User login
Painful Indurated Plaque on the Groin
The Diagnosis: Cutaneous Metastasis
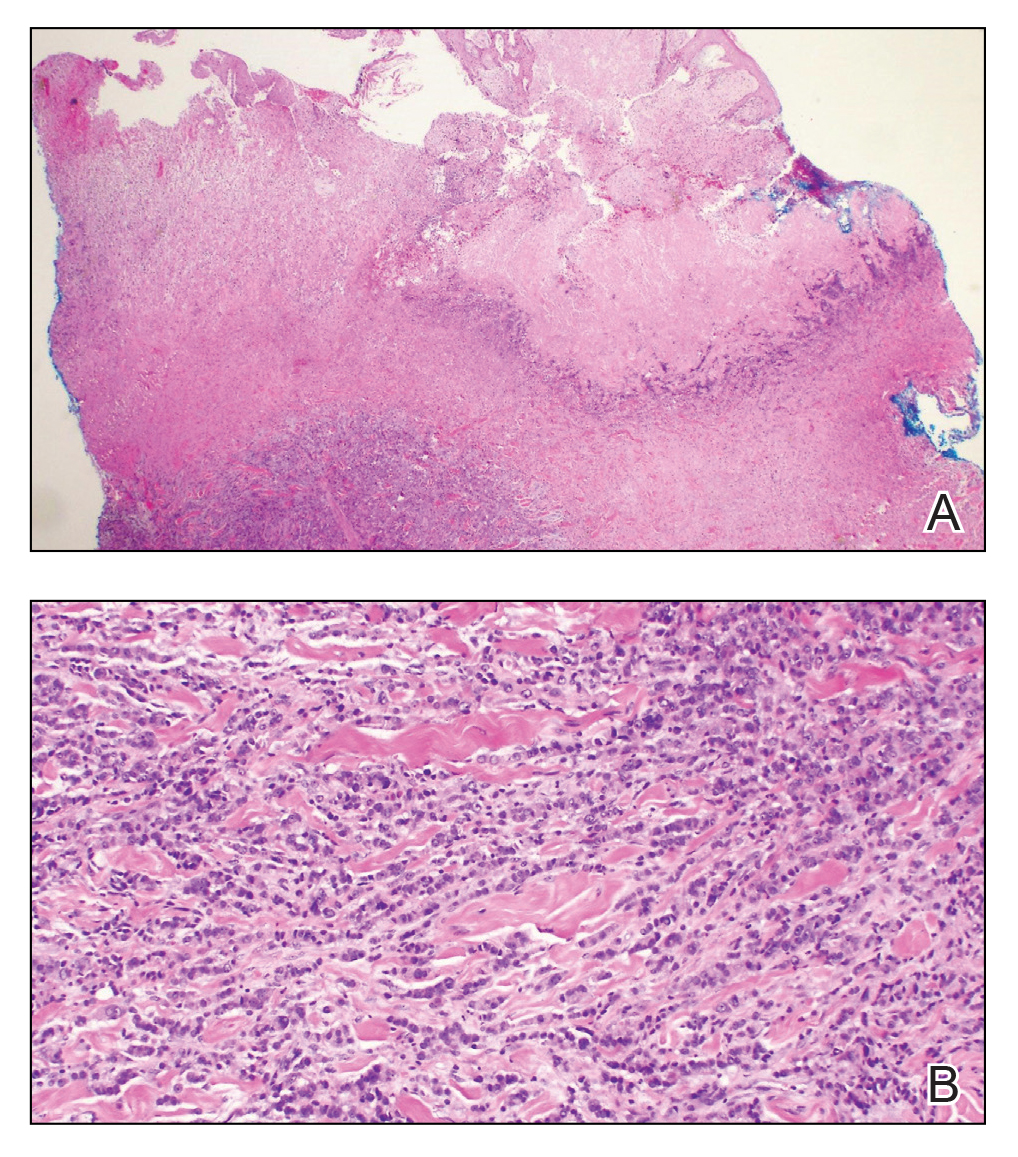
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.
Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
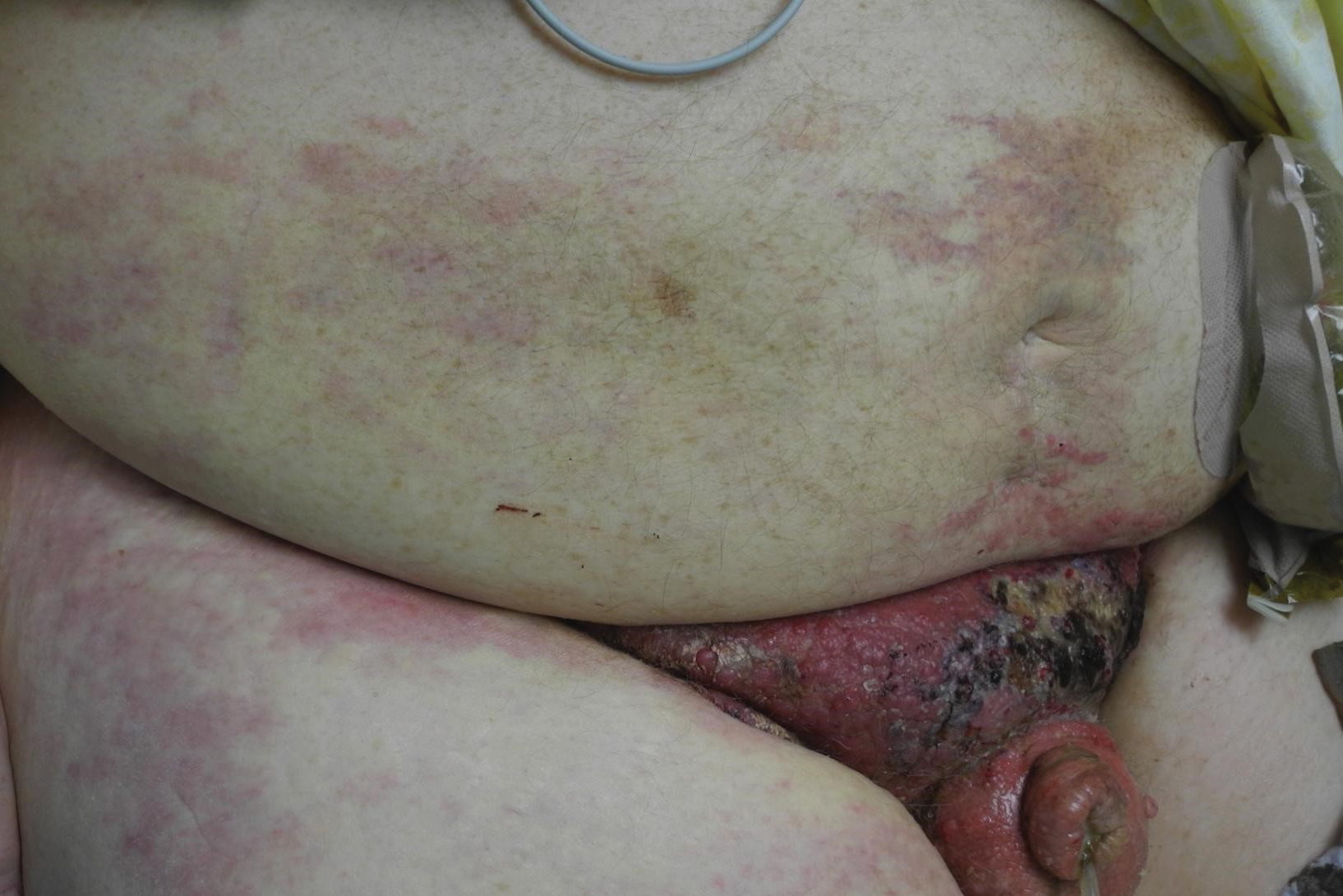
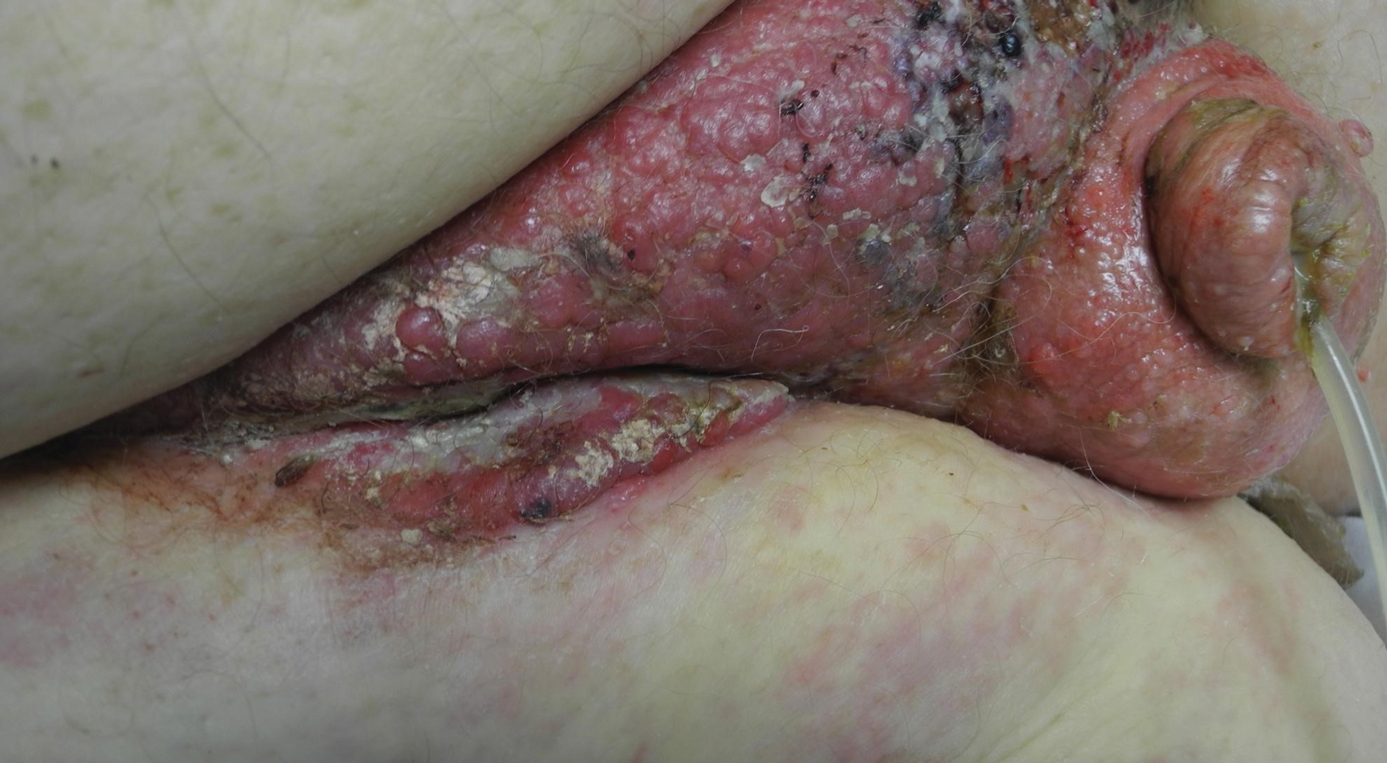
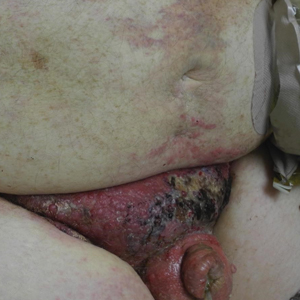
A 67-year-old man presented with a chronic lesion on the groin of 6 weeks' duration. The patient had a history of type 2 diabetes mellitus and colonic adenocarcinoma diagnosed 4 years prior that was treated with a colectomy, radiation therapy, and chemotherapy. Six weeks prior to the current presentation, the patient first sought treatment of swelling, redness, pain, and a bumpy texture on the groin. He was unsuccessfully managed by several physicians including at a long-term care facility where he was admitted and treated for presumed cellulitis. Attempted treatments included a topical antifungal, fluconazole, ciprofloxacin, metronidazole, cefepime, clindamycin, daptomycin, and vancomycin. The affected area continued to worsen along with the patient's overall health. He was transferred to the hospital for more advanced care and was evaluated by inpatient dermatology. Physical examination revealed firm, pink to red-brown, ulcerating papulonodules that coalesced into a large indurated plaque over the pubis, scrotum, penis, and inguinal folds (top). There also were red-violet, indurated plaques on the lower abdomen and bilateral proximal thighs (bottom). Punch biopsies were taken from the indurated area on the left side of the pubis--one for histopathologic evaluation and the other for bacterial, fungal, atypical mycobacterial, and Nocardia tissue cultures.
