User login
Hepatitis A is on the rise: What FPs can do
In September 2021, a community in Virginia experienced an outbreak of hepatitis A virus (HAV) that was ultimately linked to an infected food handler.1 A total of 149 cases were reported over the next 12 months; 51 were directly related to the food handler and the remainder were the result of sustained community transmission. Of the 51 people who were directly infected by the food handler, 31 were hospitalized and 3 died. This incident offers important reminders about public health surveillance and the role that family physicians can play.
Hepatitis A virus is transmitted through food and drinks that have been contaminated by small amounts of stool that contains the virus or through close contact (including sexual contact) with a person who is infected. The incubation period can range from 15 to 59 days.
HAV generally resolves in a few days to weeks, with no long-term effects. However, recent outbreaks have been associated with high hospitalization and mortality rates because of the underlying comorbidities of those infected.
An increase in incidence. The national rate of HAV infection reached a low of less than 1/100,000 in 2015 but has since increased to almost 6/100,000 in 2019. This increase is mostly due to outbreaks linked to spread among people without a fixed residence, those who use illicit drugs, and men who have sex with men.2
In the Virginia outbreak, the food handler had a risk factor for HAV and was unvaccinated. He worked at 3 different locations of a restaurant chain for a total of 16 days while infectious, preparing ready-to-eat food without using gloves. Furthermore, he delayed seeking medical care for more than 2 weeks—at which time, the nature of his employment was not disclosed.
Prevention is straightforward. HAV infection can be prevented by administration of either HAV vaccine or immune globulin within 2 weeks of exposure.3 During an HAV outbreak, vaccination is recommended for people considered to be at risk, including those without a fixed residence, those who use illicit drugs, those who travel internationally, and men who have sex with men.3
There are 3 HAV vaccines available in the United States: 2 single-antigen vaccines, Havrix and Vaqta, both approved for children and adults, and a combination vaccine (containing both HAV and hepatitis B antigens), Twinrix, which is approved for those ages 18 years and older. All are inactivated vaccines.
What you can do. The Virginia outbreak illustrates the important role that family physicians can and do play in public health. We should:
- Encourage adults with risk factors for HAV to be vaccinated.
- Ask those with an HAV diagnosis about the people they may have exposed through personal contact or occupational exposure.
- Promptly report infectious diseases that are designated “reportable” to the public health department.
- Immediately report (by telephone) when HAV and other enteric infections involve a food handler.
1. Helmick MJ, Morrow CB, White JH, et al. Widespread community transmission of Hepatitis A Virus following an outbreak at a local restaurant—Virginia, September 2021-September 2022. MMWR Morb Mortal Wkly Rep. 2023;72;362-365. doi: 10.15585/mmwr.mm7214a2
2. CDC. Hepatitis A questions and answers for health professionals. Updated July 28, 2020. Accessed April 25, 2023. www.cdc.gov/hepatitis/hav/havfaq.htm
3. Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1-38. doi: 10.15585/mmwr.rr6905a1
In September 2021, a community in Virginia experienced an outbreak of hepatitis A virus (HAV) that was ultimately linked to an infected food handler.1 A total of 149 cases were reported over the next 12 months; 51 were directly related to the food handler and the remainder were the result of sustained community transmission. Of the 51 people who were directly infected by the food handler, 31 were hospitalized and 3 died. This incident offers important reminders about public health surveillance and the role that family physicians can play.
Hepatitis A virus is transmitted through food and drinks that have been contaminated by small amounts of stool that contains the virus or through close contact (including sexual contact) with a person who is infected. The incubation period can range from 15 to 59 days.
HAV generally resolves in a few days to weeks, with no long-term effects. However, recent outbreaks have been associated with high hospitalization and mortality rates because of the underlying comorbidities of those infected.
An increase in incidence. The national rate of HAV infection reached a low of less than 1/100,000 in 2015 but has since increased to almost 6/100,000 in 2019. This increase is mostly due to outbreaks linked to spread among people without a fixed residence, those who use illicit drugs, and men who have sex with men.2
In the Virginia outbreak, the food handler had a risk factor for HAV and was unvaccinated. He worked at 3 different locations of a restaurant chain for a total of 16 days while infectious, preparing ready-to-eat food without using gloves. Furthermore, he delayed seeking medical care for more than 2 weeks—at which time, the nature of his employment was not disclosed.
Prevention is straightforward. HAV infection can be prevented by administration of either HAV vaccine or immune globulin within 2 weeks of exposure.3 During an HAV outbreak, vaccination is recommended for people considered to be at risk, including those without a fixed residence, those who use illicit drugs, those who travel internationally, and men who have sex with men.3
There are 3 HAV vaccines available in the United States: 2 single-antigen vaccines, Havrix and Vaqta, both approved for children and adults, and a combination vaccine (containing both HAV and hepatitis B antigens), Twinrix, which is approved for those ages 18 years and older. All are inactivated vaccines.
What you can do. The Virginia outbreak illustrates the important role that family physicians can and do play in public health. We should:
- Encourage adults with risk factors for HAV to be vaccinated.
- Ask those with an HAV diagnosis about the people they may have exposed through personal contact or occupational exposure.
- Promptly report infectious diseases that are designated “reportable” to the public health department.
- Immediately report (by telephone) when HAV and other enteric infections involve a food handler.
In September 2021, a community in Virginia experienced an outbreak of hepatitis A virus (HAV) that was ultimately linked to an infected food handler.1 A total of 149 cases were reported over the next 12 months; 51 were directly related to the food handler and the remainder were the result of sustained community transmission. Of the 51 people who were directly infected by the food handler, 31 were hospitalized and 3 died. This incident offers important reminders about public health surveillance and the role that family physicians can play.
Hepatitis A virus is transmitted through food and drinks that have been contaminated by small amounts of stool that contains the virus or through close contact (including sexual contact) with a person who is infected. The incubation period can range from 15 to 59 days.
HAV generally resolves in a few days to weeks, with no long-term effects. However, recent outbreaks have been associated with high hospitalization and mortality rates because of the underlying comorbidities of those infected.
An increase in incidence. The national rate of HAV infection reached a low of less than 1/100,000 in 2015 but has since increased to almost 6/100,000 in 2019. This increase is mostly due to outbreaks linked to spread among people without a fixed residence, those who use illicit drugs, and men who have sex with men.2
In the Virginia outbreak, the food handler had a risk factor for HAV and was unvaccinated. He worked at 3 different locations of a restaurant chain for a total of 16 days while infectious, preparing ready-to-eat food without using gloves. Furthermore, he delayed seeking medical care for more than 2 weeks—at which time, the nature of his employment was not disclosed.
Prevention is straightforward. HAV infection can be prevented by administration of either HAV vaccine or immune globulin within 2 weeks of exposure.3 During an HAV outbreak, vaccination is recommended for people considered to be at risk, including those without a fixed residence, those who use illicit drugs, those who travel internationally, and men who have sex with men.3
There are 3 HAV vaccines available in the United States: 2 single-antigen vaccines, Havrix and Vaqta, both approved for children and adults, and a combination vaccine (containing both HAV and hepatitis B antigens), Twinrix, which is approved for those ages 18 years and older. All are inactivated vaccines.
What you can do. The Virginia outbreak illustrates the important role that family physicians can and do play in public health. We should:
- Encourage adults with risk factors for HAV to be vaccinated.
- Ask those with an HAV diagnosis about the people they may have exposed through personal contact or occupational exposure.
- Promptly report infectious diseases that are designated “reportable” to the public health department.
- Immediately report (by telephone) when HAV and other enteric infections involve a food handler.
1. Helmick MJ, Morrow CB, White JH, et al. Widespread community transmission of Hepatitis A Virus following an outbreak at a local restaurant—Virginia, September 2021-September 2022. MMWR Morb Mortal Wkly Rep. 2023;72;362-365. doi: 10.15585/mmwr.mm7214a2
2. CDC. Hepatitis A questions and answers for health professionals. Updated July 28, 2020. Accessed April 25, 2023. www.cdc.gov/hepatitis/hav/havfaq.htm
3. Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1-38. doi: 10.15585/mmwr.rr6905a1
1. Helmick MJ, Morrow CB, White JH, et al. Widespread community transmission of Hepatitis A Virus following an outbreak at a local restaurant—Virginia, September 2021-September 2022. MMWR Morb Mortal Wkly Rep. 2023;72;362-365. doi: 10.15585/mmwr.mm7214a2
2. CDC. Hepatitis A questions and answers for health professionals. Updated July 28, 2020. Accessed April 25, 2023. www.cdc.gov/hepatitis/hav/havfaq.htm
3. Nelson NP, Weng MK, Hofmeister MG, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1-38. doi: 10.15585/mmwr.rr6905a1
Folic acid: A recommendation worth making
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
The US Preventive Services Task Force (USPSTF) recently published a draft recommendation on the use of folic acid before and during pregnancy to prevent fetal neural tube defects.1 This reaffirmation of the 2017 recommendation states that all persons planning to or who could become pregnant should take a daily supplement of folic acid.1,2 This is an “A” recommendation.
Neural tube defects are caused by a failure of the embryonic neural tube to close completely, which should occur in the first 28 days following fertilization. This is why folic acid is most effective if started at least 1 month before conception and continued for the first 2 to 3 months of pregnancy.
