User login
Group A Streptococcal Pharyngitis Diagnosis
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)

However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)

Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)

However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)

Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
It’s wintertime, peak season for GAS pharyngitis, and you’d think that this far into the 21st century we would have a foolproof process for diagnosing which among the many patients with pharyngitis have true GAS pharyngitis. Thinking back to the 1980s, we have come a long way from simple throat cultures for detecting GAS, e.g., numerous point of care (POC) Clinical Laboratory Improvement Amendments (CLIA), waved rapid antigen detection tests (RADT), and numerous highly sensitive molecular assays, e.g. nucleic acid amplification tests (NAAT). But if you think the issues surrounding management of GAS pharyngitis have been solved by these newer tests, think again.
Several good reviews1-3 are excellent resources for those wishing a refresher on GAS diagnosis/management issues. They present nitty gritty details on comparative advantages/disadvantages of the many testing options while reminding us of the nuts and bolts of GAS pharyngitis. The following are a few nuggets from these articles.
Properly collected throat specimen. A quality throat specimen involves swabbing both tonsillar pillars plus posterior pharynx without touching tongue or inner cheeks. Two swab collections increase sensitivity by almost 10% compared with a single swab. Transport media is preferred if samples will not be cultured within 24 hours. Caveat: RADT testing of a transport media-diluted sample lowers sensitivity compared with direct swab use.
Reliable GAS detection. Commercially available tests in 2025 are well studied. Culture is considered a gold standard for detecting clinically relevant GAS by CDC.4 Culture has good sensitivity (estimated 80%-90% varying among studies and by quality of specimens) and 99% specificity but requires 16-24 hours for results. RADT solves the time-delay issues and has near 100% specificity but sensitivity used to be as low as 65%, hence the 2012 Infectious Diseases Society of America guideline recommendation for backup throat culture for negative tests.5 However, current RADT have sensitivities in the 85%-90% range.3,4 So a positive RADT reliably and quickly indicates GAS antigens are present. NAAT have the highest combined sensitivity and specificity, near 100% for each, and a positive reliably indicates GAS nucleic acids are present.
So why not simply always use NAAT? First, it’s a “be careful what you wish for” scenario. NAAT can, and do, detect dead remnants and colonizing GAS way more than culture.2,3 So NAAT are overly sensitive, adding an extra layer of interpretation difficulty, ie, as many as 20% of positive NAAT detections may be carriers or dead GAS. Second, NAAT often requires special instrumentation and kits are more expensive. That said, reimbursement is often higher for NAAT.
Choice based on accuracy in detecting GAS. If time delays were not a problem, culture would still seem the answer. If more rapid detection is needed, either RADT with culture back up or NAAT could be the answer. That said, consider that in the real world, throat cultures are less sensitive and RADT are less specific than indicated by some published data.6 So, the ideal answer, it seems, would be NAAT GAS detection coupled with a confirmatory biomarker of GAS infection. Such innate immune biomarkers may be on the horizon.3
But first, pretest screening. In 2025 what do we do with a positive result? Do we prescribe antibiotics? Do we think the detected GAS bacteria/antigens/nucleic acids represent the cause of the pharyngitis? Or did we just detect dead GAS or even a carrier, while a virus is the true cause? Challenges for this decision include most pharyngitis (up to 70%) being due to viruses, not GAS, plus up to 20% of GAS detections even by less sensitive culture or RADT can be carriers, plus an added 10%-20% of RADT and NAAT detections are dead GAS. Thus, with indiscriminate testing of all pharyngitis patients, the number of truly positive GAS detections that are actually “false positives” (GAS in some form is present but not causing pharyngitis) may be almost as high as for those representing true GAS pharyngitis.
Some tool is needed to minimize testing patients who are likely to have viral pharyngitis to reduce test-positive/GAS-pharyngitis-negative scenarios. Pretest patient screening therefore is critical to increase the positive predictive value of positive GAS testing results. The history and physical can be helpful. In the simplest form of pretest screening, eliminate those younger than 3 years old* or those with viral type sign/symptoms, eg conjunctivitis, cough, coryza.7 This could cut “false” positives by as much as a half. More complete validated scoring systems are also available but remain imperfect. The most published is the McIsaac score (modified Centor score).3-5,8 (See Table and Figure.)

However, even with this validated scoring system, misdiagnoses and some antibiotic misuse will likely occur, particularly if the controversial option to treat a patient with a score above 4 without testing is used. For example, a 2004 study in patients older than 3 years old revealed that 45% with a score above 4 points did not have GAS pharyngitis. (McIsaac et al.) A 2012 study showed similar potential overdiagnosis from using the score without testing (45% with > 4 points did not have GAS pharyngitis). Of note, clinical scores of below 2 comprised up to 10% and would be neither tested nor treated. (Figure.)

Best clinical judgment. Regardless of the chosen test, we still need to interpret positive results, ie, use best clinical judgment. We know that even with pretest screening some positives tests will represent carriers or nonviable GAS. Yet true GAS pharyngitis needs antibiotic treatment to minimize nonpyogenic and pyogenic complications, plus reduce contagion/transmission risk and days of illness. Thus, we are forced to use best clinical judgment when considering if what could be GAS pharyngitis, particularly exudative pharyngitis, could actually be due to EBV, adenovirus, or gonococcus, each of which can mimic GAS findings. Differentiating these requires discussion beyond the scope of this article, but clues are often found in the history, the patient’s age, associated symptoms and distribution of tonsillopharyngeal exudate. Likewise Group C and G streptococcal pharyngitis can mimic GAS. Note: A comprehensive throat culture can identify these streptococci but requires a special order and likely a call to the laboratory.
Summary: The age-old problem persists, ie, differentiating the minority (~30%) of pharyngitis cases needing antibiotics from the majority that do not. We all wish to promptly treat true GAS pharyngitis; however our current tools remain imperfect. That said, we should strive to correctly diagnose/manage as many patients with pharyngitis as possible. I, for one, can’t wait until we get a validated biomarker that confirms GAS as the culprit in pharyngitis episodes. In the meantime, most providers likely have clinic or hospital approved pathways for managing GAS pharyngitis, many of which are at least in part based on data from sources for this discussion. If not, a firm foundation for creating one can be found in sources among the reference list below. Finally, if you think such pathways somehow interfere with patient flow, consider that a busy multi-provider private practice successfully integrated pretest screening and a pathway while maintaining patient flow and improving antibiotic stewardship.7
*Focal pharyngotonsillar GAS infection is rare in children younger than 3 years old, when GAS nasal passage infection may manifest as streptococcosis.9
Dr Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He has no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Bannerjee D, Selvarangan RS. The Evolution of Group A Streptococcus Pharyngitis Testing. Association for Diagnostics and Laboratory Medicine, 2018, Sep 1.
2. Cohen JF et al. Group A Streptococcus Pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatric Infect Dis Soc. 2024 Apr 24;13(4):250-256. doi: 10.1093/jpids/piae0223.
3. Boyanton Jr BL et al. Current Laboratory and Point-of-Care Pharyngitis Diagnostic Testing and Knowledge Gaps. J Infect Dis. 2024 Oct 23;230(Suppl 3):S182–S189. doi: 10.1093/infdis/jiae415.
4. Group A Strep Infection. Centers for Disease Control and Prevention, 2024, Mar 1.
5. Shulman ST et al. Clinical Practice Guideline for the Diagnosis and Management of Group A Streptococcal Pharyngitis: 2012 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2012 Nov 15;55(10):e86-102. doi: 10.1093/cid/cis629.
6. Rao A et al. Diagnosis and Antibiotic Treatment of Group A Streptococcal Pharyngitis in Children in a Primary Care Setting: Impact of Point-of-Care Polymerase Chain Reaction. BMC Pediatr. 2019 Jan 16;19(1):24. doi: 10.1186/s12887-019-1393-y.
7. Norton LE et al. Improving Guideline-Based Streptococcal Pharyngitis Testing: A Quality Improvement Initiative. Pediatrics. 2018 Jul;142(1):e20172033. doi: 10.1542/peds.2017-2033.
8. MD+ Calc website. Centor Score (Modified/McIsaac) for Strep Pharyngitis.
9. Langlois DM, Andreae M. Group A Streptococcal Infections. Pediatr Rev. 2011 Oct;32(10):423-9; quiz 430. doi: 10.1542/pir.32-10-423.
Predicting RSV’s Role in the Upcoming Winter Respiratory Season
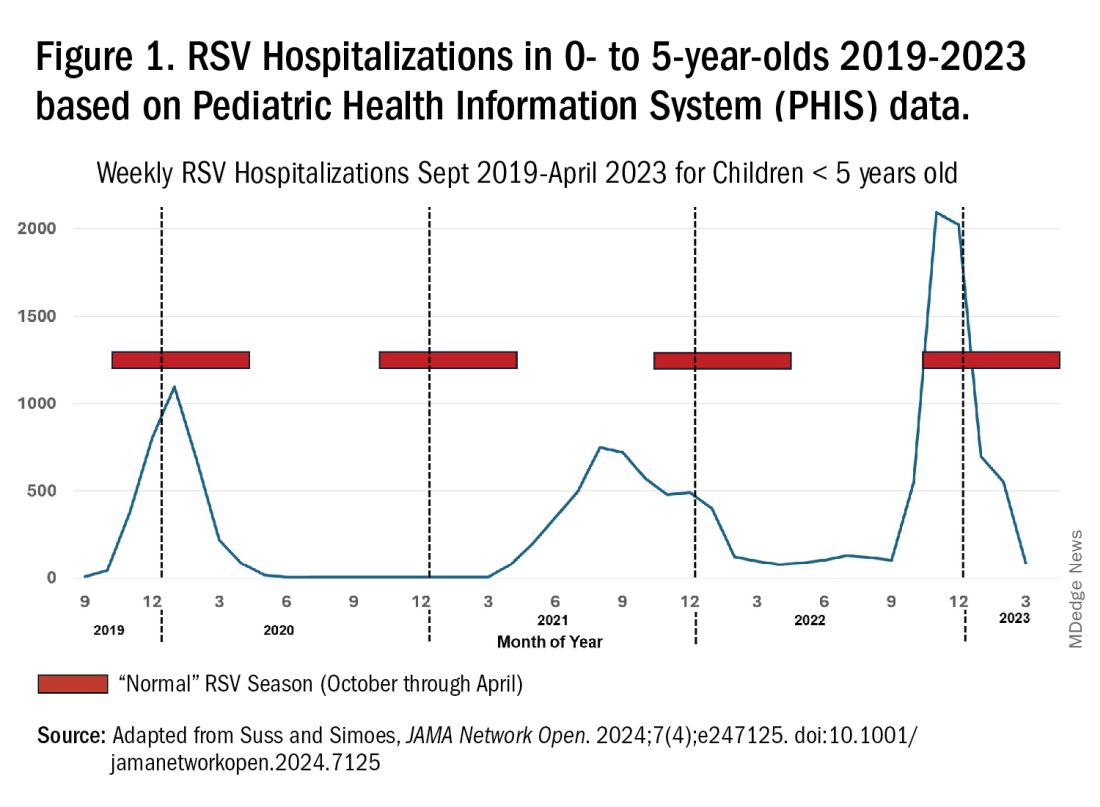
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
For children younger than 5 years old, RSV is the main drive — approximately 2,000,000 outpatient/ED visits and about 75,000 hospitalizations annually. RSV disease ranges from upper respiratory tract infections, eg, in older children and healthy adults, to more severe lower tract disease in young children and the elderly. Premature infants and high-risk groups are particularly prone to severe disease.1 Up to 300 pediatric RSV deaths occur yearly. “Normal” RSV seasons start in mid-November, peak in late December-January, and end after April. Note: More drawn out seasons occur in southern latitudes, eg Texas or Florida. But lately RSV seasons have been anything but normal.
2015-2016 to 2022-2023
RSV data from the Pediatric Health Information System (PHIS), collected at over 49 US children’s hospitals during 2015 to early 2023, show how crazy RSV seasons have been lately.2 The involved months, intensity, and duration of four prepandemic seasons were pretty “normal” (Figure 1). The 2019-2020 season started normally, peaked in January 2020, and was slowing as expected by February. But when SARS-Cov-2 restrictions kicked in during mid-March, RSV detections tanked to almost nothing (ditto other respiratory viruses). A near 14-month RSV hiatus meant that the 2020-2021 RSV season never materialized. However, RSV was not done with us in 2021. It rebounded in May with weekly hospitalizations peaking in late July; this “rebound season” lasted 9 months, not dropping to baseline until February 2022 (Figure 1).
I guess we should have expected a post-pandemic “disturbance in the Force,” as Yoda once said; but I sure didn’t see a prolonged summer/fall/early winter RSV season coming. It was like two “normal” seasons mashed up into one late-but-long season. Not to be outdone, the 2022-2023 RSV season started early (September) and hospitalizations skyrocketed to peak in November at over twice the peak number from any year since 2015, overloading hospitals (influenza and SARS-Cov-2 seasons were co-circulating). The season terminated early though (March 2023).
Okay, so RSV seasonality/intensity were weird post pandemic, but was anything else different? Some 2021-2023 data suggest more RSV disease in older children, rather than the usual younger than 18 month-olds going through their first winter.3 More medically attended RSV in older ages (2-4 years of life) may have been due to the pandemic year without RSV circulation distorting herd immunity, ie older children remained RSV naive. Other data suggest the apparent increase was really just more frequent multiplex viral testing in older children triggered by SARS-CoV-2 co-circulation.4 More data are needed to decide.
CDC 2023-2024 RESP-NET data
The 2023-2024 winter surge (Figure 2), as measured by RESP-NET’s cumulative RSV,influenza and SARS-CoV-2 hospitalization rates for 0- to 5-year-olds,5 shows that all three viruses’ seasonal months were normal-ish: late October 2023 start, late December-early January peak, and mid-May 2024 return to baseline. RSV season was approximately 22% less severe by area-under-the-curve calculations compared with 2022-2023, but still worse than prepandemic years.6
One wonders if the 2022-2023 RSV season might have been worse but for use of the limited supply of nirsevimab.7
Viral Parade
Now we ready ourselves for the 2024-2025 respiratory surge, wondering what nature has in store for us. Will the usual “respiratory virus parade” occur? Will rhinovirus and parainfluenza prevalence bump after a few weeks of schools being in session, adding to the now-usual summer/fall SARS-CoV-2 surge? Note: Twenty-seven states as of Aug. 16 had high SARS-CoV-2 detection in wastewater. Will RSV and influenza start sometime in October/November, peak in January (along with rising SARS-CoV2 activity), followed by a second parainfluenza bump as SARS-CoV-2, influenza, and RSV drop off in April/May? Further, will RSV and influenza seasons be more or less severe than the last 2 years?
Prediction
The overall 2024-2025 respiratory season will be less severe than the past 2 years and hopefully than recent prepandemic years. What is the blueprint for a milder season? First, herd immunity to non-RSV and non-influenza viruses (parainfluenza, rhinovirus, metapneumovirus, adenovirus) in older children should be normalized after 2 years back to usual social activity. So, I expect no mega-seasons from them. The emerging SARS-CoV-2 virus (LB.1) is immunologically close to its recent still-circulating ancestors (KP.2, KP.2.3, KP.3 and KP.3.1.1), so existing SARS-CoV2 herd immunity along with recommended booster vaccine uptake should keep the lid on SARS-CoV2.
Influenza Could Be the Bad News
Which type will dominate? Will a drift/shift occur or vaccine-mismatch reduce vaccine effectiveness? Can we get at least half the population influenza vaccinated, given the vaccine fatigue permeating the US population? The influenza season now underway in the Southern Hemisphere usually helps us predict our season. The Australian May-August 2024 experience (still on an upward trajectory for severity in mid-August) saw no drift/shift or vaccine mismatch. However, this 2024 season has been as severe as 2022 (their worst in a decade). That said, more than 95% has been type A (mostly H1N1 but H3N2 increased in July). So, if our overall 2024-2025 respiratory season is not milder, influenza is the most likely culprit. To reduce chances of influenza being the fly-in-the-ointment, we need to be particularly proactive with seasonal influenza vaccine which is back to the traditional trivalent formulation (one H1N1, one H3N2, and one B type).8 All of this could go out the window if avian influenza becomes more transmissible, but that seems unlikely at present.
Mild RSV Season?
RSV season should be blunted because of the increased use of both the remarkably effective CDC-recommended maternal RSV vaccine9 (one dose during pregnancy weeks 32 through 36, administered September through January) and of nirsevimab (up to 90% reduction in hospitalizations and ED visits).10 (See Figure 3.) 
I also expect residual disease to occur mostly in younger than 18 month-olds (the “normal” aged population experiencing their first winter), who received no passive immunity (mother RSV unvaccinated and child did not receive nirsevimab). Some disease will still occur in high-risk infants/children. However, unlike active vaccination strategies, a competent immune system is not required to benefit from passive antibody, whether transplacental or directly administered.
Deep Thought
What if the traditional RSV seasonal hospitalization surge fails to materialize this season? It could happen. If we could get high acceptance/uptake of maternal vaccine and infant nirsevimab, RSV season could resemble the dramatic drop in rotavirus disease the second year after rotavirus vaccine introduction. We could be asking ourselves — “What happened to RSV?”
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Missouri. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. CDC. RSV in Infants and Young Children. Respiratory Syncytial Virus Infection (RSV). June 18, 2024. https://www.cdc.gov/rsv/infants-young-children/index.html.
2. Suss RJ and Simões EAF. Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Respiratory Syncytial Virus Hospital-Based Burden of Disease in Children Younger Than 5 Years, 2015-2022. JAMA Netw Open. 2024;7(4):e247125. doi:10.1001/jamanetworkopen.2024.7125.
3. Winthrop ZA et al. Pediatric Respiratory Syncytial Virus Hospitalizations and Respiratory Support After the COVID-19 Pandemic. JAMA Netw Open. 2024;7(6):e2416852. doi:10.1001/jamanetworkopen.2024.16852.
4. Petros BA et al. Increased Pediatric RSV Case Counts Following the Emergence of SARS-CoV-2 Are Attributable to Increased Testing. medRxiv [Preprint]. 2024 Feb 12:2024.02.06.24302387. doi: 10.1101/2024.02.06.24302387.
5. Rates of Laboratory-Confirmed RSV, COVID-19, and Flu Hospitalizations from the RESP-NET Surveillance Systems. Centers for Disease Control and Prevention. https://data.cdc.gov/Public-Health-Surveillance/Rates-of-Laboratory-Confirmed-RSV-COVID-19-and-Flu/kvib-3txy/about_data.
6. CDC. Evaluating the 2023-2024 Respiratory Disease Season Outlook. CFA: Qualitative Assessments. August 14, 2024. https://www.cdc.gov/cfa-qualitative-assessments/php/data-research/2023-2024-season-outlook-retro.html.
7. Health Alert Network (HAN). Limited Availability of Nirsevimab in the United States—Interim CDC Recommendations to Protect Infants from Respiratory Syncytial Virus (RSV) during the 2023–2024 Respiratory Virus Season. October 23, 2023. https://emergency.cdc.gov/han/2023/han00499.asp.
8. CDC. Information for the 2024-2025 Flu Season. Centers for Disease Control and Prevention. March 14, 2024. https://www.cdc.gov/flu/season/faq-flu-season-2024-2025.htm.
9. Kampmann B et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480.
10. Moline HL. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season — New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb Mortal Wkly Rep. 2024;73. doi: 10.15585/mmwr.mm7309a4.
Worldwide Uptick in Invasive Group A Streptococcus Disease Post Pandemic — What Should We Know?
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.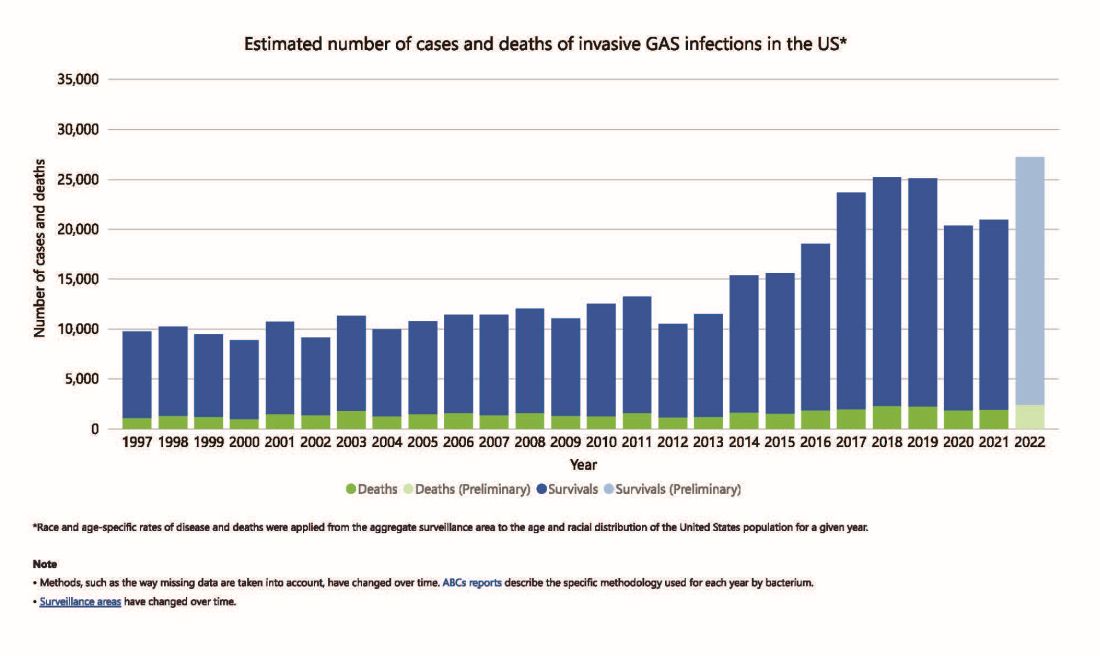
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4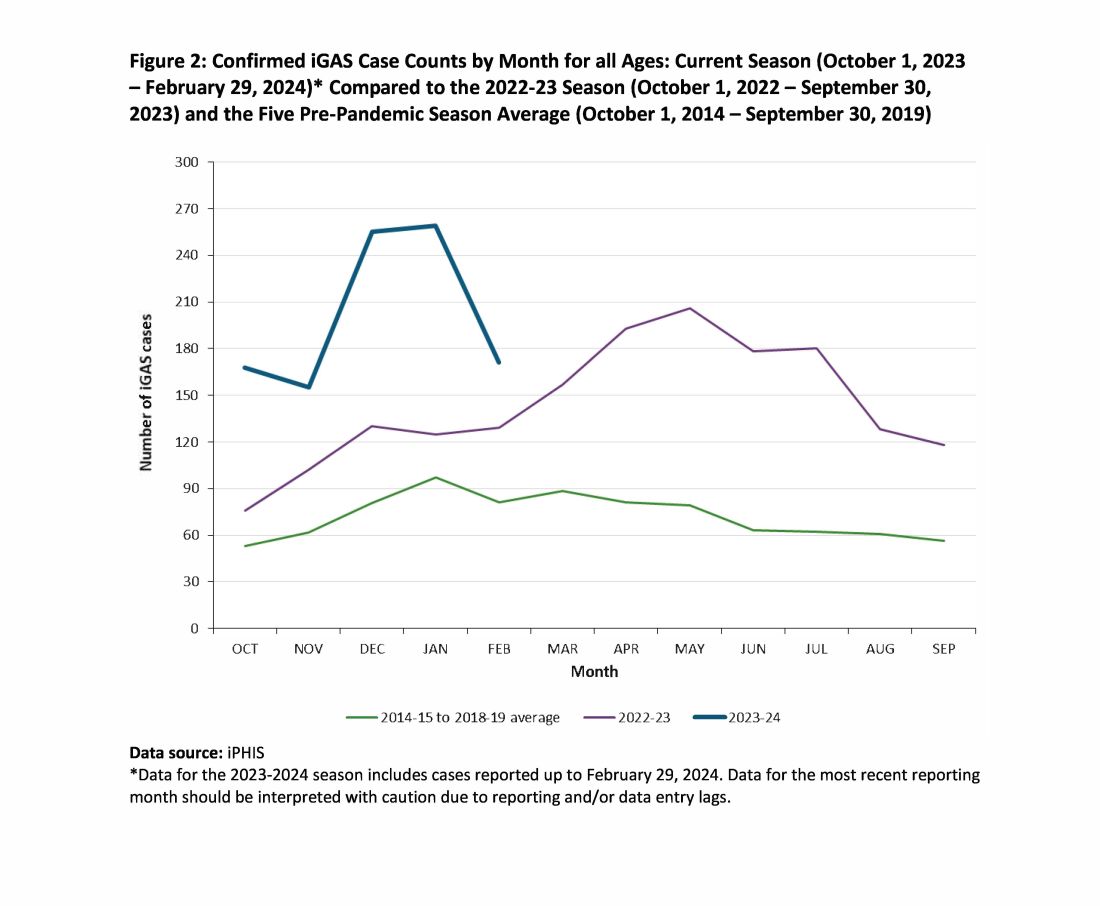
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Invasive group A streptococcus (iGAS) infections are rare (4-9 cases/100,000 US population annually) but potentially devastating (approximately 2,300 deaths annually in US), and affect all ages. Cases increase in winter-spring, paralleling the “season” of increased noninvasive GAS, e.g., pharyngitis and scarlet fever. iGAS case rates are lower in children than adults. That said, one well-known pediatric iGAS scenario has been deep cellulitis and necrotizing fasciitis during the healing phase of varicella. Other forms of iGAS include bacteremia, pneumonia (particularly when empyema is present), lymphangitis, erysipelas, and toxic shock syndrome. iGAS can occur with/after influenza but has also occurred concurrently with other viral respiratory infections.
Persons with underlying conditions (cancer or immune compromised status; chronic diseases of the heart, kidney or lung; diabetes mellitus) are at higher risk. Other subpopulations at risk for iGAS are illicit drug users, the elderly, homeless persons, nursing home residents, American Indian persons, and Alaska Native persons. Most experts feel that highly toxigenic strains of GAS are responsible for most iGAS. Indeed, most iGAS isolates produce (sometimes hyper-produce) superantigens that cause exaggerated innate immune responses, higher levels of inflammation, and often times tissue destruction, e.g., “flesh eating bacteria.” And who can forget that Jim Henson, creator of the Muppets, died of iGAS?
But why discuss iGAS in 2024? The pattern for iGAS has fluctuated more than usual in the last decade. So much so that the recent upsurge has caught the collective eye of the lay press. So, patients and friends may have questions about why and how iGAS is increasing lately. The bottom line is that no one knows for sure. However, the most recent 2 years of uptick may reflect GAS circulating at relatively high levels even when taking into account that GAS season occurs in winter-spring most years. Yet it seems likely that additional factors may have played a role in the fluctuations noted this past decade, e.g., temporary changes in societal behavior, a new GAS strain with over two dozen mutations, and possibly rapid waning of protection against GAS exotoxins.
Social Behavior Factor
The SARS-CoV-2 pandemic brought extremes of disease and death to the world and dramatic changes in social behavior. A byproduct was dramatic decreases in nearly all infectious diseases, with numerous reports of near absence of many respiratory and gastrointestinal viruses in the 2020-2021 seasons. Interestingly, we did not see a drop in human rhinovirus infections, justifying its nickname as the cockroach of viruses. Reports also emerged about drops in bacterial diseases during 2020-2021 (although not so much for STIs), including noninvasive and invasive GAS disease, and also GAS-associated deaths (lowest since 2016).1 The drop in iGAS during social restrictions makes sense because GAS is spread by direct contact with infected persons or their secretions, and social contact had dramatically decreased particularly in the first 6 months of the pandemic.
However, since 2022 and the return to “normal” social behaviors, both viral diseases (e.g., RSV, influenza, and Norovirus), and some bacterial diseases have rebounded. That said, something else must be contributing, because iGAS rates had increased 4-5 years pre pandemic. In fact, the fluctuating pattern included “normal” annual rates in the early 2000s rising in ~2015 followed by the explainable pandemic drop (by nearly 25%), and not-too-unexpected 2-year postpandemic rise. But interestingly enough, the rebound is higher than might be expected for iGAS and children were overrepresented in first year’s rise (2022 rate for pediatric iGAS was the highest since 1997) while those older than 65 were overrepresented in second year (2023).1
Emergence of M1UK
One potential factor for the prepandemic rise in iGAS infections worldwide is the emergence and worldwide spread of a new GAS emm type variant designated M1UK.2 GAS isolates can be typed into categories designated as emm types based on DNA sequence. There are more than 240 emm types, with 6 being most common — M1, M3, and M28 (each up to 20% of GAS isolates) and M12, M82, and M89 (each up to 10%). M1, M3 and M28 have also been particularly associated with invasive disease. While emm types vary year to year and region by region, the overall emm type distribution among iGAS isolates in the United States had not been unusual since the turn of the century and the US M1 strain was the same as that which had been predominant worldwide (designated M1GLOBAL). This new M1UK sublineage had emerged around 2010 and had been increasing pre pandemic. The M1UK sequence contained a specific set of 27 SNPs (single nucleoside polymorphisms, i.e., single base mutations) and was associated with an uptick in scarlet fever in the United Kingdom starting around 2010. Its prevalence increased up to around 2015 while spreading internationally. It also had enhanced expression of SpeA, a phage-encoded superantigen. Some of the M1UK mutations also appear to alter GAS metabolic processes to allow better survival (better “fitness”) compared with other GAS. So, a more virulent hardier GAS had arisen and seems a reasonable candidate for contributing to the increased iGAS rates.
Waning Antibody to GAS As Potential Factor in Rebound
No consensus exists on correlates of protection from iGAS. However, adults seem to have less noninvasive GAS than children. One potential reason is that frequent GAS re-exposure, regardless of whether disease results, likely boosts anti-GAS antibodies. Pandemic social restrictions temporarily prevented such boosts. In children with developing antibody repertoires, anti-GAS antibodies may have waned below protective levels faster during a year without frequent boosting. Thus, children were iGAS susceptible soon after pandemic restrictions were dropped (2022). Increased iGAS rates in the elderly in 2023 may have occurred because of diminished GAS exposures accelerating immune senescence with anti-GAS antibodies dropping, but less quickly than in children. These speculations are simply hypotheses until future studies can test them.
All that said, how do we use information on increased iGAS in our daily practices? In addition to standard preventive strategies for viral coinfections (e.g., varicella and influenza vaccine), reminding families about rigorous attention to wound care is the one high-risk scenario we have not yet discussed. During 2024, a time of expected increased prevalence of iGAS, early wound care needs to be fastidious. Further, share warning signs with families (e.g., rapidly expanding painful erythema), “streaks” ascending from extremity wounds, fever and a highly painful wound, darkening almost purple color within cellulitis or soft tissue infection, or loss of sensation in the middle of an otherwise painful soft tissue infection. These presentations require immediate medical attention.
If such a patient presents, the Centers for Disease Control and Prevention (CDC) recommends admission along with blood and, where possible, wound cultures. If in the context of pneumonia with pleural effusion, culturing pleural fluid is also important. Remember, leading edge cultures are not often positive for GAS, seemingly because GAS exotoxins are found at erythema’s leading edge, not the bacteria. The bacteria are somewhere more central in the inflammatory process. Despite not being prominent among recent iGAS cases, another scenario that could sneak up on you is the infected surgical wound as nascent iGAS.
Finally, remember that nationally increasing numbers of iGAS isolates are resistant to erythromycin and clindamycin, the latter usually recommended to reduce tissue damage in iGAS.3 So, it is important to be aware of susceptibility patterns in your locale and consider an ID consultation. My hope is that you do not see an iGAS case this year, but we all need to remain alert. With a high index of suspicion and rapid diagnosis, you can minimize long-term sequelae and potential fatalities.
While it is too early to tell how the rest of 2024 will turn out, preliminary indications are that GAS is circulating at higher than usual levels (30%-35% GAS positive throat swabs in early April 2024 in Kansas City area) and iGAS rates will likely also be relatively high, particularly if Ontario, Canada, data are any indication.4
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Current Group A Strep Activity, Centers for Disease Control and Prevention. April 2024. CDC webpage on current invasive GAS disease. April 2024.
2. Li Y et al. Expansion of Invasive Group A Streptococcus M1UK Lineage in Active Bacterial Core Surveillance, United States, 2019-2021 Emerg Infect Dis. 2023;29(10):2116-2120. doi: 10.3201/eid2910.230675.
3. Andreoni F et al. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J Infect Dis. 2017 Jan 15;215(2):269-277. doi: 10.1093/infdis/jiw229.
4. Group A Streptococcal Disease, Invasive (iGAS), Public Health Ontario.
Summer diarrhea – Time to think outside the box
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at pdnews@mdedge.com.
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
ICYMI articles featuring 9 important developments of the past year – and COVID is still here
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
We can’t affect most of the world’s big problems, but we can continue to do what pediatric providers have always done well – share the best science-based knowledge with families and be strong vaccine advocates.
You can read about some new aspects of science-based 2021-2022 data in this digital issue. For example, there are newer international data on the longer-acting and more effective anti-RSV monoclonal antibody nirsevimab, which may soon replace palivizumab. Closer to home, check out the article on lower antibody concentrations in infants related to the number and class of antibiotics that they had received. Measles outbreaks in areas of the world with the lowest measles vaccine uptake will likely produce more imported measles in the United States. If you have never heard of Lone-star virus, an article tells us it occurs mostly in Southern and Atlantic coastal regions; no specific treatment exists, but it is now in the differential diagnosis for endemic tick-borne febrile infections.
A bit of good news is the World Health Organization recommending a shorter course of treatment for pediatric tuberculosis. Pediatric TB has a long history of poor treatment adherence, so shorter, simpler regimens are certainly welcome. And finally, prospects for a norovirus vaccine are looking brighter with new approaches generating mucosal antibodies – a key in protection against gastrointestinal infections.
Again, no articles in this digital supplement feature SARS-CoV-2 this year, but a summer surge continues because of third-generation Omicron viruses BA.4/BA.5. The surge exists because the SARS-CoV-2 vaccine is being underutilized; plus BA.4/BA.5 is the most contagious variant yet.
A major reason deaths are not surging is COVID-19 vaccines. Having multiple vaccines authorized within 9 months of SARS-CoV-2 hitting U.S. shores is amazing despite the hiccups and politicization that accompanied implementation. Each vaccine more than met the original goal: greater than or equal to 50% effectiveness with an acceptable adverse effect profile. In the United States, two mRMA-vaccines (Moderna and Pfizer) are now authorized for use down to 6 months of age; Novavax’s more traditional protein-based vaccine was more recently given an emergency use authorization for those 18 years and older. Ongoing trials indicate that Omicron-based mRNA vaccines are highly immunogenic and safe even if blended with the original strain vaccine. Fall boosters will have an Omicron component. We need to immunize and boost enough folks so that SARS-CoV-2 variants arise infrequently, allowing high-risk persons to be able to go out in public without masks.
Dr. Harrison is professor, University of Missouri–Kansas City School of Medicine, department of medicine, infectious diseases section. He has no conflicts of interest.
To vaccinate 6-month- to 5-year-olds against SARS-CoV-2 or not to vaccinate
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
Commentary: WHO, UNICEF warn about increased risk of measles outbreaks
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
The newly released global estimate is now 25 million children (2 million more than in 2020) missing scheduled vaccines. This continues to bode badly for multiple vaccine-preventable infections, but maybe the most for measles in 2022.
Specifically for measles vaccine, global two-dose coverage was only 71%. Coverage was less than 50% in 8 countries: Chad, Guinea, Samoa, North Korea, Central African Republic, Somalia, Angola, and South Sudan. These eight areas seem ripe for outbreaks this year and indeed Somalia is having an outbreak.
Overall, worldwide measles cases increased 79% in early 2022, compared with 2021. The top 10 countries for measles cases from November 2021 to April 2022, per the World Health Organization, include Nigeria, India, Soma Ethiopia, Pakistan, DR Congo, Afghanistan, Liberia, Cameroon, and Ivory Coast.
In the United States, we have been lucky so far with only 55 cases since the start of 2021. However, MMR two-dose coverage has dropped since the pandemic’s start. The list of U.S. areas with the lowest overall two-dose MMR coverage as of 2021 were D.C. (78.9%), Houston (93.7%), Idaho (86.5%), Wisconsin (87.2%), Maryland (87.6%), Georgia (88.5%), Kentucky (88.9%), Ohio (89.6%), and Minnesota (89.8%). Only 14 states had rates over the targeted 95% rate needed for community (herd) immunity against measles (MMWR Morb Mortal Wkly Rep. 2022;71:561-8).
Two bits of good news are that there seems to be some catch-up occurring in vaccine uptake overall (including MMR) and we now have two MMR suppliers in the United States since GlaxoSmithKline’s MMR was recently approved by the Food and Drug Administration for persons over 1 year of age. Let’s all redouble our efforts at adding to the catch-up efforts.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Antibiotics use and vaccine antibody levels
This study of antibiotic use in the first 2 years of life in a reasonably standardized primary care office raises issues about antibiotic stewardship that can be the basis for counseling against antibiotics for viral infections or mild uncomplicated acute otitis media (AOM) above 6 months of age. Even unintended and previously undescribed downstream effects of antibiotics should play a role in our decisions and are another nudge toward prudent antibiotic use – for example, watchful waiting (WW) for AOM.
Some families ask for antibiotics for almost any infection while others may want antibiotics only if really necessary. But maybe patient family wishes are not the main driver, considering a report in Pediatrics (2022;150[1]:e2021055613). They analyzed over 2 million AOM episodes from billing/enrollment records from the MarketScan commercial claims research databases. They reported that, despite WW being the management of choice per American Academy of Pediatrics guidelines for uncomplicated AOM in children over 1 year of age, WW use had not increased between 2015 and 2019. Further, they noted that WW was not related to patient factors or demographics but was associated with specialty and provider. For example, WW use was five times more likely by otolaryngologists than pediatricians and less likely by nonpediatricians than pediatricians. Further, some clinicians used WW a lot, while others almost not at all (high-volume antibiotic prescribers). Of note, having a fever significantly lowered the chance of WW.
Maturing data on antibiotic-related alterations in species distribution and quantity within children’s microbiome plus potential effects on antibody responses to vaccines are ideas families need to hear. I suggest sharing these as part of anticipatory guidance at well-child checks as early in life as is feasible.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
This study of antibiotic use in the first 2 years of life in a reasonably standardized primary care office raises issues about antibiotic stewardship that can be the basis for counseling against antibiotics for viral infections or mild uncomplicated acute otitis media (AOM) above 6 months of age. Even unintended and previously undescribed downstream effects of antibiotics should play a role in our decisions and are another nudge toward prudent antibiotic use – for example, watchful waiting (WW) for AOM.
Some families ask for antibiotics for almost any infection while others may want antibiotics only if really necessary. But maybe patient family wishes are not the main driver, considering a report in Pediatrics (2022;150[1]:e2021055613). They analyzed over 2 million AOM episodes from billing/enrollment records from the MarketScan commercial claims research databases. They reported that, despite WW being the management of choice per American Academy of Pediatrics guidelines for uncomplicated AOM in children over 1 year of age, WW use had not increased between 2015 and 2019. Further, they noted that WW was not related to patient factors or demographics but was associated with specialty and provider. For example, WW use was five times more likely by otolaryngologists than pediatricians and less likely by nonpediatricians than pediatricians. Further, some clinicians used WW a lot, while others almost not at all (high-volume antibiotic prescribers). Of note, having a fever significantly lowered the chance of WW.
Maturing data on antibiotic-related alterations in species distribution and quantity within children’s microbiome plus potential effects on antibody responses to vaccines are ideas families need to hear. I suggest sharing these as part of anticipatory guidance at well-child checks as early in life as is feasible.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
This study of antibiotic use in the first 2 years of life in a reasonably standardized primary care office raises issues about antibiotic stewardship that can be the basis for counseling against antibiotics for viral infections or mild uncomplicated acute otitis media (AOM) above 6 months of age. Even unintended and previously undescribed downstream effects of antibiotics should play a role in our decisions and are another nudge toward prudent antibiotic use – for example, watchful waiting (WW) for AOM.
Some families ask for antibiotics for almost any infection while others may want antibiotics only if really necessary. But maybe patient family wishes are not the main driver, considering a report in Pediatrics (2022;150[1]:e2021055613). They analyzed over 2 million AOM episodes from billing/enrollment records from the MarketScan commercial claims research databases. They reported that, despite WW being the management of choice per American Academy of Pediatrics guidelines for uncomplicated AOM in children over 1 year of age, WW use had not increased between 2015 and 2019. Further, they noted that WW was not related to patient factors or demographics but was associated with specialty and provider. For example, WW use was five times more likely by otolaryngologists than pediatricians and less likely by nonpediatricians than pediatricians. Further, some clinicians used WW a lot, while others almost not at all (high-volume antibiotic prescribers). Of note, having a fever significantly lowered the chance of WW.
Maturing data on antibiotic-related alterations in species distribution and quantity within children’s microbiome plus potential effects on antibody responses to vaccines are ideas families need to hear. I suggest sharing these as part of anticipatory guidance at well-child checks as early in life as is feasible.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Commentary: Emerging tick-borne pathogen has spread to state of Georgia
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Just what we need – another tick-borne virus that mimics ehrlichiosis or anaplasmosis and is endemic in the lower Midwest and parts of the Southeast and Atlantic coast of the United States. Yet here it is. Human illness was first reported in northeast Missouri in 2012. It is known to be associated with Lone star ticks, with reservoirs including white tailed deer and several other mammals.
It has up to a 2-week incubation period. So, living in or having recently traveled to an endemic area is an important historical clue. Most infections present with headache, fever, fatigue, nausea, diarrhea, and/or muscle and joint pain. There may be a nonspecific rash but nothing like the classic Lyme disease or Rocky Mountain spotted fever rashes. The illness may be severe enough to lead to hospitalization, particularly when laboratory tests results, such as leukopenia, thrombocytopenia, and/or elevated liver function studies, raise the specter of other serious illnesses.
There is no commercial test, so the diagnosis is by serology and/or reverse transcription–polymerase chain reaction by the Centers for Disease Control and Prevention. Clinicians considering the diagnosis should contact their state health department for instructions on sample collection, processing, and shipment.
The good news is that it appears to be self-limited. There is no specific treatment or vaccine, so management is by supportive treatment.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.