User login
Medication-Nonadherent Hypothyroidism Requiring Frequent Primary Care Visits to Achieve Euthyroidism
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
Nonadherence to medications is an issue across health care. In endocrinology, hypothyroidism, a deficiency of thyroid hormones, is most often treated with levothyroxine and if left untreated can lead to myxedema coma, which can lead to death due to multiorgan dysfunction.1 Therefore, adherence to levothyroxine is very important in preventing fatal complications.
We present the case of a patient with persistent primary hypothyroidism who was suspected to be nonadherent to levothyroxine, although the patient consistently claimed adherence. The patient’s plasma thyrotropin (TSH) level improved to reference range after 6 weeks of weekly primary care clinic visits. After stopping the visits, his plasma TSH level increased again, so 9 more weeks of visits resumed, which again helped bring down his plasma TSH levels.
Case Presentation
A male patient aged 67 years presented to the Dayton Veterans Affairs Medical Center (VAMC) endocrinology clinic for evaluation of thyroid nodules. The patient reported no history of neck irradiation and a physical examination was unremarkable. At that time, laboratory results showed a slightly elevated plasma TSH level of 4.35 uIU/mL (reference range, 0.35-4.00 uIU/mL) and normal free thyroxine (T4) of 1.00 ng/dL (reference range, 0.74-1.46 ng/dL). Later that year, the patient underwent a total thyroidectomy at the Cincinnati VAMC for Hurthle cell variant papillary thyroid carcinoma that was noted on biopsy at the Dayton VAMC. After surgical pathology results were available, the patient started levothyroxine 200 mcg daily, although 224 mcg would have been more appropriate based on his 142 kg weight. Due to a history of arrhythmia, the goal plasma TSH level was 0.10 to 0.50 uIU/mL. The patient subsequently underwent radioactive iodine ablation. After levothyroxine dose adjustments, the patient’s plasma TSH level was noted to be within his target range at 0.28 uIU/mL 3 months postablation.
Over the next 5 years the patient had regular laboratory tests during which his plasma TSH level rose and were typically high despite adjusting levothyroxine doses between 200 mcg and 325 mcg. The patient received counseling on taking the medication in the morning on an empty stomach and waiting at least 1 hour before consuming anything, and he went to many follow-up visits at the Dayton VAMC endocrinology clinic. He reported no vomiting or diarrhea but endorsed weight gain once. The patient also had high free T4 at times and did not take extra levothyroxine before undergoing laboratory tests.
Nonadherence to levothyroxine was suspected, but the patient insisted he was adherent. He received the medication in the mail regularly, generally had 90-day refills unless a dose change was made, used a pill box, and had social support from his son, but he did not use a phone alarm to remind him to take it. A home care nurse made weekly visits to make sure the remaining levothyroxine pill counts were correct; however, the patient continued to have difficulty maintaining daily adherence at home as indicated by the nurse’s pill counts not aligning with the number of pills which should have been left if the patient was talking the pills daily.
The patient was asked to visit a local community-based outpatient clinic (CBOC) weekly (to avoid patient travel time to Dayton VAMC > 1 hour) to check pill counts and assess adherence. The patient went to the CBOC clinic for these visits, during which pill counts indicated much better but not 100% adherence. After 6 weeks of clinic visits, his plasma TSH decreased to 1.01 uIU/mL, which was within the reference range, and the patient stopped coming to the weekly clinic visits (Table). Four months later, the patient's plasma TSH levels increased to 80.72 uIU/mL. Nonadherence to levothyroxine was suspected again. He was asked to resume weekly clinic visits, and the life-threatening effects of hypothyroidism and not taking levothyroxine were discussed with the patient and his son. The patient made CBOC clinic visits for 9 weeks, after which his plasma TSH level was low at 0.23 uIU/mL.