Update on Calciphylaxis Etiopathogenesis, Diagnosis, and Management
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
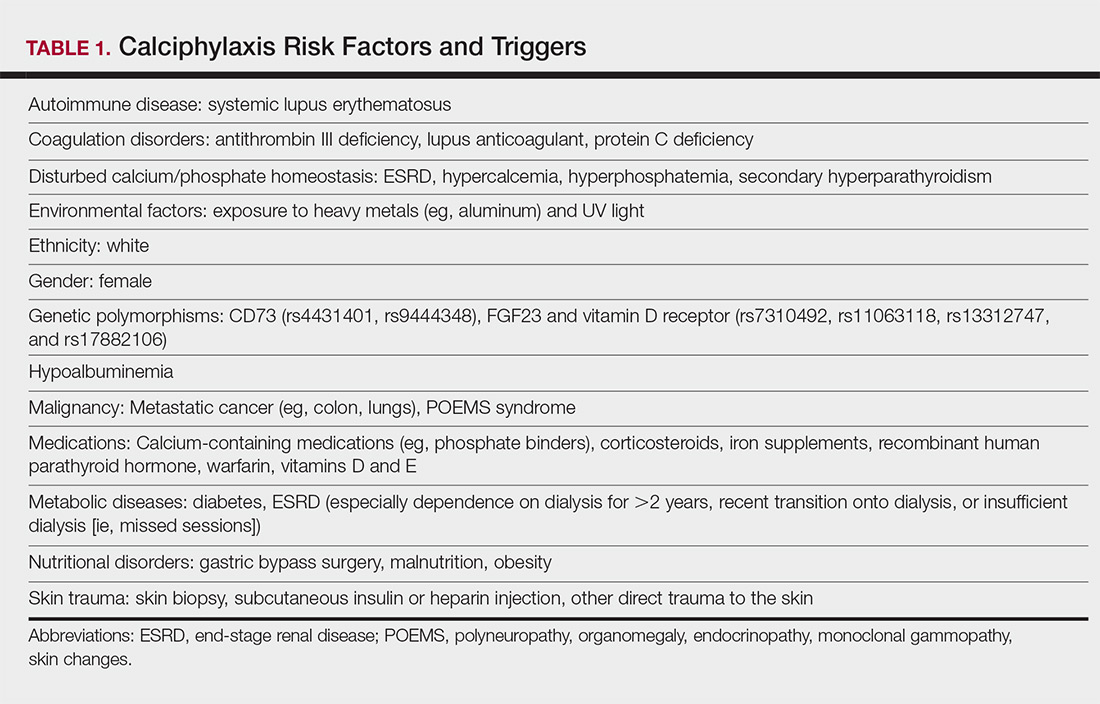
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16
Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
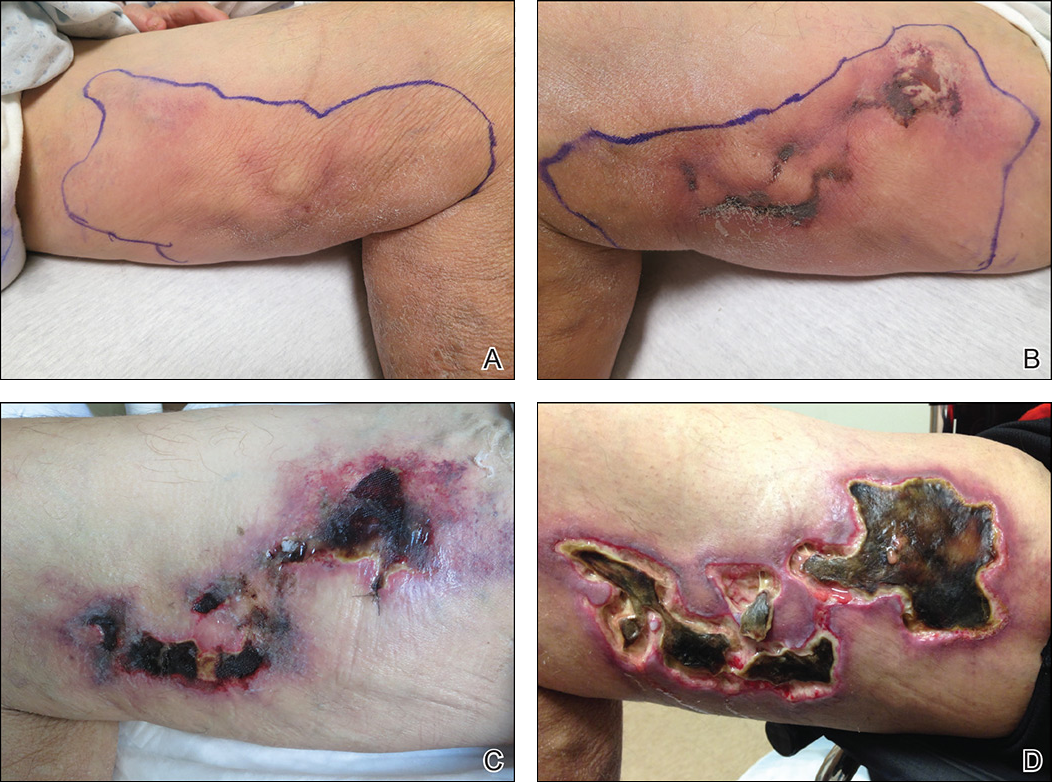
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5
Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
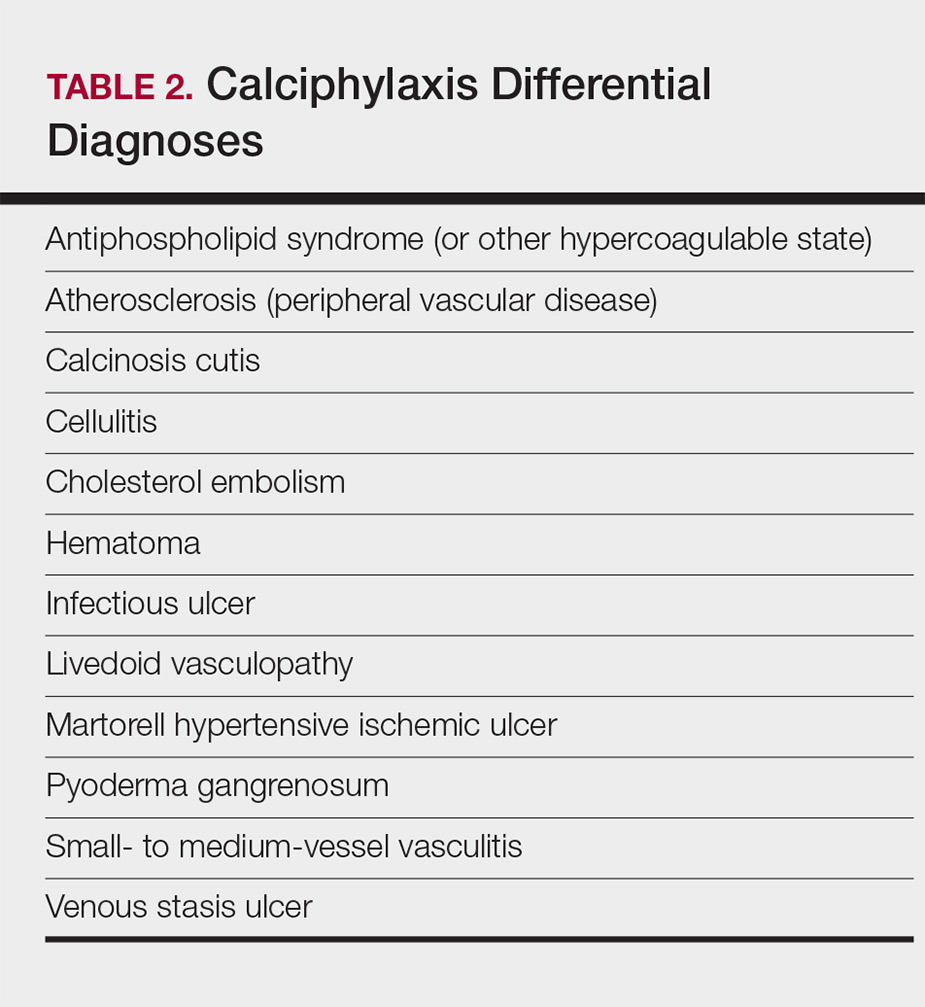
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9
Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
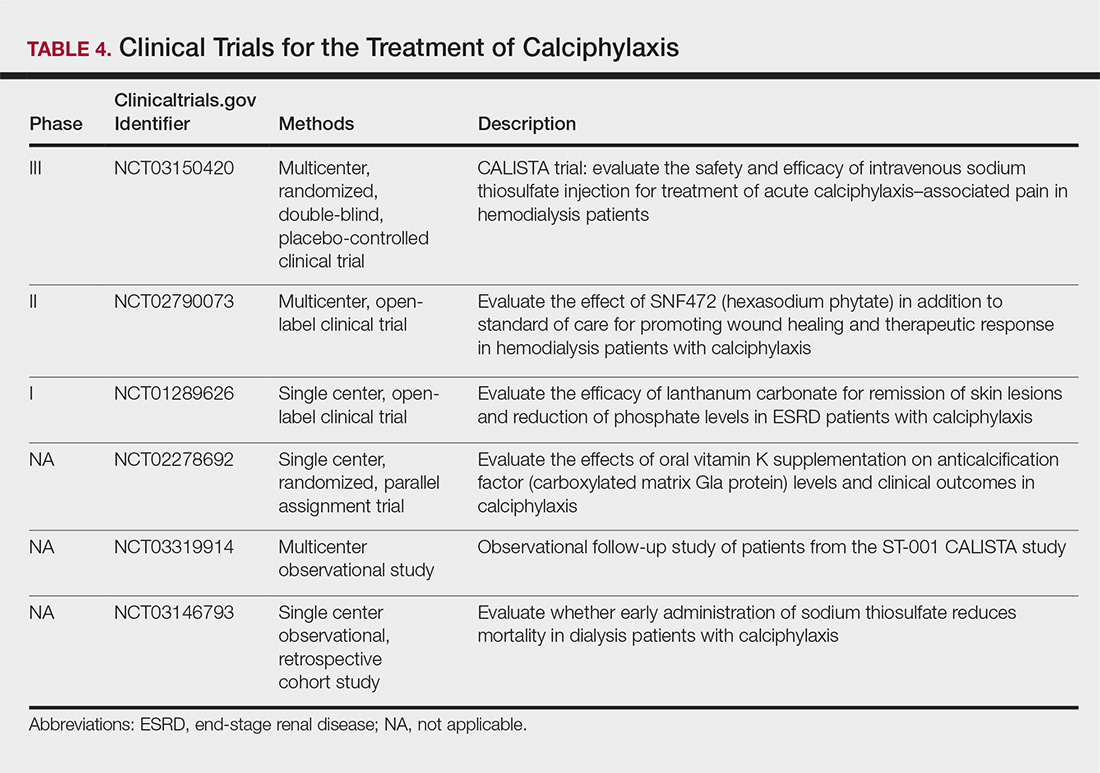
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36

Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a painful skin condition classically seen in patients with end-stage renal disease (ESRD), particularly those on chronic dialysis.1,2 It also has increasingly been reported in patients with normal renal function and calcium and phosphate homeostasis.3,4 Effective diagnosis and management of calciphylaxis remains challenging for physicians.2,5 The condition is characterized by tissue ischemia caused by calcification of cutaneous arteriolar vessels. As a result, calciphylaxis is associated with high mortality rates, ranging from 60% to 80%.5,6 Excruciating pain and nonhealing ulcers often lead to recurrent hospitalizations and infectious complications,7 and poor nutritional status, chronic pain, depression, and insomnia can further complicate recovery and lead to poor quality of life.8
We provide an update on calciphylaxis etiopathogenesis, diagnosis, and management. We also highlight some challenges faced in managing this potentially fatal condition.
Epidemiology
Calciphylaxis is considered a rare dermatosis with an estimated annual incidence of 1% to 4% in ESRD patients on dialysis. Recent data suggest that incidence of calciphylaxis is rising,5,7,9 which may stem from an increased use of calcium-based phosphate binders, an actual rise in disease incidence, and/or increased recognition of the disease.5 It is difficult to estimate the exact disease burden of calciphylaxis because the diagnostic criteria are not well defined, often leading to missed or delayed diagnosis.3,10 Furthermore, there is no centralized registry for calciphylaxis cases.3
Etiology and Pathogenesis
Calciphylaxis is thought to have a multifactorial etiology with the exact cause or trigger unknown.7 A long list of risk factors and triggers is associated with the condition (Table 1). Calciphylaxis primarily affects small arteries (40–600 μm in diameter) that become calcified due to an imbalance between inhibitors and promoters of calcification.2,11 Fetuin-A and matrix Gla protein inhibit vascular calcification and are downregulated in calciphylaxis.12,13 Dysfunctional calcium, phosphate, and parathyroid hormone regulatory pathways provide an increased substrate for the process of calcification, which causes endothelial damage and microthrombosis, resulting in tissue ischemia and infarction.14,15 Notably, there is growing interest in the role of vitamin K in the pathogenesis of calciphylaxis. Vitamin K inhibits vascular calcification, possibly by increasing the circulating levels of carboxylated matrix Gla protein.16

Clinical Features
Calciphylaxis is most commonly seen on the legs, abdomen, and buttocks.2 Patients with ESRD commonly develop proximal lesions affecting adipose-rich sites and have a poor prognosis. Distal lesions are more common in patients with nonuremic calciphylaxis, and mortality rates are lower in this population.2
Early lesions present as painful skin nodules or indurated plaques that often are rock-hard or firm to palpation with overlying mottling or a livedoid pattern (Figure, A). Early lesions progress from livedo reticularis to livedo racemosa and then to retiform purpura (Figure, B). Purpuric lesions later evolve into black eschars (Figure, C), then to necrotic, ulcerated, malodorous plaques or nodules in later stages of the disease (Figure, D). Lesions also may develop a gangrenous sclerotic appearance.2,5

Although most patients with calciphylaxis have ESRD, nonuremic patients also can develop the disease. Those with calciphylaxis who do not have renal dysfunction frequently have other risk factors for the disease and often report another notable health problem in the weeks or months prior to presentation.4 More than half of patients with calciphylaxis become bedridden or require use of a wheelchair.17 Pain is characteristically severe throughout the course of the disease; it may even precede the appearance of the skin lesions.18 Because the pain is associated with ischemia, it tends to be relatively refractory to treatment with opioids. Rare extracutaneous vascular calcifications may lead to visual impairment, gastrointestinal tract bleeding, and myopathy.5,9,19,20
Diagnosis
Considering the high morbidity and mortality associated with calciphylaxis, it is important to provide accurate and timely diagnosis; however, there currently are no validated diagnostic criteria for calciphylaxis. Careful correlation of clinical and histologic findings is required. Calciphylaxis biopsies have demonstrated medial calcification and proliferation of the intima of small- to medium-sized arteries.21 Lobular and septal panniculitis and extravascular soft-tissue calcification, particularly stippled calcification of the eccrine sweat glands, also has been seen.2,22 Special calcium stains (eg, von Kossa, Alizarin red) increase the sensitivity of biopsy by highlighting subtle areas of intravascular and extravascular calcification.5,23 Sufficient sampling of subcutaneous tissue and specimen evaluation by an experienced dermatopathologist are necessary to ensure proper interpretation of the histologic findings.