An estimated 3000 neural tube defects occur each year in the United States. Spina bifida, anencephaly, and encephalocele occur at respective rates of 3.9, 2.5, and 1.0 in 10,000 live births in the United States, which totals 7.4/10,000.3
Folic acid, if taken before and during pregnancy, can prevent about half of neural tube defects; if taken only during pregnancy, it prevents about one-third. If 50% of neural tube defects could be prevented with folic acid supplements, the number needed to treat (NNT) to prevent 1 case is about 3000.4
The case for supplementation. The recommended daily dose of folic acid is between 0.4 mg (400 μg) and 0.8 mg (800 μg), which is contained in many multivitamin products. Certain enriched cereal grain products in the United States have been fortified with folic acid for more than 2 decades, but it is unknown whether women in the United States are ingesting enough of these fortified foods to provide maximum prevention of neural tube defects. There are no known harms to mother or fetus from folic acid supplementation at recommended levels.
Room for improvement. Only 20% to 40% of people who are pregnant or trying to get pregnant, and 5% to 10% of people with an unplanned pregnancy, take folic acid supplements. Half of all pregnancies in the United States are unplanned.4 This leaves a lot of room for improvement in the prevention of neural tube defects.
An important recommendation, even if you don’t see the results. The NNT to prevent a case of neural tube defect is high; most family physicians providing perinatal care will not prevent a case during their career. And, as with most preventive interventions, we do not see the cases prevented. However, on a population-wide basis, if all women took folic acid as recommended, the number of severe birth defects prevented would be significant—making this simple recommendation worth mentioning to those of reproductive age.
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
1. USPSTF. Folic acid supplementation to prevent neural tube defects. Published February 21, 2023. Accessed March 22, 2023. https://uspreventiveservicestaskforce.org/home/getfilebytoken/sX6CTKHncTJT2nzmu7yLHh
2. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Published January 10, 2017. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
3. Mai CT, Isenburg JL, Canfield MA, et al. National population-based estimates for major birth defects, 2010-2014. Birth Defects Res. 2019;111:1420-1435. doi: 10.1002/bdr2.1589
4. Viswanathan M, Urrutia RP, Hudson KN, et al. Folic acid supplementation to prevent neural tube defects: a limited systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 230. Published February 2023. Accessed March 22, 2023. www.uspreventiveservicestaskforce.org/home/getfilebytoken/AjUYoBvpfUBDAFjHeCcfPz
5 non-COVID vaccine recommendations from ACIP
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13
Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Key takeaways from ACP’s new Tx guidelines for adults with major depressive disorder
In January 2023, the American College of Physicians (ACP) published updated recommendations on the treatment of adults with major depressive disorder (MDD).1 The ACP guidelines address initial treatment of patients in the acute phase of mild and moderate-to-severe MDD. Here’s what the ACP recommends, as well as 6 important takeaways.
Recommendations for initial treatment of those with mild or moderate-to-severe MDD center around cognitive behavioral therapy (CBT) and second-generation antidepressants (SGA). For patients in the acute phase of mild MDD, the recommendation is for monotherapy with CBT. However, if CBT is not an option due to cost and/or availability of services, the use of an SGA is acceptable.
For patients in the acute phase of moderate-to-severe MDD, either CBT, an SGA, or a combination of both is recommended.
If initial treatment does not work … Up to 70% of patients with moderate-to-severe MDD will not respond to the initial therapy chosen. If a patient does not respond to initial treatment with an SGA, consider 1 of the following:
- Switching to CBT
- Adding on CBT while continuing the SGA
- Changing to a different SGA
- Adding a second pharmacologic agent.
6 key takeaways. The full guideline should be read for a more complete discussion of the many clinical considerations of these treatment options. However, the most important points include:
• Employ shared clinical decision-making and consider the individual characteristics of each patient when making treatment decisions.
• Consider generic options when using an SGA; generic options appear to be as effective as more expensive brand-name products.
• Start with a low-dose SGA and increase gradually to an approved maximum dose before determining there has been no response.
• Monitor frequently for medication adverse effects.
• Monitor the patient for thoughts about self-harm for the first 2 months.
• Continue treatment for 4 to 9 months once remission is achieved.
A word about strength of evidence. While these recommendations are based on an extensive review of the best available evidence, most are based on low-certainty evidence—illustrating the amount of clinical research still needed on this topic. The exceptions are monotherapy with either CBT or SGA for initial treatment of moderate-to-severe MDD, both of which are based on moderate-strength evidence and received a strong recommendation. The panel felt there was insufficient evidence to assess complementary and alternative interventions including exercise and omega-3 fatty acids.
1. Qaseem A, Owens D, Etxeandia-Ikobaltzeta I, et al; Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic and pharmacologic treatments of adults in the acute phase of major depressive disorder: a living clinical guideline from the American College of Physicians. Ann Intern Med. Published online January 24, 2023. doi: 10.7326/M22-2056
In January 2023, the American College of Physicians (ACP) published updated recommendations on the treatment of adults with major depressive disorder (MDD).1 The ACP guidelines address initial treatment of patients in the acute phase of mild and moderate-to-severe MDD. Here’s what the ACP recommends, as well as 6 important takeaways.
Recommendations for initial treatment of those with mild or moderate-to-severe MDD center around cognitive behavioral therapy (CBT) and second-generation antidepressants (SGA). For patients in the acute phase of mild MDD, the recommendation is for monotherapy with CBT. However, if CBT is not an option due to cost and/or availability of services, the use of an SGA is acceptable.
For patients in the acute phase of moderate-to-severe MDD, either CBT, an SGA, or a combination of both is recommended.
If initial treatment does not work … Up to 70% of patients with moderate-to-severe MDD will not respond to the initial therapy chosen. If a patient does not respond to initial treatment with an SGA, consider 1 of the following:
- Switching to CBT
- Adding on CBT while continuing the SGA
- Changing to a different SGA
- Adding a second pharmacologic agent.
6 key takeaways. The full guideline should be read for a more complete discussion of the many clinical considerations of these treatment options. However, the most important points include:
• Employ shared clinical decision-making and consider the individual characteristics of each patient when making treatment decisions.
• Consider generic options when using an SGA; generic options appear to be as effective as more expensive brand-name products.
• Start with a low-dose SGA and increase gradually to an approved maximum dose before determining there has been no response.
• Monitor frequently for medication adverse effects.
• Monitor the patient for thoughts about self-harm for the first 2 months.
• Continue treatment for 4 to 9 months once remission is achieved.
A word about strength of evidence. While these recommendations are based on an extensive review of the best available evidence, most are based on low-certainty evidence—illustrating the amount of clinical research still needed on this topic. The exceptions are monotherapy with either CBT or SGA for initial treatment of moderate-to-severe MDD, both of which are based on moderate-strength evidence and received a strong recommendation. The panel felt there was insufficient evidence to assess complementary and alternative interventions including exercise and omega-3 fatty acids.
In January 2023, the American College of Physicians (ACP) published updated recommendations on the treatment of adults with major depressive disorder (MDD).1 The ACP guidelines address initial treatment of patients in the acute phase of mild and moderate-to-severe MDD. Here’s what the ACP recommends, as well as 6 important takeaways.
Recommendations for initial treatment of those with mild or moderate-to-severe MDD center around cognitive behavioral therapy (CBT) and second-generation antidepressants (SGA). For patients in the acute phase of mild MDD, the recommendation is for monotherapy with CBT. However, if CBT is not an option due to cost and/or availability of services, the use of an SGA is acceptable.
For patients in the acute phase of moderate-to-severe MDD, either CBT, an SGA, or a combination of both is recommended.
If initial treatment does not work … Up to 70% of patients with moderate-to-severe MDD will not respond to the initial therapy chosen. If a patient does not respond to initial treatment with an SGA, consider 1 of the following:
- Switching to CBT
- Adding on CBT while continuing the SGA
- Changing to a different SGA
- Adding a second pharmacologic agent.
6 key takeaways. The full guideline should be read for a more complete discussion of the many clinical considerations of these treatment options. However, the most important points include:
• Employ shared clinical decision-making and consider the individual characteristics of each patient when making treatment decisions.
• Consider generic options when using an SGA; generic options appear to be as effective as more expensive brand-name products.
• Start with a low-dose SGA and increase gradually to an approved maximum dose before determining there has been no response.
• Monitor frequently for medication adverse effects.
• Monitor the patient for thoughts about self-harm for the first 2 months.
• Continue treatment for 4 to 9 months once remission is achieved.
A word about strength of evidence. While these recommendations are based on an extensive review of the best available evidence, most are based on low-certainty evidence—illustrating the amount of clinical research still needed on this topic. The exceptions are monotherapy with either CBT or SGA for initial treatment of moderate-to-severe MDD, both of which are based on moderate-strength evidence and received a strong recommendation. The panel felt there was insufficient evidence to assess complementary and alternative interventions including exercise and omega-3 fatty acids.
