User login
Things We Do For No Reason: Electrolyte Testing in Pediatric Acute Gastroenteritis
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/
Acute gastroenteritis (AGE) remains a substantial cause of childhood illness and is 1 of the top 10 reasons for pediatric hospitalization nationwide. In the United States, AGE is responsible for 10% of hospital admissions and approximately 300 deaths annually.1 The American Academy of Pediatrics (AAP) and other organizations have emphasized supportive care in the management of AGE. Routine diagnostic testing has been discouraged in national guidelines except in cases of severe dehydration or an otherwise complicated course. Despite AGE guidelines, diagnostic laboratory tests are still widely used even though they have been shown to be poor predictors of dehydration. Studies have shown that high test utilization in various pediatric disease processes often influences the decision for hospitalization without improvement in patient outcome. In children with AGE, the initial and follow-up laboratory tests may not only be something that we do for no reason, but something that is associated with more risk than benefit.
An 18-month-old healthy male is brought to the emergency department (ED) with a chief complaint of 2 days of nonbloody, nonbilious emesis and watery diarrhea. He has decreased energy but smiles and plays for a few minutes. He has had decreased wet diapers. His exam is notable for mild tachycardia, mildly dry lips, and capillary refill of 3 seconds. A serum electrolyte panel is normal except for a sodium of 134 mEq/L, a bicarbonate of 16 mEq/L, and an anion gap of 18, which are flagged as abnormal by the electronic medical record. These results prompt intravenous (IV) access, a normal saline bolus, and admission on maintenance fluids overnight. The next morning, his electrolyte panel is repeated, and his sodium is 140 mEq/L and bicarbonate is 15 mEq/L. He is now drinking well with no further episodes of emesis, so he is discharged home.
WHY PHYSICIANS MIGHT THINK ELECTROLYTE TESTING IS HELPFUL
Many physicians across the United States continue to order electrolytes in AGE as a way to avoid missing severe dehydration, severe electrolyte abnormalities, or rare diagnoses, such as adrenal insufficiency or new-onset diabetes, in a child. Previous studies have revealed that bicarbonate and blood urea nitrogen (BUN) may be helpful predictors of severe dehydration. A retrospective study of 168 patients by Yilmaz et al.2 showed that BUN and bicarbonate strongly correlated with dehydration severity (P < 0.00001 and P = 0.01, respectively). A 97-patient prospective study by Vega and Avner3 showed that bicarbonate <17 can help in predicting percent body weight loss (PBWL) (sensitivity of 77% for PBWL 6-10 and 94% for PBWL >10).
In AGE, obtaining laboratory data is often considered to be the more conservative approach. Some attribute this to the medical education and legal system rewarding the uncovering of rare diagnoses,4 while others believe physicians obtain laboratory data to avoid missing severe electrolyte disorders. One author notes, “physicians who are anxious about a patient’s problem may be tempted to do something—anything—decisive in order to diminish their own anxiety.”5 Severe electrolyte derangements are common in developing countries6 but less so in the United States. A prospective pediatric dehydration study over 1 year in the United States demonstrated rates of 6% and 3% of hypo- and hypernatremia, respectively (n = 182). Only 1 patient had a sodium level >160, and this patient had an underlying genetic syndrome, and none had hyponatremia <130. Hypoglycemia was the most common electrolyte abnormality, which was present in 9.8% of patients. Electrolyte results changed management in 10.4% of patients.7
WHY ELECTROLYTE TESTING IS GENERALLY NOT HELPFUL
In AGE with or without dehydration, guidelines from the AAP and other international organizations emphasize supportive care in the management of AGE and discourage routine diagnostic testing.8-10 Yet, there continues to be wide variation in AGE management.11-13 Most AGE cases presenting to an outpatient setting or ED are uncomplicated: age >6 months, nontoxic appearance, no comorbidities, no hematochezia, diarrhea <7 days, and mild-to-moderate dehydration.
Steiner et al.14 performed a systematic meta-analysis of the precision and accuracy of symptoms, signs, and laboratory tests for evaluating dehydration in children. They concluded that a standardized clinical assessment based on physical exam (PE) findings more accurately classifies the degree of dehydration than laboratory testing. Steiner et al14 specifically analyzed the works by Yilmaz et al.2 and Vega and Avner,3 and determined that the positive likelihood ratios for >5% dehydration resulting from a BUN >45 or bicarbonate <17 were too small or had confidence intervals that were too wide to be clinically helpful alone. Therefore, Steiner et al.14 recommended that laboratory testing should not be considered definitive for dehydration.
Vega and Avner3 found that electrolyte testing is less helpful in distinguishing between <5% (mild) and 5% to 10% (moderate) dehydration compared to PBWL. Because both mild and moderate dehydration respond equally well to oral rehydration therapy (ORT),8 electrolyte testing is not helpful in managing these categories. Many studies have excluded children with hypernatremia, but generally, severe hypernatremia is uncommon in healthy patients with AGE. In most cases of mild hypernatremia, ORT is the preferred resuscitation method and is possibly safer than IV rehydration because ORT may induce less rapid shifts in intracellular water.15
Tieder et al.16 demonstrated that better hospital adherence to national recommendations to avoid diagnostic testing in children with AGE resulted in lower charges and equivalent outcomes. In this large, multicenter study among 27 children’s hospitals in the Pediatric Hospital Information System (PHIS) database, only 70% of the 188,000 patients received guideline-adherent care. Nonrecommended laboratory testing was common, especially in the admitted population. Electrolytes were measured in 22.1% of the ED and observation patients compared with 85% of admitted patients. Hospitals that were most guideline adherent in the ED demonstrated 50% lower charges. The authors estimate that standardizing AGE care and eliminating nonrecommended laboratory testing would decrease admissions by 45% and save more than $1 billion per year in direct medical costs.16 In a similar PHIS study, laboratory testing was strongly correlated with the percentage of children hospitalized for AGE at each hospital (r = 0.73, P < 0.001). Results were unchanged when excluding children <1 year of age (r = 0.75, P < 0.001). In contrast, the mean testing count was not correlated with return visits within 3 days for children discharged from the ED (r = 0.21, P = 0.235), nor was it correlated with hospital length of stay (r = −0.04, P = 0.804) or return visits within 7 days (r = 0.03, P = 0.862) for hospitalized children.12 In addition, Freedman et al.17 revealed that the clinical dehydration score is independently associated with successful ED discharge without revisits, and laboratory testing does not prevent missed cases of severe dehydration.