Despite these measures, skin biopsies may be nondiagnostic or falsely negative; therefore, when there is high clinical suspicion, it may be appropriate to move forward with a presumptive diagnosis of calciphylaxis even if the histologic findings are nondiagnostic.1,9,24 It also is worth noting that localized progression and ulceration may occur following skin biopsy, such that biopsy may even be contraindicated in certain cases (eg, penile calciphylaxis).
Standard laboratory workup for calciphylaxis includes evaluation for associated risk factors as well as exclusion of other conditions in the differential diagnosis (Table 2). Blood tests to evaluate for risk factors include liver and renal function tests, a complete metabolic panel, parathyroid hormone level, and serum albumin level.5 Elevated calcium and phosphate levels may signal disturbed calcium and phosphate homeostasis but are neither sensitive nor specific for the diagnosis.25 Complete blood cell count, blood cultures, thorough hypercoagulability workup (including but not limited to antiphospholipid antibodies, proteins C and S, factor V Leiden, antithrombin III, homocysteine, methylenetetrahydrofolate reductase mutation, and cryoglobulins), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibody testing may be relevant to help identify contributing factors or mimickers of calciphylaxis.5 Various imaging modalities also have been used to evaluate for the presence of soft-tissue calcification in areas of suspected calciphylaxis, including radiography, mammography, computed tomography, ultrasonography, nuclear bone scintigraphy, and spectroscopy.2,26,27 Unfortunately, there currently is no standardized reproducible imaging modality for reliable diagnosis of calciphylaxis. Ultimately, histologic and radiographic findings should always be interpreted in the context of relevant clinical findings.2,9

Prevention
Reduction of the net calcium phosphorus product may help reduce the risk of calciphylaxis in ESRD patients, which can be accomplished by using non–calcium-phosphate binders, adequate dialysis, and restricting use of vitamin D and vitamin K antagonists.2,5 There are limited data regarding the benefits of using bisphosphonates and cinacalcet in ESRD patients on dialysis to prevent calciphylaxis.28,29
Management
Management of calciphylaxis is multifactorial. Besides dermatology and nephrology, specialists in pain management, wound care, plastic surgery, and nutrition are critical partners in management.1,5,9,30 Nephrologists can help optimize calcium and phosphate balance and ensure adequate dialysis. Pain specialists can aid in creating aggressive multiagent pain regimens that target the neuropathic/ischemic and physical aspects of calciphylaxis pain. When appropriate, nutrition specialists can help establish high-protein, low-phosphorus diets, and wound specialists can provide access to advanced wound dressings and adjunctive hyperbaric oxygen therapy. Plastic surgeons can provide conservative debridement procedures in a subset of patients, usually those with distal stable disease.
The limited understanding of the etiopathogenesis of calciphylaxis and the lack of data on its management are reflected in the limited treatment options for the disease (Table 3).2,5,9 There are no formal algorithms for the treatment of calciphylaxis. Therapeutic trials are scarce, and most of the current treatment recommendations are based on small retrospective reports or case series. Sodium thiosulfate has been the most widely used treatment option since 2004, when its use in calciphylaxis was first reported.31 Sodium thiosulfate chelates calcium and is thought to have antioxidant and vasodilatory properties.32 There are a few promising clinical trials and large-scale studies (Table 4) that aim to evaluate the efficacy of existing treatments (eg, sodium thiosulfate) as well as novel treatment options such as lanthanum carbonate, SNF472 (hexasodium phytate), and vitamin K.33-36


Prognosis
Calciphylaxis is a potentially fatal condition with a poor prognosis and a median survival rate of approximately 1 year following the appearance of skin lesions.37-39 Patients with proximal lesions and those on peritoneal dialysis (as opposed to hemodialysis) have a worse prognosis.40 Mortality rates are estimated to be 30% at 6 months, 50% at 12 months, and 80% at 2 years, with sepsis secondary to infection of cutaneous ulcers being the leading cause of death.37-39 The impact of calciphylaxis on patient quality of life and activities of daily living is severe.8,17
Future Directions
Multi-institution cohort studies and collaborative registries are needed to provide updated information related to the epidemiology, diagnosis, treatment, morbidity, and mortality associated with calciphylaxis and to help formulate evidence-based diagnostic criteria. Radiographic and histologic studies, as well as other tools for early and accurate diagnosis of calciphylaxis, should be studied for feasibility, accuracy, and reproducibility. The incidence of nonuremic calciphylaxis points toward pathogenic pathways besides those based on the bone-mineral axis. Basic science research directed at improving understanding of the pathophysiology of calciphylaxis would be helpful in devising new treatment strategies targeting these pathways. Establishment of a collaborative, multi-institutional calciphylaxis working group would enable experts to formulate therapeutic guidelines based on current evidence. Such a group could facilitate initiation of large prospective studies to establish the efficacy of existing and new treatment modalities for calciphylaxis. A working group within the Society for Dermatology Hospitalists has been tasked with addressing these issues and is currently establishing a multicenter calciphylaxis database.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146.
- Nigwekar SU, Thadhani RI, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Davis JM. The relationship between obesity and calciphylaxis: a review of the literature. Ostomy Wound Manage. 2016;62:12-18.
- Bajaj R, Courbebaisse M, Kroshinsky D, et al. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93:1202-1212.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. 2016;351:217-227.
- Westphal SG, Plumb T. Calciphylaxis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018. https://www.ncbi.nlm.nih.gov/books/NBK519020. Accessed November 12, 2018.
- Riemer CA, El-Azhary RA, Wu KL, et al. Underreported use of palliative care and patient-reported outcome measures to address reduced quality of life in patients with calciphylaxis: a systematic review. Br J Dermatol. 2017;177:1510-1518.
- Nigwekar SU. Calciphylaxis. Curr Opin Nephrol Hypertens. 2017;26:276-281.
- Fine A, Fontaine B. Calciphylaxis: the beginning of the end? Perit Dial Int. 2008;28:268-270.
- Lin WT, Chao CM. Tumoral calcinosis in renal failure. QJM. 2014;107:387.
- Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357-366.
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78-81.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:267-276.
- Nigwekar SU, Bloch DB, Nazarian RM, et al. Vitamin K-dependent carboxylation of matrix gla protein influences the risk of calciphylaxis. J Am Soc Nephrol. 2017;28:1717-1722.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579.
- Polizzotto MN, Bryan T, Ashby MA, et al. Symptomatic management of calciphylaxis: a case series and review of the literature. J Pain Symptom Manage. 2006;32:186-190.
- Gupta N, Haq KF, Mahajan S, et al. Gastrointestinal bleeding secondary to calciphylaxis. Am J Case Rep. 2015;16:818-822.
- Edelstein CL, Wickham MK, Kirby PA. Systemic calciphylaxis presenting as a painful, proximal myopathy. Postgrad Med J. 1992;68:209-211.
- Mochel MC, Arakari RY, Wang G, et al. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586.
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802.
- Cassius C, Moguelet P, Monfort JB, et al. Calciphylaxis in haemodialysed patients: diagnostic value of calcifications in cutaneous biopsy. Br J Dermatol. 2018;178:292-293.
- Sreedhar A, Sheikh HA, Scagliotti CJ, et al. Advanced-stage calciphylaxis: think before you punch. Cleve Clin J Med. 2016;83:562-564.
- Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nation-wide registry. Nephrol Dial Transplant. 2017;32:126-132.
- Paul S, Rabito CA, Vedak P, et al. The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol. 2017;153:101-103.
- Shmidt E, Murthy NS, Knudsen JM, et al. Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol. 2012;67:1296-1301.
- EVOLVE Trial Investigators; Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482-2494.
- Rogers NM, Teubner DJO, Coates PT. Calcific uremic arteriolopathy: advances in pathogenesis and treatment. Semin Dial. 2007;20:150-157.
- Nigwekar SU. Multidisciplinary approach to calcific uremic arteriolopathy. Curr Opin Nephrol Hypertens. 2015;24:531-537.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Chen NX, O’Neill K, Akl NK, et al. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449:151-156.
- Chan MR, Ghandour F, Murali NS, et al. Pilot study of the effect of lanthanum carbonate in patients with calciphylaxis: a Wisconsin Network for Health Research (WiNHR) study. J Nephrol Ther. 2014;4:1000162.
- Perelló J, Gómez M, Ferrer MD, et al. SNF472, a novel inhibitor of vascular calcification, could be administered during hemodialysis to attain potentially therapeutic phytate levels. J Nephrol. 2018;31:287-296.
- Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J. 2018;11:528-529.
- Caluwe R, Vandecasteele S, Van Vlem B, et al. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29:1385-1390.
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429.
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241.
Practice Points
- Maintain a high index of suspicion for calciphylaxis in patients with end-stage renal disease on chronic dialysis presenting with severely painful livedoid plaques or retiform purpura, particularly in fat-rich body sites.
- Skin biopsies may be limited by biopsy site, inadequate biopsy depth, missed areas of microcalcification, and absence of definitive histologic criteria. Special calcium stains and review by an experienced dermatopathologist may lower the rate of false-negative biopsies.
- In cases where the most likely clinical diagnosis is calciphylaxis, treatment should be initiated even if definitive histopathology findings are lacking.
- Treatment should be multimodal, including elimination of risk factors, intravenous sodium thiosulfate, agents addressing calcium-phosphate metabolism, and surgical debridement, if indicated.
Purpura Fulminans in the Setting of Escherichia coli Septicemia
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
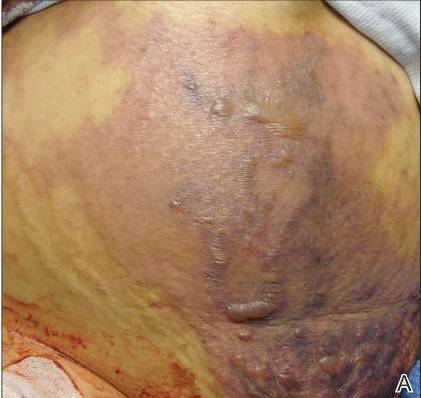
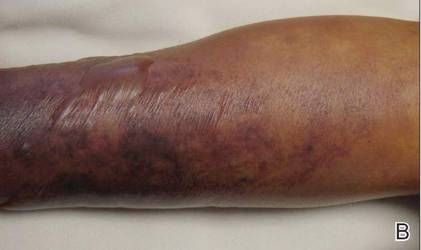
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.
Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.


Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
To the Editor:
Purpura fulminans is a severe and rapidly fatal thrombotic disorder that can occur in association with either hereditary or acquired deficiencies of the natural anticoagulants protein C and protein S.1 It most commonly results from the acute inflammatory response and subsequent disseminated intravascular coagulation (DIC) seen in severe bacterial septicemia. Excessive bleeding, retiform purpura, and skin necrosis may develop as a result of the coagulopathies of typical DIC.1Neisseria meningitidis, Streptococcus, and Staphylococcus frequently are implicated as pathogens, but Escherichia coli–associated purpura fulminans in adults is rare.2,3 We report a case of purpura fulminans in the setting of E coli septicemia.
A 62-year-old woman with a history of end-stage liver disease secondary to alcoholic liver cirrhosis diagnosed 13 years prior complicated by ascites and esophageal varices presented to a primary care clinic for evaluation of a recent-onset nontender lesion on the left buttock. She was hypotensive with a blood pressure of 62/48 mmHg. The patient was prescribed ciprofloxacin 250 mg twice daily and hydrocodone/acetominophen 5 mg/325 mg twice daily as needed for pain management and was discharged. Six hours later, the patient presented to the emergency department with new onset symptoms of confusion and dark-colored spots on the abdomen and lower legs, which her family members noted had developed shortly after the patient took ciprofloxacin. In the emergency department, the patient was noted to be hypotensive and febrile with a severe metabolic acidosis. She was intubated for respiratory failure and received intravenous fluid resuscitation, broad-spectrum antibiotics, and vasopressors. Blood cultures were obtained, and the dermatology department was consulted.
On physical examination, extensive purpuric, reticulated, and stellate plaques with central necrosis and hemorrhagic bullae were noted on the abdomen (Figure, A) and bilateral lower legs (Figure, B) extending onto the thighs. The patient was coagulopathic with persistent sanguineous oozing at intravenous sites and bilateral nares. A small erythematous ulcer with overlying black eschar was noted on the left medial buttock.


Laboratory test results showed new-onset thrombocytopenia, prolonged prothrombin time/international normalized ratio and partial thromboplastin time, and low fibrinogen levels, which confirmed a diagnosis of acute DIC. Blood cultures were positive for gram-negative rods in 4 out of 4 bottles within 12 hours of being drawn. Further testing identified the microorganism as E coli, and antibiotic susceptibility testing revealed it was sensitive to most antibiotics.
The patient was clinically diagnosed with purpura fulminans secondary to severe E coli septicemia and DIC. This life-threatening disorder is considered a medical emergency with a high mortality rate. Laboratory findings supporting DIC include the presence of schistocytes on a peripheral blood smear, thrombocytopenia, positive plasma protamine paracoagulation test, low fibrinogen levels, and positive fibrin degradation products. Reported cases of purpura fulminans in the setting of E coli septicemia are rare, and meningococcemia is the most common presentation.2,3 Bacterial components (eg, lipopolysaccharides found in the cell walls of gram-negative bacteria) may contribute to the progression of septicemia. Increased levels of endotoxin lipopolysaccharide can lead to septic shock and organ dysfunction.4 However, the release of lipooligosaccharides is associated with the development of meningococcal septicemia, and the lipopolysaccharide levels are directly correlated with prognosis in patients without meningitis.5-7
Human activated protein C concentrate (and its precursor, protein C concentrate) replacement therapy has been shown to improve outcomes in patients with meningococcemia-associated–purpura fulminans and severe sepsis, respectively.8 Heparin may be considered in the treatment of patients with purpura fulminans in addition to the replacement of any missing clotting factors or blood products.9 The international guidelines for the management of severe sepsis and septic shock include early quantitative resuscitation of the patient during the first 6 hours after recognition of sepsis, performing blood cultures before antibiotic therapy, and administering broad-spectrum antimicrobial therapy within 1 hour of recognition of septic shock.10 The elapsed time from triage to the actual administration of appropriate antimicrobials are primary determinants of patient mortality.11 Therefore, physicians must act quickly to stabilize the patient.
Gram-positive bacteria and gram-negative diplococci are common infectious agents implicated in purpura fulminans. Escherichia coli rarely has been identified as the inciting agent for purpura fulminans in adults. The increasing frequency of E coli strains that produce extended-spectrum β-lactamases—enzymes that mediate resistance to extended-spectrum (third generation) cephalosporins (eg, ceftazidime, cefotaxime, ceftriaxone) and monobactams (eg, aztreonam)—complicates matters further when deciding on appropriate antibiotics. Patients who have infections from extended-spectrum β-lactamase strains will require more potent carbapenems (eg, meropenem, imipenem) for treatment of infections. Despite undergoing treatment for septicemia, our patient went into cardiac arrest within 24 hours of presentation to the emergency department and died a few hours later. Physicians should consider E coli as an inciting agent of purpura fulminans and consider appropriate empiric antibiotics with gram-negative coverage to include E coli.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.
- Madden RM, Gill JC, Marlar RA. Protein C and protein S levels in two patients with acquired purpura fulminans. Br J Haematol. 1990;75:112-117.
- Nolan J, Sinclair R. Review of management of purpura fulminans and two case reports. Br J Anaesth. 2001;86:581-586.
- Huemer GM, Bonatti H, Dunst KM. Purpura fulminans due to E. coli septicemia. Wien Klin Wochenschr. 2004;116:82.
- Pugin J. Recognition of bacteria and bacterial products by host immune cells in sepsis. In: Vincent JL, ed. Yearbook of Intensive Care and Emergency Medicine. Berlin, Germany: Springer-Verlag; 1997:11-12.
- Brandtzaeg P, Oktedalen O, Kierulf P, et al. Elevated VIP and endotoxin plasma levels in human gram-negative septic shock. Regul Pept. 1989;24:37-44.
- Brandtzaeg P, Kierulf P, Gaustad P, et al. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195-204.
- Brandtzaeg P, Ovstebøo R, Kierulf P. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J Infect Dis. 1992;166:650-652.
- Hodgson A, Ryan T, Moriarty J, et al. Plasma exchange as a source of protein C for acute onset protein C pathway failure. Br J Haematol. 2002;116:905-908.
- Feinstein DI. Diagnosis and management of disseminated intravascular coagulation: the role of heparin therapy. Blood. 1982;60:284-287.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign guidelines committee including the pediatric subgroup. Crit Care Med. 2013;41:580-637.
- Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053.