User login
Newer 3D lung models starting to remake research
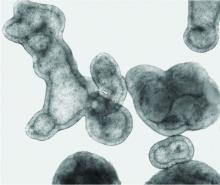
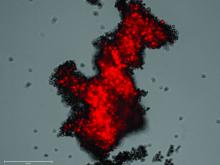
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Brodalumab suicide risk similar to other biologics, postmarket study finds
.
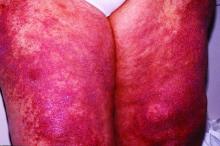
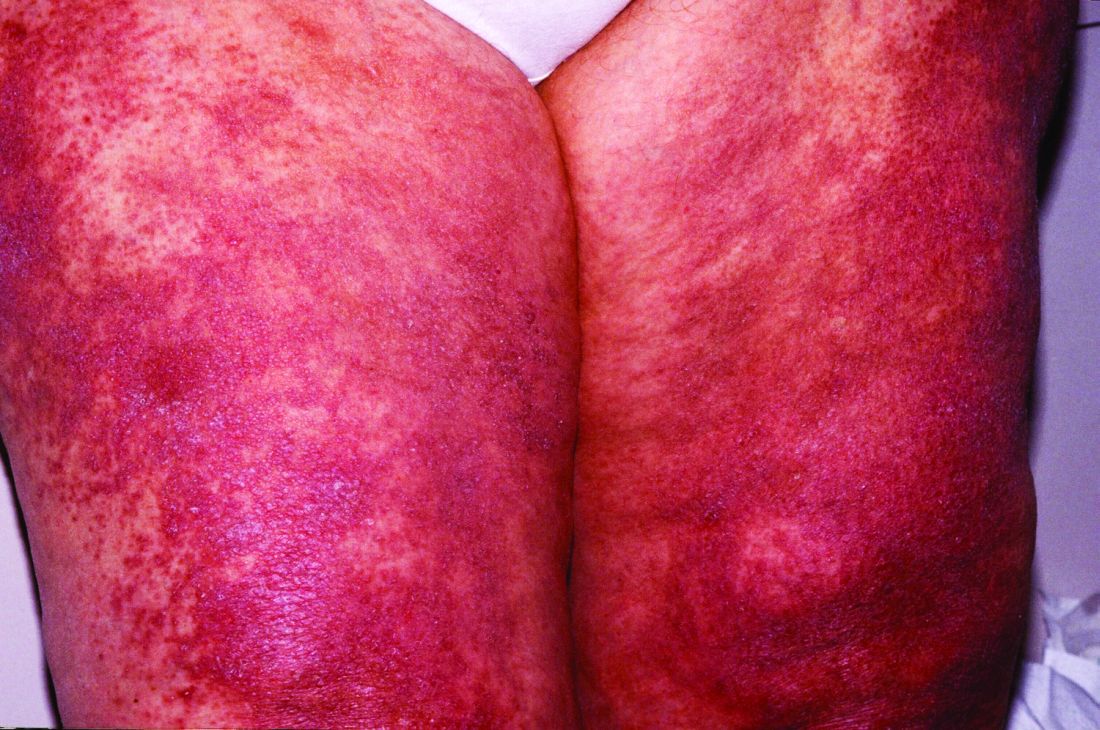
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
Litifilimab meets primary endpoint in phase 2 lupus trial
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Asian patients with psoriasis have shortest visits, study shows
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
(NAMCS) from 2010 to 2016.
Yet the reasons for the difference are unclear and in need of further research, said the investigators and dermatologists who were asked to comment on the research.
The study covered over 4 million visits for psoriasis and found that the mean duration of visits for Asian patients was 9.2 minutes, compared with 15.7 minutes for Hispanic or Latino patients, 20.7 minutes for non-Hispanic Black patients, and 15.4 minutes for non-Hispanic White patients.
The mean duration of visits with Asian patients was 39.9% shorter, compared with visits with White patients (beta coefficient, –5,747; 95% confidence interval, –11.026 to –0.469; P = .03), and 40.6% shorter, compared with visits with non-Asian patients combined (beta coefficient, –5.908; 95% CI, –11.147 to –0.669, P = .03), April W. Armstrong, MD, MPH, professor of dermatology and director of the psoriasis program at the University of Southern California, Los Angeles, and Kevin K. Wu, MD, a dermatology resident at USC, said in a research letter published in JAMA Dermatology.
“The etiology of these differences is unclear,” they wrote. “It is possible that factors such as unconscious bias, cultural differences in communication, or residual confounding may be responsible for the observed findings.”
Their findings came from multivariable linear regression analyses that adjusted for age, sex, type of visit (new or follow-up), visit complexity based on the number of reasons for the visit, insurance status (such as private insurance or Medicaid), psoriasis severity on the basis of systemic psoriasis treatment or phototherapy, and complex topical regimens (three or more topical agents).
Commenting on the results, Deborah A. Scott, MD, codirector of the skin of color dermatology program at Brigham and Women’s Hospital and assistant professor at Harvard Medical School, both in Boston, said in an interview that visit length “is a reasonable parameter to look at among many others” when investigating potential disparities in care.
“They’re equating [shorter visit times] with lack of time spent counseling patients,” said Dr. Scott, who was not involved in the research. But there are “many variables” that can affect visit time, such as language differences, time spent with interpreters, and differences in patient educational levels.
Clarissa Yang, MD, dermatologist-in-chief at Tufts Medical Center, Boston, agreed. “We’re worried about there being a quality of care issue. However, there could also be differences culturally in how [the patients] interact with their physicians – their styles and the questions they ask,” she said in an interview. “The study is a good first step to noting that there may be a disparity,” and there is a need to break down the differences “into more granularity.”
Previous research, the authors wrote, has found that Asian patients were less likely to receive counseling from physicians, compared with White patients. And “paradoxically,” they noted, Asian individuals tend to present with more severe psoriasis than patients of other races and ethnicities.
Dr. Scott said the tendency to present with more severe psoriasis has been documented in patients with skin of color broadly – likely because of delays in recognition and treatment.
Race and ethnicity in the study were self-reported by patients, and missing data were imputed by NAMCS researchers using a sequential regression method. Patients who did not report race and ethnicity may have different characteristics affecting visit duration than those who did report the information, Dr. Armstrong and Dr. Wu said in describing their study’s limitations.
Other differences found
In addition to visit length, they found significant differences in mean age and in the use of complex topical regimens. The mean ages of Asian, Hispanic or Latino, and non-Hispanic Black patients were 37.2, 44.7, and 33.3 years, respectively. Complex topical regimens were prescribed to 11.8% of Asian patients, compared with 1.5% of Black and 1.1% of White patients.
For practicing dermatologists, knowing for now that Asian patients have shorter visits “may bring to light some consciousness to how we practice,” Dr. Yang noted. “We may counsel differently, we may spend differing amounts of time – for reasons still unknown. But being generally aware can help us to shift any unconscious bias that may be there.”
Dermatologists, Dr. Armstrong and Dr. Wu wrote, “need to allow sufficient time to develop strong physician-patient communication regardless of patient background.”
The NAMCS – administered by the Center for Disease Control and Prevention’s National Center for Health Statistics – collects data on a sample of visits provided by non–federally employed office-based physicians.
Dr. Armstrong disclosed receiving personal fees from AbbVie and Regeneron for research funding and serving as a scientific adviser and speaker for additional pharmaceutical and therapeutic companies. Dr. Wu, Dr. Scott, and Dr. Yang did not report any disclosures.
FROM JAMA DERMATOLOGY
Anti-BDCA2 antibody meets primary endpoint in phase 2 cutaneous lupus trial
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab improved scores on a validated measure of skin disease activity in an international phase 2 trial of patients with cutaneous lupus erythematosus (CLE).
Improvements in Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores in patients randomly assigned to receive subcutaneous litifilimab were superior to changes in patients randomly assigned to placebo over the trial period of 16 weeks. The double-blind study was published in the New England Journal of Medicine.
“This validated measure is working, and it’s very important to now go into phase 3 using the instrument that worked in phase 2 to measure improvement in the skin,” Victoria P. Werth, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and lead author of the study, said in an interview.
Research on lupus erythematosus has focused on systemic lupus erythematosus (SLE), with few randomized controlled trials addressing CLE, she said, and no Food and Drug Administration–approved treatments for CLE in the last 50 years.
Asked to comment on the results, Alisa Femia, MD, associate professor and director of autoimmune connective tissue disease in the department of dermatology at New York University, who was not involved in the research, said it is “exciting to have a trial that specifically investigates the effect of a drug on cutaneous lupus, as well-designed investigations into this potentially disfiguring disease are relatively sparse and novel treatment pathways are needed.”
The investigational drug targets blood dendritic cell antigen 2 (BDCA2) – a receptor expressed solely on the surface of plasmacytoid dendritic cells (pDCs) – and inhibits the production of type 1 interferon and other inflammatory cytokines and chemokines believed to play a major role in the pathogenesis of cutaneous and systemic lupus, the investigators said.
Rheumatologist Edward Vital, MD, who leads a lupus research group at the University of Leeds (England), said he’s most interested in how the therapy works. The “idea [has been] that pDCs are the main source of type 1 interferon. But there’s a lot of data emerging at present that suggests there are many other sources of interferons, and the drug may work in other ways,” Dr. Vital, an associate professor at the university, said in an interview. He was not involved with the study.
“Maybe pDCs have other important roles. Or maybe other cells are targeted by the therapy, too,” he said. “Understanding this will help us understand the pathogenesis of lupus and which patients will benefit the most.”
Improvements in CLASI-A scores
Across 54 centers, the study enrolled 132 patients with primarily moderate to severe active subacute CLE or chronic CLE (including discoid lupus erythematosus), or both subacute and chronic CLE with or without systemic manifestations. Active CLE was defined as a score of at least 8 on CLASI-A, which measures erythema and scaling or hypertrophy in 13 skin regions.
Patients were randomly assigned to receive placebo or litifilimab at doses of 50 mg, 150 mg, or 450 mg subcutaneously at weeks 0, 2, 4, 8, and 12. Mean CLASI-A scores at baseline for placebo and each of the dosage groups were 16.5, 15.2, 18.4, and 16.5, respectively.
The investigators used a test of dose-response to assess response across the four groups on the basis of the percent change in CLASI-A scores from baseline to 16 weeks, the primary endpoint. The percent changes in CLASI-A score were –38.8 ± 7.5 in the 50-mg group; –47.9 ± 7.5 in the 150-mg group; –42.5 ± 5.5 in the 450-mg group; and –14.5 ± 6.4 in the placebo group. (Negative value indicates improvement from baseline.)
When compared with placebo, the change in CLASI-A scores in each of the litifilimab groups was –24.3 percentage points for the 50-mg dose (95% confidence interval, –43.7 to –4.9); –33.4 percentage points for the 150-mg dose (95% CI, –52.7 to –14.1); and –28.0 percentage points for the 450-mg dose (95% CI, –44.6 to –11.4).
“All three dosages caused a similar skin response,” said Dr. Werth. “And importantly, the placebo response is fairly low, much lower than in SLE trials, possibly because the background therapies tend to be less overall [including with slightly lower doses of prednisone]. So we can really see the broad effect of the drug.”
Just under half of participants – 42%-48% of patients receiving litifilimab and 42% of those in the placebo group – had concomitant SLE with low to moderate disease activity as measured by the Systemic Lupus Erythematosus Disease Activity Index 2000. Patients could meet SLE criteria based on previous findings, and “didn’t have to have active SLE,” Dr. Werth noted.
The trial allowed background therapy as long as treatment had begun at least 12 weeks before randomization, with a stable dose starting at least 4 weeks before randomization and maintained throughout the trial period.