Discussion
There are multiple important causes to consider in patients with persistent hypothyroidism. One is medication nonadherence, which was most likely seen in the patient in this case. Missing even 1 day of levothyroxine can affect TSH and thyroid hormone levels for several days due to the long half-life of the medication.2 Hepp and colleagues found that patients with hypothyroidism were significantly more likely to be nonadherent to levothyroxine if they had comorbid conditions such as type 2 diabetes or were obese.3 Another study of levothyroxine adherence found that the most common reason for missing doses was forgetfulness.4 However, memory and cognition impairments can also be symptoms of hypothyroidism itself; Haskard-Zolnierek and colleagues found a significant association between nonadherence to levothyroxine and self-reported brain fog in patients with hypothyroidism.5
Another cause of persistent hypothyroidism is malabsorption. Absorption of levothyroxine can be affected by intestinal malabsorption due to inflammatory bowel disease, lactose intolerance, or gastrointestinal infection, as well as several foods, drinks (eg, coffee), medications, vitamins, and supplements (eg, proton-pump inhibitors and calcium).2,6 Levothyroxine is absorbed mainly at the jejunum and upper ileum, so any pathologies or ingested items that would directly or indirectly affect absorption at those sites can affect levothyroxine absorption.2
A liquid levothyroxine formulation can help with malabsorption.2 Alternatively, weight gain may lead to a need for increasing the dosage of levothyroxine.2,6 Other factors that can affect TSH levels include Addison disease, dysregulation of the hypothalamic-pituitary-thyroid axis, and TSH heterophile antibodies.2
Research describes methods that have effectively treated hypothyroidism in patients struggling with levothyroxine adherence. Two case reports describe weekly visits for levothyroxine administration successfully treating uncontrolled hypothyroidism.7,8 A meta-analysis found that while weekly levothyroxine tablets led to a higher mean TSH level than daily use, weekly use still led to reference-range TSH levels, suggesting that weekly levothyroxine may be a helpful alternative for nonadherent patients.9 Alternatively, patients taking levothyroxine tablets have been shown to forget to take their medication more frequently compared to those taking the liquid formulation.10,11 Additionally, a study by El Helou and colleagues found that adherence to levothyroxine was significantly improved when patients had endocrinology visits once a month and when the endocrinologist provided information about hypothyroidism.12
Another method that may improve adherence to levothyroxine is telehealth visits. This would be especially helpful for patients who live far from the clinic or do not have the time, transportation, or financial means to visit the clinic for weekly visits to assess medication adherence. Additionally, patients may be afraid of admitting to a health care professional that they are nonadherent. Clinicians must be tactful when asking about adherence to make the patient feel comfortable with admitting to nonadherence if their cognition is not impaired. Then, a patient-led conversation can occur regarding realistic ways the patient feels they can work toward adherence.
To our knowledge, the patient in this case report had no symptoms of intestinal malabsorption, and weight gain was not thought to be the issue, as levothyroxine dosage was adjusted multiple times. His plasma TSH levels returned to reference range after weekly pill count visits for 6 weeks and after weekly pill count visits for 9 weeks. Therefore, nonadherence to levothyroxine was suspected to be the cause of frequently elevated plasma TSH levels despite the patient’s insistence on adherence. While the patient did not report memory issues, cognitive impairments due to hypothyroidism may have been contributing to his probable nonadherence. Additionally, he had comorbidities, such as type 2 diabetes mellitus and obesity, which may have made adherence more difficult.
Levothyroxine was also only prescribed in daily tablet form, so the frequency and formulation may have also contributed to nonadherence. While the home nurse was originally sent to assess the patient’s adherence, the care team could have had the nurse start giving the patient weekly levothyroxine once nonadherence was determined to be a likely issue. The patient’s adherence only improved when he went to the clinic for pill counts but not when the home nurse came to his house weekly; this could be because the patient knew he had to invest the time to physically go to clinic visits for pill checks, motivating him to increase adherence.
Conclusions
This case reports a patient with frequently high plasma TSH levels achieving normalization of plasma TSH levels after weekly medication adherence checks at a primary care clinic. Weekly visits to a clinic seem impractical compared to weekly dosing with a visiting nurse; however, after review of the literature, this may be an approach to consider in the future. This strategy may especially help in cases of persistent abnormal plasma TSH levels in which no etiology can be found other than suspected medication nonadherence. Knowing their medication use will be checked at weekly clinic visits may motivate patients to be adherent.
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
1. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550-1562. doi:10.1016/S0140-6736(17)30703-1
2. Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289-1301. doi:10.1007/s40618-017-0706-y
3. Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912-919. doi:10.1080/13696998.2018.1484749
4. Shakya Shrestha S, Risal K, Shrestha R, Bhatta RD. Medication Adherence to Levothyroxine Therapy among Hypothyroid Patients and their Clinical Outcomes with Special Reference to Thyroid Function Parameters. Kathmandu Univ Med J (KUMJ). 2018;16(62):129-137.
5. Haskard-Zolnierek K, Wilson C, Pruin J, Deason R, Howard K. The Relationship Between Brain Fog and Medication Adherence for Individuals With Hypothyroidism. Clin Nurs Res. 2022;31(3):445-452. doi:10.1177/10547738211038127
6. McNally LJ, Ofiaeli CI, Oyibo SO. Treatment-refractory hypothyroidism. BMJ. 2019;364:l579. Published 2019 Feb 25. doi:10.1136/bmj.l579
7. Nakano Y, Hashimoto K, Ohkiba N, et al. A Case of Refractory Hypothyroidism due to Poor Compliance Treated with the Weekly Intravenous and Oral Levothyroxine Administration. Case Rep Endocrinol. 2019;2019:5986014. Published 2019 Feb 5. doi:10.1155/2019/5986014
8. Kiran Z, Shaikh KS, Fatima N, Tariq N, Baloch AA. Levothyroxine absorption test followed by directly observed treatment on an outpatient basis to address long-term high TSH levels in a hypothyroid patient: a case report. J Med Case Rep. 2023;17(1):24. Published 2023 Jan 25. doi:10.1186/s13256-023-03760-0
9. Chiu HH, Larrazabal R Jr, Uy AB, Jimeno C. Weekly Versus Daily Levothyroxine Tablet Replacement in Adults with Hypothyroidism: A Meta-Analysis. J ASEAN Fed Endocr Soc. 2021;36(2):156-160. doi:10.15605/jafes.036.02.07
10. Cappelli C, Castello R, Marini F, et al. Adherence to Levothyroxine Treatment Among Patients With Hypothyroidism: A Northeastern Italian Survey. Front Endocrinol (Lausanne). 2018;9:699. Published 2018 Nov 23. doi:10.3389/fendo.2018.00699
11. Bocale R, Desideri G, Barini A, et al. Long-Term Adherence to Levothyroxine Replacement Therapy in Thyroidectomized Patients. J Clin Med. 2022;11(15):4296. Published 2022 Jul 24. doi:10.3390/jcm11154296
12. El Helou S, Hallit S, Awada S, et al. Adherence to levothyroxine among patients with hypothyroidism in Lebanon. East Mediterr Health J. 2019;25(3):149-159. Published 2019 Apr 25. doi:10.26719/emhj.18.022
Monitoring Thyrotropin in Veterans With Thyroid Nodules
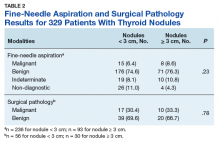
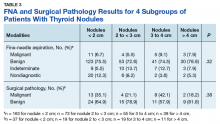
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
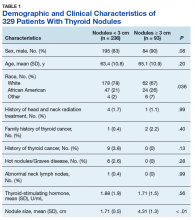
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
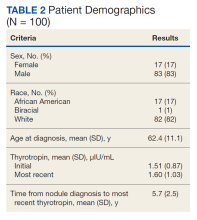
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
Prevalence of Cancer in Thyroid Nodules In the Veteran Population (FULL)
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.