User login
Even mild COVID tied to vascular impairment
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
In a small prospective study, participants who previously had COVID-19, even those with mild illness, had significantly decreased CVR, compared with never-infected individuals.
Results also showed cerebral blood flow (CBF) was greater in never-infected versus previously infected participants, and whole-brain CVR was lower in previously infected versus never-infected participants. Although CVR was also smaller in those with versus those without post-COVID neurologic conditions, the difference was not considered significant.
“It is important to remember that while our findings were statistically significant, we had a relatively small sample size – 25 total participants – and so we encourage future larger studies in this domain to see if these results are reproducible at a larger scale,” lead author Andrew Callen, MD, assistant professor of radiology, Neuroradiology Section, University of Colorado at Denver, Aurora, said in an interview.
“In a practical sense, it may encourage treating clinicians to be more aggressive with preventative neurovascular and cardiovascular health measures and/or screening in this patient population,” Dr. Callen said.
The findings were published online in the American Journal of Roentgenology.
Endothelial dysfunction
The acute phase SARS-CoV-2 infection “is associated with strokes that have features of both vascular inflammation and thromboembolism,” the investigators note.
Moreover, following the acute phase of infection, up to three-quarters of patients “experience persistent neurologic symptoms not attributable to another diagnosis, including headache, difficulty concentrating, vision changes, disequilibrium, and fatigue,” they write.
Preliminary studies “suggest a potential role for endothelial and circulatory dysfunction” in these symptoms, they add.
The researchers note that vessel wall imaging is an MRI technique that can detect and characterize arterial vascular inflammation and may differentiate vasculitic arterial pathology from atherosclerotic pathology.
Dr. Callen conducted previous research assessing cerebral vasoreactivity in women living with HIV. He noted that this is a population at a much higher risk of stroke, compared with uninfected individuals with otherwise similar cardiovascular risk factors, even when their viral load is controlled with antiretroviral therapies.
Evidence has pointed to chronic endothelial dysfunction in these individuals, and endothelial function and dysfunction can be measured through vasoreactivity testing, Dr. Callen said.
“As the COVID pandemic progressed, not only did we observe an increased rate of stroke in individuals acutely infected with COVID, but histopathological evidence began to emerge which suggested that the COVID-19 virus had tropism to and often damaged the vascular endothelium,” he noted.
This emerging evidence prompted Dr. Callen to wonder whether “individuals previously infected with COVID might also demonstrate long-term impairment in cerebral vasoreactivity or if we might see abnormalities using high resolution vessel wall imaging.”
In the current study, 15 individuals with prior SARS-CoV-2 infection (11 women, 4 men; mean age, 43 years) were compared with 10 never-infected individuals (8 women, 2 men; mean age, 43 years) who functioned as the control group.
The previously infected individuals, of whom three had prior critical infection and 12 had prior mild infection, were assessed, on average, about 8 months after infection. Of this group, seven had various post-COVID neurologic conditions, including headache, memory impairment, insomnia, depression, disequilibrium, fatigue, personality change, phantosmias (detecting smells that aren’t present), dysgeusia (taste disorder), and tinnitus.
All participants underwent MRI and vessel wall imaging. The MRI included arterial spin labeling perfusion imaging with acetazolamide stimulus to measure CBF and calculate CVR. The vessel wall imaging examinations used a contrast-enhanced black-blood 3D T1-weighted sequence.
Imaging data
Prior to acetazolamide administration, the mean whole-cortex CBF did not differ significantly between never-infected and previously infected participants. However, following the acetazolamide administration, the mean whole-cortex CBF was greater in never-infected participants (73.8 mL/100 g/min vs. 60.5 mL/100 g/min, respectively; P = .04).
Moreover, the mean whole-brain CVR was greater in never-infected participants, compared with previously infected participants (27.8 mL/100 g/min vs. 19.1 mL/100 g/min; P < .001).
After adjusting for age and sex, researchers found that prior infection was associated with a lower whole-brain CVR (–8.9 mL/100 g/min; 95% confidence interval, 4.6-13.3 ml/100g/min; P < .001).
Previously infected individuals also showed significantly lower CVR, even after the researchers excluded those with prior critical illness.
A nonsignificant difference was found in previously infected participants, with smaller CVR in participants with versus without post-COVID neurologic symptoms (16.9 vs. 21.0 mL/100 g/min; P = .22).
In addition, 40% of the previously infected participants versus 10% of the never-infected participants had at least one vessel wall imaging abnormality – but the difference was not deemed significant (P = .18). Notably, “all detected vessel wall imaging abnormalities were morphologically consistent with atherosclerosis rather than vasculitis,” the investigators said.
Dr. Callen said it is “unknown whether the lack of statistical significance in the differences in vasoreactivity impairment with those living with long COVID symptoms is due to a lack of a biomechanistic correlation or due to statistical underpowering.”
If it is the latter, “it may emphasize the role of vascular health in those living with long COVID symptoms and potentially all individuals living with COVID,” he added.
Independent risk factor?
Commenting on the study for this article, Jared Narvid, MD, associate professor of neuroradiology, University of California, San Francisco, said it “adds to the literature suggesting a correlation between COVID-19 infection and measures of cerebrovascular abnormality.”
Dr. Narvid, who was not involved with the research, added that “although it is a small case-control study, it is well executed and should encourage scientists to further study whether COVID-19 infection represents an independent risk factor for cerebrovascular disease.”
The investigators agree. “Future studies are needed to determine the clinical implications arising from SARS-CoV-2–associated CVR impairment,” they write.