1. Qaseem A, Owens D, Etxeandia-Ikobaltzeta I, et al; Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic and pharmacologic treatments of adults in the acute phase of major depressive disorder: a living clinical guideline from the American College of Physicians. Ann Intern Med. Published online January 24, 2023. doi: 10.7326/M22-2056
1. Qaseem A, Owens D, Etxeandia-Ikobaltzeta I, et al; Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic and pharmacologic treatments of adults in the acute phase of major depressive disorder: a living clinical guideline from the American College of Physicians. Ann Intern Med. Published online January 24, 2023. doi: 10.7326/M22-2056
CDC updates guidance on opioid prescribing in adults
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
Latent TB: The case for vigilance
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
The US Preventive Services Task Force (USPSTF) recently released draft recommendations on screening for tuberculosis (TB).1 The USPSTF continues to recommend screening for latent TB infection (LTBI) in those at high risk.
Why is this important? Up to one-quarter of the world’s population has been infected with TB, according to World Health Organization (WHO) estimates. In 2021, active TB was diagnosed in 10.6 million people, and it caused 1.6 million deaths.2 Worldwide, TB is still a major cause of mortality: It is the 13th leading cause of death and is the leading cause of infectious disease mortality in non-COVID years.
Although the rate of active TB in the United States has been declining for decades (from 30.7/100,000 in 1960 to 2.4/100,000 in 2021), 7882 cases were reported in 2021, and an estimated 13 million people in the United States have LTBI.3 If not treated, 5% to 10% of LTBI cases will progress to active TB. This risk is higher in those with certain medical conditions.3 People born outside the United States currently account for 71.4% of reported TB cases in the United States.3
To reduce the morbidity and mortality of TB, the Centers for Disease Control and Prevention (CDC), WHO, and USPSTF all recommend screening for and treating LTBI. An effective approach to TB control also includes early detection and completion of treatment for active TB, as well as testing contacts of active TB cases.
Who should be screened? Those at high risk for LTBI include those who were born in, or who have resided in, countries with high rates of TB (eg, Latin America, the Caribbean, Africa, Asia, Eastern Europe, and Russia); those who have lived in a correctional facility or homeless shelter; household and other close contacts of active TB cases; and health care workers who provide care to patients with TB.
Some chronic medical conditions can increase risk for progression to active TB in those with LTBI. Patients who should be tested for LTBI as part of their routine care include those who are HIV positive; are receiving immunosuppressive therapy (chemotherapy, biological immune suppressants); have received an organ transplant; have silicosis; use illicit injected drugs; and/or have had a gastrectomy or jejunoileal bypass.
In addition, local communities may have populations or geographic regions in which TB rates are high. Family physicians can obtain this information from their state or local health departments.
There are 2 screening tests for LTBI: TB blood tests (interferon-gamma release assays [IGRAs]) and the Mantoux tuberculin skin test (TST). Two TB blood tests are available in the United States: QuantiFERON-TB Gold Plus (QFT-Plus) and T-SPOT.TB test (T-Spot).
There are advantages and disadvantages to both types of tests. A TST requires accurate administration and interpretation and 2 clinic visits, 48 to 72 hours apart. The cutoff on a positive test (5, 10, or 15 mm) depends on the patient’s age and risk.4 An IGRA should be processed within 8 to 32 hours and is more expensive. However, a major advantage is that it is more specific, because it is unaffected by previous vaccination with bacille Calmette-Guérin or by most nontuberculous mycobacteria infections.
To rule out active TB ... If a TB screening test is positive, the recommended work-up is to ask about TB symptoms and perform a chest x-ray to rule out active pulmonary TB. Sputum collection for acid-fast smear and culture should be ordered for anyone with a suspicious chest x-ray, respiratory symptoms consistent with TB, or HIV infection.
Treatment for LTBI markedly reduces the risk for active TB. There are 4 options:
- Isoniazid (INH) plus rifapentine (RPT) once per week for 3 months.
- Rifampin (RIF) daily for 4 months.
- INH plus RIF daily for 3 months.
- INH daily for 6 or 9 months.
Details about the variables to consider in choosing a regimen are described on the CDC website.4,5
Know your resources. Local and state public health departments should have TB control programs and are sources of information on TB diagnosis and treatment; they also can assist with follow-up of TB contacts.6 Although LTBI is a reportable condition only in young children, any suspicion of community spread of active TB should be reported to the public health department.
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
1. USPSTF. Latent tuberculosis infection in adults: screening. Draft recommendation statement. Published November 22, 2022. Accessed December 14, 2022. www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/latent-tuberculosis-infection-adults
2. WHO. Tuberculosis: key facts. Updated October 27, 2022. Accessed December 14, 2022. www.who.int/news-room/fact-sheets/detail/tuberculosis
3. CDC. Tuberculosis: data and statistics. Updated November 29, 2022. Accessed December 14, 2022. www.cdc.gov/tb/statistics/default.htm
4. CDC. Latent TB infection: a guide for primary health care providers. Updated February 3, 2021. Accessed December 14, 2022. www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf
5. CDC. Treatment regimens for latent TB infection. Updated February 13, 2020. Accessed December 14, 2022. www.cdc.gov/tb/topic/treatment/ltbi.htm
6. CDC. TB control offices. Updated March 28, 2022. Accessed December 14, 2022. www.cdc.gov/tb/links/tboffices.htm
Whom to screen for anxiety and depression: Updated USPSTF recommendations
In September 2022, the US Preventive Services Task Force (USPSTF) released 2 sets of draft recommendations on screening for 3 mental health conditions in adults: anxiety, depression, and suicide risk.1,2 These draft recommendations are summarized in TABLE 11-4 along with finalized recommendations on the same topics for children and adolescents, published in October 2022.3,4
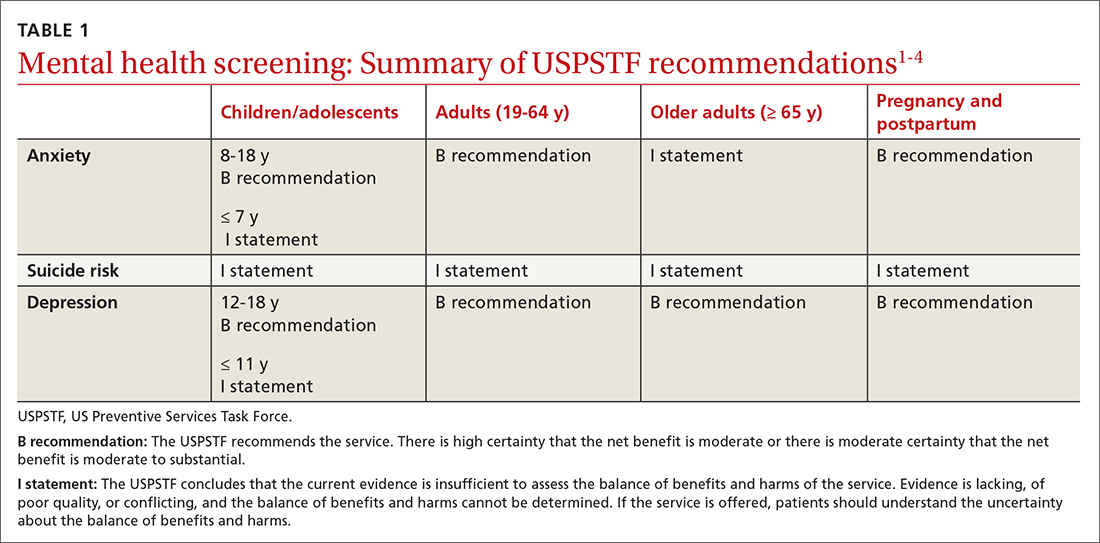
The recommendations on depression and suicide risk screening in adults are updates of previous recommendations (2016 for depression and 2014 for suicide risk) with no major changes. Screening for anxiety is a topic addressed for the first time this year for adults and for children and adolescents.1,3
The recommendations are fairly consistent between age groups. A “B” recommendation supports screening for major depression in all patients starting at age 12 years, including during pregnancy and the postpartum period. (See TABLE 1 for grade definitions.) For all age groups, evidence was insufficient to recommend screening for suicide risk. A “B” recommendation was also assigned to screening for anxiety in those ages 8 to 64 years. The USPSTF believes the evidence is insufficient to make a recommendation on screening for anxiety among adults ≥ 65 years of age.
The anxiety disorders common to both children and adults included in the USPSTF recommendations are generalized anxiety disorder, social anxiety disorder, panic disorder, separation anxiety disorder, phobias, selective mutism, and anxiety type not specified. For adults, the USPSTF also includes substance/medication-induced anxiety and anxiety due to other medical conditions.
Adults with anxiety often present with generalized complaints such as sleep disturbance, pain, and other somatic disorders that can remain undiagnosed for years. The USPSTF cites a lifetime prevalence of anxiety disorders of 26.4% for men and 40.4% for women, although the data used are 10 years old.5 The cited rate of generalized anxiety in pregnancy is 8.5% to 10.5%, and in the postpartum period, 4.4% to 10.8%.6
The data on direct benefits and harms of screening for anxiety in adults through age 64 are sparse. Nevertheless, the USPSTF deemed that screening tests for anxiety have adequate accuracy and that psychological interventions for anxiety result in moderate reduction of anxiety symptoms. Pharmacologic interventions produce a small benefit, although there is a lack of evidence for pharmacotherapy in pregnant and postpartum women. There is even less evidence of benefit for treatment in adults ≥ 65 years of age.1
How anxiety screening tests compare
Screening tests for anxiety in adults reviewed by the USPSTF included the Generalized Anxiety Disorder (GAD) scale and the Edinburgh Postnatal Depression Scale (EPDS) anxiety subscale.1 The most studied tools are the GAD-2 and GAD-7.
