be confirmed with either ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) before starting treatment. (For its recommendation, the USPSTF used the HBP definition from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [TABLE 1].2,3)
Ensuring accurate blood-pressure measurements. More than 30% of adults in the United States have HBP, with prevalence increasing with age (TABLE 2).2 Only about half of this population has HBP under control.4 This modifiable condition contributes to more than 360,000 deaths annually.2 However, while treatment of true HBP results in substantial benefits, it is important not to over-diagnose HBP and over-treat it.
Studies have shown that 15% to 30% of individuals diagnosed with HBP in a clinical setting will have blood pressure in the normal range when measurements are taken outside of the doctor’s office.1 This discrepancy can be due to measurement error, regression to the mean, recent caffeine ingestion by the patient, or isolated clinical hypertension wherein the stress and anxiety caused by clinic visits elevates blood pressure transiently.
With this in mind, the USPSTF recommends that OMBP-detected HBP be confirmed with either ABPM or HBPM. Of these 2 follow-up methods, ABPM is supported by stronger evidence and is preferred. The USPSTF includes HBPM as an alternative because ABPM equipment may not always be available—or affordable—and using the equipment may present logistical challenges.
Starting off on the right foot
Screening for HBP in a clinical setting is more accurate if conducted according to recommended procedures: use an appropriately sized cuff; take the measurement at least 5 minutes after the patient’s arrival while he or she is seated with legs uncrossed and the cuffed arm is at the level of the heart; and record the mean of 2 separate measurements. There appears to be no real difference in the accuracy of automated vs manual sphygmomanometers.
Optimal frequency of screening varies. While the USPSTF found little evidence to support any particular overall screening frequency, it recommends annual screening for those who are 40 years of age or older and those ages 18 to 39 who are obese or overweight, are African American, or who have high-normal blood pressure (TABLE 3).1 Screening every 3 to 5 years is recommended for individuals not in these categories.
Initial steps in treating HBP. The Task Force also commented on which medications to use when initiating HBP treatment (after lifestyle and dietary interventions). Non-African Americans should receive a thiazide diuretic, calcium channel blocker, angiotensin-converting enzyme inhibitor, or angiotensin-receptor blocker. African Americans should begin treatment with a thiazide diuretic or calcium channel blocker. These recommendations appear to have been adopted from the Eighth Joint National Committee, since the accompanying evidence report for the USPSTF’s update did not address this issue.5
Don't forget patient support
Patient support is key. As of June 2015, the Community Preventive Services Task Force (CPSTF) recommends self-measured blood pressure monitoring combined with additional support as a means of improving blood pressure control in those with HBP.4
Supportive measures include things such as patient counseling on medications and health behavior changes (eg, diet and exercise); education on HBP and blood pressure self-management; and use of secure electronic or Web-based tools such as text or e-mail reminders to measure blood pressure, show up for appointments, or communicate blood pressure readings to healthcare providers. Patients who participate in home self-measurement of blood pressure with additional support lower their systolic blood pressure, on average, 1.4 mm Hg more than those who do not.4
Remaining questions
The new USPSTF recommendation leaves several issues unaddressed. For one thing, the Affordable Care Act mandates that commercial health insurance plans provide services with an A or B Task Force recommendation to patients with no copayments. So does the new HBP recommendation mean payers have to make ABPM and HBPM available to patients at no charge?
There are other questions, too. If HBP detected by OMBP is not confirmed when ABPM is performed, should ABPM be repeated, and if so, at what interval? What is the role of emerging technologies that use devices other than arm cuffs to measure blood pressure?
Despite these uncertainties, the new USPSTF and CPSTF recommendations refine the longstanding in-office–only approach to diagnosing and monitoring HBP and advocate newer technologies that could help improve diagnostic accuracy, avoid over-diagnosis and over-treatment, and improve patient adherence to treatment goals.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. US Preventive Services Task Force. High blood pressure in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/high-blood-pressure-in-adults-screening. Accessed November 24, 2015.
2. Piper MA, Evans CV, Burda BU, et al. Screening for high blood pressure in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK269495/. Accessed November 24, 2015.
3. US Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: http://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Accessed November 24, 2015.
4. Community Preventive Services Task Force. Cardiovascular disease prevention and control: self-measured blood pressure monitoring interventions for improved blood pressure control — when combined with additional support. Available at: http://www.thecommunityguide.org/cvd/SMBP-additional.html. Accessed November 24, 2015.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.