The study was funded by a University of Colorado department of radiology Faculty Development Seed Grant. The investigators and Dr. Narvid report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
Prior psychological distress tied to ‘long-COVID’ conditions
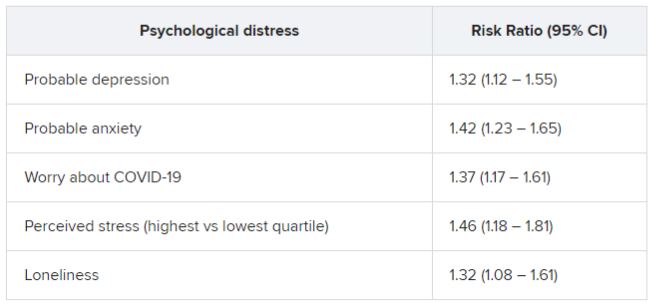
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Subtle visual dysfunctions often precede early-stage psychosis
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Distorted time perception during the pandemic tied to stress, poor mental health
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL TRAUMA: THEORY, RESEARCH, PRACTICE, AND POLICY
Stable, long-term opioid therapy safer than tapering?
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.
The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
New panic disorder model flags risk for recurrence, persistence
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
Investigators based in France and the United States analyzed data for almost 800 patients with DSM-IV–diagnosed PD.
Results showed that having a “general psychopathology factor,” defined as the shared effects of all comorbid conditions, or PD liability, significantly and independently predicted 3-year recurrence or persistence of PD symptoms.
Having a lower physical health-related quality of life (QOL), a greater number of stressful life events, and not seeking treatment at baseline were also significant and independent predictors.
“This integrative model could help clinicians to identify individuals at high risk of recurrence or persistence of panic disorder and provide content for future research,” Valentin Scheer, MD, MPH, a resident in psychiatry at AP-HP, Assistance Publique, Hôpitaux de Paris, and colleagues wrote.
The findings were published online in the Journal of Clinical Psychiatry.
Integration needed
PD is a disabling disorder with a “chronic course” – and a recurrence rate ranging from 25% to 50%, the investigators noted.
“Because of the heterogeneous course of PD, there is a need to develop a comprehensive predictive model of recurrence or persistence,” they wrote. This could “help practitioners adapt therapeutic strategies and develop prevention strategies in high-risk individuals.”
Most previous studies that have investigated risk factors for PD recurrence and persistence have relied on clinical samples, often with limited sample sizes.
Moreover, each risk factor, when considered individually, accounts for only a “small proportion” of the variance in risk, the researchers noted. The co-occurrence of these risk factors “suggests the need to combine them into a broad multivariable model.”
However, currently proposed integrative models do not identify independent predictors or mitigate the influence of confounding variables. To fill this gap, the investigators conducted a study using structural equation modeling “to take into account multiple correlations across predictors.”
They drew on data from 775 participants (mean age, 40 years) in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). For the current analysis, they examined two waves of NESARC (2001-2002 and 2004-2005) to “build a comprehensive model” of the 3-year recurrence or persistence of PD.
The researchers used a “latent variable approach” that simultaneously examined the effect of the following five groups of potential predictors of recurrence or persistence: PD severity, severity of comorbidity, family history of psychiatric disorders, sociodemographic characteristics, and treatment-seeking behavior.
They also distinguished between risk factors responsible for recurrence and those responsible for persistence.
Psychiatric diagnoses were determined on the basis of the Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV. Participants also completed Version 2 of the Short Form 12-Item Health Survey, which assesses both mental and physical QOL over the previous 4 weeks.
Early treatment needed
Among participants with a 12-month diagnosis of PD at wave 1, 13% had persistent PD and 27.6% had recurrent PD during the 3-year period. The mean duration of illness was 9.5 years.
A greater number of lifetime panic attacks, the presence of any Axis I or II comorbid disorder, and any Axis I disorder, especially social anxiety disorder, were significantly associated with 3-year risk for recurrence and for persistence.
Sweating, choking, paresthesias, the comorbid disorders of mania/hypomania and general anxiety disorder, nicotine dependence, lower mental and physical QOL scores, and exposure to a greater number of stressful life events in the previous year were all significantly associated with 3-year risk for recurrence.
Only variables shown with a P value were statistically significant, “with the a priori fixed at .05,” the researchers noted.
A combination of psychopathology factors, such as the shared effect of all comorbid psychiatric conditions, PD liability, lower physical health-related QOL, more life stressors during the past year, and not seeking treatment at baseline “significantly and independently” predicted recurrence or persistence of symptoms between the two waves (all Ps < .05), the investigators reported.
One study limitation cited was that several psychiatric disorders known to be associated with PD recurrence or persistence, such as borderline personality disorder, were not examined. Additionally, the study used a 3-year follow-up period – and the results might have differed for other follow-up time frames, the researchers noted.
Nevertheless, the findings constitute a “comprehensive model” to predict recurrence and persistence of PD, they wrote. Moreover, early treatment-seeking behavior “should be promoted, as it may reduce the risk of recurrence.”
Not much new?
Commenting on the study, Peter Roy-Byrne, MD, professor of psychiatry, University of Washington, Seattle, noted, “there is not much that is new here.”
Dr. Roy-Byrne, who was not involved with the study, said that a “general theme for years has been that more severe illness, whether you measure it by greater number of other Axis I disorders or symptom severity or a general psychopathology factor, usually predicts worse outcome – here codified as persistence and recurrence.”
Greater stress and reluctance to seek treatment may also predict worse outcomes, he noted.
In addition, the study “did not examine another very important factor: the degree of social connection/social support that someone has,” Dr. Roy-Byrne said. However, “perhaps some of this was contained in specific life events.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Diagnostic criterion may hide borderline personality disorder
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared characteristics of almost 400 psychiatric outpatients diagnosed with BPD. About half of the participants met the suicidality/self-injury diagnostic criterion for the disorder, while the other half did not.
Results showed no differences between the two groups in degree of impairment in occupational or social functioning, comorbid psychiatric disorders, history of childhood trauma, or severity of depression, anxiety, or anger.
“Just because a person doesn’t engage in self-harm or suicidal behavior doesn’t mean that the person is free of borderline personality disorder,” lead author Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“Clinicians need to screen for borderline personality disorder in patients with other suggestive symptoms, even if those patients don’t self-harm, just as they would for similar patients who do self-harm,” said Dr. Zimmerman, who is also the director of the Outpatient Division at the Partial Hospital Program, Rhode Island Hospital.
The findings were published online in Psychological Medicine.