Continue to: The sensitivity and specificity...
The sensitivity and specificity of each test depends on the cutoff used. With the GAD-2, a cutoff of 2 or more resulted in a sensitivity of 94% and a specificity of 68% for detecting generalized anxiety.7 A cutoff of 3 or more resulted in a sensitivity of 81% and a specificity of 86%.7 The GAD-7, using 10 as a cutoff, achieves a sensitivity of 79% and a specificity of 89%.7 Given the similar performance of the 2 options, the GAD-2 (TABLE 28,9) is probably preferable for use in primary care because of its ease of administration.
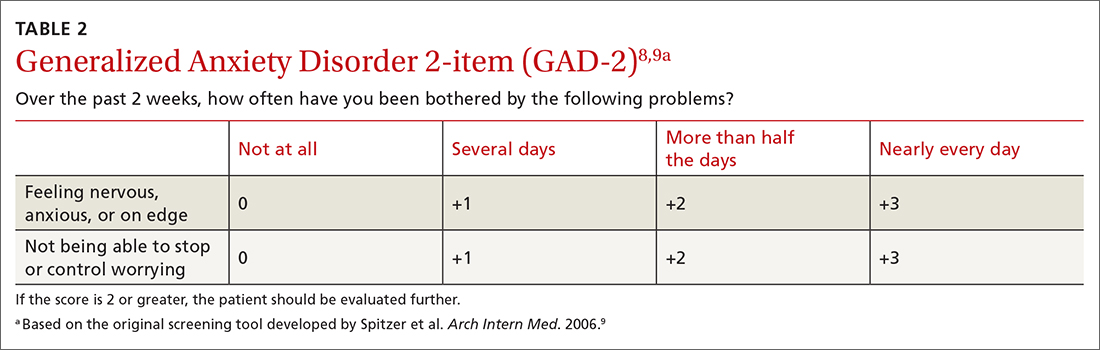
The tests evaluated by the USPSTF for anxiety screening in children and adolescents ≥ 8 years of age included the Screen for Child Anxiety Related Disorders (SCARED) and the Patient Health Questionnaire–Adolescent (PHQ-A).3 These tools ask more questions than the adult screening tools do: 41 for the SCARED and 13 for the PHQ-A. The sensitivity of SCARED for generalized anxiety disorder was 64% and the specificity was 63%.10 The sensitivity of the PHQ-A was 50% and the specificity was 98%.10
Various versions of all of these screening tools can be easily located on the internet. Search for them using the acronyms.
Screening for major depression
The depression screening tests the USPSTF examined were various versions of the Patient Health Questionnaire (PHQ), the Center for Epidemiologic Studies Depression Scale (CES-D), the Geriatric Depression Scale (GDS) in older adults, and the EPDS in postpartum and pregnant persons.7
A 2-question version of the PHQ was found to have a sensitivity of 91% with a specificity of 67%. The 9-question PHQ was found to have a similar sensitivity (88%) but better specificity (85%).7 TABLE 311 lists the 2 questions in the PHQ-2 and explains how to score the results.
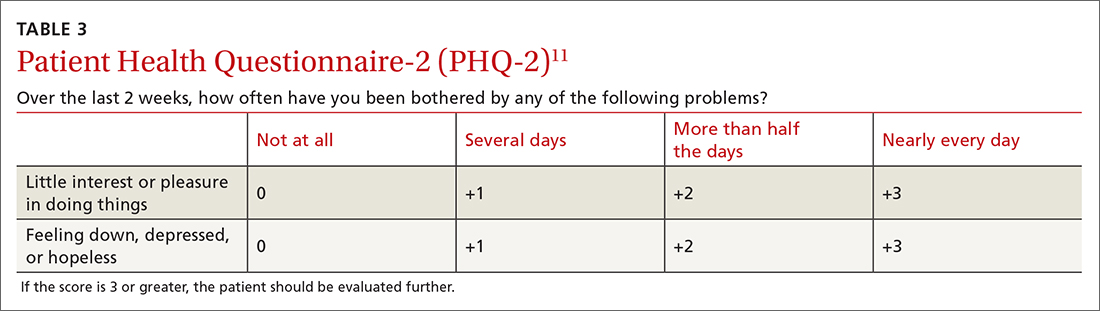
Continue to: The most commonly...
The most commonly studied screening tool for adolescents is the PHQ-A. Its sensitivity is 73% and specificity is 94%.12
The GAD-2 and PHQ-2 have the same possible answers and scores and can be combined into a 4-question screening tool to assess for anxiety and depression. If an initial screen for anxiety or depression (or both) is positive, further diagnostic testing and follow-up are needed.
Frequency of screening
The USPSTF recognized that limited information on the frequency of screening for both anxiety and depression does not support any recommendation on this matter. It suggested screening everyone once and then basing the need for subsequent screening tests on clinical judgment after considering risk factors and life events, with periodic rescreening of those at high risk. Finally, USPSTF recognized the many challenges to implementing screening tests for mental health conditions in primary care practice, but offered little practical advice on how to do this.
Suicide risk screening
As for the evidence on benefits and harms of screening for suicide risk in all age groups, the USPSTF still regards it as insufficient to make a recommendation. The lack of evidence applies to all aspects of screening, including the accuracy of the various screening tools and the potential benefits and harms of preventive interventions.2,7
Next steps
The recommendations on screening for depression, suicide risk, and anxiety in adults have been published as a draft, and the public comment period will be over by the time of this publication. The USPSTF generally takes 6 to 9 months to consider all the public comments and to publish final recommendations. The final recommendations on these topics for children and adolescents have been published since drafts were made available last April. There were no major changes between the draft and final versions.
1. USPSTF. Screening for anxiety in adults. Draft recommendation statement. Published September 20, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/anxiety-adults-screening
2. USPSTF. Screening for depression and suicide risk in adults. Updated September 14, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-update-summary/screening-depression-suicide-risk-adults
3. USPSTF. Anxiety in children and adolescents: screening. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
4. USPSTF. Depression and suicide risk in children and adolescents: screening. Final recommendation statement. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-depression-suicide-risk-children-adolescents
5. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
6. Misri S, Abizadeh J, Sanders S, et al. Perinatal generalized anxiety disorder: assessment and treatment. J Womens Health (Larchmt). 2015;24:762-770. doi: 10.1089/jwh.2014.5150
7. O’Connor E, Henninger M, Perdue LA, et al. Screening for depression, anxiety, and suicide risk in adults: a systematic evidence review for the US Preventive Services Task Force. Accessed November 22, 2022. www.uspreventiveservicestaskforce.org/home/getfilebytoken/dpG5pjV5yCew8fXvctFJNK
8. Sapra A, Bhandari P, Sharma S, et al. Using Generalized Anxiety Disorder-2 (GAD-2) and GAD-7 in a primary care setting. Cureus. 2020;12:e8224. doi: 10.7759/cureus.8224
9. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. doi: 10.1001/archinte.166.10.1092
10. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for anxiety in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1445-1455. doi: 10.1001/jama.2022.16303
11. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284‐1292. doi: 10.1097/01.MLR.0000093487.78664.3C
12. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for depression and suicide risk in children and adolescents: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1543-1556. doi:10.1001/jama.2022.16310
In September 2022, the US Preventive Services Task Force (USPSTF) released 2 sets of draft recommendations on screening for 3 mental health conditions in adults: anxiety, depression, and suicide risk.1,2 These draft recommendations are summarized in TABLE 11-4 along with finalized recommendations on the same topics for children and adolescents, published in October 2022.3,4

The recommendations on depression and suicide risk screening in adults are updates of previous recommendations (2016 for depression and 2014 for suicide risk) with no major changes. Screening for anxiety is a topic addressed for the first time this year for adults and for children and adolescents.1,3
The recommendations are fairly consistent between age groups. A “B” recommendation supports screening for major depression in all patients starting at age 12 years, including during pregnancy and the postpartum period. (See TABLE 1 for grade definitions.) For all age groups, evidence was insufficient to recommend screening for suicide risk. A “B” recommendation was also assigned to screening for anxiety in those ages 8 to 64 years. The USPSTF believes the evidence is insufficient to make a recommendation on screening for anxiety among adults ≥ 65 years of age.
The anxiety disorders common to both children and adults included in the USPSTF recommendations are generalized anxiety disorder, social anxiety disorder, panic disorder, separation anxiety disorder, phobias, selective mutism, and anxiety type not specified. For adults, the USPSTF also includes substance/medication-induced anxiety and anxiety due to other medical conditions.
Adults with anxiety often present with generalized complaints such as sleep disturbance, pain, and other somatic disorders that can remain undiagnosed for years. The USPSTF cites a lifetime prevalence of anxiety disorders of 26.4% for men and 40.4% for women, although the data used are 10 years old.5 The cited rate of generalized anxiety in pregnancy is 8.5% to 10.5%, and in the postpartum period, 4.4% to 10.8%.6
The data on direct benefits and harms of screening for anxiety in adults through age 64 are sparse. Nevertheless, the USPSTF deemed that screening tests for anxiety have adequate accuracy and that psychological interventions for anxiety result in moderate reduction of anxiety symptoms. Pharmacologic interventions produce a small benefit, although there is a lack of evidence for pharmacotherapy in pregnant and postpartum women. There is even less evidence of benefit for treatment in adults ≥ 65 years of age.1
How anxiety screening tests compare
Screening tests for anxiety in adults reviewed by the USPSTF included the Generalized Anxiety Disorder (GAD) scale and the Edinburgh Postnatal Depression Scale (EPDS) anxiety subscale.1 The most studied tools are the GAD-2 and GAD-7.
Continue to: The sensitivity and specificity...
The sensitivity and specificity of each test depends on the cutoff used. With the GAD-2, a cutoff of 2 or more resulted in a sensitivity of 94% and a specificity of 68% for detecting generalized anxiety.7 A cutoff of 3 or more resulted in a sensitivity of 81% and a specificity of 86%.7 The GAD-7, using 10 as a cutoff, achieves a sensitivity of 79% and a specificity of 89%.7 Given the similar performance of the 2 options, the GAD-2 (TABLE 28,9) is probably preferable for use in primary care because of its ease of administration.

The tests evaluated by the USPSTF for anxiety screening in children and adolescents ≥ 8 years of age included the Screen for Child Anxiety Related Disorders (SCARED) and the Patient Health Questionnaire–Adolescent (PHQ-A).3 These tools ask more questions than the adult screening tools do: 41 for the SCARED and 13 for the PHQ-A. The sensitivity of SCARED for generalized anxiety disorder was 64% and the specificity was 63%.10 The sensitivity of the PHQ-A was 50% and the specificity was 98%.10
Various versions of all of these screening tools can be easily located on the internet. Search for them using the acronyms.
Screening for major depression
The depression screening tests the USPSTF examined were various versions of the Patient Health Questionnaire (PHQ), the Center for Epidemiologic Studies Depression Scale (CES-D), the Geriatric Depression Scale (GDS) in older adults, and the EPDS in postpartum and pregnant persons.7
A 2-question version of the PHQ was found to have a sensitivity of 91% with a specificity of 67%. The 9-question PHQ was found to have a similar sensitivity (88%) but better specificity (85%).7 TABLE 311 lists the 2 questions in the PHQ-2 and explains how to score the results.

Continue to: The most commonly...
The most commonly studied screening tool for adolescents is the PHQ-A. Its sensitivity is 73% and specificity is 94%.12
The GAD-2 and PHQ-2 have the same possible answers and scores and can be combined into a 4-question screening tool to assess for anxiety and depression. If an initial screen for anxiety or depression (or both) is positive, further diagnostic testing and follow-up are needed.
Frequency of screening
The USPSTF recognized that limited information on the frequency of screening for both anxiety and depression does not support any recommendation on this matter. It suggested screening everyone once and then basing the need for subsequent screening tests on clinical judgment after considering risk factors and life events, with periodic rescreening of those at high risk. Finally, USPSTF recognized the many challenges to implementing screening tests for mental health conditions in primary care practice, but offered little practical advice on how to do this.
Suicide risk screening
As for the evidence on benefits and harms of screening for suicide risk in all age groups, the USPSTF still regards it as insufficient to make a recommendation. The lack of evidence applies to all aspects of screening, including the accuracy of the various screening tools and the potential benefits and harms of preventive interventions.2,7
Next steps
The recommendations on screening for depression, suicide risk, and anxiety in adults have been published as a draft, and the public comment period will be over by the time of this publication. The USPSTF generally takes 6 to 9 months to consider all the public comments and to publish final recommendations. The final recommendations on these topics for children and adolescents have been published since drafts were made available last April. There were no major changes between the draft and final versions.
In September 2022, the US Preventive Services Task Force (USPSTF) released 2 sets of draft recommendations on screening for 3 mental health conditions in adults: anxiety, depression, and suicide risk.1,2 These draft recommendations are summarized in TABLE 11-4 along with finalized recommendations on the same topics for children and adolescents, published in October 2022.3,4

The recommendations on depression and suicide risk screening in adults are updates of previous recommendations (2016 for depression and 2014 for suicide risk) with no major changes. Screening for anxiety is a topic addressed for the first time this year for adults and for children and adolescents.1,3
The recommendations are fairly consistent between age groups. A “B” recommendation supports screening for major depression in all patients starting at age 12 years, including during pregnancy and the postpartum period. (See TABLE 1 for grade definitions.) For all age groups, evidence was insufficient to recommend screening for suicide risk. A “B” recommendation was also assigned to screening for anxiety in those ages 8 to 64 years. The USPSTF believes the evidence is insufficient to make a recommendation on screening for anxiety among adults ≥ 65 years of age.
The anxiety disorders common to both children and adults included in the USPSTF recommendations are generalized anxiety disorder, social anxiety disorder, panic disorder, separation anxiety disorder, phobias, selective mutism, and anxiety type not specified. For adults, the USPSTF also includes substance/medication-induced anxiety and anxiety due to other medical conditions.
Adults with anxiety often present with generalized complaints such as sleep disturbance, pain, and other somatic disorders that can remain undiagnosed for years. The USPSTF cites a lifetime prevalence of anxiety disorders of 26.4% for men and 40.4% for women, although the data used are 10 years old.5 The cited rate of generalized anxiety in pregnancy is 8.5% to 10.5%, and in the postpartum period, 4.4% to 10.8%.6
The data on direct benefits and harms of screening for anxiety in adults through age 64 are sparse. Nevertheless, the USPSTF deemed that screening tests for anxiety have adequate accuracy and that psychological interventions for anxiety result in moderate reduction of anxiety symptoms. Pharmacologic interventions produce a small benefit, although there is a lack of evidence for pharmacotherapy in pregnant and postpartum women. There is even less evidence of benefit for treatment in adults ≥ 65 years of age.1
How anxiety screening tests compare
Screening tests for anxiety in adults reviewed by the USPSTF included the Generalized Anxiety Disorder (GAD) scale and the Edinburgh Postnatal Depression Scale (EPDS) anxiety subscale.1 The most studied tools are the GAD-2 and GAD-7.
Continue to: The sensitivity and specificity...
The sensitivity and specificity of each test depends on the cutoff used. With the GAD-2, a cutoff of 2 or more resulted in a sensitivity of 94% and a specificity of 68% for detecting generalized anxiety.7 A cutoff of 3 or more resulted in a sensitivity of 81% and a specificity of 86%.7 The GAD-7, using 10 as a cutoff, achieves a sensitivity of 79% and a specificity of 89%.7 Given the similar performance of the 2 options, the GAD-2 (TABLE 28,9) is probably preferable for use in primary care because of its ease of administration.

The tests evaluated by the USPSTF for anxiety screening in children and adolescents ≥ 8 years of age included the Screen for Child Anxiety Related Disorders (SCARED) and the Patient Health Questionnaire–Adolescent (PHQ-A).3 These tools ask more questions than the adult screening tools do: 41 for the SCARED and 13 for the PHQ-A. The sensitivity of SCARED for generalized anxiety disorder was 64% and the specificity was 63%.10 The sensitivity of the PHQ-A was 50% and the specificity was 98%.10
Various versions of all of these screening tools can be easily located on the internet. Search for them using the acronyms.
Screening for major depression
The depression screening tests the USPSTF examined were various versions of the Patient Health Questionnaire (PHQ), the Center for Epidemiologic Studies Depression Scale (CES-D), the Geriatric Depression Scale (GDS) in older adults, and the EPDS in postpartum and pregnant persons.7
A 2-question version of the PHQ was found to have a sensitivity of 91% with a specificity of 67%. The 9-question PHQ was found to have a similar sensitivity (88%) but better specificity (85%).7 TABLE 311 lists the 2 questions in the PHQ-2 and explains how to score the results.

Continue to: The most commonly...
The most commonly studied screening tool for adolescents is the PHQ-A. Its sensitivity is 73% and specificity is 94%.12
The GAD-2 and PHQ-2 have the same possible answers and scores and can be combined into a 4-question screening tool to assess for anxiety and depression. If an initial screen for anxiety or depression (or both) is positive, further diagnostic testing and follow-up are needed.
Frequency of screening
The USPSTF recognized that limited information on the frequency of screening for both anxiety and depression does not support any recommendation on this matter. It suggested screening everyone once and then basing the need for subsequent screening tests on clinical judgment after considering risk factors and life events, with periodic rescreening of those at high risk. Finally, USPSTF recognized the many challenges to implementing screening tests for mental health conditions in primary care practice, but offered little practical advice on how to do this.
Suicide risk screening
As for the evidence on benefits and harms of screening for suicide risk in all age groups, the USPSTF still regards it as insufficient to make a recommendation. The lack of evidence applies to all aspects of screening, including the accuracy of the various screening tools and the potential benefits and harms of preventive interventions.2,7
Next steps
The recommendations on screening for depression, suicide risk, and anxiety in adults have been published as a draft, and the public comment period will be over by the time of this publication. The USPSTF generally takes 6 to 9 months to consider all the public comments and to publish final recommendations. The final recommendations on these topics for children and adolescents have been published since drafts were made available last April. There were no major changes between the draft and final versions.