Most patients had moderate to severe CLE at baseline “despite approximately 90% having received concomitant background therapy and 80% of those participants having received antimalarial drugs, either alone or with other agents,” Dr. Werth and coinvestigators wrote.
CLASI-A has been shown to correlate to patients’ quality of life, Dr. Werth emphasized in the interview.
Most of the reported side effects in the phase 2 CLE trial were mild or moderate. The treatment was associated with three cases of hypersensitivity, three cases of oral herpes infection, and one case of herpes zoster infection. One case of herpes zoster meningitis occurred 4 months after the last dose of litifilimab.
Approximately 10% of study participants who reported race and ethnicity were Black or African American.
Phase 3 trials
The trial was one part of a two-part phase 2 study of litifilimab, named the LILAC trial, sponsored by Biogen. The other part, which will be published separately, involved patients who had SLE with active joint and skin manifestations.
Biogen is currently enrolling patients in phase 3 studies – the TOPAZ-1 and TOPAZ-2 studies – to evaluate the efficacy and safety of the drug in patients with active SLE. As secondary endpoints, both trials will measure the percentage of participants with a CLASI-A score of at least 10 at baseline who achieve improvement in the score, including a 50% improvement from baseline to week 16, Nathalie Franchimont, MD, PhD, of Biogen, a coauthor of the NEJM study, said in an email.
Biogen also has “plans to initiate a pivotal study in CLE this year,” she said.
With respect to the newly published phase 2 study, Dr. Femia said that, while “conclusions about the magnitude of efficacy are difficult to extrapolate in this trial design, there’s reason for cautious optimism.” There is “good theoretical basis to be optimistic about a drug such as litifilimab, that ultimately reduces type 1 interferon response,” she added.
Anifrolumab, a type 1 interferon receptor monoclonal antibody marketed as Saphnelo, was approved by the FDA for SLE in July 2021, but CLE subtypes were not characterized in trials and CLE was not studied independently of SLE, the authors pointed out in their NEJM article.
The study was supported by Biogen. In addition to working with Biogen, Dr. Werth serves as a consultant to Gilead Sciences and other pharmaceutical companies. Dr. Vital has research grants and has received honoraria from AstraZeneca. Dr. Femia disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Anxiety in COPD: Consequential, but often overlooked
Anand S. Iyer, MD, MSPH, frequently hears his patients with chronic obstructive pulmonary disease (COPD) express fear and hopelessness and describe panic and other symptoms of anxiety. He sees anxiety affect the course of COPD, worsening symptoms and outcomes.
“I had questions about what we are doing [to help patients], so I began looking into the role of palliative care to help patients assess and manage these complex emotional and psychological symptoms,” said Dr. Iyer, assistant professor in the division of pulmonology, allergy, and critical care medicine at the University of Alabama at Birmingham.
His research is now focused on the integration of palliative care principles in COPD care. For Dr. Iyer and others engaged in research and/or patient care, finding ways of identifying and managing anxiety in patients with COPD – and other chronic lung diseases – is a calling of growing urgency.
More has been published about anxiety in patients with COPD than in other pulmonary conditions – and . Prevalence rates vary from about 1 in 4, to 1 in 2 or higher, depending on the instruments used and whether clinical DSM-based diagnoses are made, Dr. Iyer said.
A 2013 systematic review of 10 studies that utilized clinical interviews based on DSM criteria, for instance, found a prevalence of clinical anxiety of 10%-55% among inpatients and 13%-46% among outpatients with COPD. The results were similar, investigators said, to studies using self-report screening tools (Respiratory Care 2013;58[5]:858-66).
In the 16 years since an ACCP workshop panel on anxiety and depression in COPD reported higher prevalence rates than for other chronic diseases and detailed a host of problems and research needs (CHEST. 2008;134;43S-56), investigators have more fully documented links to COPD outcomes, showing, for instance, that anxiety predicts exacerbations, hospitalizations, poorer adherence to therapies, poorer quality of life and higher mortality.
Dr. Iyer and other experts say anxiety is still too often a neglected comorbidity. “It’s still underdiagnosed and therefore undertreated,” said Nick Hanania, MD, MS, professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston.
The literature on optimal approaches for management remains limited, and the role of pharmacotherapy for anxiety (and depression) in the context of COPD has not been well investigated. But there have been some advances: Screening tools have been further studied, questionnaires specific to COPD have been developed, and pulmonary rehabilitation (PR) and cognitive behavioral therapy (CBT) have both been shown to be effective in decreasing anxiety.
Researchers and academic clinicians are talking, meanwhile, about how to have to important conversations about anxiety with patients who have COPD and other chronic lung conditions, and how improve care in the face of significant health system challenges.
Understanding anxiety in COPD
Anxiety is often intertwined with dyspnea in a bidirectional and complex relationship, but anxiety in COPD is not always acute or limited to times of acute exacerbations.
“There’s not only the acute experience of shortness of breath or a lung change episode, but there’s an anticipation that can occur, psychologically and socially,” said Lauren Garvin, PhD, of the department of psychiatry at the University of Iowa, Iowa City. Patients worry, “what if I’m short of breath in a particular situation? What if my devices fail when I’m out somewhere?”
Patients are often living “in a state of heightened surveillance of the body,” she noted, which can be exhausting and can impact functioning.
It’s also important to appreciate that anxiety is “a continuum of experience,” said Karin Hoth, PhD, associate professor of psychiatry at the medical school, whose research includes projects focusing on psychological adjustment in COPD.
“Research historically categorizes anxiety as ‘have or don’t have.’ But there’s a continuum of experience that we’re moving toward understanding and recognizing in research,” she said. “Anxiety is part of a patient’s whole experience, no matter where one falls on the continuum.”
Female sex, current smoking, greater airflow restrictions – and in some studies, younger age – have all been associated with a greater risk of anxiety in COPD. (It may well be that women receive more attention, leaving men with higher rates of undiagnosed anxiety, Dr. Hoth said.)
Dr. Iyer stresses the complex relationship between smoking – the No. 1 cause of COPD – and anxiety. Smoking has been associated in multiple studies with an increased risk of anxiety (Brain and Behavior. 2013;3[3]:302-26), he said. (A study led by Dr. Iyer found a similar frequency of anxiety symptoms in smokers with and without COPD [Journal of Psychosomatic Research. 2019;118:18-26].)
Some patients with COPD and anxiety may smoke in order to ease their anxiety, he said, making management of anxiety an important part of the smoking cessation desired for COPD improvement.
COPD medications such as bronchodilators may cause transient symptoms of anxiety, but these are rare and short-lived, Dr. Iyer said.
Screening tools and conversations
“It’s not just us not thinking about anxiety that’s the problem, it’s also patients thinking that it’s just the disease [causing their anxiety symptoms],” said Dr. Hanania, a member of the 2006 ACCP panel and an author of numerous papers on COPD and anxiety and depression. “There’s quite a bit of overlap between COPD symptoms and anxiety and depression symptoms, and unless you use structured questionnaires, you may not pick it up,” he said.
Screening tools include the Generalized Anxiety Disorder 7-item (GAD-7) scale, the PHQ-9 for depression and anxiety, and the longer Primary Care Evaluation of Mental Disorders (PRIME-MD). The Hospital Anxiety and Depression Scale (HADS), Dr. Iyer noted, has been well validated for use in ambulatory settings.
Validated screening tools specific to anxiety in COPD are also now an option. Abebaw M. Yohannes, PhD, MSc, FCCP, professor in the department of physical therapy at Azuza Pacific University in Orange, Calif., and the author of numerous studies on COPD and anxiety, developed one of these tools – the 10-item Anxiety Inventory for Respiratory Disease (AIR) scale – out of concern that other surveys contain overlapping somatic symptoms (Chest. 2013;144[5]:1587-96).
“We removed the physical symptoms [of anxiety] that often manifest in patients with COPD,” he said.
Dr. Iyer said screening tools can effectively “highlight which person might be dealing with high levels of anxiety symptoms that might meet a threshold of clinical significance and require collaborative or interprofessional management,” including with psychologists and psychiatrists.
They can also open the door to conversation with patients. “I’ll often bluntly ask, do you feel anxious? Do you feel scared, or hopeless about what the future holds for you?,” he said. “Anxiety about the future plays a big role, and helping patients navigate the illness and understand early how it might look … can ease the level of anxiety.”
Asking patients about their experiences in managing their symptoms and about their psychological and emotional well-being can help to normalize anxiety – and it can be therapeutic, said Dr. Hoth and Dr. Garvin. Asking “how it’s going with the things that really matter in [their] life” is often a good question, they said.
Patients “won’t be offended if you ask,” said Dr. Hoth. “They view their mood and [whole] well-being as part of their medical condition.”
Time is a challenge, she said, but “conversation can be done little by little, as part of a philosophy of engaging the patient around their whole functioning, even if there’s not [a need or] a route to refer just then.”
Such early and integrated conversation borrows from the palliative care model. “Palliative care is a specialty, but it can also be an approach to care,” Dr. Iyer said. He is leading a National Institutes of Health–funded study on nurse-coach–led early palliative care for older adults with COPD and wants to see training opportunities for pulmonologists to learn basic palliative care skills that would equip them to better guide management of mild-moderate anxiety and other complex symptoms.
Pulmonary rehabilitation
For many patients with COPD who have anxiety and/or depressive symptoms, referral for nonpharmacologic therapies such as psychotherapy, cognitive-behavioral therapy (CBT), and pulmonary rehabilitation (PR) is “one of the best things you can do,” Dr. Iyer said.
“If patients haven’t done pulmonary rehabilitation, get them in. And if they have done it before, get them back into it again,” he emphasized. “Accredited programs give a holistic approach to improving your strength, your breathlessness, your mindset and understanding of your breathlessness, and your own levels of security.”
Studies addressing the impact of PR and CBT on anxiety have been mostly small and observational but have yielded encouraging findings. A 2017 review reported that PR and CBT were effective in the treatment of anxiety and dyspnea, in the short term, in the majority of 47 studies (JAMA. 2017;18[12]:1096.e1-1096.e17). And a 2019 systematic review and meta-analysis focused on PR reported that, across 11 studies comprising 734 patients, PR conferred significant benefits for anxiety and depression compared with usual care (CHEST. 2019;156[1]:80-91).
Dr. Yohannes, Dr. Hanania, and colleagues recently reported on 734 patients with clinically stable COPD who completed a community-based 8-week PR program of 2 hours a week: 1 hour of exercise and 1 hour of education, the latter of which covered anxiety, panic management, and relaxation.
Patients who had severe dyspnea and comorbid anxiety and depression prior to PR – one-third of the group compared with 20% having anxiety alone and 5% having depression alone – had the most significant improvements in dyspnea scores and anxiety and depression scores (Respir Med. Apr 9. doi: 10.1016/j.rmed.2022.106850.)
The problem is, pulmonary rehabilitation is under-reimbursed and not widely accessible. It’s logistically challenging for patients to attend therapy 2-3 times a week. And according to a recently published study by Dr. Yohannes, Dr. Hanania, and colleagues, patients with more anxiety and dyspnea may be at higher risk of dropping out (Respir Med. 2022 Jan 20. doi: 10.1016/j.rmed.2022.10674). Moreover, Dr. Iyer said, there is a shortage of programs that are accredited.
Telehealth may help on some of these fronts. The efficacy of real-time video PR for COPD is being investigated in a randomized NIH trial (now in the recruitment phase) led by pulmonologist Surya P. Bhatt, MD, also at the University of Alabama at Birmingham.
Researchers also need to investigate issues of sustainability – to learn what “works best in the long run,” Dr. Iyer said.
Dr. Yohannes and Dr. Hanania are encouraged by a recent finding that patients with COPD who completed 8 weeks of PR maintained improvements in anxiety and quality-of-life scores at 2 years. (Improvements in dyspnea and other outcomes did not persist.) (CHEST. 2021;159[3]:967-74). Prospective studies contrasting maintenance programs with no maintenance following PR, are needed, they wrote.
Understanding psychological interventions
Dr. Hoth and Dr. Garvin advise their pulmonologist colleagues to feel as confident as possible in describing for patients what CBT and other psychological therapies entail.
“A person [with COPD] who is experiencing something on the continuum of anxiety might be really turning inward and [assessing] unwanted internal experiences” and accompanying thoughts, sensations, emotional impacts and behaviors, Dr. Garvin said.
Among the goals, she said, are to “make shifts around those internal experiences that might invoke some more tolerance or that might shift their relationship with the experiences, or even with the diagnosis itself and all the uncertainties it carries.”
Psychological therapies can involve social support, or “breath and grounding work,” she said. “There are lots of different approaches from different providers.”
Dr. Yohannes advocates incorporating principles of CBT into PR. “In the absence of one-on-one or group [stand-alone] CBT … the principles are worth incorporating as part of the education piece [of PR],” he said. “CBT helps patients to refocus their attention. … and gives them self-confidence to engage in exercise and to function a bit more in their daily activities.”
None of those interviewed for this story reported having any relevant conflicts of interest.
Anand S. Iyer, MD, MSPH, frequently hears his patients with chronic obstructive pulmonary disease (COPD) express fear and hopelessness and describe panic and other symptoms of anxiety. He sees anxiety affect the course of COPD, worsening symptoms and outcomes.
“I had questions about what we are doing [to help patients], so I began looking into the role of palliative care to help patients assess and manage these complex emotional and psychological symptoms,” said Dr. Iyer, assistant professor in the division of pulmonology, allergy, and critical care medicine at the University of Alabama at Birmingham.
His research is now focused on the integration of palliative care principles in COPD care. For Dr. Iyer and others engaged in research and/or patient care, finding ways of identifying and managing anxiety in patients with COPD – and other chronic lung diseases – is a calling of growing urgency.
More has been published about anxiety in patients with COPD than in other pulmonary conditions – and . Prevalence rates vary from about 1 in 4, to 1 in 2 or higher, depending on the instruments used and whether clinical DSM-based diagnoses are made, Dr. Iyer said.
A 2013 systematic review of 10 studies that utilized clinical interviews based on DSM criteria, for instance, found a prevalence of clinical anxiety of 10%-55% among inpatients and 13%-46% among outpatients with COPD. The results were similar, investigators said, to studies using self-report screening tools (Respiratory Care 2013;58[5]:858-66).
In the 16 years since an ACCP workshop panel on anxiety and depression in COPD reported higher prevalence rates than for other chronic diseases and detailed a host of problems and research needs (CHEST. 2008;134;43S-56), investigators have more fully documented links to COPD outcomes, showing, for instance, that anxiety predicts exacerbations, hospitalizations, poorer adherence to therapies, poorer quality of life and higher mortality.
Dr. Iyer and other experts say anxiety is still too often a neglected comorbidity. “It’s still underdiagnosed and therefore undertreated,” said Nick Hanania, MD, MS, professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston.
The literature on optimal approaches for management remains limited, and the role of pharmacotherapy for anxiety (and depression) in the context of COPD has not been well investigated. But there have been some advances: Screening tools have been further studied, questionnaires specific to COPD have been developed, and pulmonary rehabilitation (PR) and cognitive behavioral therapy (CBT) have both been shown to be effective in decreasing anxiety.
Researchers and academic clinicians are talking, meanwhile, about how to have to important conversations about anxiety with patients who have COPD and other chronic lung conditions, and how improve care in the face of significant health system challenges.
Understanding anxiety in COPD
Anxiety is often intertwined with dyspnea in a bidirectional and complex relationship, but anxiety in COPD is not always acute or limited to times of acute exacerbations.
“There’s not only the acute experience of shortness of breath or a lung change episode, but there’s an anticipation that can occur, psychologically and socially,” said Lauren Garvin, PhD, of the department of psychiatry at the University of Iowa, Iowa City. Patients worry, “what if I’m short of breath in a particular situation? What if my devices fail when I’m out somewhere?”
Patients are often living “in a state of heightened surveillance of the body,” she noted, which can be exhausting and can impact functioning.
It’s also important to appreciate that anxiety is “a continuum of experience,” said Karin Hoth, PhD, associate professor of psychiatry at the medical school, whose research includes projects focusing on psychological adjustment in COPD.
“Research historically categorizes anxiety as ‘have or don’t have.’ But there’s a continuum of experience that we’re moving toward understanding and recognizing in research,” she said. “Anxiety is part of a patient’s whole experience, no matter where one falls on the continuum.”
Female sex, current smoking, greater airflow restrictions – and in some studies, younger age – have all been associated with a greater risk of anxiety in COPD. (It may well be that women receive more attention, leaving men with higher rates of undiagnosed anxiety, Dr. Hoth said.)
Dr. Iyer stresses the complex relationship between smoking – the No. 1 cause of COPD – and anxiety. Smoking has been associated in multiple studies with an increased risk of anxiety (Brain and Behavior. 2013;3[3]:302-26), he said. (A study led by Dr. Iyer found a similar frequency of anxiety symptoms in smokers with and without COPD [Journal of Psychosomatic Research. 2019;118:18-26].)
Some patients with COPD and anxiety may smoke in order to ease their anxiety, he said, making management of anxiety an important part of the smoking cessation desired for COPD improvement.
COPD medications such as bronchodilators may cause transient symptoms of anxiety, but these are rare and short-lived, Dr. Iyer said.
Screening tools and conversations
“It’s not just us not thinking about anxiety that’s the problem, it’s also patients thinking that it’s just the disease [causing their anxiety symptoms],” said Dr. Hanania, a member of the 2006 ACCP panel and an author of numerous papers on COPD and anxiety and depression. “There’s quite a bit of overlap between COPD symptoms and anxiety and depression symptoms, and unless you use structured questionnaires, you may not pick it up,” he said.
Screening tools include the Generalized Anxiety Disorder 7-item (GAD-7) scale, the PHQ-9 for depression and anxiety, and the longer Primary Care Evaluation of Mental Disorders (PRIME-MD). The Hospital Anxiety and Depression Scale (HADS), Dr. Iyer noted, has been well validated for use in ambulatory settings.
Validated screening tools specific to anxiety in COPD are also now an option. Abebaw M. Yohannes, PhD, MSc, FCCP, professor in the department of physical therapy at Azuza Pacific University in Orange, Calif., and the author of numerous studies on COPD and anxiety, developed one of these tools – the 10-item Anxiety Inventory for Respiratory Disease (AIR) scale – out of concern that other surveys contain overlapping somatic symptoms (Chest. 2013;144[5]:1587-96).
“We removed the physical symptoms [of anxiety] that often manifest in patients with COPD,” he said.
Dr. Iyer said screening tools can effectively “highlight which person might be dealing with high levels of anxiety symptoms that might meet a threshold of clinical significance and require collaborative or interprofessional management,” including with psychologists and psychiatrists.
They can also open the door to conversation with patients. “I’ll often bluntly ask, do you feel anxious? Do you feel scared, or hopeless about what the future holds for you?,” he said. “Anxiety about the future plays a big role, and helping patients navigate the illness and understand early how it might look … can ease the level of anxiety.”
Asking patients about their experiences in managing their symptoms and about their psychological and emotional well-being can help to normalize anxiety – and it can be therapeutic, said Dr. Hoth and Dr. Garvin. Asking “how it’s going with the things that really matter in [their] life” is often a good question, they said.
Patients “won’t be offended if you ask,” said Dr. Hoth. “They view their mood and [whole] well-being as part of their medical condition.”
Time is a challenge, she said, but “conversation can be done little by little, as part of a philosophy of engaging the patient around their whole functioning, even if there’s not [a need or] a route to refer just then.”
Such early and integrated conversation borrows from the palliative care model. “Palliative care is a specialty, but it can also be an approach to care,” Dr. Iyer said. He is leading a National Institutes of Health–funded study on nurse-coach–led early palliative care for older adults with COPD and wants to see training opportunities for pulmonologists to learn basic palliative care skills that would equip them to better guide management of mild-moderate anxiety and other complex symptoms.
Pulmonary rehabilitation
For many patients with COPD who have anxiety and/or depressive symptoms, referral for nonpharmacologic therapies such as psychotherapy, cognitive-behavioral therapy (CBT), and pulmonary rehabilitation (PR) is “one of the best things you can do,” Dr. Iyer said.
“If patients haven’t done pulmonary rehabilitation, get them in. And if they have done it before, get them back into it again,” he emphasized. “Accredited programs give a holistic approach to improving your strength, your breathlessness, your mindset and understanding of your breathlessness, and your own levels of security.”
Studies addressing the impact of PR and CBT on anxiety have been mostly small and observational but have yielded encouraging findings. A 2017 review reported that PR and CBT were effective in the treatment of anxiety and dyspnea, in the short term, in the majority of 47 studies (JAMA. 2017;18[12]:1096.e1-1096.e17). And a 2019 systematic review and meta-analysis focused on PR reported that, across 11 studies comprising 734 patients, PR conferred significant benefits for anxiety and depression compared with usual care (CHEST. 2019;156[1]:80-91).
Dr. Yohannes, Dr. Hanania, and colleagues recently reported on 734 patients with clinically stable COPD who completed a community-based 8-week PR program of 2 hours a week: 1 hour of exercise and 1 hour of education, the latter of which covered anxiety, panic management, and relaxation.
Patients who had severe dyspnea and comorbid anxiety and depression prior to PR – one-third of the group compared with 20% having anxiety alone and 5% having depression alone – had the most significant improvements in dyspnea scores and anxiety and depression scores (Respir Med. Apr 9. doi: 10.1016/j.rmed.2022.106850.)
The problem is, pulmonary rehabilitation is under-reimbursed and not widely accessible. It’s logistically challenging for patients to attend therapy 2-3 times a week. And according to a recently published study by Dr. Yohannes, Dr. Hanania, and colleagues, patients with more anxiety and dyspnea may be at higher risk of dropping out (Respir Med. 2022 Jan 20. doi: 10.1016/j.rmed.2022.10674). Moreover, Dr. Iyer said, there is a shortage of programs that are accredited.
Telehealth may help on some of these fronts. The efficacy of real-time video PR for COPD is being investigated in a randomized NIH trial (now in the recruitment phase) led by pulmonologist Surya P. Bhatt, MD, also at the University of Alabama at Birmingham.
Researchers also need to investigate issues of sustainability – to learn what “works best in the long run,” Dr. Iyer said.
Dr. Yohannes and Dr. Hanania are encouraged by a recent finding that patients with COPD who completed 8 weeks of PR maintained improvements in anxiety and quality-of-life scores at 2 years. (Improvements in dyspnea and other outcomes did not persist.) (CHEST. 2021;159[3]:967-74). Prospective studies contrasting maintenance programs with no maintenance following PR, are needed, they wrote.
Understanding psychological interventions
Dr. Hoth and Dr. Garvin advise their pulmonologist colleagues to feel as confident as possible in describing for patients what CBT and other psychological therapies entail.
“A person [with COPD] who is experiencing something on the continuum of anxiety might be really turning inward and [assessing] unwanted internal experiences” and accompanying thoughts, sensations, emotional impacts and behaviors, Dr. Garvin said.
Among the goals, she said, are to “make shifts around those internal experiences that might invoke some more tolerance or that might shift their relationship with the experiences, or even with the diagnosis itself and all the uncertainties it carries.”
Psychological therapies can involve social support, or “breath and grounding work,” she said. “There are lots of different approaches from different providers.”
Dr. Yohannes advocates incorporating principles of CBT into PR. “In the absence of one-on-one or group [stand-alone] CBT … the principles are worth incorporating as part of the education piece [of PR],” he said. “CBT helps patients to refocus their attention. … and gives them self-confidence to engage in exercise and to function a bit more in their daily activities.”
None of those interviewed for this story reported having any relevant conflicts of interest.
Anand S. Iyer, MD, MSPH, frequently hears his patients with chronic obstructive pulmonary disease (COPD) express fear and hopelessness and describe panic and other symptoms of anxiety. He sees anxiety affect the course of COPD, worsening symptoms and outcomes.
“I had questions about what we are doing [to help patients], so I began looking into the role of palliative care to help patients assess and manage these complex emotional and psychological symptoms,” said Dr. Iyer, assistant professor in the division of pulmonology, allergy, and critical care medicine at the University of Alabama at Birmingham.
His research is now focused on the integration of palliative care principles in COPD care. For Dr. Iyer and others engaged in research and/or patient care, finding ways of identifying and managing anxiety in patients with COPD – and other chronic lung diseases – is a calling of growing urgency.
More has been published about anxiety in patients with COPD than in other pulmonary conditions – and . Prevalence rates vary from about 1 in 4, to 1 in 2 or higher, depending on the instruments used and whether clinical DSM-based diagnoses are made, Dr. Iyer said.
A 2013 systematic review of 10 studies that utilized clinical interviews based on DSM criteria, for instance, found a prevalence of clinical anxiety of 10%-55% among inpatients and 13%-46% among outpatients with COPD. The results were similar, investigators said, to studies using self-report screening tools (Respiratory Care 2013;58[5]:858-66).
In the 16 years since an ACCP workshop panel on anxiety and depression in COPD reported higher prevalence rates than for other chronic diseases and detailed a host of problems and research needs (CHEST. 2008;134;43S-56), investigators have more fully documented links to COPD outcomes, showing, for instance, that anxiety predicts exacerbations, hospitalizations, poorer adherence to therapies, poorer quality of life and higher mortality.
Dr. Iyer and other experts say anxiety is still too often a neglected comorbidity. “It’s still underdiagnosed and therefore undertreated,” said Nick Hanania, MD, MS, professor of medicine and director of the Airways Clinical Research Center at Baylor College of Medicine, Houston.
The literature on optimal approaches for management remains limited, and the role of pharmacotherapy for anxiety (and depression) in the context of COPD has not been well investigated. But there have been some advances: Screening tools have been further studied, questionnaires specific to COPD have been developed, and pulmonary rehabilitation (PR) and cognitive behavioral therapy (CBT) have both been shown to be effective in decreasing anxiety.
Researchers and academic clinicians are talking, meanwhile, about how to have to important conversations about anxiety with patients who have COPD and other chronic lung conditions, and how improve care in the face of significant health system challenges.
Understanding anxiety in COPD
Anxiety is often intertwined with dyspnea in a bidirectional and complex relationship, but anxiety in COPD is not always acute or limited to times of acute exacerbations.
“There’s not only the acute experience of shortness of breath or a lung change episode, but there’s an anticipation that can occur, psychologically and socially,” said Lauren Garvin, PhD, of the department of psychiatry at the University of Iowa, Iowa City. Patients worry, “what if I’m short of breath in a particular situation? What if my devices fail when I’m out somewhere?”
Patients are often living “in a state of heightened surveillance of the body,” she noted, which can be exhausting and can impact functioning.
It’s also important to appreciate that anxiety is “a continuum of experience,” said Karin Hoth, PhD, associate professor of psychiatry at the medical school, whose research includes projects focusing on psychological adjustment in COPD.
“Research historically categorizes anxiety as ‘have or don’t have.’ But there’s a continuum of experience that we’re moving toward understanding and recognizing in research,” she said. “Anxiety is part of a patient’s whole experience, no matter where one falls on the continuum.”
Female sex, current smoking, greater airflow restrictions – and in some studies, younger age – have all been associated with a greater risk of anxiety in COPD. (It may well be that women receive more attention, leaving men with higher rates of undiagnosed anxiety, Dr. Hoth said.)
Dr. Iyer stresses the complex relationship between smoking – the No. 1 cause of COPD – and anxiety. Smoking has been associated in multiple studies with an increased risk of anxiety (Brain and Behavior. 2013;3[3]:302-26), he said. (A study led by Dr. Iyer found a similar frequency of anxiety symptoms in smokers with and without COPD [Journal of Psychosomatic Research. 2019;118:18-26].)
Some patients with COPD and anxiety may smoke in order to ease their anxiety, he said, making management of anxiety an important part of the smoking cessation desired for COPD improvement.
COPD medications such as bronchodilators may cause transient symptoms of anxiety, but these are rare and short-lived, Dr. Iyer said.
Screening tools and conversations
“It’s not just us not thinking about anxiety that’s the problem, it’s also patients thinking that it’s just the disease [causing their anxiety symptoms],” said Dr. Hanania, a member of the 2006 ACCP panel and an author of numerous papers on COPD and anxiety and depression. “There’s quite a bit of overlap between COPD symptoms and anxiety and depression symptoms, and unless you use structured questionnaires, you may not pick it up,” he said.
Screening tools include the Generalized Anxiety Disorder 7-item (GAD-7) scale, the PHQ-9 for depression and anxiety, and the longer Primary Care Evaluation of Mental Disorders (PRIME-MD). The Hospital Anxiety and Depression Scale (HADS), Dr. Iyer noted, has been well validated for use in ambulatory settings.
Validated screening tools specific to anxiety in COPD are also now an option. Abebaw M. Yohannes, PhD, MSc, FCCP, professor in the department of physical therapy at Azuza Pacific University in Orange, Calif., and the author of numerous studies on COPD and anxiety, developed one of these tools – the 10-item Anxiety Inventory for Respiratory Disease (AIR) scale – out of concern that other surveys contain overlapping somatic symptoms (Chest. 2013;144[5]:1587-96).
“We removed the physical symptoms [of anxiety] that often manifest in patients with COPD,” he said.
Dr. Iyer said screening tools can effectively “highlight which person might be dealing with high levels of anxiety symptoms that might meet a threshold of clinical significance and require collaborative or interprofessional management,” including with psychologists and psychiatrists.
They can also open the door to conversation with patients. “I’ll often bluntly ask, do you feel anxious? Do you feel scared, or hopeless about what the future holds for you?,” he said. “Anxiety about the future plays a big role, and helping patients navigate the illness and understand early how it might look … can ease the level of anxiety.”
Asking patients about their experiences in managing their symptoms and about their psychological and emotional well-being can help to normalize anxiety – and it can be therapeutic, said Dr. Hoth and Dr. Garvin. Asking “how it’s going with the things that really matter in [their] life” is often a good question, they said.
Patients “won’t be offended if you ask,” said Dr. Hoth. “They view their mood and [whole] well-being as part of their medical condition.”
Time is a challenge, she said, but “conversation can be done little by little, as part of a philosophy of engaging the patient around their whole functioning, even if there’s not [a need or] a route to refer just then.”
Such early and integrated conversation borrows from the palliative care model. “Palliative care is a specialty, but it can also be an approach to care,” Dr. Iyer said. He is leading a National Institutes of Health–funded study on nurse-coach–led early palliative care for older adults with COPD and wants to see training opportunities for pulmonologists to learn basic palliative care skills that would equip them to better guide management of mild-moderate anxiety and other complex symptoms.
Pulmonary rehabilitation
For many patients with COPD who have anxiety and/or depressive symptoms, referral for nonpharmacologic therapies such as psychotherapy, cognitive-behavioral therapy (CBT), and pulmonary rehabilitation (PR) is “one of the best things you can do,” Dr. Iyer said.
“If patients haven’t done pulmonary rehabilitation, get them in. And if they have done it before, get them back into it again,” he emphasized. “Accredited programs give a holistic approach to improving your strength, your breathlessness, your mindset and understanding of your breathlessness, and your own levels of security.”
Studies addressing the impact of PR and CBT on anxiety have been mostly small and observational but have yielded encouraging findings. A 2017 review reported that PR and CBT were effective in the treatment of anxiety and dyspnea, in the short term, in the majority of 47 studies (JAMA. 2017;18[12]:1096.e1-1096.e17). And a 2019 systematic review and meta-analysis focused on PR reported that, across 11 studies comprising 734 patients, PR conferred significant benefits for anxiety and depression compared with usual care (CHEST. 2019;156[1]:80-91).
Dr. Yohannes, Dr. Hanania, and colleagues recently reported on 734 patients with clinically stable COPD who completed a community-based 8-week PR program of 2 hours a week: 1 hour of exercise and 1 hour of education, the latter of which covered anxiety, panic management, and relaxation.
Patients who had severe dyspnea and comorbid anxiety and depression prior to PR – one-third of the group compared with 20% having anxiety alone and 5% having depression alone – had the most significant improvements in dyspnea scores and anxiety and depression scores (Respir Med. Apr 9. doi: 10.1016/j.rmed.2022.106850.)
The problem is, pulmonary rehabilitation is under-reimbursed and not widely accessible. It’s logistically challenging for patients to attend therapy 2-3 times a week. And according to a recently published study by Dr. Yohannes, Dr. Hanania, and colleagues, patients with more anxiety and dyspnea may be at higher risk of dropping out (Respir Med. 2022 Jan 20. doi: 10.1016/j.rmed.2022.10674). Moreover, Dr. Iyer said, there is a shortage of programs that are accredited.
Telehealth may help on some of these fronts. The efficacy of real-time video PR for COPD is being investigated in a randomized NIH trial (now in the recruitment phase) led by pulmonologist Surya P. Bhatt, MD, also at the University of Alabama at Birmingham.
Researchers also need to investigate issues of sustainability – to learn what “works best in the long run,” Dr. Iyer said.
Dr. Yohannes and Dr. Hanania are encouraged by a recent finding that patients with COPD who completed 8 weeks of PR maintained improvements in anxiety and quality-of-life scores at 2 years. (Improvements in dyspnea and other outcomes did not persist.) (CHEST. 2021;159[3]:967-74). Prospective studies contrasting maintenance programs with no maintenance following PR, are needed, they wrote.
Understanding psychological interventions
Dr. Hoth and Dr. Garvin advise their pulmonologist colleagues to feel as confident as possible in describing for patients what CBT and other psychological therapies entail.
“A person [with COPD] who is experiencing something on the continuum of anxiety might be really turning inward and [assessing] unwanted internal experiences” and accompanying thoughts, sensations, emotional impacts and behaviors, Dr. Garvin said.
Among the goals, she said, are to “make shifts around those internal experiences that might invoke some more tolerance or that might shift their relationship with the experiences, or even with the diagnosis itself and all the uncertainties it carries.”
Psychological therapies can involve social support, or “breath and grounding work,” she said. “There are lots of different approaches from different providers.”
Dr. Yohannes advocates incorporating principles of CBT into PR. “In the absence of one-on-one or group [stand-alone] CBT … the principles are worth incorporating as part of the education piece [of PR],” he said. “CBT helps patients to refocus their attention. … and gives them self-confidence to engage in exercise and to function a bit more in their daily activities.”
None of those interviewed for this story reported having any relevant conflicts of interest.
Study finds higher risk of skin cancer after childhood organ transplant
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
FROM JAMA DERMATOLOGY
Obesity and lung disease: Much more than BMI
The diverse effects of obesity on lung health and disease are increasingly being teased apart, with researchers honing in on the impact of metabolic dysfunction, circulating inflammatory factors produced by adipose tissue, lipid handling, and other factors – in addition to body mass index – that are associated with the obese state.
“The bird’s eye view is that obesity completely changes lung health. It’s something we’ve only recently begun to appreciate,” said Anne E. Dixon, MA, BM, BCh, director of the Vermont Lung Center at the University of Vermont, Burlington, who is focused on the research field of obesity and lung disease.
Structural, mechanical effects of obesity on lung function are better known and appreciated. Accumulation of fat in the mediastinum and abdominal and thoracic cavities causes reductions in lung volume, in functional residual capacity, and in the compliance of the lungs, chest wall, and entire respiratory system, for instance.
Yet obesity is more than a state of increased BMI, and “what we’ve begun to understand is that [its impact on the lungs and respiratory health] is much more complicated than just a mechanical problem,” said Dr. Dixon, also director of pulmonary and critical care medicine at the University of Vermont Medical Center and professor of medicine at the medical college.
With obesity, adipose tissue changes not only in quantity, but in function, producing proinflammatory cytokines and hormones – such as leptin, tumor necrosis factor-alpha (TNF-alpha), and interleukin-6 – that can have direct effects on the lung. Insulin resistance, which is common with obesity, is also seemingly deleterious. And obesity-associated changes in immune function, lipid handling, diet, and the gut microbiome may also impact lung health and disease, she said.
Dr. Dixon, who wrote about these changes in a 2018 review article in the journal CHEST and another 2019 piece in Expert Review of Respiratory Medicine, has developed a research program focused on obesity and lung disease and has edited a book and organized international conferences on the topic. (CHEST 2018;153[3]:702-9 and Exper Rev Respir Med. 2018;12[9]:755-67.)
“The more I do, the more I realize that there are multiple obesity-associated changes involved, and that [our current high level of] obesity is like a huge population-level natural experiment ... on lung health,” she told this news organization.
Associations between lung disease and the metabolic and other disturbances of obesity are most established in asthma research and have taken hold in the realm of sleep-disordered breathing. But as the prevalence of obesity continues to grow, its role in other lung diseases such as chronic obstructive pulmonary disorder (COPD) and, most recently, pulmonary arterial hypertension (PAH), is getting attention in academia.
And certainly, COVID-19 has highlighted an “urgent need” to better understand how obesity increases susceptibility to severe viral infections, Dr. Dixon added.
Here are some glimpses into current thinking and some examples of research that may have preventive and therapeutic implications in the future:
OSA and OHS
“With sleep apnea we tend to focus on anatomic considerations, but there may be relationships or interactions between obesity and neuromuscular function and neuroventilatory control,” Susheel P. Patil, MD, PhD, director of the sleep medicine program for University Hospitals and assistant professor at Case Western Reserve University, Cleveland, said in an interview.
Some studies suggest, for instance, that TNF-alpha can increase obstructive sleep apnea (OSA) susceptibility and severity through its neuroventilatory modulating properties during sleep. And the potential for additional proinflammatory cytokines produced by adipose tissue to similarly affect upper airway neuroventilatory control is an “intriguing line” of inquiry for researchers in the sleep apnea space, he said.
Leptin is of interest particularly in obesity hypoventilation syndrome (OHS), which is characterized by chronic daytime hypercapnia. Best known as a satiety hormone, leptin is produced by adipose tissue and suppresses appetite at the central nervous system level. But it has long been known that leptin also affects ventilation and the control of breathing.
When transported across the blood-brain barrier, leptin increases the hypercapnic ventilatory response, Babak Mokhlesi, MD, MSc, codirector of the Rush Lung Center and chief of pulmonary, critical care, and sleep medicine at Rush University Medical Center in Chicago, said in an interview.
Research suggests that patients with OHS may have resistance to leptin at the central nervous system level – with leptin not reaching the sites of ventilatory control. This is a “prevailing theory” and could explain why these patients “do not augment their ventilation to maintain homeostasis, normal levels of CO2,” Dr. Mokhlesi said.
“Why some patients with severe obesity develop CO2 retention while others do not is not fully understood,” he said, noting that patients with OHS can normalize their CO2 quickly when instructed to take deep breaths. “What we know is that the centers in the brain responsible for augmenting ventilation when CO2 goes up are somehow blunted.”
In a study of obese mice led by Vsevolod Y. Polotsky, MD, PhD, of Johns Hopkins University, Baltimore – and highlighted by Dr. Mokhlesi as an example of important, recent research – leptin delivered intranasally alleviated hypoventilation (and upper-airway obstruction), while intraperitoneally administered leptin did not, seemingly overcoming “central leptin deficiency.” (Am J Respir Crit Care Med. 2019;199[6]:773-83).
“This proved that there is some level of resistance in this animal model ... and has potential for therapeutics in the future,” Dr. Mokhlesi said.
Understanding the role of insulin resistance in OSA is another research focus. Some data suggest that insulin resistance, which is more common in obesity, is more prevalent in populations with OSA, Dr. Patil said. Researchers have discussed a bidirectional relationship for years, but it’s likely that insulin resistance is a precursor, he said.
In a mechanistic study published in 2016, Dr. Patil and his coinvestigators found that obese individuals with insulin resistance but without frank diabetes or sleep apnea demonstrated preclinical elevations in pharyngeal collapsibility during sleep. The findings suggest that insulin resistance could play a causal role in OSA pathogenesis by “generating requisite elevations in pharyngeal collapsibility,” they wrote (Eur. Respir J. 2016;47[6]:1718-26).
More recently, Dr. Patil noted in the interview, there is increasing appreciation in academia that the type of fat may be important to predicting OSA. “Visceral fat has a completely different cytokine-secretion profile than subcutaneous fat ... it is the more metabolically active fat that may secondarily impact upper airway function though a neuroinflammatory mechanism,” he said. “That is one of the working hypotheses today.”
Asthma
Research has so roundly suggested that metabolic dysfunction contributes to severe, poorly controlled asthma that there’s recent and growing interest in targeting metabolic dysfunction as part of the treatment of obese asthma, said Dr. Dixon, whose own research in obesity and lung disease has focused on asthma.
Data from animal models and some epidemiologic studies have suggested that drugs used to treat type 2 diabetes mellitus, such as glucagon-like peptide receptor-1 (GLPR-1) agonists and metformin, may help control asthma. In one recent study – cited by Dr. Dixon in a 2022 review of obesity and asthma – people with obesity and asthma who were prescribed GLPR-1 agonists for diabetes had fewer asthma exacerbations compared with those who took other medications for diabetes (Semin Respir Crit Care Med. 2022 Feb 17. doi: 10.1055/s-0042-1742384).
There is also research interest in targeting the pro-inflammatory adipokine interleukin 6 (IL-6), since increased circulating levels of IL-6 correlate with asthma severity, and in addressing oxidative stress in asthma through treatment with a mitochondrially targeted antioxidant, she said. Oxidative stress is increased in the airways of people with obesity, and researchers believe it may contribute to the pathophysiology of obese asthma through effects on airway nitric oxide levels.
(Her own research work at the University of Vermont has found associations between poor asthma control and high levels of leptin, and similar associations involving low levels of adiponectin, an anti-inflammatory adipokine that has been shown to downregulate eosinophil recruitment in the airways.)
Weight loss has been shown in mostly small, single-center studies to improve asthma control, but short of weight loss, researchers are also investigating the role of poor dietary quality. Thus far, data suggest that it’s the composition of the diet, and not just its contribution to weight gain, that could be impactful, Dr. Dixon said.
More basic research questions cited by Dr. Dixon include the extent to which adipose tissue inflammation causes inflammation in the lungs. “It’s a little unclear whether all the metabolic dysfunction associated with poor asthma control is causing inflammation in the lungs,” she said, though “we’ve done some work here that shows mediators produced by the adipose tissue could be impacting production of inflammatory mediators by the airway epithelium.”
Overall, she said, “the big questions [in asthma] are, how does adipose tissue affect the airway? Is it through direct effects? Through effects on the immune system? And obesity is affected by diet and the gut microbiome – how can these be [impacting] the airway?”
Obesity “is associated with so many changes – the gut, the immune system, and metabolic dysfunction, in addition to airway mechanics,” she said, “that I no longer think, as I did when I came to this, that it’s just one thing. It’s probably all of these things together.”
In the meantime, questions about potential shared pathways for the development of obesity and asthma remain. “Obesity is a risk factor for developing asthma, but it’s also entirely possible that asthma is a risk factor for developing obesity,” she said. (Some data from pediatric populations, she noted, suggest that nonobese children with asthma are at increased risk of developing obesity.)
Also important, Dr. Dixon said, is “emerging literature in the last 5-10 years” that suggests that people with obesity are more susceptible to the effects of air pollution. Research involving inner-city schoolchildren with asthma, for instance, has shown that those with obesity had worse symptoms with air pollution exposure than did those who were not obese.
Pulmonary arterial hypertension
Some research has looked at adipose tissue–produced substances in PAH, but the most well-established association in obesity and PAH involves insulin resistance.
“I don’t think we’re certain as a community that obesity [in general] is the problem – it’s not itself considered a risk factor for PAH,” Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., said in an interview. She noted that it’s “hard to dissect obesity” apart.
Researchers are “more confident,” she said, “that insulin resistance – one feature of obesity [in some people] – is associated with worse outcomes in PAH.” Metabolic disease resembling insulin resistance is common in PAH and is believed to contribute to pulmonary vascular disease and right ventricular (RV) failure – the main cause of mortality in PAH – at least in part because of increased oxidative stress.
Dr. Hemnes led a mechanistic phase II clinical trial of metformin in PAH in which the drug was associated with improved RV fractional area change and reduced RV lipid deposition (J Am Heart Assoc. 2020;9[22]:e018349), and she’s now leading a National Institutes of Health–funded multicenter trial looking at the impact of metformin and an exercise intervention on 6-minute walk distance and World Health Organization functional class in PAH.
At the Rush Lung Center, in the meantime, Dr. Mokhlesi is utilizing animal models of OSA and OHS to explore the effect of hypoxia and nighttime hypercapnia on the development of PAH. “I think the jury is still out as to whether obesity itself is a major risk factor, but if so, by what mechanism?” he said. “Is it worsening [sleep-disordered breathing], which then worsens PAH?”
COPD
The focus in COPD has traditionally been on underweight, but the relationship between obesity and COPD has increasingly been recognized in the last 10-15 years, said Frits M. E. Franssen, MD, PhD, of CIRO, a research institute in Horn, the Netherlands, that treats COPD and other chronic lung diseases, and of the department of respiratory medicine at Maastricht University.
Researchers like Dr. Franssen are trying, for one, to understand obesity’s impact on COPD pathophysiology and to tease apart the impact of both conditions on disease severity and patient-related outcomes such as exercise capacity and exercise-related symptoms.
When Dr. Franssen’s group compared responses to weight-bearing exercise (6-min. walk test) and weight-supported exercise (cycling) in obese and normal weight COPD patients matched for age, gender, and degree of airflow limitation, the researchers found that walking capacity was significantly reduced while cycling capacity was preserved in the obese group (Respirology. 2016;21[3]:483-8).
Exercise-related symptoms (dyspnea and leg fatigue) were largely comparable between the obese and normal-weight COPD patients in both exercise modalities. However, in other studies, dyspnea ratings during cycling – at any given level of ventilation – have been lower in obese patients, indicating that “additional fat mass may have a beneficial effect on lung functioning [in non–weight-bearing exercise],” he said in an interview.
Dr. Franssen’s group also has assessed body composition in overweight and obese patients with COPD and found that a significant number have low muscle mass. These patients had worse lung function, exercise tolerance, and muscle strength compared to patients with comparable BMI and normal muscle mass (Respir Res. 2021 Mar 25. doi: 10.1186/s12931-021-01689-w).
“We’d always thought that obese patients have normal muscle mass ... but now we know it can be dramatically low,” he said. In assessing obesity and formulating any weight loss plans, “we’re now interested not only in weight but in the distribution of fat mass and fat-free mass ... and in maintaining muscle mass in patients who are [prescribed dietary interventions].”
Paradoxically, in patients with severe COPD, obesity is associated with prolonged survival, while in patients with mild to moderate COPD, obesity is associated with increased mortality risk, he noted.
The impact of adipose tissue and the chronic inflammation and metabolic disturbances that characterize obesity are currently largely unexplored, he said. Researchers have not yet studied what optimal weights may be for patients with COPD. “And we’re interested in the questions, are body weight and body composition the result of the disease, or [are they] determining the type of COPD one will get?” Dr. Franssen said.
Patients with COPD who are obese have “more of the phenotype of chronic bronchitis,” he noted, “while typical emphysema patients are normally underweight.”
The diverse effects of obesity on lung health and disease are increasingly being teased apart, with researchers honing in on the impact of metabolic dysfunction, circulating inflammatory factors produced by adipose tissue, lipid handling, and other factors – in addition to body mass index – that are associated with the obese state.
“The bird’s eye view is that obesity completely changes lung health. It’s something we’ve only recently begun to appreciate,” said Anne E. Dixon, MA, BM, BCh, director of the Vermont Lung Center at the University of Vermont, Burlington, who is focused on the research field of obesity and lung disease.
Structural, mechanical effects of obesity on lung function are better known and appreciated. Accumulation of fat in the mediastinum and abdominal and thoracic cavities causes reductions in lung volume, in functional residual capacity, and in the compliance of the lungs, chest wall, and entire respiratory system, for instance.
Yet obesity is more than a state of increased BMI, and “what we’ve begun to understand is that [its impact on the lungs and respiratory health] is much more complicated than just a mechanical problem,” said Dr. Dixon, also director of pulmonary and critical care medicine at the University of Vermont Medical Center and professor of medicine at the medical college.
With obesity, adipose tissue changes not only in quantity, but in function, producing proinflammatory cytokines and hormones – such as leptin, tumor necrosis factor-alpha (TNF-alpha), and interleukin-6 – that can have direct effects on the lung. Insulin resistance, which is common with obesity, is also seemingly deleterious. And obesity-associated changes in immune function, lipid handling, diet, and the gut microbiome may also impact lung health and disease, she said.
Dr. Dixon, who wrote about these changes in a 2018 review article in the journal CHEST and another 2019 piece in Expert Review of Respiratory Medicine, has developed a research program focused on obesity and lung disease and has edited a book and organized international conferences on the topic. (CHEST 2018;153[3]:702-9 and Exper Rev Respir Med. 2018;12[9]:755-67.)
“The more I do, the more I realize that there are multiple obesity-associated changes involved, and that [our current high level of] obesity is like a huge population-level natural experiment ... on lung health,” she told this news organization.
Associations between lung disease and the metabolic and other disturbances of obesity are most established in asthma research and have taken hold in the realm of sleep-disordered breathing. But as the prevalence of obesity continues to grow, its role in other lung diseases such as chronic obstructive pulmonary disorder (COPD) and, most recently, pulmonary arterial hypertension (PAH), is getting attention in academia.
And certainly, COVID-19 has highlighted an “urgent need” to better understand how obesity increases susceptibility to severe viral infections, Dr. Dixon added.
Here are some glimpses into current thinking and some examples of research that may have preventive and therapeutic implications in the future:
OSA and OHS
“With sleep apnea we tend to focus on anatomic considerations, but there may be relationships or interactions between obesity and neuromuscular function and neuroventilatory control,” Susheel P. Patil, MD, PhD, director of the sleep medicine program for University Hospitals and assistant professor at Case Western Reserve University, Cleveland, said in an interview.
Some studies suggest, for instance, that TNF-alpha can increase obstructive sleep apnea (OSA) susceptibility and severity through its neuroventilatory modulating properties during sleep. And the potential for additional proinflammatory cytokines produced by adipose tissue to similarly affect upper airway neuroventilatory control is an “intriguing line” of inquiry for researchers in the sleep apnea space, he said.
Leptin is of interest particularly in obesity hypoventilation syndrome (OHS), which is characterized by chronic daytime hypercapnia. Best known as a satiety hormone, leptin is produced by adipose tissue and suppresses appetite at the central nervous system level. But it has long been known that leptin also affects ventilation and the control of breathing.
When transported across the blood-brain barrier, leptin increases the hypercapnic ventilatory response, Babak Mokhlesi, MD, MSc, codirector of the Rush Lung Center and chief of pulmonary, critical care, and sleep medicine at Rush University Medical Center in Chicago, said in an interview.
Research suggests that patients with OHS may have resistance to leptin at the central nervous system level – with leptin not reaching the sites of ventilatory control. This is a “prevailing theory” and could explain why these patients “do not augment their ventilation to maintain homeostasis, normal levels of CO2,” Dr. Mokhlesi said.
“Why some patients with severe obesity develop CO2 retention while others do not is not fully understood,” he said, noting that patients with OHS can normalize their CO2 quickly when instructed to take deep breaths. “What we know is that the centers in the brain responsible for augmenting ventilation when CO2 goes up are somehow blunted.”
In a study of obese mice led by Vsevolod Y. Polotsky, MD, PhD, of Johns Hopkins University, Baltimore – and highlighted by Dr. Mokhlesi as an example of important, recent research – leptin delivered intranasally alleviated hypoventilation (and upper-airway obstruction), while intraperitoneally administered leptin did not, seemingly overcoming “central leptin deficiency.” (Am J Respir Crit Care Med. 2019;199[6]:773-83).
“This proved that there is some level of resistance in this animal model ... and has potential for therapeutics in the future,” Dr. Mokhlesi said.
Understanding the role of insulin resistance in OSA is another research focus. Some data suggest that insulin resistance, which is more common in obesity, is more prevalent in populations with OSA, Dr. Patil said. Researchers have discussed a bidirectional relationship for years, but it’s likely that insulin resistance is a precursor, he said.
In a mechanistic study published in 2016, Dr. Patil and his coinvestigators found that obese individuals with insulin resistance but without frank diabetes or sleep apnea demonstrated preclinical elevations in pharyngeal collapsibility during sleep. The findings suggest that insulin resistance could play a causal role in OSA pathogenesis by “generating requisite elevations in pharyngeal collapsibility,” they wrote (Eur. Respir J. 2016;47[6]:1718-26).
More recently, Dr. Patil noted in the interview, there is increasing appreciation in academia that the type of fat may be important to predicting OSA. “Visceral fat has a completely different cytokine-secretion profile than subcutaneous fat ... it is the more metabolically active fat that may secondarily impact upper airway function though a neuroinflammatory mechanism,” he said. “That is one of the working hypotheses today.”
Asthma
Research has so roundly suggested that metabolic dysfunction contributes to severe, poorly controlled asthma that there’s recent and growing interest in targeting metabolic dysfunction as part of the treatment of obese asthma, said Dr. Dixon, whose own research in obesity and lung disease has focused on asthma.
Data from animal models and some epidemiologic studies have suggested that drugs used to treat type 2 diabetes mellitus, such as glucagon-like peptide receptor-1 (GLPR-1) agonists and metformin, may help control asthma. In one recent study – cited by Dr. Dixon in a 2022 review of obesity and asthma – people with obesity and asthma who were prescribed GLPR-1 agonists for diabetes had fewer asthma exacerbations compared with those who took other medications for diabetes (Semin Respir Crit Care Med. 2022 Feb 17. doi: 10.1055/s-0042-1742384).
There is also research interest in targeting the pro-inflammatory adipokine interleukin 6 (IL-6), since increased circulating levels of IL-6 correlate with asthma severity, and in addressing oxidative stress in asthma through treatment with a mitochondrially targeted antioxidant, she said. Oxidative stress is increased in the airways of people with obesity, and researchers believe it may contribute to the pathophysiology of obese asthma through effects on airway nitric oxide levels.
(Her own research work at the University of Vermont has found associations between poor asthma control and high levels of leptin, and similar associations involving low levels of adiponectin, an anti-inflammatory adipokine that has been shown to downregulate eosinophil recruitment in the airways.)
Weight loss has been shown in mostly small, single-center studies to improve asthma control, but short of weight loss, researchers are also investigating the role of poor dietary quality. Thus far, data suggest that it’s the composition of the diet, and not just its contribution to weight gain, that could be impactful, Dr. Dixon said.
More basic research questions cited by Dr. Dixon include the extent to which adipose tissue inflammation causes inflammation in the lungs. “It’s a little unclear whether all the metabolic dysfunction associated with poor asthma control is causing inflammation in the lungs,” she said, though “we’ve done some work here that shows mediators produced by the adipose tissue could be impacting production of inflammatory mediators by the airway epithelium.”
Overall, she said, “the big questions [in asthma] are, how does adipose tissue affect the airway? Is it through direct effects? Through effects on the immune system? And obesity is affected by diet and the gut microbiome – how can these be [impacting] the airway?”
Obesity “is associated with so many changes – the gut, the immune system, and metabolic dysfunction, in addition to airway mechanics,” she said, “that I no longer think, as I did when I came to this, that it’s just one thing. It’s probably all of these things together.”
In the meantime, questions about potential shared pathways for the development of obesity and asthma remain. “Obesity is a risk factor for developing asthma, but it’s also entirely possible that asthma is a risk factor for developing obesity,” she said. (Some data from pediatric populations, she noted, suggest that nonobese children with asthma are at increased risk of developing obesity.)
Also important, Dr. Dixon said, is “emerging literature in the last 5-10 years” that suggests that people with obesity are more susceptible to the effects of air pollution. Research involving inner-city schoolchildren with asthma, for instance, has shown that those with obesity had worse symptoms with air pollution exposure than did those who were not obese.
Pulmonary arterial hypertension
Some research has looked at adipose tissue–produced substances in PAH, but the most well-established association in obesity and PAH involves insulin resistance.
“I don’t think we’re certain as a community that obesity [in general] is the problem – it’s not itself considered a risk factor for PAH,” Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., said in an interview. She noted that it’s “hard to dissect obesity” apart.
Researchers are “more confident,” she said, “that insulin resistance – one feature of obesity [in some people] – is associated with worse outcomes in PAH.” Metabolic disease resembling insulin resistance is common in PAH and is believed to contribute to pulmonary vascular disease and right ventricular (RV) failure – the main cause of mortality in PAH – at least in part because of increased oxidative stress.
Dr. Hemnes led a mechanistic phase II clinical trial of metformin in PAH in which the drug was associated with improved RV fractional area change and reduced RV lipid deposition (J Am Heart Assoc. 2020;9[22]:e018349), and she’s now leading a National Institutes of Health–funded multicenter trial looking at the impact of metformin and an exercise intervention on 6-minute walk distance and World Health Organization functional class in PAH.
At the Rush Lung Center, in the meantime, Dr. Mokhlesi is utilizing animal models of OSA and OHS to explore the effect of hypoxia and nighttime hypercapnia on the development of PAH. “I think the jury is still out as to whether obesity itself is a major risk factor, but if so, by what mechanism?” he said. “Is it worsening [sleep-disordered breathing], which then worsens PAH?”
COPD
The focus in COPD has traditionally been on underweight, but the relationship between obesity and COPD has increasingly been recognized in the last 10-15 years, said Frits M. E. Franssen, MD, PhD, of CIRO, a research institute in Horn, the Netherlands, that treats COPD and other chronic lung diseases, and of the department of respiratory medicine at Maastricht University.
Researchers like Dr. Franssen are trying, for one, to understand obesity’s impact on COPD pathophysiology and to tease apart the impact of both conditions on disease severity and patient-related outcomes such as exercise capacity and exercise-related symptoms.
When Dr. Franssen’s group compared responses to weight-bearing exercise (6-min. walk test) and weight-supported exercise (cycling) in obese and normal weight COPD patients matched for age, gender, and degree of airflow limitation, the researchers found that walking capacity was significantly reduced while cycling capacity was preserved in the obese group (Respirology. 2016;21[3]:483-8).
Exercise-related symptoms (dyspnea and leg fatigue) were largely comparable between the obese and normal-weight COPD patients in both exercise modalities. However, in other studies, dyspnea ratings during cycling – at any given level of ventilation – have been lower in obese patients, indicating that “additional fat mass may have a beneficial effect on lung functioning [in non–weight-bearing exercise],” he said in an interview.
Dr. Franssen’s group also has assessed body composition in overweight and obese patients with COPD and found that a significant number have low muscle mass. These patients had worse lung function, exercise tolerance, and muscle strength compared to patients with comparable BMI and normal muscle mass (Respir Res. 2021 Mar 25. doi: 10.1186/s12931-021-01689-w).
“We’d always thought that obese patients have normal muscle mass ... but now we know it can be dramatically low,” he said. In assessing obesity and formulating any weight loss plans, “we’re now interested not only in weight but in the distribution of fat mass and fat-free mass ... and in maintaining muscle mass in patients who are [prescribed dietary interventions].”
Paradoxically, in patients with severe COPD, obesity is associated with prolonged survival, while in patients with mild to moderate COPD, obesity is associated with increased mortality risk, he noted.
The impact of adipose tissue and the chronic inflammation and metabolic disturbances that characterize obesity are currently largely unexplored, he said. Researchers have not yet studied what optimal weights may be for patients with COPD. “And we’re interested in the questions, are body weight and body composition the result of the disease, or [are they] determining the type of COPD one will get?” Dr. Franssen said.
Patients with COPD who are obese have “more of the phenotype of chronic bronchitis,” he noted, “while typical emphysema patients are normally underweight.”
The diverse effects of obesity on lung health and disease are increasingly being teased apart, with researchers honing in on the impact of metabolic dysfunction, circulating inflammatory factors produced by adipose tissue, lipid handling, and other factors – in addition to body mass index – that are associated with the obese state.
“The bird’s eye view is that obesity completely changes lung health. It’s something we’ve only recently begun to appreciate,” said Anne E. Dixon, MA, BM, BCh, director of the Vermont Lung Center at the University of Vermont, Burlington, who is focused on the research field of obesity and lung disease.
Structural, mechanical effects of obesity on lung function are better known and appreciated. Accumulation of fat in the mediastinum and abdominal and thoracic cavities causes reductions in lung volume, in functional residual capacity, and in the compliance of the lungs, chest wall, and entire respiratory system, for instance.
Yet obesity is more than a state of increased BMI, and “what we’ve begun to understand is that [its impact on the lungs and respiratory health] is much more complicated than just a mechanical problem,” said Dr. Dixon, also director of pulmonary and critical care medicine at the University of Vermont Medical Center and professor of medicine at the medical college.
With obesity, adipose tissue changes not only in quantity, but in function, producing proinflammatory cytokines and hormones – such as leptin, tumor necrosis factor-alpha (TNF-alpha), and interleukin-6 – that can have direct effects on the lung. Insulin resistance, which is common with obesity, is also seemingly deleterious. And obesity-associated changes in immune function, lipid handling, diet, and the gut microbiome may also impact lung health and disease, she said.
Dr. Dixon, who wrote about these changes in a 2018 review article in the journal CHEST and another 2019 piece in Expert Review of Respiratory Medicine, has developed a research program focused on obesity and lung disease and has edited a book and organized international conferences on the topic. (CHEST 2018;153[3]:702-9 and Exper Rev Respir Med. 2018;12[9]:755-67.)
“The more I do, the more I realize that there are multiple obesity-associated changes involved, and that [our current high level of] obesity is like a huge population-level natural experiment ... on lung health,” she told this news organization.
Associations between lung disease and the metabolic and other disturbances of obesity are most established in asthma research and have taken hold in the realm of sleep-disordered breathing. But as the prevalence of obesity continues to grow, its role in other lung diseases such as chronic obstructive pulmonary disorder (COPD) and, most recently, pulmonary arterial hypertension (PAH), is getting attention in academia.
And certainly, COVID-19 has highlighted an “urgent need” to better understand how obesity increases susceptibility to severe viral infections, Dr. Dixon added.
Here are some glimpses into current thinking and some examples of research that may have preventive and therapeutic implications in the future:
OSA and OHS
“With sleep apnea we tend to focus on anatomic considerations, but there may be relationships or interactions between obesity and neuromuscular function and neuroventilatory control,” Susheel P. Patil, MD, PhD, director of the sleep medicine program for University Hospitals and assistant professor at Case Western Reserve University, Cleveland, said in an interview.
Some studies suggest, for instance, that TNF-alpha can increase obstructive sleep apnea (OSA) susceptibility and severity through its neuroventilatory modulating properties during sleep. And the potential for additional proinflammatory cytokines produced by adipose tissue to similarly affect upper airway neuroventilatory control is an “intriguing line” of inquiry for researchers in the sleep apnea space, he said.
Leptin is of interest particularly in obesity hypoventilation syndrome (OHS), which is characterized by chronic daytime hypercapnia. Best known as a satiety hormone, leptin is produced by adipose tissue and suppresses appetite at the central nervous system level. But it has long been known that leptin also affects ventilation and the control of breathing.
When transported across the blood-brain barrier, leptin increases the hypercapnic ventilatory response, Babak Mokhlesi, MD, MSc, codirector of the Rush Lung Center and chief of pulmonary, critical care, and sleep medicine at Rush University Medical Center in Chicago, said in an interview.
Research suggests that patients with OHS may have resistance to leptin at the central nervous system level – with leptin not reaching the sites of ventilatory control. This is a “prevailing theory” and could explain why these patients “do not augment their ventilation to maintain homeostasis, normal levels of CO2,” Dr. Mokhlesi said.
“Why some patients with severe obesity develop CO2 retention while others do not is not fully understood,” he said, noting that patients with OHS can normalize their CO2 quickly when instructed to take deep breaths. “What we know is that the centers in the brain responsible for augmenting ventilation when CO2 goes up are somehow blunted.”
In a study of obese mice led by Vsevolod Y. Polotsky, MD, PhD, of Johns Hopkins University, Baltimore – and highlighted by Dr. Mokhlesi as an example of important, recent research – leptin delivered intranasally alleviated hypoventilation (and upper-airway obstruction), while intraperitoneally administered leptin did not, seemingly overcoming “central leptin deficiency.” (Am J Respir Crit Care Med. 2019;199[6]:773-83).
“This proved that there is some level of resistance in this animal model ... and has potential for therapeutics in the future,” Dr. Mokhlesi said.
Understanding the role of insulin resistance in OSA is another research focus. Some data suggest that insulin resistance, which is more common in obesity, is more prevalent in populations with OSA, Dr. Patil said. Researchers have discussed a bidirectional relationship for years, but it’s likely that insulin resistance is a precursor, he said.
In a mechanistic study published in 2016, Dr. Patil and his coinvestigators found that obese individuals with insulin resistance but without frank diabetes or sleep apnea demonstrated preclinical elevations in pharyngeal collapsibility during sleep. The findings suggest that insulin resistance could play a causal role in OSA pathogenesis by “generating requisite elevations in pharyngeal collapsibility,” they wrote (Eur. Respir J. 2016;47[6]:1718-26).
More recently, Dr. Patil noted in the interview, there is increasing appreciation in academia that the type of fat may be important to predicting OSA. “Visceral fat has a completely different cytokine-secretion profile than subcutaneous fat ... it is the more metabolically active fat that may secondarily impact upper airway function though a neuroinflammatory mechanism,” he said. “That is one of the working hypotheses today.”
Asthma
Research has so roundly suggested that metabolic dysfunction contributes to severe, poorly controlled asthma that there’s recent and growing interest in targeting metabolic dysfunction as part of the treatment of obese asthma, said Dr. Dixon, whose own research in obesity and lung disease has focused on asthma.
Data from animal models and some epidemiologic studies have suggested that drugs used to treat type 2 diabetes mellitus, such as glucagon-like peptide receptor-1 (GLPR-1) agonists and metformin, may help control asthma. In one recent study – cited by Dr. Dixon in a 2022 review of obesity and asthma – people with obesity and asthma who were prescribed GLPR-1 agonists for diabetes had fewer asthma exacerbations compared with those who took other medications for diabetes (Semin Respir Crit Care Med. 2022 Feb 17. doi: 10.1055/s-0042-1742384).
There is also research interest in targeting the pro-inflammatory adipokine interleukin 6 (IL-6), since increased circulating levels of IL-6 correlate with asthma severity, and in addressing oxidative stress in asthma through treatment with a mitochondrially targeted antioxidant, she said. Oxidative stress is increased in the airways of people with obesity, and researchers believe it may contribute to the pathophysiology of obese asthma through effects on airway nitric oxide levels.
(Her own research work at the University of Vermont has found associations between poor asthma control and high levels of leptin, and similar associations involving low levels of adiponectin, an anti-inflammatory adipokine that has been shown to downregulate eosinophil recruitment in the airways.)
Weight loss has been shown in mostly small, single-center studies to improve asthma control, but short of weight loss, researchers are also investigating the role of poor dietary quality. Thus far, data suggest that it’s the composition of the diet, and not just its contribution to weight gain, that could be impactful, Dr. Dixon said.
More basic research questions cited by Dr. Dixon include the extent to which adipose tissue inflammation causes inflammation in the lungs. “It’s a little unclear whether all the metabolic dysfunction associated with poor asthma control is causing inflammation in the lungs,” she said, though “we’ve done some work here that shows mediators produced by the adipose tissue could be impacting production of inflammatory mediators by the airway epithelium.”
Overall, she said, “the big questions [in asthma] are, how does adipose tissue affect the airway? Is it through direct effects? Through effects on the immune system? And obesity is affected by diet and the gut microbiome – how can these be [impacting] the airway?”
Obesity “is associated with so many changes – the gut, the immune system, and metabolic dysfunction, in addition to airway mechanics,” she said, “that I no longer think, as I did when I came to this, that it’s just one thing. It’s probably all of these things together.”
In the meantime, questions about potential shared pathways for the development of obesity and asthma remain. “Obesity is a risk factor for developing asthma, but it’s also entirely possible that asthma is a risk factor for developing obesity,” she said. (Some data from pediatric populations, she noted, suggest that nonobese children with asthma are at increased risk of developing obesity.)
Also important, Dr. Dixon said, is “emerging literature in the last 5-10 years” that suggests that people with obesity are more susceptible to the effects of air pollution. Research involving inner-city schoolchildren with asthma, for instance, has shown that those with obesity had worse symptoms with air pollution exposure than did those who were not obese.
Pulmonary arterial hypertension
Some research has looked at adipose tissue–produced substances in PAH, but the most well-established association in obesity and PAH involves insulin resistance.
“I don’t think we’re certain as a community that obesity [in general] is the problem – it’s not itself considered a risk factor for PAH,” Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., said in an interview. She noted that it’s “hard to dissect obesity” apart.
Researchers are “more confident,” she said, “that insulin resistance – one feature of obesity [in some people] – is associated with worse outcomes in PAH.” Metabolic disease resembling insulin resistance is common in PAH and is believed to contribute to pulmonary vascular disease and right ventricular (RV) failure – the main cause of mortality in PAH – at least in part because of increased oxidative stress.
Dr. Hemnes led a mechanistic phase II clinical trial of metformin in PAH in which the drug was associated with improved RV fractional area change and reduced RV lipid deposition (J Am Heart Assoc. 2020;9[22]:e018349), and she’s now leading a National Institutes of Health–funded multicenter trial looking at the impact of metformin and an exercise intervention on 6-minute walk distance and World Health Organization functional class in PAH.
At the Rush Lung Center, in the meantime, Dr. Mokhlesi is utilizing animal models of OSA and OHS to explore the effect of hypoxia and nighttime hypercapnia on the development of PAH. “I think the jury is still out as to whether obesity itself is a major risk factor, but if so, by what mechanism?” he said. “Is it worsening [sleep-disordered breathing], which then worsens PAH?”
COPD
The focus in COPD has traditionally been on underweight, but the relationship between obesity and COPD has increasingly been recognized in the last 10-15 years, said Frits M. E. Franssen, MD, PhD, of CIRO, a research institute in Horn, the Netherlands, that treats COPD and other chronic lung diseases, and of the department of respiratory medicine at Maastricht University.
Researchers like Dr. Franssen are trying, for one, to understand obesity’s impact on COPD pathophysiology and to tease apart the impact of both conditions on disease severity and patient-related outcomes such as exercise capacity and exercise-related symptoms.
When Dr. Franssen’s group compared responses to weight-bearing exercise (6-min. walk test) and weight-supported exercise (cycling) in obese and normal weight COPD patients matched for age, gender, and degree of airflow limitation, the researchers found that walking capacity was significantly reduced while cycling capacity was preserved in the obese group (Respirology. 2016;21[3]:483-8).
Exercise-related symptoms (dyspnea and leg fatigue) were largely comparable between the obese and normal-weight COPD patients in both exercise modalities. However, in other studies, dyspnea ratings during cycling – at any given level of ventilation – have been lower in obese patients, indicating that “additional fat mass may have a beneficial effect on lung functioning [in non–weight-bearing exercise],” he said in an interview.
Dr. Franssen’s group also has assessed body composition in overweight and obese patients with COPD and found that a significant number have low muscle mass. These patients had worse lung function, exercise tolerance, and muscle strength compared to patients with comparable BMI and normal muscle mass (Respir Res. 2021 Mar 25. doi: 10.1186/s12931-021-01689-w).
“We’d always thought that obese patients have normal muscle mass ... but now we know it can be dramatically low,” he said. In assessing obesity and formulating any weight loss plans, “we’re now interested not only in weight but in the distribution of fat mass and fat-free mass ... and in maintaining muscle mass in patients who are [prescribed dietary interventions].”
Paradoxically, in patients with severe COPD, obesity is associated with prolonged survival, while in patients with mild to moderate COPD, obesity is associated with increased mortality risk, he noted.
The impact of adipose tissue and the chronic inflammation and metabolic disturbances that characterize obesity are currently largely unexplored, he said. Researchers have not yet studied what optimal weights may be for patients with COPD. “And we’re interested in the questions, are body weight and body composition the result of the disease, or [are they] determining the type of COPD one will get?” Dr. Franssen said.
Patients with COPD who are obese have “more of the phenotype of chronic bronchitis,” he noted, “while typical emphysema patients are normally underweight.”
Lenabasum improved skin symptoms in dermatomyositis, but future is uncertain
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
An – some of it statistically significant – in a phase 2, double-blind, randomized, controlled study.
Patients taking lenabasum experienced greater reductions in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) – a validated outcome designed to assess inflammatory skin involvement in the rare autoimmune disease – and improvements in patient-reported and biomarker outcomes, compared with those on placebo, dermatologist Victoria P. Werth, MD, and coinvestigators reported.
And in a recently completed phase 3 trial, reported by the manufacturer, a subpopulation of patients with active skin disease and no active muscle disease again showed greater reductions in CDASI activity scores – a secondary outcome in the trial.
However, the phase 3 DETERMINE trial produced negative findings overall. It enrolled a more heterogeneous group of patients – including those with both muscle weakness and skin involvement – and its primary outcome measure was a broader composite measure, the Total Improvement Score. The trial failed to meet this primary endpoint, Corbus Pharmaceuticals, the developer of lenabasum, announced in a press release in June 2021.
The phase 3 results are “frustrating” for patients with symptomatic and refractory skin manifestations of dermatomyositis (DM), given the promising findings from the phase 2 trial and from an open-label extension study, said Dr. Werth, professor of dermatology and medicine, University of Pennsylvania, Philadelphia, and principal investigator and coprincipal investigator of the phase 2 and phase 3 studies, respectively.
Dr. Werth is scheduled to present the results from the phase 3 trial at the annual European Alliance of Associations for Rheumatology meeting in June.
“With lenabasum, we have a therapy that doesn’t work for every patient, but does work for quite a number of them,” Dr. Werth said in an interview. “It’s oral, it’s not really that immunosuppressing, and there aren’t many side effects. Right now, patients are often being managed with steroids ... we really need treatments that are not as toxic.”
Robert Spiera, MD, a rheumatologist who led trials of lenabasum for treatment of diffuse cutaneous systemic sclerosis (dcSSc), agreed. “The CB2 agonist strategy is appealing because it’s nonimmunosuppressing and has both anti-inflammatory and antifibrotic properties,” he said in an interview. “I wouldn’t want to give up on it ... especially [for patients] with scleroderma and dermatomyositis who are treated with substantial drugs that are associated with morbidity.”
Lenabasum, he said, has proven to be “incredibly safe, and incredibly safe in the long term.”
While the phase 2 trial of the drug for dcSSc showed clear benefit over placebo, the phase 3 trial did not meet its primary endpoint using the American College of Rheumatology Combined Response Index in Diffuse Cutaneous Systemic Sclerosis.
It allowed background immunosuppressant therapy to reflect real-world clinical practice, and “there was such a high response rate to [that therapy, largely mycophenolate] that there was little room to show benefit beyond that,” said Dr. Spiera, director of the vasculitis and scleroderma program, Hospital for Special Surgery, New York.
The drug led to more improvement in the small subset of participants who were not receiving background immunotherapy during the trial, he noted.
Corbus is currently “seeking a partnership to further explore the drug” for treatment in different subpopulations, according to a company spokesperson. Results of a phase 2 trial of lenabasum for the treatment of systemic lupus erythematosus – with a pain rating as the primary outcome measure – are expected soon.
Phase 2 findings
The single-center phase 2 trial of lenabasum for DM enrolled 22 adults with minimal muscle involvement as evidenced by normal maximal resistance on muscle testing at entry and throughout the study. Most were taking immunosuppressant medication, and all had CDASI scores of at least 20, with mean scores in the severe range (> 26). Symptoms registered on patient-reported outcome measures were moderate to severe.
Patients received a half-dose of lenabasum (20 mg daily) for 1 month and a full dose (20 mg twice daily) for 2 months, or placebo, and were followed for an additional month without dosing.
Starting at day 43 – approximately 2 weeks after the dose was increased – there was “a trend for the change from baseline CDASI to be greater” in the lenabasum group, compared with those on placebo, Dr. Werth and colleagues reported. The differences reached statistical significance on day 113 (P = .038), a month after patients discontinued lenabasum, “suggesting that the modulation of the inflammatory response by lenabasum continued beyond its last dose.”
Five of the 11 patients treated with lenabasum (45%), and none of those on placebo, achieved at least a 40% reduction in the CDASI activity score by the end of the study.
Patients in the lenabasum group also had greater improvement in the Skindex-29 Symptoms scores – an objective measure of itch – and improvements in other secondary efficacy outcomes, including pain, though these did not reach statistical significance.
Skin biopsies before and after treatment showed significant reductions in inflammatory cytokines relevant to DM pathogenesis. Patients treated with the CB2 agonist had a downward trend in the CD4+ T cell population, which correlated with decreased CDASI activity scores, for instance, and a decrease in IL-31 protein expression, which correlated with decreased Skindex-29 Symptoms scores, the investigators reported.
There were no serious adverse events related to the CB2 agonist, and no treatment discontinuations.
The main part of the phase 2 trial, conducted from 2015 to 2017, was followed by a 3-year, open-label extension, in which 20 of the 22 patients took lenabasum 20 mg twice a day. The drug continued to be safe and well tolerated, and the CDASI activity score and other outcomes improved through year 1 and remained stable thereafter, according to a poster presented by Dr. Werth at the 2021 EULAR meeting.
After 1 year in the open-label extension, 60%-70% of patients had achieved mild skin disease, and 75% had achieved at least a 40% reduction in CDASI activity.
“A lot of patients, even if they weren’t completely cleared, were much happier in terms of their itch,” said Dr. Werth, also chief of dermatology, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia. “It’s been difficult for a lot of them now that they’re off the long-term extension ... a lot of them are flaring.”
The future
In the lab, with funding from the National Institutes of Health, Dr. Werth is continuing to investigate how lenabasum may be working in DM. A paper just published in the open access journal Arthritis Research & Therapy describes CB2 receptor distribution and up-regulation on key immune cells in the skin and blood, and how, in DM skin, its highest expression is on dendritic cells.
Through both mechanistic and more clinical research, “it’s important to understand the characteristics of the people [lenabasum] worked in or didn’t work in,” she said.
And in clinical trials, it’s important to capture meaningful improvement from the patient perspective. “It may be,” she noted, “that more global, systemic assessments are not the way to go for autoimmune skin disease.”
For dcSSc, Dr. Spiera said, it’s possible that a CB2 agonist may be helpful for patients who have been on immunosuppressants, particularly mycophenolate, for more than 6 months “and are still struggling.”
The phase 2 trial in DM was funded by the National Institutes of Health, the Department of Veterans Affairs, and Corbus Pharmaceuticals. The phase 3 trials in DM and in dcSSc were funded by Corbus. Dr. Werth disclosed grant support from Corbus and several other pharmaceutical companies. Dr. Spiera disclosed that he has received grant support or consulting fees from Roche-Genentech, GlaxoSmithKline, and several other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Topical treatment for EB recommended for approval in the EU
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.
A topical (EMA’s) Committee for Medicinal Products for Human Use.
“The benefit of Filsuvez is its ability to promote healing of EB partial thickness wounds,” the EMA said in an announcement on April 22. “It is thought to work by modulating inflammatory mediators and stimulating keratinocyte differentiation and migration, thereby promoting wound health and closure,” the statement adds.
The recommended indication for the product – developed by Amryt Pharmaceuticals DAC and currently designated as an orphan drug – is for the treatment of partial-thickness wounds associated with dystrophic and junctional EB in patients aged 6 months and older. The recommendation for approval came after the EMA sought and received external advice from independent physicians treating EB and from patients with the rare disease.
The most common side effects, according to the EMA announcement, are wound complications, application site reactions, wound infections, pruritus, and hypersensitivity reactions.
In February 2022, the Food and Drug Administration declined to approve the company’s new drug application as it was presented and asked the company to submit additional evidence of effectiveness for Oleogel-S10 in EB, the company announced at that time. The statement noted that the company was committed to working with the FDA to identify "the most expeditious pathway towards a potential approval.”
The company’s pivotal phase 3 trial enrolled 223 patients with EB, including 156 pediatric patients. The patients variously had three types of EB. The trial has two components: A 3-month, double-blind, randomized controlled phase, which has been completed, and an ongoing 24-month open-label, single-arm phase. The trial is being performed at 58 sites in 28 countries.
Results from the randomized controlled phase, reported in 2020, include a statistically significant increase in the proportion of patients achieving complete closure of an EB target wound within 45 days: 41.3% in the Oleogel-S10 group and 28.9% in the control group (P = .013). (Target wounds measured 10 cm² to 50 cm² and were present for at least 21 days but less than 9 months.) The safety profile of the treatment gel was acceptable and was well tolerated, compared with the control gel, according to Amryt’s press release. The results were presented at the European Academy of Dermatology and Venereology Congress in October 2020.
Data from a 12-month interim analysis of the follow-up phase were presented at the annual meeting of the American Academy of Dermatology in March 2022. Results showed further reductions in total body surface area percentage wounding to 5.4% among (from 7.4% at the end of the double-blind period and 12.1% at the beginning of the study) among the patients who continued treatment and who underwent assessment, according to a company press release. Treatment was well tolerated, and no new safety signals were identified, the release said.
A decision by the European Commission is expected within the next 2 months.
A version of this article first appeared on Medscape.com.