A ‘polythetic diagnosis’
Dr. Zimmerman noted the impetus for conducting the study originated with a patient he saw who had all of the features of BPD except for self-harm and suicidality. However, because she didn’t have those two features, she was told by her therapist she could not have BPD.
“This sparked the idea that perhaps there are other individuals whose BPD may not be recognized because they don’t engage in self-harm or suicidal behavior,” Dr. Zimmerman said.
“Most individuals with BPD don’t present for treatment saying, ‘I’m here because I don’t have a sense of myself’ or ‘I feel empty inside’ – but they do say, ‘I’m here because I’m cutting myself’ or ‘I’m suicidal,’ ” he added.
The investigators wondered if there were other “hidden” cases of BPD that were being missed by therapists.
They had previously analyzed each diagnostic criterion for BPD to ascertain its sensitivity. “We had been interested in wanting to see whether there was a criterion so frequent in BPD that every patient with BPD has it,” Dr. Zimmerman said.
BPD is a “polythetic diagnosis,” he added. It is “based on a list of features, with a certain minimum number of those features necessary to make the diagnosis rather than one specific criterion.”
His group’s previous research showed affective instability criterion to be present in more than 90% of individuals with BPD. “It had a very high negative predictive value, meaning that if you didn’t have affective instability, you didn’t have the disorder,” he said.
“Given the clinical and public health significance of suicidal and self-harm behavior in patients with BPD, an important question is whether the absence of this criterion, which might attenuate the likelihood of recognizing and diagnosing the disorder, identifies a subgroup of patients with BPD who are ‘less borderline’ than patients with BPD who do not manifest this criterion,” the investigators write.
The researchers wanted to see if a similar finding applied to self-injury and suicidal behavior and turned to the Rhode Island Methods to Improve Diagnostic Assessment and Services (MIDAS) project to compare the demographic and clinical characteristics of patients with BPD who do and do not engage in repeated suicidal and self-harm behavior.
MIDAS project
The study population was derived from 3,800 psychiatric outpatients who had been evaluated in the MIDAS project with semi-structured diagnostic interviews.
Of these, 390 patients were diagnosed with BPD. Since the suicidality/self-harm item was not rated in one patient, the analyzed sample consisted of 389 individuals with BPD (28.3% male; mean age, 32.6 years; 86.3% White). A little more than half the participants (54%) met the BPD suicidality/ self-harm criterion.
Only one-fifth (20.5%) of patients with BPD presented with a chief complaint that was related to a feature of BPD and had received BPD as their principal diagnosis.
Patients who met the suicidality/self-injury criterion were almost twice as likely to be diagnosed with BPD as the principal diagnosis, compared with those who did not have that criterion (24.8% vs. 14.5%, respectively; P < .01).
On the other hand, there was no difference in the mean number of BPD criteria that were met, other than suicidality/self-harm, between those who did and did not present with suicidality/self-harm (5.5 ± 1.2 vs. 5.7 ± 0.8, t = 1.44). The investigators note that this finding was “not significant.”
There also was no difference between patients who did and did not meet the criterion in number of psychiatric diagnoses at time of evaluation (3.4 ± 1.9 vs. 3.5 ± 1.8, t = 0.56).
Hidden BPD
Similarly, there was no difference in any specific Axis I or personality disorder – except for generalized anxiety disorder (GAD) and histrionic personality disorder. Both were more frequent in the patients who did not meet the suicidality/self-injury criterion. However, after controlling for age, the group difference in GAD was no longer deemed significant (F = 3.45, P = .064).
By contrast, histrionic personality disorder remained significant with age as the covariate (F = 6.03, P = .015).
“The patients who met the suicidality/self-injury criterion were significantly more likely to have been hospitalized and reported more suicidal ideation at the time of the evaluation,” the researchers write. Both variables remained significant even after including age as a covariate.
There were no between-group differences on severity of depression, anxiety, or anger at initial evaluation nor were there differences in social functioning, adolescent social functioning, likelihood of persistent unemployment or receiving disability payments, childhood trauma, or neglect.
“I suspect that there are a number of individuals whose BPD is not recognized because they don’t have the more overt feature of self-injury or suicidal behavior,” said Dr. Zimmerman, noting that these patients might be considered as having “hidden” BPD.
“Repeated self-injurious and suicidal behavior is not synonymous with BPD, and clinicians should be aware that the absence of these behaviors does not rule out a diagnosis of BPD,” he added.
Stigmatizing diagnosis?
Monica Carsky, PhD, clinical assistant professor of psychology in psychiatry and senior fellow, Personality Disorders Institute, Weill Cornell Medical College, New York, said the study “will be particularly useful in the education of clinicians about the characteristics of individuals with BPD.”
Dr. Carsky, who is also an adjunct assistant professor in the NYU Postdoctoral Program in Psychoanalysis and Psychotherapy, was not involved with the study. She noted that other factors “can contribute to misdiagnosis of the borderline patients who do not have suicidality/self-harm.”
Clinicians and patients “may see BPD as a stigmatizing diagnosis so that clinicians become reluctant to make, share, and explain this personality disorder diagnosis,” she said.
Dr. Carsky suggested that increasing use of the Alternate Model for Personality Disorders in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), which first rates the severity level of personality by assessing identity and relationship problems and then notes traits of specific personality disorders, “will help clinicians who dread telling patients they are ‘borderline.’ ”
No source of study funding has been reported. The investigators and Dr. Carsky reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LAIAs safer, more effective than oral antipsychotics for schizophrenia
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and they carry no increased risk for adverse events, new research shows.
Investigators analyzed data for more than 70,000 patients with schizophrenia and found that, compared with OAs, LAIAs were associated with lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempts.
Among patients treated fully with LAIAs, there were fewer hospitalizations for somatic disorders and cardiovascular diseases and less extrapyramidal symptoms (EPS), compared with those treated fully with OAs. In addition, among those for whom treatment with LAIAs was initiated early, the reduction in these outcome events was greater in comparison with patients for whom treatment was initiated later.