1. USPSTF. Screening for anxiety in adults. Draft recommendation statement. Published September 20, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/anxiety-adults-screening
2. USPSTF. Screening for depression and suicide risk in adults. Updated September 14, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-update-summary/screening-depression-suicide-risk-adults
3. USPSTF. Anxiety in children and adolescents: screening. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
4. USPSTF. Depression and suicide risk in children and adolescents: screening. Final recommendation statement. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-depression-suicide-risk-children-adolescents
5. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
6. Misri S, Abizadeh J, Sanders S, et al. Perinatal generalized anxiety disorder: assessment and treatment. J Womens Health (Larchmt). 2015;24:762-770. doi: 10.1089/jwh.2014.5150
7. O’Connor E, Henninger M, Perdue LA, et al. Screening for depression, anxiety, and suicide risk in adults: a systematic evidence review for the US Preventive Services Task Force. Accessed November 22, 2022. www.uspreventiveservicestaskforce.org/home/getfilebytoken/dpG5pjV5yCew8fXvctFJNK
8. Sapra A, Bhandari P, Sharma S, et al. Using Generalized Anxiety Disorder-2 (GAD-2) and GAD-7 in a primary care setting. Cureus. 2020;12:e8224. doi: 10.7759/cureus.8224
9. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. doi: 10.1001/archinte.166.10.1092
10. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for anxiety in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1445-1455. doi: 10.1001/jama.2022.16303
11. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284‐1292. doi: 10.1097/01.MLR.0000093487.78664.3C
12. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for depression and suicide risk in children and adolescents: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1543-1556. doi:10.1001/jama.2022.16310
1. USPSTF. Screening for anxiety in adults. Draft recommendation statement. Published September 20, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/anxiety-adults-screening
2. USPSTF. Screening for depression and suicide risk in adults. Updated September 14, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-update-summary/screening-depression-suicide-risk-adults
3. USPSTF. Anxiety in children and adolescents: screening. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-anxiety-children-adolescents
4. USPSTF. Depression and suicide risk in children and adolescents: screening. Final recommendation statement. Published October 11, 2022. Accessed November 22, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/screening-depression-suicide-risk-children-adolescents
5. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
6. Misri S, Abizadeh J, Sanders S, et al. Perinatal generalized anxiety disorder: assessment and treatment. J Womens Health (Larchmt). 2015;24:762-770. doi: 10.1089/jwh.2014.5150
7. O’Connor E, Henninger M, Perdue LA, et al. Screening for depression, anxiety, and suicide risk in adults: a systematic evidence review for the US Preventive Services Task Force. Accessed November 22, 2022. www.uspreventiveservicestaskforce.org/home/getfilebytoken/dpG5pjV5yCew8fXvctFJNK
8. Sapra A, Bhandari P, Sharma S, et al. Using Generalized Anxiety Disorder-2 (GAD-2) and GAD-7 in a primary care setting. Cureus. 2020;12:e8224. doi: 10.7759/cureus.8224
9. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. doi: 10.1001/archinte.166.10.1092
10. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for anxiety in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1445-1455. doi: 10.1001/jama.2022.16303
11. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire‐2: validity of a two‐item depression screener. Med Care. 2003;41:1284‐1292. doi: 10.1097/01.MLR.0000093487.78664.3C
12. Viswanathan M, Wallace IF, Middleton JC, et al. Screening for depression and suicide risk in children and adolescents: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:1543-1556. doi:10.1001/jama.2022.16310
Why you (still) shouldn’t prescribe hormone therapy for disease prevention
On November 1, the US Preventive Services Task Force (USPSTF) published updated recommendations (and a supporting evidence report) for the use of hormone therapy in postmenopausal women for the prevention of chronic medical conditions, such as heart disease, cancer, and osteoporosis.1,2 The USPSTF continues to recommend against the use of either estrogen or combined estrogen plus progesterone for this purpose.
A bit of context. These recommendations apply to asymptomatic postmenopausal women and do not apply to those who are unable to manage menopausal symptoms (eg, hot flashes or vaginal dryness) with other interventions, or to those who have premature or surgically caused menopause.
This update is a reconfirmation of USPSTF’s 2017 recommendations on this topic. These recommendations are consistent with those of multiple other organizations, including the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American College of Physicians, and the American Heart Association.
A look at the evidence. The evidence report included data from 20 randomized clinical trials and 3 cohort studies that examined the use of oral or transdermal hormone therapy. The most commonly used therapy was oral conjugated equine estrogen 0.625 mg/d, with or without medroxyprogesterone acetate 2.5 mg/d. The strongest evidence is from the Women’s Health Initiative, which included postmenopausal women ages 50 to 79 years and had follow-up of 7.2 years for the estrogen-only trial, 5.6 years for the estrogen plus progestin trial, and a long-term follow-up of up to 20.7 years.2,3
Benefits and harms of hormone therapy. Among postmenopausal women, use of estrogen alone was associated with absolute reduction in risk for fractures (–388 per 10,000 women), diabetes (–134), and breast cancer (–52) and an absolute increase in risk for urinary incontinence (+ 885 per 10,000 women), gallbladder disease (+ 377), stroke (+ 79), and venous thromboembolism (+ 77). Use of estrogen plus progestin was associated with reduced risk for fractures (–230 per 10,000 women), diabetes (–78), and colorectal cancer (–34) and an increased risk for urinary incontinence ( + 562 per 10,000 women), gallbladder disease (+ 260), venous thromboembolism (+ 120), dementia (+ 88), stroke (+ 52), and breast cancer (+ 51).2,3
Lingering questions. The USPSTF felt that the evidence is too limited to answer the following: (1) Are the potential benefits and harms of hormone therapy affected by participants’ age or by the timing of therapy initiation in relation to menopause onset? and (2) Do different types, doses, or delivery modes of hormone therapy affect benefits and harms?1
The bottom line. In asymptomatic, healthy, postmenopausal women, do not prescribe hormone therapy to try to prevent chronic conditions.
1. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Final recommendation statement. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/menopausal-hormone-therapy-preventive-medication
2. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Evidence summary. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-summary28/menopausal-hormone-therapy-preventive-medication
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040
On November 1, the US Preventive Services Task Force (USPSTF) published updated recommendations (and a supporting evidence report) for the use of hormone therapy in postmenopausal women for the prevention of chronic medical conditions, such as heart disease, cancer, and osteoporosis.1,2 The USPSTF continues to recommend against the use of either estrogen or combined estrogen plus progesterone for this purpose.
A bit of context. These recommendations apply to asymptomatic postmenopausal women and do not apply to those who are unable to manage menopausal symptoms (eg, hot flashes or vaginal dryness) with other interventions, or to those who have premature or surgically caused menopause.
This update is a reconfirmation of USPSTF’s 2017 recommendations on this topic. These recommendations are consistent with those of multiple other organizations, including the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American College of Physicians, and the American Heart Association.
A look at the evidence. The evidence report included data from 20 randomized clinical trials and 3 cohort studies that examined the use of oral or transdermal hormone therapy. The most commonly used therapy was oral conjugated equine estrogen 0.625 mg/d, with or without medroxyprogesterone acetate 2.5 mg/d. The strongest evidence is from the Women’s Health Initiative, which included postmenopausal women ages 50 to 79 years and had follow-up of 7.2 years for the estrogen-only trial, 5.6 years for the estrogen plus progestin trial, and a long-term follow-up of up to 20.7 years.2,3
Benefits and harms of hormone therapy. Among postmenopausal women, use of estrogen alone was associated with absolute reduction in risk for fractures (–388 per 10,000 women), diabetes (–134), and breast cancer (–52) and an absolute increase in risk for urinary incontinence (+ 885 per 10,000 women), gallbladder disease (+ 377), stroke (+ 79), and venous thromboembolism (+ 77). Use of estrogen plus progestin was associated with reduced risk for fractures (–230 per 10,000 women), diabetes (–78), and colorectal cancer (–34) and an increased risk for urinary incontinence ( + 562 per 10,000 women), gallbladder disease (+ 260), venous thromboembolism (+ 120), dementia (+ 88), stroke (+ 52), and breast cancer (+ 51).2,3
Lingering questions. The USPSTF felt that the evidence is too limited to answer the following: (1) Are the potential benefits and harms of hormone therapy affected by participants’ age or by the timing of therapy initiation in relation to menopause onset? and (2) Do different types, doses, or delivery modes of hormone therapy affect benefits and harms?1
The bottom line. In asymptomatic, healthy, postmenopausal women, do not prescribe hormone therapy to try to prevent chronic conditions.
On November 1, the US Preventive Services Task Force (USPSTF) published updated recommendations (and a supporting evidence report) for the use of hormone therapy in postmenopausal women for the prevention of chronic medical conditions, such as heart disease, cancer, and osteoporosis.1,2 The USPSTF continues to recommend against the use of either estrogen or combined estrogen plus progesterone for this purpose.
A bit of context. These recommendations apply to asymptomatic postmenopausal women and do not apply to those who are unable to manage menopausal symptoms (eg, hot flashes or vaginal dryness) with other interventions, or to those who have premature or surgically caused menopause.
This update is a reconfirmation of USPSTF’s 2017 recommendations on this topic. These recommendations are consistent with those of multiple other organizations, including the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, the American College of Physicians, and the American Heart Association.
A look at the evidence. The evidence report included data from 20 randomized clinical trials and 3 cohort studies that examined the use of oral or transdermal hormone therapy. The most commonly used therapy was oral conjugated equine estrogen 0.625 mg/d, with or without medroxyprogesterone acetate 2.5 mg/d. The strongest evidence is from the Women’s Health Initiative, which included postmenopausal women ages 50 to 79 years and had follow-up of 7.2 years for the estrogen-only trial, 5.6 years for the estrogen plus progestin trial, and a long-term follow-up of up to 20.7 years.2,3
Benefits and harms of hormone therapy. Among postmenopausal women, use of estrogen alone was associated with absolute reduction in risk for fractures (–388 per 10,000 women), diabetes (–134), and breast cancer (–52) and an absolute increase in risk for urinary incontinence (+ 885 per 10,000 women), gallbladder disease (+ 377), stroke (+ 79), and venous thromboembolism (+ 77). Use of estrogen plus progestin was associated with reduced risk for fractures (–230 per 10,000 women), diabetes (–78), and colorectal cancer (–34) and an increased risk for urinary incontinence ( + 562 per 10,000 women), gallbladder disease (+ 260), venous thromboembolism (+ 120), dementia (+ 88), stroke (+ 52), and breast cancer (+ 51).2,3
Lingering questions. The USPSTF felt that the evidence is too limited to answer the following: (1) Are the potential benefits and harms of hormone therapy affected by participants’ age or by the timing of therapy initiation in relation to menopause onset? and (2) Do different types, doses, or delivery modes of hormone therapy affect benefits and harms?1
The bottom line. In asymptomatic, healthy, postmenopausal women, do not prescribe hormone therapy to try to prevent chronic conditions.
1. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Final recommendation statement. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/menopausal-hormone-therapy-preventive-medication
2. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Evidence summary. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-summary28/menopausal-hormone-therapy-preventive-medication
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040
1. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Final recommendation statement. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/menopausal-hormone-therapy-preventive-medication
2. USPSTF. Hormone therapy in postmenopausal persons: primary prevention of chronic conditions. Evidence summary. Published November 1, 2022. Accessed November 14, 2022. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-summary28/menopausal-hormone-therapy-preventive-medication
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040
Syphilis screening: Who and when
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
The US Preventive Services Task Force (USPSTF) published updated recommendations on screening for syphilis on September 27.1 The Task Force continues to recommend screening for all adolescents and adults who are at increased risk for infection. (As part of previous recommendations, the USPSTF also advocates screening all pregnant women for syphilis early in their pregnancy to prevent congenital syphilis.2)
Who is at increased risk? Men who have sex with men (MSM), those with HIV or other sexually transmitted infections (STIs), those who use illicit drugs, and those with a history of incarceration, sex work, or military service are considered to be at increased risk for syphilis. Additionally, since state and local health departments collect and publish STI incidence data, it’s important to stay up to date on how common syphilis is in one’s community and tailor screening practices accordingly.
Men account for more than 80% of all primary and secondary syphilis infections, and MSM account for 53% of cases in men.3 The highest rates of syphilis are in men ages 25-29 years and 30-34 years (58.1 and 55.7 cases per 100,000, respectively).3
Why screening is important. Primary and secondary syphilis rates have increased steadily from an all-time low of 2.1 per 100,000 in 2000 to 12.7 per 100,000 in 2020.4 There were 171,074 cases reported in 2021.5
If not detected and treated, syphilis will progress from the primary and secondary stages to a latent form. About one-third of those with latent syphilis will develop tertiary syphilis, which can affect every organ system and cause multiple neurologic disorders.
How to screen. Syphilis screening typically involves a 2-step process. The first test that should be performed is a Venereal Disease Research Laboratory (VDRL) or rapid plasma reagin (RPR) test. This is followed by a treponemal antibody test if the initial test is positive. While the VDRL and RPR tests have high sensitivity, many other conditions can cause a false-positive result, necessitating confirmation with the more specific antibody test.
As far as frequency, the Task Force suggests screening annually for those at continued risk and more frequently (every 3 or 6 months) for those at highest risk.
Treatment for primary, secondary, and early latent syphilis (< 1 year’s duration) is a single intramuscular (IM) injection of benzathine penicillin, 2.4 million units. For late latent syphilis or syphilis of unknown duration, treatment is benzathine penicillin, 2.4 million units, administered in 3 weekly IM doses.
Treatment for those with penicillin allergies depends on the stage of syphilis and whether or not the patient is pregnant. Refer to the STD treatment guidelines for guidance.6
The CDC recommends presumptive treatment for anyone who has had sexual contact in the past 90 days with a person who’s been given a diagnosis of primary, secondary, or early latent syphilis.6
And finally, remember that all STIs are reportable to your local health department, which can assist with contract tracing and treatment follow-up.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.
1. USPSTF. Syphilis infection in nonpregnant adolescents and adults: Screening. Final recommendation statement. September 27, 2022. Accessed October 25, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-nonpregnant-adults-adolescents-screening
2. USPSTF. Syphilis infection in pregnant women: screening. Final recommendation statement. September 4, 2018. Accessed October 25, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-pregnancy-screening
3. CDC. Sexually transmitted disease surveillance 2020: syphilis. Updated August 22, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/figures/2020-STD-Surveillance-Syphilis.pptx
4. CDC. Sexually transmitted disease surveillance 2020. Table 1: Sexually transmitted diseases—reported cases and rates of reported cases, United States, 1941-2020. Updated April 12, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2020/tables/1.htm
5. CDC. Preliminary 2021 STD surveillance data. Updated September 1, 2022. Accessed October 25, 2022. www.cdc.gov/std/statistics/2021/default.htm
6. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recommend Rep. 2021;70:1-187.
Vaccine update for the 2022-23 influenza season
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.
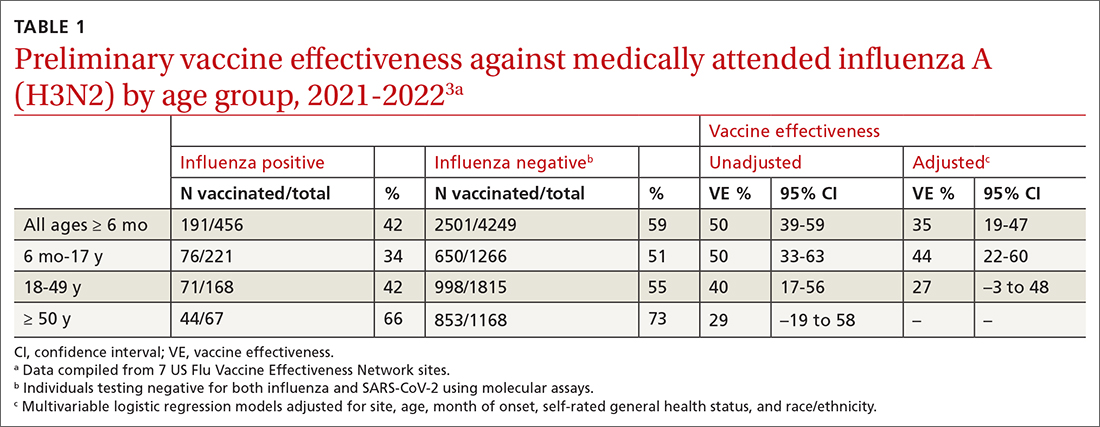
The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).
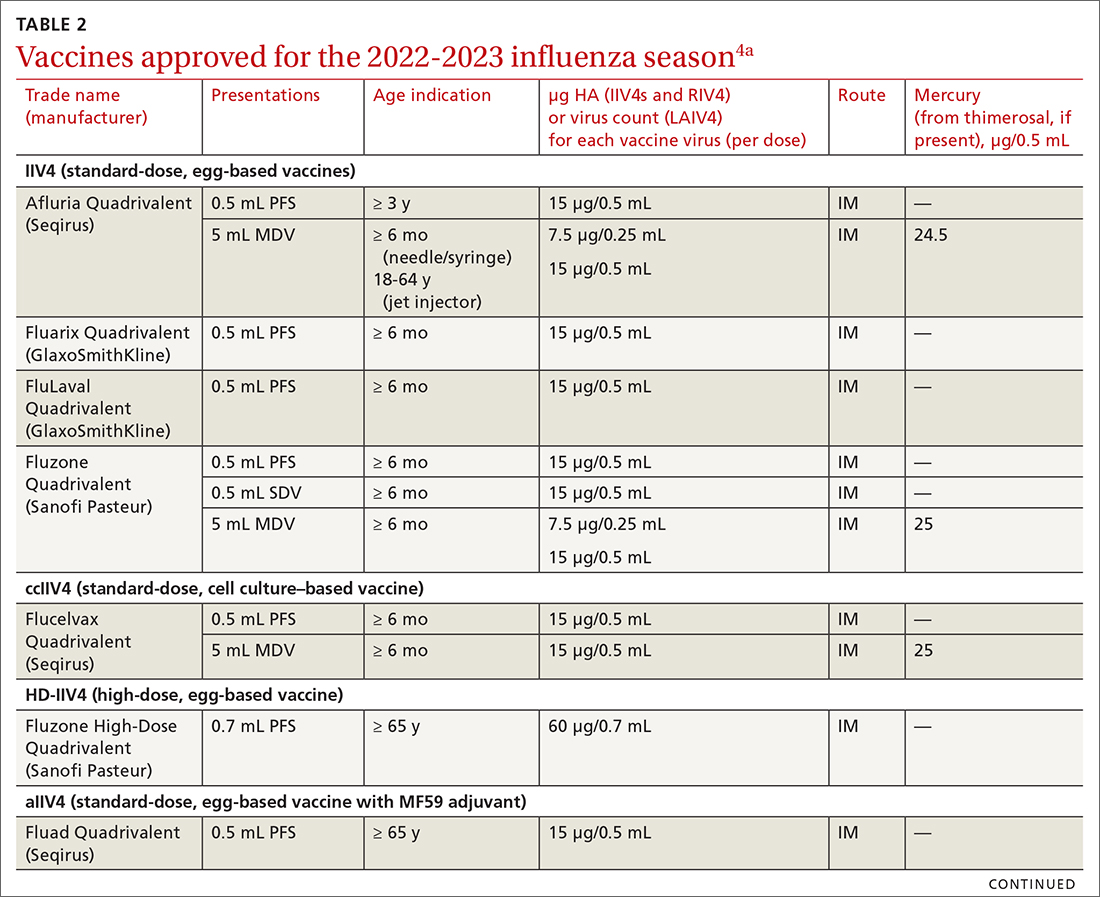
Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).
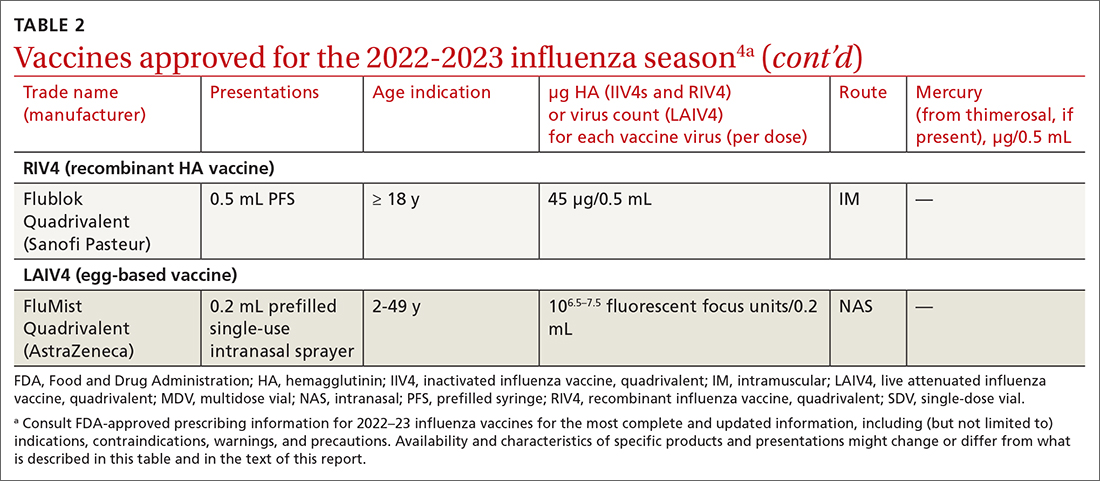
Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.
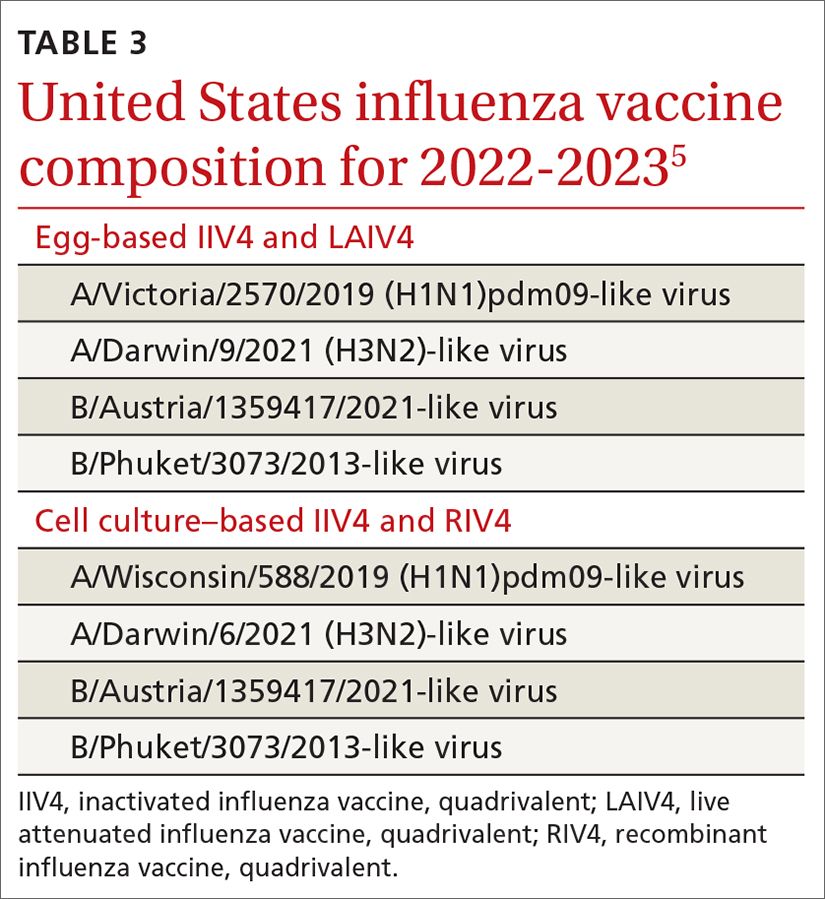
All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.
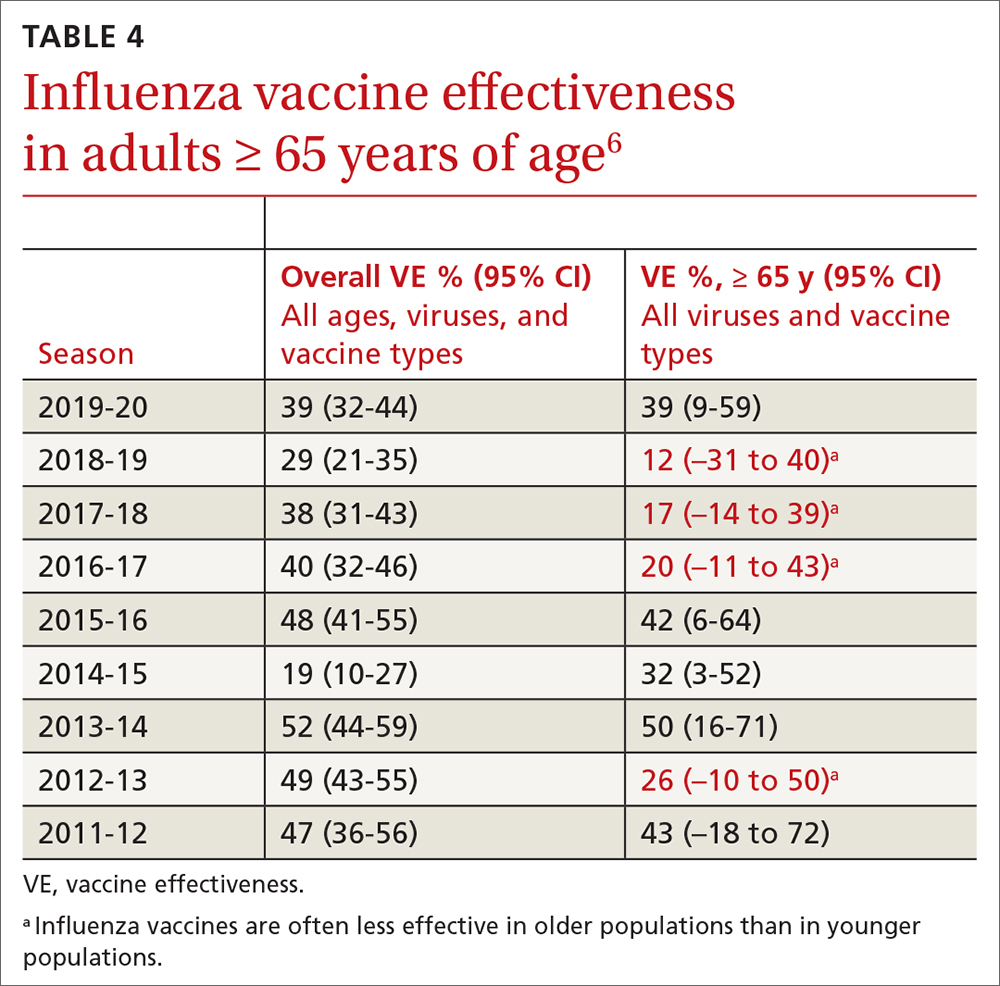
One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf