User login
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
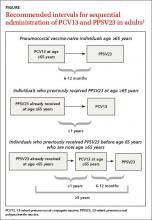
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
In August 2014, the Advisory Committee on Immunization Practices (ACIP) decided to add the 13-valent pneumococcal conjugate vaccine (PCV13) to the routine immunization schedule for adults ages 65 years and older; previously, it had recommended that these patients receive only the 23-valent pneumococcal polysaccharide vaccine (PPSV23).1 The US Food and Drug Administration (FDA) had approved PCV13 for use in adults ages 50 years and older in late 2011. The delay between FDA approval and this new ACIP recommendation occurred for 2 reasons: The epidemiology of pneumococcal disease (pneumonia, meningitis, and bacteremia) in older adults is evolving due to the widespread use of PCV13 in children, and a large clinical trial looking at the efficacy of this vaccine in individuals 65 and older was still underway.
Pneumococcal disease in older adults remains a problem
Routine use of the 7-valent pneumococcal conjugate vaccine (PCV7) in children began in 2000. In 2010, the vaccine was expanded to include 6 more antigens (PCV13). The routine use of this vaccine has markedly reduced pneumococcal disease in children and, by way of indirect protection, in adults. Between 2010 and 2013, the incidence of invasive pneumococcal disease (eg, meningitis and bacteremia) caused by the 13 serotypes in the vaccine had decreased by 50% in adults ages 65 years and older.1 However, in this age group, there are still more than 13,000 cases of invasive pneumococcal disease each year.1 Approximately 20% of these cases—and 10% of cases community-acquired pneumonia (CAP) in this age group—are still caused by one of the PCV13 serotypes. This epidemiology left ACIP to consider whether to recommend PCV13 for older adults even though the incidence of pneumococcal disease was declining without the use of the vaccine. ACIP took a middle-of-the-road position on August 13, 2014 by recommending the vaccine now but agreeing to reexamine the issue again in 2018.1
PCV13 substantially cuts the rate of pneumococcal disease
In June 2014, ACIP reviewed the results of a large randomized, placebo-controlled clinical trial of PCV13 in 85,000 adults ages 65 years and older that was conducted in the Netherlands from 2008 to 2013.1 PCV13 reduced the rate of disease caused by the vaccine serotypes by 45.6% for pneumonia and 75% for invasive pneumococcal disease.
Because the population in this study was PPSV23-naïve, the added advantage of PCV13 in patients who have been vaccinated with PPSV23 has not been determined. Twelve of the 13 serotypes in PCV13 are in PPSV23. And while PPSV23 can protect against invasive pneumococcal disease, its effectiveness against CAP is less well proven.
Using modeling that took into consideration anticipated rates of vaccination with both PCV13 and PPSV23 in adults and children, the Centers for Disease Control and Prevention estimated that adding PCV13 to the adult immunization schedule would prevent 230 cases of invasive pneumococcal disease and 12,000 cases of CAP over the lifetime of a cohort of 65 year olds.1 With time, however, and the increasing indirect protection from routine use of PCV13 in children, these numbers would decline.
Timing of administration depends on patients’ vaccine history
Adults 65 years of age and older should receive both PCV13 and PPSV23, but not at the same time. In those who have not received any pneumococcal vaccine, the preferred sequence is to first administer PCV13 and then PPSV23 6 to 12 months later (FIGURE); the minimum acceptable interval between PCV13 and PPSV23 is 8 weeks.1 If PPSV23 is administered first, PCV13 should not be given until at least 12 months after the PPSV23 dose. This is because the immune response to PCV13 is not as robust when PCV13 follows PPSV23.
For patients who have been vaccinated with PPSV23 before age 65, PCV13 should be administered at least 12 months after PPSV23, followed by another dose of PPSV23 that should be administered 6 to 12 months after PCV13, but no sooner than 5 years since the previous PPSV23 (FIGURE).
Coadministration of PCV13 with trivalent influenza vaccine results in a slight decrease in the immune response to each vaccine;1 this is unlikely to be clinically important. Coadministration with other vaccines has not been studied.
Who’ll reimburse for the PCV13 vaccine? One issue that could delay the use of both vaccines in older adults is that currently, Medicare pays for only one pneumococcal vaccine in patients who are 65 and older. The Centers for Medicare and Medicaid Services will attempt to amend this policy, but how quickly this will occur is unknown.
Different recommendations for patients at higher risk
There are 2 sets of recommendations for use of pneumococcal vaccines: one for routine use for most patients, and a separate set of recommendations for those with conditions that put them at higher risk of infections and/or complications from pneumococcal disease.1-4 PPSV23 is recommended for children (starting at age 2 years) and adults with certain high-risk medical conditions, such as chronic heart, lung, or liver disease, and diabetes; functional or anatomical asplenia; or immunocompromising conditions such as human immunodeficiency virus infection, chronic renal failure, leukemia, or lymphoma.3 PPSV23 should be repeated 5 years after the first dose in patients with asplenia, those who are immunocompromised, and for everyone age 65 and older who received it before age 65. No more than 3 doses of PPSV23 should be given to anyone.
PCV13 is recommended for previously unvaccinated children and adults who have cochlear implants, cerebrospinal fluid leaks, functional or anatomical asplenia, or are immunocompromised.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.
1. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822-825.
2. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61:816-819.
3. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6-18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:521-524.
4. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59:1-18.