User login
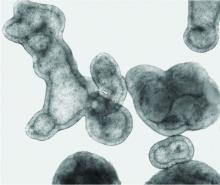
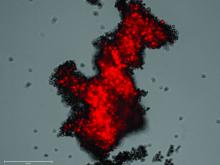
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.