Nonrecommended and often unnecessary laboratory testing in AGE results in IV procedures that are sometimes repeated because of abnormal values. “Shotgun testing,” or ordering a panel of labs, can result in abnormal laboratory values in healthy patients. Deyo et al.
WHY ELECTROLYTE TESTING MIGHT BE HELPFUL
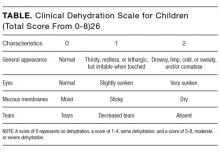
Electrolyte panels may be useful in assessing children with severe dehydration (scores of 5-8 on the Clinical Dehydration Scale (CDS) or more than 10% weight loss) or in complicated cases of AGE (those that do not meet the criteria of age >6 months, nontoxic appearance, no comorbidities, no hematochezia, and diarrhea <7 days) to guide IV fluid management and correct markedly abnormal electrolytes.14
Electrolyte panels may also rarely uncover disease processes, such as new-onset diabetes, hemolytic uremic syndrome, adrenal insufficiency, or inborn errors of metabolism, allowing for early diagnosis and preventing adverse outcomes. Suspicion to investigate such entities should arise during a thorough history and PE instead of routinely screening all children with symptoms of AGE. One should also have a higher level of concern for other disease processes when clinical recovery does not occur within the expected amount of time; symptoms usually resolve within 2 to 3 days but sometimes will last up to a week.
WHAT WE SHOULD DO INSTEAD
RECOMMENDATIONS
- Perform a thorough history and PE to diagnose AGE.8
- Clinical assessment of dehydration should be performed upon initial presentation and repeatedly with vital signs throughout the stay using a validated CDS to classify the patient’s initial dehydration severity and monitor improvement. Obtain a current patient weight and compare with previously recorded weights, if available.25,26
- Laboratory testing in patients with AGE should not be performed unless a patient is classified as severely dehydrated, is toxic appearing, has a comorbidity that increases the likelihood of complications, or is not improving as expected.
- Rehydration via ORT is preferred to an IV in mild and moderate dehydration.15
- If initial testing is performed and indicates an expected value indicative of dehydration, do not repeat testing to demonstrate normalization as long as the child is clinically improving as expected.
CONCLUSION
Children presenting with mild-to-moderate dehydration should be treated with supportive measures in accordance with current guidelines. Electrolyte panels very rarely provide clinical information that cannot be garnered through a thorough history and PE. As in our clinical scenario, the laboratory values obtained may have led to potential harm, including overdiagnosis, painful procedures, and psychological distress. Without testing, the patient likely could have been appropriately treated with ORT and discharged from the ED.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosure
The authors have nothing to disclose.
1. Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35-40. PubMed
2. Yilmaz K, Karabocuoglu M, Citak A, Uzel N. Evaluation of laboratory tests in dehydrated children with acute gastroenteritis. J Paediatr Child Health. 2002;38(3):226-228. PubMed
3. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179-182. PubMed
4. Jha S. Stop hunting for zebras in Texas: end the diagnostic culture of “rule-out”. BMJ. 2014;348:g2625. PubMed
5. Mold JW, Stein HF. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314(8):512-514. PubMed
6. Shahrin L, Chisti MJ, Huq S, et al. Clinical Manifestations of Hyponatremia and Hypernatremia in Under-Five Diarrheal Children in a Diarrhea Hospital. J Trop Pediatr. 2016;62(3):206-212. PubMed
7. Wathen JE, MacKenzie T, Bothner JP. Usefulness of the serum electrolyte panel in the management of pediatric dehydration treated with intravenously administered fluids. Pediatrics. 2004;114(5):1227-1234. PubMed
8. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97(3):424-435. PubMed
9. National Collaborating Centre for Women’s and Children’s Health. Diarrhoea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment and Management in Children Younger than 5 Years. London: RCOG Press; 2009. PubMed
10. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-152. PubMed
11. Freedman SB, Gouin S, Bhatt M, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127(2):e287-e295. PubMed
12. Lind CH, Hall M, Arnold DH, et al. Variation in Diagnostic Testing and Hospitalization Rates in Children With Acute Gastroenteritis. Hosp Pediatr. 2016;6(12):714-721. PubMed
13. Powell EC, Hampers LC. Physician variation in test ordering in the management of gastroenteritis in children. Arch Pediatr Adolesc Med. 2003;157(10):978-983. PubMed
14. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291(22):2746-2754. PubMed
15. Sandhu BK, European Society of Pediatric Gastroenterology H, Nutrition Working Group on Acute D. Practical guidelines for the management of gastroenteritis in children. J Pediatr Gastroenterol Nutr. 2001;33(suppl 2):S36-S39.
16. Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124(6):e1081-e1087. PubMed
17. Freedman SB, DeGroot JM, Parkin PC. Successful discharge of children with gastroenteritis requiring intravenous rehydration. J Emerg Med. 2014;46(1):9-20. PubMed
18. Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23-44. PubMed
19. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. PubMed
20. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. PubMed
21. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. PubMed
22. McMurtry CM, Noel M, Chambers CT, McGrath PJ. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30(6):780-788. PubMed
23. von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5(5):241-249. PubMed
24. American Academy of Pediatrics. Section on Hospital Medicine. Rauch DA, Gershel JC. Caring for the hospitalized child: a handbook of inpatient pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
25. Bailey B, Gravel J, Goldman RD, Friedman JN, Parkin PC. External validation of the clinical dehydration scale for children with acute gastroenteritis. Acad Emerg Med. 2010;17(6):583-588. PubMed
26. Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201-207. PubMed
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/
Acute gastroenteritis (AGE) remains a substantial cause of childhood illness and is 1 of the top 10 reasons for pediatric hospitalization nationwide. In the United States, AGE is responsible for 10% of hospital admissions and approximately 300 deaths annually.1 The American Academy of Pediatrics (AAP) and other organizations have emphasized supportive care in the management of AGE. Routine diagnostic testing has been discouraged in national guidelines except in cases of severe dehydration or an otherwise complicated course. Despite AGE guidelines, diagnostic laboratory tests are still widely used even though they have been shown to be poor predictors of dehydration. Studies have shown that high test utilization in various pediatric disease processes often influences the decision for hospitalization without improvement in patient outcome. In children with AGE, the initial and follow-up laboratory tests may not only be something that we do for no reason, but something that is associated with more risk than benefit.
An 18-month-old healthy male is brought to the emergency department (ED) with a chief complaint of 2 days of nonbloody, nonbilious emesis and watery diarrhea. He has decreased energy but smiles and plays for a few minutes. He has had decreased wet diapers. His exam is notable for mild tachycardia, mildly dry lips, and capillary refill of 3 seconds. A serum electrolyte panel is normal except for a sodium of 134 mEq/L, a bicarbonate of 16 mEq/L, and an anion gap of 18, which are flagged as abnormal by the electronic medical record. These results prompt intravenous (IV) access, a normal saline bolus, and admission on maintenance fluids overnight. The next morning, his electrolyte panel is repeated, and his sodium is 140 mEq/L and bicarbonate is 15 mEq/L. He is now drinking well with no further episodes of emesis, so he is discharged home.
WHY PHYSICIANS MIGHT THINK ELECTROLYTE TESTING IS HELPFUL
Many physicians across the United States continue to order electrolytes in AGE as a way to avoid missing severe dehydration, severe electrolyte abnormalities, or rare diagnoses, such as adrenal insufficiency or new-onset diabetes, in a child. Previous studies have revealed that bicarbonate and blood urea nitrogen (BUN) may be helpful predictors of severe dehydration. A retrospective study of 168 patients by Yilmaz et al.2 showed that BUN and bicarbonate strongly correlated with dehydration severity (P < 0.00001 and P = 0.01, respectively). A 97-patient prospective study by Vega and Avner3 showed that bicarbonate <17 can help in predicting percent body weight loss (PBWL) (sensitivity of 77% for PBWL 6-10 and 94% for PBWL >10).
In AGE, obtaining laboratory data is often considered to be the more conservative approach. Some attribute this to the medical education and legal system rewarding the uncovering of rare diagnoses,4 while others believe physicians obtain laboratory data to avoid missing severe electrolyte disorders. One author notes, “physicians who are anxious about a patient’s problem may be tempted to do something—anything—decisive in order to diminish their own anxiety.”5 Severe electrolyte derangements are common in developing countries6 but less so in the United States. A prospective pediatric dehydration study over 1 year in the United States demonstrated rates of 6% and 3% of hypo- and hypernatremia, respectively (n = 182). Only 1 patient had a sodium level >160, and this patient had an underlying genetic syndrome, and none had hyponatremia <130. Hypoglycemia was the most common electrolyte abnormality, which was present in 9.8% of patients. Electrolyte results changed management in 10.4% of patients.7
WHY ELECTROLYTE TESTING IS GENERALLY NOT HELPFUL
In AGE with or without dehydration, guidelines from the AAP and other international organizations emphasize supportive care in the management of AGE and discourage routine diagnostic testing.8-10 Yet, there continues to be wide variation in AGE management.11-13 Most AGE cases presenting to an outpatient setting or ED are uncomplicated: age >6 months, nontoxic appearance, no comorbidities, no hematochezia, diarrhea <7 days, and mild-to-moderate dehydration.
Steiner et al.14 performed a systematic meta-analysis of the precision and accuracy of symptoms, signs, and laboratory tests for evaluating dehydration in children. They concluded that a standardized clinical assessment based on physical exam (PE) findings more accurately classifies the degree of dehydration than laboratory testing. Steiner et al14 specifically analyzed the works by Yilmaz et al.2 and Vega and Avner,3 and determined that the positive likelihood ratios for >5% dehydration resulting from a BUN >45 or bicarbonate <17 were too small or had confidence intervals that were too wide to be clinically helpful alone. Therefore, Steiner et al.14 recommended that laboratory testing should not be considered definitive for dehydration.
Vega and Avner3 found that electrolyte testing is less helpful in distinguishing between <5% (mild) and 5% to 10% (moderate) dehydration compared to PBWL. Because both mild and moderate dehydration respond equally well to oral rehydration therapy (ORT),8 electrolyte testing is not helpful in managing these categories. Many studies have excluded children with hypernatremia, but generally, severe hypernatremia is uncommon in healthy patients with AGE. In most cases of mild hypernatremia, ORT is the preferred resuscitation method and is possibly safer than IV rehydration because ORT may induce less rapid shifts in intracellular water.15
Tieder et al.16 demonstrated that better hospital adherence to national recommendations to avoid diagnostic testing in children with AGE resulted in lower charges and equivalent outcomes. In this large, multicenter study among 27 children’s hospitals in the Pediatric Hospital Information System (PHIS) database, only 70% of the 188,000 patients received guideline-adherent care. Nonrecommended laboratory testing was common, especially in the admitted population. Electrolytes were measured in 22.1% of the ED and observation patients compared with 85% of admitted patients. Hospitals that were most guideline adherent in the ED demonstrated 50% lower charges. The authors estimate that standardizing AGE care and eliminating nonrecommended laboratory testing would decrease admissions by 45% and save more than $1 billion per year in direct medical costs.16 In a similar PHIS study, laboratory testing was strongly correlated with the percentage of children hospitalized for AGE at each hospital (r = 0.73, P < 0.001). Results were unchanged when excluding children <1 year of age (r = 0.75, P < 0.001). In contrast, the mean testing count was not correlated with return visits within 3 days for children discharged from the ED (r = 0.21, P = 0.235), nor was it correlated with hospital length of stay (r = −0.04, P = 0.804) or return visits within 7 days (r = 0.03, P = 0.862) for hospitalized children.12 In addition, Freedman et al.17 revealed that the clinical dehydration score is independently associated with successful ED discharge without revisits, and laboratory testing does not prevent missed cases of severe dehydration.
Nonrecommended and often unnecessary laboratory testing in AGE results in IV procedures that are sometimes repeated because of abnormal values. “Shotgun testing,” or ordering a panel of labs, can result in abnormal laboratory values in healthy patients. Deyo et al.
WHY ELECTROLYTE TESTING MIGHT BE HELPFUL
Electrolyte panels may be useful in assessing children with severe dehydration (scores of 5-8 on the Clinical Dehydration Scale (CDS) or more than 10% weight loss) or in complicated cases of AGE (those that do not meet the criteria of age >6 months, nontoxic appearance, no comorbidities, no hematochezia, and diarrhea <7 days) to guide IV fluid management and correct markedly abnormal electrolytes.14
Electrolyte panels may also rarely uncover disease processes, such as new-onset diabetes, hemolytic uremic syndrome, adrenal insufficiency, or inborn errors of metabolism, allowing for early diagnosis and preventing adverse outcomes. Suspicion to investigate such entities should arise during a thorough history and PE instead of routinely screening all children with symptoms of AGE. One should also have a higher level of concern for other disease processes when clinical recovery does not occur within the expected amount of time; symptoms usually resolve within 2 to 3 days but sometimes will last up to a week.
WHAT WE SHOULD DO INSTEAD
RECOMMENDATIONS
- Perform a thorough history and PE to diagnose AGE.8
- Clinical assessment of dehydration should be performed upon initial presentation and repeatedly with vital signs throughout the stay using a validated CDS to classify the patient’s initial dehydration severity and monitor improvement. Obtain a current patient weight and compare with previously recorded weights, if available.25,26
- Laboratory testing in patients with AGE should not be performed unless a patient is classified as severely dehydrated, is toxic appearing, has a comorbidity that increases the likelihood of complications, or is not improving as expected.
- Rehydration via ORT is preferred to an IV in mild and moderate dehydration.15
- If initial testing is performed and indicates an expected value indicative of dehydration, do not repeat testing to demonstrate normalization as long as the child is clinically improving as expected.
CONCLUSION
Children presenting with mild-to-moderate dehydration should be treated with supportive measures in accordance with current guidelines. Electrolyte panels very rarely provide clinical information that cannot be garnered through a thorough history and PE. As in our clinical scenario, the laboratory values obtained may have led to potential harm, including overdiagnosis, painful procedures, and psychological distress. Without testing, the patient likely could have been appropriately treated with ORT and discharged from the ED.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosure
The authors have nothing to disclose.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but that may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/
Acute gastroenteritis (AGE) remains a substantial cause of childhood illness and is 1 of the top 10 reasons for pediatric hospitalization nationwide. In the United States, AGE is responsible for 10% of hospital admissions and approximately 300 deaths annually.1 The American Academy of Pediatrics (AAP) and other organizations have emphasized supportive care in the management of AGE. Routine diagnostic testing has been discouraged in national guidelines except in cases of severe dehydration or an otherwise complicated course. Despite AGE guidelines, diagnostic laboratory tests are still widely used even though they have been shown to be poor predictors of dehydration. Studies have shown that high test utilization in various pediatric disease processes often influences the decision for hospitalization without improvement in patient outcome. In children with AGE, the initial and follow-up laboratory tests may not only be something that we do for no reason, but something that is associated with more risk than benefit.
An 18-month-old healthy male is brought to the emergency department (ED) with a chief complaint of 2 days of nonbloody, nonbilious emesis and watery diarrhea. He has decreased energy but smiles and plays for a few minutes. He has had decreased wet diapers. His exam is notable for mild tachycardia, mildly dry lips, and capillary refill of 3 seconds. A serum electrolyte panel is normal except for a sodium of 134 mEq/L, a bicarbonate of 16 mEq/L, and an anion gap of 18, which are flagged as abnormal by the electronic medical record. These results prompt intravenous (IV) access, a normal saline bolus, and admission on maintenance fluids overnight. The next morning, his electrolyte panel is repeated, and his sodium is 140 mEq/L and bicarbonate is 15 mEq/L. He is now drinking well with no further episodes of emesis, so he is discharged home.
WHY PHYSICIANS MIGHT THINK ELECTROLYTE TESTING IS HELPFUL
Many physicians across the United States continue to order electrolytes in AGE as a way to avoid missing severe dehydration, severe electrolyte abnormalities, or rare diagnoses, such as adrenal insufficiency or new-onset diabetes, in a child. Previous studies have revealed that bicarbonate and blood urea nitrogen (BUN) may be helpful predictors of severe dehydration. A retrospective study of 168 patients by Yilmaz et al.2 showed that BUN and bicarbonate strongly correlated with dehydration severity (P < 0.00001 and P = 0.01, respectively). A 97-patient prospective study by Vega and Avner3 showed that bicarbonate <17 can help in predicting percent body weight loss (PBWL) (sensitivity of 77% for PBWL 6-10 and 94% for PBWL >10).
In AGE, obtaining laboratory data is often considered to be the more conservative approach. Some attribute this to the medical education and legal system rewarding the uncovering of rare diagnoses,4 while others believe physicians obtain laboratory data to avoid missing severe electrolyte disorders. One author notes, “physicians who are anxious about a patient’s problem may be tempted to do something—anything—decisive in order to diminish their own anxiety.”5 Severe electrolyte derangements are common in developing countries6 but less so in the United States. A prospective pediatric dehydration study over 1 year in the United States demonstrated rates of 6% and 3% of hypo- and hypernatremia, respectively (n = 182). Only 1 patient had a sodium level >160, and this patient had an underlying genetic syndrome, and none had hyponatremia <130. Hypoglycemia was the most common electrolyte abnormality, which was present in 9.8% of patients. Electrolyte results changed management in 10.4% of patients.7
WHY ELECTROLYTE TESTING IS GENERALLY NOT HELPFUL
In AGE with or without dehydration, guidelines from the AAP and other international organizations emphasize supportive care in the management of AGE and discourage routine diagnostic testing.8-10 Yet, there continues to be wide variation in AGE management.11-13 Most AGE cases presenting to an outpatient setting or ED are uncomplicated: age >6 months, nontoxic appearance, no comorbidities, no hematochezia, diarrhea <7 days, and mild-to-moderate dehydration.
Steiner et al.14 performed a systematic meta-analysis of the precision and accuracy of symptoms, signs, and laboratory tests for evaluating dehydration in children. They concluded that a standardized clinical assessment based on physical exam (PE) findings more accurately classifies the degree of dehydration than laboratory testing. Steiner et al14 specifically analyzed the works by Yilmaz et al.2 and Vega and Avner,3 and determined that the positive likelihood ratios for >5% dehydration resulting from a BUN >45 or bicarbonate <17 were too small or had confidence intervals that were too wide to be clinically helpful alone. Therefore, Steiner et al.14 recommended that laboratory testing should not be considered definitive for dehydration.
Vega and Avner3 found that electrolyte testing is less helpful in distinguishing between <5% (mild) and 5% to 10% (moderate) dehydration compared to PBWL. Because both mild and moderate dehydration respond equally well to oral rehydration therapy (ORT),8 electrolyte testing is not helpful in managing these categories. Many studies have excluded children with hypernatremia, but generally, severe hypernatremia is uncommon in healthy patients with AGE. In most cases of mild hypernatremia, ORT is the preferred resuscitation method and is possibly safer than IV rehydration because ORT may induce less rapid shifts in intracellular water.15
Tieder et al.16 demonstrated that better hospital adherence to national recommendations to avoid diagnostic testing in children with AGE resulted in lower charges and equivalent outcomes. In this large, multicenter study among 27 children’s hospitals in the Pediatric Hospital Information System (PHIS) database, only 70% of the 188,000 patients received guideline-adherent care. Nonrecommended laboratory testing was common, especially in the admitted population. Electrolytes were measured in 22.1% of the ED and observation patients compared with 85% of admitted patients. Hospitals that were most guideline adherent in the ED demonstrated 50% lower charges. The authors estimate that standardizing AGE care and eliminating nonrecommended laboratory testing would decrease admissions by 45% and save more than $1 billion per year in direct medical costs.16 In a similar PHIS study, laboratory testing was strongly correlated with the percentage of children hospitalized for AGE at each hospital (r = 0.73, P < 0.001). Results were unchanged when excluding children <1 year of age (r = 0.75, P < 0.001). In contrast, the mean testing count was not correlated with return visits within 3 days for children discharged from the ED (r = 0.21, P = 0.235), nor was it correlated with hospital length of stay (r = −0.04, P = 0.804) or return visits within 7 days (r = 0.03, P = 0.862) for hospitalized children.12 In addition, Freedman et al.17 revealed that the clinical dehydration score is independently associated with successful ED discharge without revisits, and laboratory testing does not prevent missed cases of severe dehydration.
Nonrecommended and often unnecessary laboratory testing in AGE results in IV procedures that are sometimes repeated because of abnormal values. “Shotgun testing,” or ordering a panel of labs, can result in abnormal laboratory values in healthy patients. Deyo et al.
WHY ELECTROLYTE TESTING MIGHT BE HELPFUL
Electrolyte panels may be useful in assessing children with severe dehydration (scores of 5-8 on the Clinical Dehydration Scale (CDS) or more than 10% weight loss) or in complicated cases of AGE (those that do not meet the criteria of age >6 months, nontoxic appearance, no comorbidities, no hematochezia, and diarrhea <7 days) to guide IV fluid management and correct markedly abnormal electrolytes.14
Electrolyte panels may also rarely uncover disease processes, such as new-onset diabetes, hemolytic uremic syndrome, adrenal insufficiency, or inborn errors of metabolism, allowing for early diagnosis and preventing adverse outcomes. Suspicion to investigate such entities should arise during a thorough history and PE instead of routinely screening all children with symptoms of AGE. One should also have a higher level of concern for other disease processes when clinical recovery does not occur within the expected amount of time; symptoms usually resolve within 2 to 3 days but sometimes will last up to a week.
WHAT WE SHOULD DO INSTEAD
RECOMMENDATIONS
- Perform a thorough history and PE to diagnose AGE.8
- Clinical assessment of dehydration should be performed upon initial presentation and repeatedly with vital signs throughout the stay using a validated CDS to classify the patient’s initial dehydration severity and monitor improvement. Obtain a current patient weight and compare with previously recorded weights, if available.25,26
- Laboratory testing in patients with AGE should not be performed unless a patient is classified as severely dehydrated, is toxic appearing, has a comorbidity that increases the likelihood of complications, or is not improving as expected.
- Rehydration via ORT is preferred to an IV in mild and moderate dehydration.15
- If initial testing is performed and indicates an expected value indicative of dehydration, do not repeat testing to demonstrate normalization as long as the child is clinically improving as expected.
CONCLUSION
Children presenting with mild-to-moderate dehydration should be treated with supportive measures in accordance with current guidelines. Electrolyte panels very rarely provide clinical information that cannot be garnered through a thorough history and PE. As in our clinical scenario, the laboratory values obtained may have led to potential harm, including overdiagnosis, painful procedures, and psychological distress. Without testing, the patient likely could have been appropriately treated with ORT and discharged from the ED.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosure
The authors have nothing to disclose.
1. Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35-40. PubMed
2. Yilmaz K, Karabocuoglu M, Citak A, Uzel N. Evaluation of laboratory tests in dehydrated children with acute gastroenteritis. J Paediatr Child Health. 2002;38(3):226-228. PubMed
3. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179-182. PubMed
4. Jha S. Stop hunting for zebras in Texas: end the diagnostic culture of “rule-out”. BMJ. 2014;348:g2625. PubMed
5. Mold JW, Stein HF. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314(8):512-514. PubMed
6. Shahrin L, Chisti MJ, Huq S, et al. Clinical Manifestations of Hyponatremia and Hypernatremia in Under-Five Diarrheal Children in a Diarrhea Hospital. J Trop Pediatr. 2016;62(3):206-212. PubMed
7. Wathen JE, MacKenzie T, Bothner JP. Usefulness of the serum electrolyte panel in the management of pediatric dehydration treated with intravenously administered fluids. Pediatrics. 2004;114(5):1227-1234. PubMed
8. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97(3):424-435. PubMed
9. National Collaborating Centre for Women’s and Children’s Health. Diarrhoea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment and Management in Children Younger than 5 Years. London: RCOG Press; 2009. PubMed
10. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-152. PubMed
11. Freedman SB, Gouin S, Bhatt M, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127(2):e287-e295. PubMed
12. Lind CH, Hall M, Arnold DH, et al. Variation in Diagnostic Testing and Hospitalization Rates in Children With Acute Gastroenteritis. Hosp Pediatr. 2016;6(12):714-721. PubMed
13. Powell EC, Hampers LC. Physician variation in test ordering in the management of gastroenteritis in children. Arch Pediatr Adolesc Med. 2003;157(10):978-983. PubMed
14. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291(22):2746-2754. PubMed
15. Sandhu BK, European Society of Pediatric Gastroenterology H, Nutrition Working Group on Acute D. Practical guidelines for the management of gastroenteritis in children. J Pediatr Gastroenterol Nutr. 2001;33(suppl 2):S36-S39.
16. Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124(6):e1081-e1087. PubMed
17. Freedman SB, DeGroot JM, Parkin PC. Successful discharge of children with gastroenteritis requiring intravenous rehydration. J Emerg Med. 2014;46(1):9-20. PubMed
18. Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23-44. PubMed
19. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. PubMed
20. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. PubMed
21. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. PubMed
22. McMurtry CM, Noel M, Chambers CT, McGrath PJ. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30(6):780-788. PubMed
23. von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5(5):241-249. PubMed
24. American Academy of Pediatrics. Section on Hospital Medicine. Rauch DA, Gershel JC. Caring for the hospitalized child: a handbook of inpatient pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
25. Bailey B, Gravel J, Goldman RD, Friedman JN, Parkin PC. External validation of the clinical dehydration scale for children with acute gastroenteritis. Acad Emerg Med. 2010;17(6):583-588. PubMed
26. Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201-207. PubMed
1. Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35-40. PubMed
2. Yilmaz K, Karabocuoglu M, Citak A, Uzel N. Evaluation of laboratory tests in dehydrated children with acute gastroenteritis. J Paediatr Child Health. 2002;38(3):226-228. PubMed
3. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179-182. PubMed
4. Jha S. Stop hunting for zebras in Texas: end the diagnostic culture of “rule-out”. BMJ. 2014;348:g2625. PubMed
5. Mold JW, Stein HF. The cascade effect in the clinical care of patients. N Engl J Med. 1986;314(8):512-514. PubMed
6. Shahrin L, Chisti MJ, Huq S, et al. Clinical Manifestations of Hyponatremia and Hypernatremia in Under-Five Diarrheal Children in a Diarrhea Hospital. J Trop Pediatr. 2016;62(3):206-212. PubMed
7. Wathen JE, MacKenzie T, Bothner JP. Usefulness of the serum electrolyte panel in the management of pediatric dehydration treated with intravenously administered fluids. Pediatrics. 2004;114(5):1227-1234. PubMed
8. Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97(3):424-435. PubMed
9. National Collaborating Centre for Women’s and Children’s Health. Diarrhoea and Vomiting Caused by Gastroenteritis: Diagnosis, Assessment and Management in Children Younger than 5 Years. London: RCOG Press; 2009. PubMed
10. Guarino A, Ashkenazi S, Gendrel D, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132-152. PubMed
11. Freedman SB, Gouin S, Bhatt M, et al. Prospective assessment of practice pattern variations in the treatment of pediatric gastroenteritis. Pediatrics. 2011;127(2):e287-e295. PubMed
12. Lind CH, Hall M, Arnold DH, et al. Variation in Diagnostic Testing and Hospitalization Rates in Children With Acute Gastroenteritis. Hosp Pediatr. 2016;6(12):714-721. PubMed
13. Powell EC, Hampers LC. Physician variation in test ordering in the management of gastroenteritis in children. Arch Pediatr Adolesc Med. 2003;157(10):978-983. PubMed
14. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291(22):2746-2754. PubMed
15. Sandhu BK, European Society of Pediatric Gastroenterology H, Nutrition Working Group on Acute D. Practical guidelines for the management of gastroenteritis in children. J Pediatr Gastroenterol Nutr. 2001;33(suppl 2):S36-S39.
16. Tieder JS, Robertson A, Garrison MM. Pediatric hospital adherence to the standard of care for acute gastroenteritis. Pediatrics. 2009;124(6):e1081-e1087. PubMed
17. Freedman SB, DeGroot JM, Parkin PC. Successful discharge of children with gastroenteritis requiring intravenous rehydration. J Emerg Med. 2014;46(1):9-20. PubMed
18. Deyo RA. Cascade effects of medical technology. Annu Rev Public Health. 2002;23:23-44. PubMed
19. Coon ER, Quinonez RA, Moyer VA, Schroeder AR. Overdiagnosis: how our compulsion for diagnosis may be harming children. Pediatrics. 2014;134(5):1013-1023. PubMed
20. Florin TA, French B, Zorc JJ, Alpern ER, Shah SS. Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics. 2013;132(2):237-244. PubMed
21. Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44-54. PubMed
22. McMurtry CM, Noel M, Chambers CT, McGrath PJ. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30(6):780-788. PubMed
23. von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5(5):241-249. PubMed
24. American Academy of Pediatrics. Section on Hospital Medicine. Rauch DA, Gershel JC. Caring for the hospitalized child: a handbook of inpatient pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; 2013.
25. Bailey B, Gravel J, Goldman RD, Friedman JN, Parkin PC. External validation of the clinical dehydration scale for children with acute gastroenteritis. Acad Emerg Med. 2010;17(6):583-588. PubMed
26. Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201-207. PubMed
©2018 Society of Hospital Medicine
Prolonged IV Instead of Oral Antibiotics
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A previously healthy 6‐year‐old boy presented to the emergency room with 3 days of right lower leg pain and fevers up to 102F. The leg pain had progressed until he refused to walk. The patient and family did not recall any trauma to the leg. In the emergency department, he had a blood culture drawn. Because he had elevated inflammatory markers and a negative x‐ray of his right leg, a magnetic resonance imaging scan of the right leg was obtained that revealed right tibial osteomyelitis. He was taken to the operating room for debridement. After obtaining blood and bone cultures, he was started on intravenous (IV) vancomycin. His blood and surgical cultures grew methicillin‐resistant Staphylococcus aureus, sensitive to clindamycin. Subsequent blood cultures were negative, and his inflammatory markers trended down shortly after starting therapy. As he clinically improved, a peripherally inserted central catheter (PICC) was placed, and he was discharged home to complete a 6‐week course of IV vancomycin.
BACKGROUND
Osteoarticular infections (osteomyelitis and septic arthritis) are common problems in the pediatric population, affecting 1/2000 children annually and accounting for approximately 1% of all pediatric hospitalizations.[1, 2] Osteomyelitis can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. The most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis), particularly in young children, due to the highly vascular nature of the developing bone. Long bones, such as the femur, tibia, and humerus, are most commonly involved. Treatment of acute osteomyelitis requires prolonged administration of antimicrobial agents. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone.[3]
WHY YOU MIGHT THINK PARENTERAL ANTIBIOTICS AT DISCHARGE IS SUPERIOR TO ENTERAL THERAPY
In the United States, a large proportion of children with hematogenous osteomyelitis are discharged from the hospital with long‐term parenteral intravenous antibiotics through a PICC line.[3] The medical community historically favored parenteral therapy for young children with serious bacterial infections given concerns regarding impaired enteral absorption. As a result, children with osteomyelitis were initially stabilized in the hospital and discharged with parenteral therapy through a PICC line to continue or complete care, even when the organism was susceptible to a viable oral alternative such as clindamycin or cephalexin. Recommendations regarding the safety and timing to transition to oral antibiotics have been lacking. There is also extreme variation in practice in route of administration (oral vs prolonged IV therapy) in patients being discharged from the hospital with osteomyelitis.[3, 4] The most recent Infectious Diseases Society of America (IDSA) guidelines do not clearly state when transition to oral antibiotics may be safe. Specifically, they state that if patients are stable and without ongoing bacteremia, they can transition to oral therapy to complete a 4‐ to 6‐week course.[5]
WHY LONG‐TERM PARENTERAL ANTIBIOTICS MAY NOT BE SUPERIOR
The use of PICC lines has increased substantially in recent years. This has led to an increasing awareness of complications associated with PICC lines. As a result, guidelines for the appropriate use of PICC lines have been established in adults by collaborators at the University of Michigan.[6] Mounting evidence has called into question whether longer parenteral therapy is truly a more conservative or safer approach for the treatment of osteomyelitis.[3, 4, 7, 8, 9] Providing antibiotics via a PICC line in both the inpatient and outpatient settings may not be as benign as once accepted and may not improve outcomes in osteomyelitis as expected.
Costs and Potential Harms Associated With PICC Lines
PICC lines are known to have complications in the hospital including infection and thrombotic events,[10] but these events are not isolated to the hospital setting. Multiple studies have shown outpatient PICC line complication rates ranging from 29% to 41% depending on the type of catheter, the population, and the indication for use.[8, 10, 11, 12, 13] In a recently published study by Keren et al. looking specifically at children with osteomyelitis, emergency department visits and readmissions for PICC line complications occurred in 15% of patients discharged with a PICC line.[4] Given the potential complications and complexity that are inherent in outpatient parenteral therapy, the ISDA has even published guidelines regarding its use.[9] In addition, the cost of IV antibiotics, including administration costs, need for sedation in some children for line placement, and cost of the antibiotic itself, is significantly higher compared to oral therapy. In studies looking at early conversion to oral antibiotics versus prolonged intravenous antibiotics for complicated skin and soft tissue infections, as well as perforated appendicitis, oral antibiotics were more cost effective with an average savings of 30% to 50% and >$4000 respectively.[14, 15]
Patient Outcomes Are Similar When Comparing Parenteral and Enteral Therapy
In addition to increased costs and medication‐related complications, treatment of osteomyelitis with parenteral antibiotics through a PICC line does not improve clinical outcomes. As early as 1997, evidence emerged that an early transition to enteral therapy for osteomyelitis in children may be safe.[16] In 2010, the same group published a larger randomized study with the intent of determining overall treatment duration for osteomyelitis. This study involved 131 culture‐positive cases of osteomyelitis randomized to either a short‐term (20 days) or long‐term (30 days) oral antibiotics following 2 to 4 days of parenteral therapy. In this study, outcomes were favorable and similar despite such a short course of parenteral antibiotics and regardless of the overall treatment duration.[17] Although the aim of this study was not to compare oral and parenteral antibiotics, all patients in this large cohort were treated successfully with early transition to oral therapy.
In 2009, Zaoutis et al. published a large, multicenter, retrospective study of 1969 children with culture‐positive osteomyelitis treated with either prolonged IV therapy (defined as a central line placed before discharge) or oral therapy (no central line placed). They found a 4% incidence of treatment failure in the oral therapy group compared to a 5% incidence in the prolonged IV therapy group. They concluded that early transition to oral therapy was not associated with an increased risk of treatment failure.[3]
More recently, Keren et al. published a comparative effectiveness study using propensity scorebased matching to adjust for confounding variables. This retrospective study included 2060 children without comorbid conditions, ages 2 months to 18 years, with both culture‐positive and culture‐negative acute hematogenous osteomyelitis. Propensity‐based matching used logistic regression to compare patient‐level characteristics including age, race, insurance, length of stay, location of infection, surgical procedures, and isolation of causative pathogens. The rates of treatment failure were nearly identical in the oral therapy (5.0%) and PICC line (6.0%) groups. Similarly, in across‐hospital (risk difference, 0.3% [95% confidence interval {CI}: 0.1% to 2.5%]) and within‐hospital (risk difference, 0.6% [95% CI: 0.2% to 3.0%]) matched analyses, children in the oral therapy group did not have more treatment failures than those in the PICC line group. In the same comparisons, both adverse drug reactions and all treatment‐related events were significantly more likely to occur in children treated with long‐term parenteral antibiotics.[4]
Other studies have looked at the treatment of culture‐negative osteoarticular infections in children and have similarly found favorable outcomes in transitioning to oral therapy after a short course of parenteral treatment.[18]
In short, enteral therapy has similar treatment outcomes for culture‐positive and culture‐negative osteomyelitis without the complications associated with parenteral treatment via a PICC line.
WHEN TO CONSIDER PROLONGED PARENTERAL ANTIBIOTICS
The studies indicating the safe transition to oral antibiotics discussed above all excluded children with certain comorbid conditions. Although this varied from study to study, exclusions were as general in some as not previously healthy, and others were as specific as hematologic malignancies, immunocompromised states, sickle cell disease, malabsorption, and penetrating injuries. Also, although we know blood cultures obtained in children with osteomyelitis are positive in only approximately half of the patients,[19] the studies cited do not contain information for their study populations regarding the duration of bacteremia or endovascular complications, such as septic thrombophlebitis, which are well described in the literature.[20, 21] There are limited data on optimal treatment of children with prolonged bacteremia and endovascular complications. Because studies generally involved previously healthy children and do not specifically address these potential complications, the safety of early oral transition in complicated cases is not clear. The current IDSA and Red Book Committee on Infectious Diseases recommend intravenous therapy for bacteremia and endovascular infections with methicillin‐resistant S aureus.[5, 22] Clinical judgement should be used when treating children with comorbid illnesses who experience persistent bacteremia >48 hours, or who have endovascular complications.
WHAT YOU SHOULD DO INSTEAD
For children with acute hematogenous osteomyelitis who are either culture negative and improve on empiric therapy, or who have culture results (blood or tissue) that are susceptible to a reasonable oral antibiotic agent and who have clinical improvement on initial IV antibiotic therapy, a growing body of evidence indicates that the benefit of early transition to oral antibiotics outweighs the risks of continuing with parenteral therapy. Discharging children on oral antibiotics does not increase their risk of treatment failure but seems to decrease the risk of therapy‐associated complications, including increased healthcare utilization with return visits to the emergency department or the hospital. The possible exceptions to early transition to enteral antibiotics are prolonged bacteremia or endovascular infection, though there are insufficient data in the literature indicating benefits or risks of one administration route over the other.
RECOMMENDATIONS
- Previously healthy children with acute hematogenous osteomyelitis, without endovascular complications, should be transitioned to enteral antibiotics when they are showing signs of clinical improvement, as defined by: resolution of fever, improving physical exam, ability to take oral medications, and decreasing C‐reactive protein.
- The choice of oral antibiotics should be based on the organism's antibiotic susceptibility. If cultures are negative and the child has improved on empiric IV therapy, transition to an oral regimen with similar spectrum is acceptable.
- Patients with acute osteomyelitis should have close follow‐up after discharge from the hospital, within 1 to 2 weeks, to ensure continued improvement on therapy.
CONCLUSION
Early transition to oral antibiotics should be used in children with acute, uncomplicated osteomyelitis. A growing body of evidence shows that early transition to oral antibiotics does not increase the risk of treatment failure and can obviate the need for an outpatient PICC line. Oral antibiotics do not carry the risk of potential complications and complexity that are inherent in outpatient parenteral therapy. The transition to oral therapy should occur prior to discharge from the hospital after clinical improvement. Close follow‐up is essential to ensure successful treatment in children with acute osteomyelitis.
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
- . Osteomyelitis. In: Feigin RD, Cherry JD, Kaplan, SL, Demmler‐Harrison, GJ, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. Philadelphia, PA: Saunders Elsevier; 2009.
- . Osteomyelitis in children. Curr Opin Pediatr. 2002;14:112–115.
- , , , , , . Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642.
- , , , et al.; Pediatric Research in Inpatient Settings Network. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120–128.
- , , , et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin‐resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292.
- , , , et al.; Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) Panel. The Michigan appropriateness guide for intravenous catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 suppl):S1–S40.
- , . Intravenous antibiotic durations for common bacterial infections in children: when is enough enough? J Hosp Med. 2014;9(9):604–609.
- , , , , , . Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis. Pediatrics. 2006;117:1210–1215.
- , , , et al; IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38(12):1651–1672.
- , , , . Frequency of Peripherally Inserted Central Catheter Complications in Children. Pediatr Infect Dis J. 2012;31(5):519–521.
- , , , , . Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr. 2013;167(5):429–435.
- , , , , . Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2007;46:247–251.
- , , . Experience using peripherally inserted central venous catheters for outpatient parenteral antibiotic therapy in children at a community hospital. Pediatr Infect Dis J. 2008;27:1069–1072.
- , , , , , . Economic burden of inpatient and outpatient antibiotic treatment for methicillin‐resistant Staphylococcus aureus complicated skin and soft‐tissue infections: a comparison of linezolid, vancomycin, and daptomycin. Clinicoecon Outcomes Res. 2013;5:447–457.
- , , , et al. Postoperative antibiotic therapy for children with perforated appendicitis: long course of intravenous antibiotics versus early conversion to an oral regimen. Am J Surg. 2008;195(2):141–143.
- , , . Simplified treatment of acute staphylococcal osteomyelitis of childhood. The Finnish Study Group. Pediatrics. 1997;99(6):846–850.
- , , , ; Osteomyelitis‐Septic Arthritis Study Group. Short‐ versus long‐term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture‐positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128.
- , , , . Significance of negative cultures in the treatment of acute hematogenous bone and joint infections in children. J Ped Infect Dis. 2013;2(2):119–125.
- , . Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435.
- , , , . Venous thrombosis and thromboembolism in children with osteomyelitis. J Pediatr. 2006;149(4):537–541.
- , , , et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117(5):1673–1679.
- , , , . Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
© 2016 Society of Hospital Medicine