The results “reinforced that LAIAs were associated with a lower risk of hospitalizations, disease relapses, and suicide attempts than OAs, and this association remained during subsequent treatment periods,” wrote the investigators, led by Esther Chan, PhD, Center for Safe Medication Practice and Research, department of pharmacology and pharmacy, University of Hong Kong.
The findings were published online in JAMA Network Open.
Misclassification common
“Current clinical guidelines on the use of LAIAs are derived mainly from randomized clinical trials in which strict inclusion criteria limit generalizability,” the investigators noted. In addition, most of these trials were of “relatively short duration,” so long-term observational studies are “important to establish the safety and effectiveness of LAIAs.”
Moreover, most studies have been based on Western populations, and the findings may not be generalizable to Asian populations, which have been less studied.
Previous studies were also often subject to “misclassification of exposure,” since patients treated with LAIAs alone and those treated concurrently with LAIAs and OAs were categorized together as “LAIA” users and were compared with users of OAs alone, the researchers noted.
To investigate the long-term safety and efficacy of LAIAs, they assessed data from the Clinical Data Analysis and Reporting System, an electronic health records database of the Hong Kong Hospital Authority. They identified 70,396 patients with schizophrenia (52.8% women; mean age, 44.2 years).
The investigators used a self-controlled case series design – “within-individual comparison, based on a case-only approach” – to analyze the data. This model uses incidence rate ratios derived by “comparing the rate of outcomes between exposed and unexposed or reference periods for the same individual.” In this approach, “only people with both the exposure and the outcome are eligible.”
To be included, a patient had to have received at least one OA and LAIA and had to have had at least one outcome event during the observation period of January 2004 or the date of first schizophrenia diagnosis (whichever came later) through December 2019 or death (whichever came first).
The observation period was further subdivided into four periods: a nontreatment period, a period in which OAs were used alone, a period in which LAIAs were used alone, and a period in which a combination of OAs and LAIAs were used together.
Primary outcomes included health care use and disease relapses, such as hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, and incident suicide attempt. Secondary outcomes included hospitalizations for somatic disorders, hospitalizations for cardiovascular diseases, and EPS.
LAIAs superior
Of the total study population, 23,719 patients (33.7%) were prescribed both OAs and LAIAs (mean age, 41.7 years). Of these participants, 15.4% died during the observation period.
The mean duration of follow-up was 12.5 years. The mean duration of exposure to OAs alone was 5 years; for LAIA exposure alone, 1.4 years; and for OA plus LAIA exposure, 4.4 years.
During the observation period, almost all individuals (92.8%) had one or more ED visits, and most (88.4%) had one or more hospitalizations for any psychiatric disorder. Over three-quarters (77.5%) were hospitalized for schizophrenia, and a small percentage (6.1%) had an incident suicide attempt.
Almost all patients experienced EPS (93.5%); over half (64.9%) were hospitalized for somatic disorders; and 15.6% were hospitalized for cardiovascular diseases.
After adjustment, compared with OAs, use of LAIAs was associated with a significantly lower risk for most outcomes.
There were no differences between LAIAs and OAs regarding ED visits.
The reduction in EPS suggests that LAIAs “were not associated with a higher risk of those adverse events than OAs,” the investigators write.
When the patients were stratified by initiation time of LAIAs, early initiators had 76% fewer hospitalizations for schizophrenia during LAIA vs OA treatment (IRR, 0.24; 95% confidence interval, 0.21-0 .27), while late initiators of LAIAs had 55% fewer hospitalizations for schizophrenia (IRR, 0.45; 95% CI, 0.40-0.49), “suggesting that early LAIA initiators could have greater reduction in disease relapse,” the researchers noted.
Participants with comorbid substance use had a significantly lower risk for hospitalizations for any cause, hospitalizations for psychiatric disorders, hospitalizations for schizophrenia, hospitalizations for somatic disorders, incident suicide attempts, and EPS during the time they were treated with LAIAs versus the time they were treated with OAs.
Older adults (> 65 years) treated with LAIAs had a lower risk for ED visits, hospitalizations for any cause, hospitalizations for psychiatric disorders, and hospitalizations for schizophrenia. They were not at increased risk for hospitalizations for somatic disorders or cardiovascular diseases.
There was, however, a higher risk for EPS during initial treatment with LAIAs, so “caution should be exercised when initiating LAIAs” in older individuals, the investigators wrote.
Cited study limitations include the fact that pooled estimates were used for all LAIAs, rather than for individual antipsychotics. Additionally, the dose of these antipsychotics “was not accounted for because the disease information was recorded in a different way for LAIAs and OAs.”
Proactive approach
Commenting on the study, Brittany Gouse, MD, assistant professor at Boston University School of Medicine and a psychiatrist in the wellness and recovery after psychosis program, Boston Medical Center, said the study “adds to the growing body of evidence supporting the longitudinal benefits” of LAIAs for patients with schizophrenia spectrum disorders.
“In particular, there may be a unique benefit to transitioning to long-acting injectable antipsychotics within the first 2 years of illness,” said Dr. Gouse, who coauthored an accompanying editorial and was not involved with the research.
She also called the study “important,” because it suggests that early initiation of LAIAs “may serve as an important tool to reduce morbidity and bridge the premature mortality gap in schizophrenia.”
Dr. Gouse emphasized the importance of prioritizing “tertiary prevention” right from the onset of psychotic illness.
“Clinicians must take a proactive approach and include long-acting antipsychotics in shared decision-making conversations with patients within the first few years of illness,” she said.
The study was funded by the Excellent Young Scientists Fund of the National Natural Science Foundation of China. Dr. Chan received grants from the National Natural Science Foundation of China during the conduct of the study; nonfinancial support from the Wellcome Trust; grants from the Research Grants Council, the Research Fund Secretariat of the Food and Health Bureau, the National Health and Medical Research Council (Australia), the narcotics division of the Security Bureau of Hong Kong SAR, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Janssen, Pfizer, Takeda, and Novartis; and personal fees from Pfizer, Novartis, and the Hong Kong SAR Hospital Authority outside the submitted work. The editorialists reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
A ‘promising target’ to improve outcomes in late-life depression
A new study sheds light on the neurologic underpinnings of late-life depression (LLD) with apathy and its frequently poor response to treatment.
Investigators headed by Faith Gunning, PhD, of the Institute of Geriatric Psychiatry, Weill Cornell Medicine, New York, analyzed baseline and posttreatment brain MRIs and functional MRIs (fMRIs) of older adults with depression who participated in a 12-week open-label nonrandomized clinical trial of escitalopram. Participants had undergone clinical and cognitive assessments.
Disturbances were found in resting state functional connectivity (rsFC) between the salience network (SN) and other large-scale networks that support goal-directed behavior, especially in patients with depression who also had features of apathy.
“This study suggests that, among older adults with depression, distinct network abnormalities may be associated with apathy and poor response to first-line pharmacotherapy and may serve as promising targets for novel interventions,” the investigators write.
The study was published online in JAMA Network Open.
A leading cause of disability
LLD is a “leading cause of disability and medical morbidity in older adulthood,” with one-third to one-half of patients with LLD also suffering from apathy, the authors write.
Older adults with depression and comorbid apathy have poorer outcomes, including lower remission rates and poorer response to first-line antidepressants, compared with those with LLD but who do not have apathy.
Despite the high prevalence of apathy in people with depression, “little is known about its optimal treatment and, more broadly, about the brain-based mechanisms of apathy,” the authors note.
An “emerging hypothesis” points to the role of a compromised SN and its large-scale connections between apathy and poor treatment response in LLD.
The SN (which includes the insula and the dorsal anterior cingulate cortex) “attributes motivational value to a stimulus” and “dynamically coordinates the activity of other large-scale networks, including the executive control network and default mode network (DMN).”
Preliminary studies of apathy in patients with depression report reduced volume in structures of the SN and suggest disruption in functional connectivity among the SN, DMN, and the executive control network; but the mechanisms linking apathy to poor antidepressant response in LLD “are not well understood.”
“Connectometry” is a “novel approach to diffusion MRI analysis that quantifies the local connectome of white matter pathways.” It has been used along with resting-state imagery, but it had not been used in studying apathy.
The researchers investigated the functional connectivity of the SN, hypothesizing that alterations in connectivity among key nodes of the SN and other core circuits that modulate goal-directed behavior (DMN and the executive control network) were implicated in individuals with depression and apathy.
They applied connectometry to “identify pathway-level disruptions in structural connectivity,” hypothesizing that compromise of frontoparietal and frontolimbic pathways would be associated with apathy in patients with LLD.
They also wanted to know whether apathy-related network abnormalities were associated with antidepressant response after 12 weeks of pharmacotherapy with the selective serotonin reuptake inhibitor escitalopram.
Emerging model
The study included 40 older adults (65% women; mean [SD] age, 70.0 [6.6] years) with DSM-IV–diagnosis major depressive disorder (without psychotic features) who were from a single-group, open-label escitalopram treatment trial.
The Hamilton-Depression (HAM-D) scale was used to assess depression, while the Apathy Evaluation Scale was used to assess apathy. On the Apathy Evaluation Scale, a score of greater than 40.5 represents “clinically significant apathy.” Participants completed these tests at baseline and after 12 weeks of escitalopram treatment.
They also completed a battery of neuropsychological tests to assess cognition and underwent MRI imaging. fMRI was used to map group differences in rsFC of the SN, and diffusion connectometry was used to “evaluate pathway-level disruptions in structural connectivity.”
Of the participants, 20 had clinically significant apathy. There were no differences in age, sex, educational level, or the severity of depression at baseline between those who did and those who did not have apathy.
Compared with participants with depression but not apathy, those with depression and comorbid apathy had lower rsFC of salience network seeds (specifically, the dorsolateral prefrontal cortex [DLPFC], premotor cortex, midcingulate cortex, and paracentral lobule).
They also had greater rsFC in the lateral temporal cortex and temporal pole (z > 2.7; Bonferroni-corrected threshold of P < .0125).
Additionally, participants with apathy had lower structural connectivity in the splenium, cingulum, and fronto-occipital fasciculus, compared with those without apathy (t > 2.5; false discovery rate–corrected P = .02).
Of the 27 participants who completed escitalopram treatment; 16 (59%) achieved remission (defined as an HAM-D score <10). Participants with apathy had poorer response to escitalopram treatment.
Lower insula-DLPFC/midcingulate cortex rsFC was associated with less improvement in depressive symptoms (HAM-D percentage change, beta [df] = .588 [26]; P = .001) as well as a greater likelihood that the participant would not achieve remission after treatment (odds ratio, 1.041; 95% confidence interval, 1.003-1.081; P = .04).
In regression models, lower insula-DLPFC/midcingulate cortex rsFC was found to be a mediator of the association between baseline apathy and persistence of depression.
The SN findings were also relevant to cognition. Lower dorsal anterior cingulate-DLPFC/paracentral rsFC was found to be associated with residual cognitive difficulties on measures of attention and executive function (beta [df] = .445 [26] and beta [df] = .384 [26], respectively; for each, P = .04).
“These findings support an emerging model of apathy, which proposes that apathy may arise from dysfunctional interactions among core networks (that is, SN, DMN, and executive control) that support motivated behavior,” the investigators write.
“This may cause a failure of network integration, leading to difficulties with salience processing, action planning, and behavioral initiation that manifests clinically as apathy,” they conclude.
One limitation they note was the lack of longitudinal follow-up after acute treatment and a “relatively limited neuropsychological battery.” Therefore, they could not “establish the persistence of treatment differences nor the specificity of cognitive associations.”
The investigators add that “novel interventions that modulate interactions among affected circuits may help to improve clinical outcomes in this distinct subgroup of older adults with depression, for whom few effective treatments exist.”
Commenting on the study, Helen Lavretsy, MD, professor of psychiatry in residence and director of the Late-Life Mood, Stress, and Wellness Research Program and the Integrative Psychiatry Clinic, Jane and Terry Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, said, the findings “can be used in future studies targeting apathy and the underlying neural mechanisms of brain connectivity.” Dr. Lavretsy was not involved with the study.
The study was supported by grants from the National Institute of Mental Health. Dr. Gunning reported receiving grants from the National Institute of Mental Health during the conduct of the study and grants from Akili Interactive. The other authors’ disclosures are listed on the original article. Dr. Lavretsky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study sheds light on the neurologic underpinnings of late-life depression (LLD) with apathy and its frequently poor response to treatment.
Investigators headed by Faith Gunning, PhD, of the Institute of Geriatric Psychiatry, Weill Cornell Medicine, New York, analyzed baseline and posttreatment brain MRIs and functional MRIs (fMRIs) of older adults with depression who participated in a 12-week open-label nonrandomized clinical trial of escitalopram. Participants had undergone clinical and cognitive assessments.
Disturbances were found in resting state functional connectivity (rsFC) between the salience network (SN) and other large-scale networks that support goal-directed behavior, especially in patients with depression who also had features of apathy.
“This study suggests that, among older adults with depression, distinct network abnormalities may be associated with apathy and poor response to first-line pharmacotherapy and may serve as promising targets for novel interventions,” the investigators write.
The study was published online in JAMA Network Open.
A leading cause of disability
LLD is a “leading cause of disability and medical morbidity in older adulthood,” with one-third to one-half of patients with LLD also suffering from apathy, the authors write.
Older adults with depression and comorbid apathy have poorer outcomes, including lower remission rates and poorer response to first-line antidepressants, compared with those with LLD but who do not have apathy.
Despite the high prevalence of apathy in people with depression, “little is known about its optimal treatment and, more broadly, about the brain-based mechanisms of apathy,” the authors note.
An “emerging hypothesis” points to the role of a compromised SN and its large-scale connections between apathy and poor treatment response in LLD.
The SN (which includes the insula and the dorsal anterior cingulate cortex) “attributes motivational value to a stimulus” and “dynamically coordinates the activity of other large-scale networks, including the executive control network and default mode network (DMN).”
Preliminary studies of apathy in patients with depression report reduced volume in structures of the SN and suggest disruption in functional connectivity among the SN, DMN, and the executive control network; but the mechanisms linking apathy to poor antidepressant response in LLD “are not well understood.”
“Connectometry” is a “novel approach to diffusion MRI analysis that quantifies the local connectome of white matter pathways.” It has been used along with resting-state imagery, but it had not been used in studying apathy.
The researchers investigated the functional connectivity of the SN, hypothesizing that alterations in connectivity among key nodes of the SN and other core circuits that modulate goal-directed behavior (DMN and the executive control network) were implicated in individuals with depression and apathy.
They applied connectometry to “identify pathway-level disruptions in structural connectivity,” hypothesizing that compromise of frontoparietal and frontolimbic pathways would be associated with apathy in patients with LLD.
They also wanted to know whether apathy-related network abnormalities were associated with antidepressant response after 12 weeks of pharmacotherapy with the selective serotonin reuptake inhibitor escitalopram.
Emerging model
The study included 40 older adults (65% women; mean [SD] age, 70.0 [6.6] years) with DSM-IV–diagnosis major depressive disorder (without psychotic features) who were from a single-group, open-label escitalopram treatment trial.
The Hamilton-Depression (HAM-D) scale was used to assess depression, while the Apathy Evaluation Scale was used to assess apathy. On the Apathy Evaluation Scale, a score of greater than 40.5 represents “clinically significant apathy.” Participants completed these tests at baseline and after 12 weeks of escitalopram treatment.
They also completed a battery of neuropsychological tests to assess cognition and underwent MRI imaging. fMRI was used to map group differences in rsFC of the SN, and diffusion connectometry was used to “evaluate pathway-level disruptions in structural connectivity.”
Of the participants, 20 had clinically significant apathy. There were no differences in age, sex, educational level, or the severity of depression at baseline between those who did and those who did not have apathy.
Compared with participants with depression but not apathy, those with depression and comorbid apathy had lower rsFC of salience network seeds (specifically, the dorsolateral prefrontal cortex [DLPFC], premotor cortex, midcingulate cortex, and paracentral lobule).
They also had greater rsFC in the lateral temporal cortex and temporal pole (z > 2.7; Bonferroni-corrected threshold of P < .0125).
Additionally, participants with apathy had lower structural connectivity in the splenium, cingulum, and fronto-occipital fasciculus, compared with those without apathy (t > 2.5; false discovery rate–corrected P = .02).
Of the 27 participants who completed escitalopram treatment; 16 (59%) achieved remission (defined as an HAM-D score <10). Participants with apathy had poorer response to escitalopram treatment.
Lower insula-DLPFC/midcingulate cortex rsFC was associated with less improvement in depressive symptoms (HAM-D percentage change, beta [df] = .588 [26]; P = .001) as well as a greater likelihood that the participant would not achieve remission after treatment (odds ratio, 1.041; 95% confidence interval, 1.003-1.081; P = .04).
In regression models, lower insula-DLPFC/midcingulate cortex rsFC was found to be a mediator of the association between baseline apathy and persistence of depression.
The SN findings were also relevant to cognition. Lower dorsal anterior cingulate-DLPFC/paracentral rsFC was found to be associated with residual cognitive difficulties on measures of attention and executive function (beta [df] = .445 [26] and beta [df] = .384 [26], respectively; for each, P = .04).
“These findings support an emerging model of apathy, which proposes that apathy may arise from dysfunctional interactions among core networks (that is, SN, DMN, and executive control) that support motivated behavior,” the investigators write.
“This may cause a failure of network integration, leading to difficulties with salience processing, action planning, and behavioral initiation that manifests clinically as apathy,” they conclude.
One limitation they note was the lack of longitudinal follow-up after acute treatment and a “relatively limited neuropsychological battery.” Therefore, they could not “establish the persistence of treatment differences nor the specificity of cognitive associations.”
The investigators add that “novel interventions that modulate interactions among affected circuits may help to improve clinical outcomes in this distinct subgroup of older adults with depression, for whom few effective treatments exist.”
Commenting on the study, Helen Lavretsy, MD, professor of psychiatry in residence and director of the Late-Life Mood, Stress, and Wellness Research Program and the Integrative Psychiatry Clinic, Jane and Terry Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, said, the findings “can be used in future studies targeting apathy and the underlying neural mechanisms of brain connectivity.” Dr. Lavretsy was not involved with the study.
The study was supported by grants from the National Institute of Mental Health. Dr. Gunning reported receiving grants from the National Institute of Mental Health during the conduct of the study and grants from Akili Interactive. The other authors’ disclosures are listed on the original article. Dr. Lavretsky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study sheds light on the neurologic underpinnings of late-life depression (LLD) with apathy and its frequently poor response to treatment.
Investigators headed by Faith Gunning, PhD, of the Institute of Geriatric Psychiatry, Weill Cornell Medicine, New York, analyzed baseline and posttreatment brain MRIs and functional MRIs (fMRIs) of older adults with depression who participated in a 12-week open-label nonrandomized clinical trial of escitalopram. Participants had undergone clinical and cognitive assessments.
Disturbances were found in resting state functional connectivity (rsFC) between the salience network (SN) and other large-scale networks that support goal-directed behavior, especially in patients with depression who also had features of apathy.
“This study suggests that, among older adults with depression, distinct network abnormalities may be associated with apathy and poor response to first-line pharmacotherapy and may serve as promising targets for novel interventions,” the investigators write.
The study was published online in JAMA Network Open.
A leading cause of disability
LLD is a “leading cause of disability and medical morbidity in older adulthood,” with one-third to one-half of patients with LLD also suffering from apathy, the authors write.
Older adults with depression and comorbid apathy have poorer outcomes, including lower remission rates and poorer response to first-line antidepressants, compared with those with LLD but who do not have apathy.
Despite the high prevalence of apathy in people with depression, “little is known about its optimal treatment and, more broadly, about the brain-based mechanisms of apathy,” the authors note.
An “emerging hypothesis” points to the role of a compromised SN and its large-scale connections between apathy and poor treatment response in LLD.
The SN (which includes the insula and the dorsal anterior cingulate cortex) “attributes motivational value to a stimulus” and “dynamically coordinates the activity of other large-scale networks, including the executive control network and default mode network (DMN).”
Preliminary studies of apathy in patients with depression report reduced volume in structures of the SN and suggest disruption in functional connectivity among the SN, DMN, and the executive control network; but the mechanisms linking apathy to poor antidepressant response in LLD “are not well understood.”
“Connectometry” is a “novel approach to diffusion MRI analysis that quantifies the local connectome of white matter pathways.” It has been used along with resting-state imagery, but it had not been used in studying apathy.
The researchers investigated the functional connectivity of the SN, hypothesizing that alterations in connectivity among key nodes of the SN and other core circuits that modulate goal-directed behavior (DMN and the executive control network) were implicated in individuals with depression and apathy.
They applied connectometry to “identify pathway-level disruptions in structural connectivity,” hypothesizing that compromise of frontoparietal and frontolimbic pathways would be associated with apathy in patients with LLD.
They also wanted to know whether apathy-related network abnormalities were associated with antidepressant response after 12 weeks of pharmacotherapy with the selective serotonin reuptake inhibitor escitalopram.
Emerging model
The study included 40 older adults (65% women; mean [SD] age, 70.0 [6.6] years) with DSM-IV–diagnosis major depressive disorder (without psychotic features) who were from a single-group, open-label escitalopram treatment trial.
The Hamilton-Depression (HAM-D) scale was used to assess depression, while the Apathy Evaluation Scale was used to assess apathy. On the Apathy Evaluation Scale, a score of greater than 40.5 represents “clinically significant apathy.” Participants completed these tests at baseline and after 12 weeks of escitalopram treatment.
They also completed a battery of neuropsychological tests to assess cognition and underwent MRI imaging. fMRI was used to map group differences in rsFC of the SN, and diffusion connectometry was used to “evaluate pathway-level disruptions in structural connectivity.”
Of the participants, 20 had clinically significant apathy. There were no differences in age, sex, educational level, or the severity of depression at baseline between those who did and those who did not have apathy.
Compared with participants with depression but not apathy, those with depression and comorbid apathy had lower rsFC of salience network seeds (specifically, the dorsolateral prefrontal cortex [DLPFC], premotor cortex, midcingulate cortex, and paracentral lobule).
They also had greater rsFC in the lateral temporal cortex and temporal pole (z > 2.7; Bonferroni-corrected threshold of P < .0125).
Additionally, participants with apathy had lower structural connectivity in the splenium, cingulum, and fronto-occipital fasciculus, compared with those without apathy (t > 2.5; false discovery rate–corrected P = .02).
Of the 27 participants who completed escitalopram treatment; 16 (59%) achieved remission (defined as an HAM-D score <10). Participants with apathy had poorer response to escitalopram treatment.
Lower insula-DLPFC/midcingulate cortex rsFC was associated with less improvement in depressive symptoms (HAM-D percentage change, beta [df] = .588 [26]; P = .001) as well as a greater likelihood that the participant would not achieve remission after treatment (odds ratio, 1.041; 95% confidence interval, 1.003-1.081; P = .04).
In regression models, lower insula-DLPFC/midcingulate cortex rsFC was found to be a mediator of the association between baseline apathy and persistence of depression.
The SN findings were also relevant to cognition. Lower dorsal anterior cingulate-DLPFC/paracentral rsFC was found to be associated with residual cognitive difficulties on measures of attention and executive function (beta [df] = .445 [26] and beta [df] = .384 [26], respectively; for each, P = .04).
“These findings support an emerging model of apathy, which proposes that apathy may arise from dysfunctional interactions among core networks (that is, SN, DMN, and executive control) that support motivated behavior,” the investigators write.
“This may cause a failure of network integration, leading to difficulties with salience processing, action planning, and behavioral initiation that manifests clinically as apathy,” they conclude.
One limitation they note was the lack of longitudinal follow-up after acute treatment and a “relatively limited neuropsychological battery.” Therefore, they could not “establish the persistence of treatment differences nor the specificity of cognitive associations.”
The investigators add that “novel interventions that modulate interactions among affected circuits may help to improve clinical outcomes in this distinct subgroup of older adults with depression, for whom few effective treatments exist.”
Commenting on the study, Helen Lavretsy, MD, professor of psychiatry in residence and director of the Late-Life Mood, Stress, and Wellness Research Program and the Integrative Psychiatry Clinic, Jane and Terry Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, said, the findings “can be used in future studies targeting apathy and the underlying neural mechanisms of brain connectivity.” Dr. Lavretsy was not involved with the study.
The study was supported by grants from the National Institute of Mental Health. Dr. Gunning reported receiving grants from the National Institute of Mental Health during the conduct of the study and grants from Akili Interactive. The other authors’ disclosures are listed on the original article. Dr. Lavretsky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Sexual assault flagged as a possible psychosis trigger
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
A new study sheds light on some of the risk factors for the development of psychosis, including the potentially causative role of sexual assault.
Investigators conducted an exposome-wide association analysis on more than 155,000 individuals. Of more than 140 correlates of psychotic experiences that they identified, they narrowed it down to 36 variables, which they further explored using Mendelian randomization analysis.
On the other hand, having experienced a physical violent crime, cannabis use, and prolonged worry after embarrassment showed a pleiotropic association and appeared to be an aftereffect of psychotic experience.
“From a public health perspective, we need more investment in comprehensive strategies to prevent traumatic experiences at the population level to decrease the burden of psychosis,” senior author Sinan Gülöksüz, MD, PhD, associate professor in the department of psychiatry and neuropsychiatry, Maastricht University Medical Center, the Netherlands, said in an interview.
“From a clinical perspective, clinicians should be aware of the harmful influence of traumatic experiences on mental health and address this through interventions such as trauma-informed care,” he said.
The study was published online in JAMA Psychiatry.
‘Disentangling’ cause and effect
“Previous research has shown associations between psychosis and a few environmental factors, such as substance use, urbanicity, pregnancy complications, and traumatic experiences, but research has so far investigated only a few specific environmental factors by singling them out in individual studies,” Dr. Gülöksüz said.
“Yet, environment is a much more complex and interactive network that includes many factors shaping our health – where we live, what we eat, our lifestyle preferences and habits such as exercise and smoking, and our social surrounding,” he continued. “Rarely has it been possible to understand whether these environmental factors have causal roles in developing psychosis.”
To investigate the question, the researchers turned to the UK Biobank, one of the largest population-based datasets in the world. The current study focused on individuals with completed data on mental questionnaires that assessed psychotic experiences (n = 155,247; mean [SD] age, 55.94 [7.74] years; 57% female).
They began by conducting an exposome-wide association study, using logistic regression analyses with psychotic experiences as the outcome and adjusting all analyses for age and sex.
“Initially, we identified many associations between environmental factors and psychotic experiences in this large cohort,” Dr. Gülöksüz reported.
In the final multivariable model, variables associated with psychotic experiences were further analyzed using “genetically informed approaches to probe potential associations.”
The researchers utilized Mendelian randomization (MR) methodology “to disentangle cause and effect in this observational study,” Dr. Gülöksüz said. “This method reduces confounding and reverse causation in observational studies by using genetic variants that have been passed on from generation to generation randomly as instruments.”
MR analysis “has allowed us to assess whether these associations reflect potentially causal influences of environmental factors on psychotic experiences,” he added.
Well-studied and unexplored risk factors
The researchers identified 162 variables associated with psychotic experiences in the discovery dataset and were able to replicate 148. When these 148 variables were subjected to multivariable analyses, 36 were found to be statistically significantly associated with psychotic experiences. Of these variables, 28 had “significant genetic overlap” with psychotic experiences.
When the researchers conducted one-sample MR analyses, they found forward associations with three variables and reverse associations with three variables.
Forward associations were found with ever having experienced sexual assault (odds ratio [OR], 1.32; 95% confidence interval [CI], 1.14-1.52; P = 2.67), and forward associations (with pleiotropy) were found with ever having experienced a physically violent crime and risk-taking behavior (OR, 1.25, 95% CI, 1.11-1.41; P = 3.28 and OR, 1.21, 95% CI, 1.08-1.35; P = 1.34, respectively).
“The allele scores for these 3 variables explained 0.03% to 0.23% variance of the corresponding variable” and the F statistics “ranged from 21.53 to 181.84, indicating that the results did not suffer from a weak-instrument bias,” the authors reported.
The researchers calculated an instrument based on increasing psychotic experiences risk allele scores and found that these scores explained 0.14% variance of psychotic experiences (F statistic, 19.26).
Using that calculation, they found a reverse association with having experienced a physically violent crime (OR, 1.08; 95% CI, 1.04-1.13; P = 3.92 × 10-4), cannabis use (OR, 1.11; 95% CI, 1.06-1.15; P = 2.64 × 10-6), and worrying too long after embarrassment (OR, 1.06; 95% CI, 1.03-1.10; P = 3.96 × 10-4). They then validated these associations.
The presence of all five correlates was associated with tenfold increased odds of psychotic experiences (OR, 10.63; 95% CI, 8.27-13.65, P = 1.2 × 10-114).
“Associations with psychotic experiences were found with both well-studied and unexplored multiple correlated variables,” the authors stated.
Era of ‘big data’
In a comment, Chirag Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, who was not involved with the study, said he thought the study was “a nice example of a data-driven and comprehensive study of the environment coupled with attempts to triangulate evidence from genetics, made possible by biobank data.
“To guide public health policies and implementation of prevention strategies for psychosis, we need more systematic analyses and triangulate evidence with genetically informed methods to identify potentially modifiable risk factors in the era of ‘big data,’ ” he said.
“For instance, traumatic experiences contribute to poor mental and physical health, including psychosis,” Dr. Gülöksüz added.
The Kootstra Talent Fellowship, the Ophelia Research Project, and the Vidi Award from the Netherlands Scientific Organization provided funding to individual investigators. Dr. Gülöksüz and coauthors declared no relevant financial conflicts. Dr. Patel served as a reviewer on the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY