User login
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
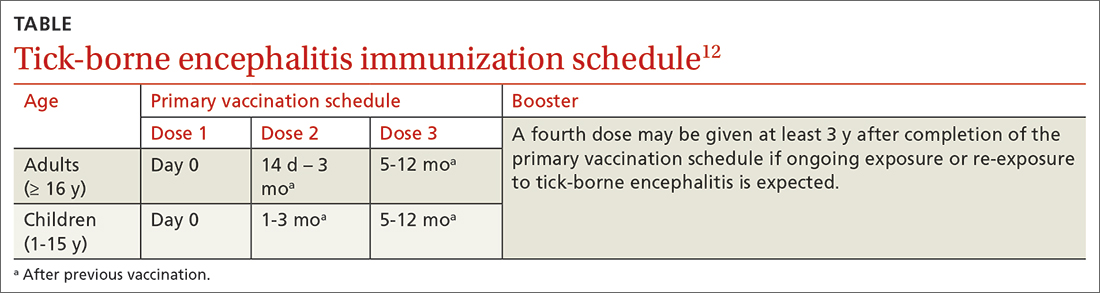
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13
Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
Much of the work of the Advisory Committee on Immunization Practices (ACIP) in 2022 was devoted to vaccines to protect against coronavirus disease 2019 (COVID-19); details about the 4 available products can be found on the Centers for Disease Control and Prevention’s COVID vaccine website (www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html).1,2 However, ACIP also issued recommendations about 5 other (non-COVID) vaccines last year, and those are the focus of this Practice Alert.
A second MMR vaccine option
The United States has had only 1 measles, mumps, and rubella (MMR) vaccine approved for use since 1978: M-M-R II (Merck). In June 2022, the US Food and Drug Administration (FDA) approved a second MMR vaccine, PRIORIX (GlaxoSmithKline Biologicals), which ACIP now recommends as an option when MMR vaccine is indicated.3
ACIP considers the 2 MMR options fully interchangeable.3 Both vaccines produce similar levels of immunogenicity and the safety profiles are also equivalent—including the rate of febrile seizures 6 to 11 days after vaccination, estimated at 3.3 to 8.7 per 10,000 doses.4 Since PRIORIX has been used in other countries since 1997, the MMR workgroup was able to include 13 studies on immunogenicity and 4 on safety in its evidence assessment; these are summarized on the CDC website.4
It is desirable to have multiple manufacturers of recommended vaccines to prevent shortages if there a disruption in the supply chain of 1 manufacturer, as well as to provide competition for cost control. A second MMR vaccine is therefore a welcome addition to the US vaccine supply. However, there remains only 1 combination measles, mumps, rubella, and varicella vaccine approved for use in the United States: ProQuad (Merck).
Pneumococcal vaccine recommendations are revised and simplified
Adults. Last year, ACIP made recommendations regarding 2 new vaccine options for use against pneumococcal infections in adults: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). These have been described in detail in a CDC publication and summarized in a recent Practice Alert.5,6
ACIP revised and simplified its recommendations on vaccination to prevent pneumococcal disease in adults as follows5:
1. Maintained the cutoff of age 65 years for universal pneumococcal vaccination
2. Recommended pneumococcal vaccination (with either PCV15 or PCV20) for all adults ages 65 years and older and for those younger than 65 years with chronic medical conditions or immunocompromise
3. Recommended that if PCV15 is used, it should be followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23, Merck).
These revisions created a number of uncertain clinical situations, since patients could have already started and/or completed their pneumococcal vaccination with previously available products, including PCV7, PCV13, and PPSV23. At the October 2022 ACIP meeting, the pneumococcal workgroup addressed a number of “what if” clinical questions. These clinical considerations will soon be published in the Morbidity and Mortality Weekly Report (MMWR) but also can be reviewed by looking at the October ACIP meeting materials.7 The main considerations are summarized below7:
- For those who have previously received PCV7, either PCV15 or PCV20 should be given.
- If PPSV23 was inadvertently administered first, it should be followed by PCV15 or PCV20 at least 1 year later.
- Adults who have only received PPSV23 should receive a dose of either PCV20 or PCV15 at least 1 year after their last PPSV23 dose. When PCV15 is used in those with a history of PPSV23 receipt, it need not be followed by another dose of PPSV23.
- Adults who have received PCV13 only are recommended to complete their pneumococcal vaccine series by receiving either a dose of PCV20 at least 1 year after the PCV13 dose or PPSV23 as previously recommended.
- Shared clinical decision-making is recommended regarding administration of PCV20 for adults ages ≥ 65 years who have completed their recommended vaccine series with both PCV13 and PPSV23 but have not received PCV15 or PCV20. If a decision to administer PCV20 is made, a dose of PCV20 is recommended at least 5 years after the last pneumococcal vaccine dose.
Continue to: Children
Children. In 2022, PCV15 was licensed for use in children and adolescents ages 6 weeks to 17 years. PCV15 contains all the serotypes in the PCV13 vaccine, plus 22F and 33F. In June 2022, ACIP adopted recommendations regarding the use of PCV15 in children. The main recommendation is that PCV13 and PCV15 can be used interchangeably. The recommended schedule for PCV use in children and the catch-up schedule have not changed, nor has the use of PPSV23 in children with underlying medical conditions.8,9
Those who have been vaccinated with PCV13 do not need to be revaccinated with PCV15, and an incomplete series of PCV13 can be completed with PCV15. It is anticipated that in 2023, PCV20 will be FDA approved for use in children and adolescents, and this will probably change the recommendations for the use of PPSV23 in children with underlying medical conditions. The recommended routine immunization and catch-up immunization schedules are published on the CDC website,9 and the pneumococcal-specific recommendations are described in a recent MMWR.8
Preferential choice for influenza vaccine in those ≥ 65 years
The ACIP now recommends 1 of 3 influenza vaccines be used preferentially in those ages 65 years and older: the high-dose quadrivalent vaccine (HD-IIV4), Fluzone; the adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad; or the recombinant quadrivalent influenza vaccine (RIV4), Flublok. However, if none of these options are available, a standard-dose vaccine is acceptable.
Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. The RIV4 is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. These 3 products produce better antibody levels and improved clinical outcomes in older adults compared to other, standard-dose flu vaccines, but there is no convincing evidence that any 1 of these is more effective than the others. A more in-depth discussion of flu vaccines and the considerations that went into this preferential recommendation were described in a previous Practice Alert.10
Updates for 2 travel vaccines
Tick-borne encephalitis (TBE). A TBE vaccine (Ticovac; Pfizer) has been available in other countries for more than 20 years, with no serious safety concerns identified. The vaccine was approved for use in the United States by the FDA in August 2021, and in early 2022, the ACIP made 3 recommendations for its use (to be discussed shortly).
TBE is a neuroinvasive flavivirus spread by ticks in parts of Europe and Asia. There are 3 main subtypes of the virus, and they cause serious illness, with a fatality rate of 1% to 20% and a sequelae rate of 10% to 50%.11 TBE infection is rare among US travelers, with only 11 cases documented between 2001 and 2020. There were 9 cases within the US military between 2006 and 2020.11
The TBE vaccine contains inactivated TBE virus, which is produced in chick embryo cells. It is administered in 3 doses over a 12-month timeframe, and those with continued exposure should receive a booster after 3 years.12 (See TABLE12 for administration schedule.) More information about the vaccine, contraindications, and rates of adverse reactions is available in the FDA package insert.13

Continue to: The ACIP has made...
The ACIP has made the following recommendations for the TBE vaccine11,12:
1. Vaccination is recommended for laboratory workers with a potential for exposure to TBE virus.
2. TBE vaccine also is recommended for individuals who are moving abroad or traveling to a TBE-endemic area and who will have extensive exposure to ticks based on their planned outdoor activities and itinerary.
3. TBE vaccine can be considered for people traveling or moving to a TBE-endemic area who might engage in outdoor activities in areas where ticks are likely to be found. The decision to vaccinate should be based on an assessment of the patient’s planned activities and itinerary, risk factors for a poorer medical outcome, and personal perception and tolerance of risk.
Cholera. ACIP now recommends CVD 103-HgR (PaxVax, VAXCHORA), a single-dose, live attenuated oral cholera vaccine, for travelers as young as 2 years who plan to visit an area that has active cholera transmission.14 In February 2022, ACIP expanded its recommendation for adults ages 18 to 64 years to include children and adolescents ages 2 to 17 years. This followed a 2020 FDA approval for the vaccine in the younger age group. Details about the vaccine were described in an MMWR publication.14
Cholera is caused by toxigenic bacteria. Infection occurs by ingestion of contaminated water or food and can be prevented by consumption of safe water and food, along with good sanitation and handwashing. Cholera produces a profuse watery diarrhea that can rapidly lead to death in 50% of those infected who do not receive rehydration therapy.15 Cholera is endemic is many countries and can cause large outbreaks. The World Health Organization estimates that 1 to 4 million cases of cholera and 21,000 to 143,000 related deaths occur globally each year.16
Staying current is moreimportant than ever
Vaccines are one of the most successful public health interventions of the past century, and maintaining a robust vaccine approval and safety monitoring system is an important priority. However, to gain the most benefit from vaccines, physicians need to stay current on vaccine recommendations—something that is becoming increasingly difficult to accomplish as the options expand. Consulting the literature and visiting the CDC’s website (www.cdc.gov) with frequency can be helpful to that end.
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1
1. CDC. Summary document for interim clinical considerations for use of COVID-19 vaccines currently authorized or approved in the US. Published December 6, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf
2. CDC. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. Published December 8, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
3. Krow-Lucal E, Marin M, Shepersky L, et al. Measles, mumps, rubella vaccine (PRIORIX): recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1465-1470. doi: 10.15585/mmwr.mm7146a1
4. CDC. ACIP evidence to recommendations framework for use of PRIORIX for prevention of measles, mumps, and rubella. Updated October 27, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/recs/grade/mmr-PRIORIX-etr.html
5. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:109-117. doi: 10.15585/mmwr.mm7104a1
6. Campos-Outcalt D. Vaccine update: the latest recommendations from ACIP. J Fam Pract. 2022;71:80-84. doi: 10.12788/jfp.0362
7. Kobayashi M. Proposed updates to clinical guidance on pneumococcal vaccine use among adults. Presented to the ACIP on October 19, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-10-19-20/04-Pneumococcal-Kobayashi-508.pdf
8. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine among US children: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1174-1181. doi: 10.15585/mmwr.mm7137a3
9. CDC. Immunization schedules. Updated February 17, 2022. Accessed February 6, 2022. www.cdc.gov/vaccines/schedules/hcp/index.html
10. Campos-Outcalt D. Vaccine update for the 2022-2023 influenza season. J Fam Pract. 2022;71:362-365. doi: 10.12788/jfp.0487
11. Hills S. Tick-borne encephalitis. Presented to the ACIP on February 23, 2022. Accessed February 2, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-TBE-Hills-508.pdf
12. CDC. Tick-borne encephalitis. Updated March 11, 2022. Accessed February 2, 2023. www.cdc.gov/tick-borne-encephalitis/
13. Ticovac. Package insert. Pfizer; 2022. Accessed February 6, 2023. www.fda.gov/media/151502/download
14. Collins JP, Ryan ET, Wong KK, et al. Cholera vaccine: recommendations of the Advisory Committee on Immunization Practices, 2022. MMWR Recomm Rep. 2022;71:1-8. doi: 10.15585/mmwr.rr7102a1
15. Global Task Force on Cholera Control. Cholera outbreak response field manual. Published October 2019. Accessed February 16, 2023. www.gtfcc.org/wp-content/uploads/2020/05/gtfcc-cholera-outbreak-response-field-manual.pdf
16. WHO. Health topics: cholera. Accessed February 16, 2023. www.who.int/health-topics/cholera#tab=tab_1