User login
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
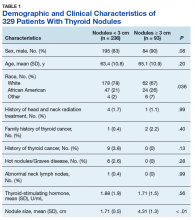
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
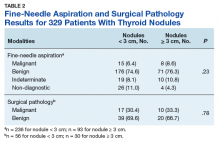
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
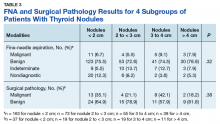
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
Thyroid nodules are identified incidentally in 4% to 10% of the general population in the US.1,2 Clinicians and patients often are concerned about potential malignancy when thyroid nodules are identified because 5% to 15% of nodules will be cancerous.1 The most common form of cancer is papillary carcinoma followed by follicular carcinoma.2 Initially, serum thyroid-stimulating hormone (TSH) levels and thyroid ultrasound are used to evaluate a thyroid nodule because both tests can reveal vital information about malignancy potential.3 Ultrasound characteristics, such as macrocalcifications, hypoechogenicity, absence of halo, increased vascularity, and irregular nodular margins, increase suspicion for malignancy and warrant further investigation.3
Ultrasound-guided fine-needle aspiration (FNA) is the modality of choice for evaluation of thyroid nodules with sensitivity and specificity > 90%.2,4 Most patients receive a definitive diagnosis with this test; however, about 25% of cases are indeterminate based on the Bethesda System and require surgical investigation.3
Currently, it is well accepted clinical practice to refer all nodules > 4 cm for surgical intervention regardless of malignancy risk factors or the mass effect of the nodule.3-6 The preference for surgery—rather than FNA—is because of the notable false negative rate with FNA in larger nodules; studies have described false negative rates for FNA close to 10%.7,8 In contrast, Megwalu recently reported a FNA false negative rate of 0%.9
The risk of malignancy associated with nodule size has been researched for many years, but studies have produced conflicting results. In this retrospective cohort study, the authors compared malignancy rates between patients with nodules ≥ 3 cm and those with nodules < 3 cm.
Methods
The authors performed a retrospective chart review of the medical records of 329 patients presenting for thyroid nodule evaluation found on physical exam or incidentally identified with imaging at the Dayton Veteran Affairs Medical Center from January 2000 to May 2016. Data collection included sex, age, race, personal history of neck radiation treatment, family history of thyroid cancer, personal history of thyroid cancer, hot nodules/Graves disease, abnormal neck lymph nodes, and serum TSH levels. The authors looked for an association between TSH level and cancer. Hot thyroid nodules are known to have low risk of malignancy.
All patients aged 18 to 99 years with a thyroid nodule evaluated with FNA were included in the study. Patients were divided into 2 groups, those with nodules ≥ 3 cm and those with nodules < 3 cm. For nodules requiring subsequent biopsies, only the initial nodule biopsy was included in our study. The 3-cm cutoff was selected based on previous studies.1,5,10 Patients who did not undergo a FNA study were excluded. Indications for surgery were positive FNA results, suspicious imaging, size of nodule, or patient preference.
Means and standard deviations are reported for continuous variables and counts and percentages for categorical variables. We used the Mann-Whitney test for comparisons involving continuous variables with 2 groups and the Kruskal-Wallis test for 4 groups. The chi-square test—corrected for continuity if necessary—was used to compare 2 categorical variables. We used multiple logistic regression to adjust for demographic and clinical variables other than nodule size that were related to malignancy. Inferences were made at the 0.05 level of significance.
Results
A total of 329 patients with thyroid nodules were identified: 236 were < 3 cm and 93 were ≥ 3 cm. The 2 groups differed on race, with more white patients in the < 3-cm nodule group (78% vs 67%, P = .036) (Table 1).
Prevalence of cancer based on FNA in nodules < 3 cm was 6.4% (95% CI, 3.6%–10.3%) and nodules ≥ 3 cm was 8.6% (95% CI, 3.8%–16.2%; P = .23) (Table 2).
When divided into 4 subgroups, cancer using FNA was found in 35.1% of nodules < 2 cm, 21.1% of nodules 2 cm to < 3 cm, 42.1% of nodules 3 cm to 4 cm, and 18.2% of nodules > 4 cm (P = .32) (Table 3).
Surgical pathology results showed 17 cases of papillary carcinoma in nodules < 3 cm, whereas there were 9 cases of papillary carcinoma and 1 case of follicular carcinoma in nodules > 3 cm. When correlated with the cytology results, 10 cases were reported as benign, 11 were malignant, and 6 samples were non-diagnostic.
There were 30 nondiagnostic FNA samples: 7 patients had surgery, 19 were monitored with serial imaging, 2 were lost to follow-up, and 2 expired for other reasons. Of the 19 patients who were monitored with serial imaging, the nodules were stable and did not require repeat sampling.
Discussion
The authors found no relationship between thyroid nodule size and malignancy over a 16-year period in a veteran population, either with FNA or surgical pathology. The lack of relationship persists when adjusted for the only nonthyroid variable on which the 2 groups differed (race).
The finding of no relationship between larger thyroid nodule size and cancer is consistent with other studies. In a 10-year chart review of 695 patients at Walter Reed Army Medical Center, Burch and colleagues found a malignancy rate of 18.6% but no association between thyroid nodule size and malignancy.11 They concluded that nodules ≥ 4 cm did not increase malignancy risk. In a 3-year retrospective study of 326 patients, Mangister and colleagues reported that the malignancy rate was higher in nodules < 3 cm (48.4%) compared with nodules ≥ 3 cm (33.3%).10 This study concluded that the malignancy potential of thyroid nodules peaked at 2 cm and decreased at > 3 cm. Kamran and colleagues reported a nonlinear relationship between nodule size and malignancy with a threshold of 2 cm, beyond which there was no increased risk of malignancy.1
Conversely, in a prospective study Kuru and colleagues followed 571 patients who had undergone thyroidectomy and found that nodules ≥ 4 cm were associated with increased malignancy risk compared with nodules < 4 cm. However, with a cutoff of 3 cm there was no relationship.5 Discrepancies among studies might be because of variability in patient demographics and the prevalence of thyroid cancer in a specific institution. Although the majority of thyroid nodules are seen in females, the current study’s population was predominantly male and entirely veteran. Consequently, interpretation of these studies highlight the need to individualize clinical decision-making for each patient.
Limitations
This study has several limitations. It was conducted at a single institution with a group of veterans, which limits the ability to generalize its results to the general population. Second, data omissions are likely in retrospective chart reviews, and ensuring accuracy of data collection could be challenging. Third, all thyroid nodules found to be benign with cytology did not undergo surgical intervention to confirm the diagnosis; therefore, only 93 of 329 nodules were evaluated with the definitive diagnostic test. Therefore, selection bias was introduced into the nodule size comparisons when surgical intervention was used to measure the outcome. However, because false negative rates for FNA is low, likely few malignant nodules were missed. In addition, all patients with thyroid nodules are not referred for surgery because of potential complications.
Conclusion
This study strongly suggests there is no increased or decreased cancer risk for thyroid nodules ≥ 3 cm compared with those < 3 cm. Current clinical practice is to refer patients with larger nodules for surgical evaluation. In a large systemic review, Shin and colleagues reported higher pretest probability of malignancy in larger nodules and recommended consideration of surgical intervention for nodules > 3 cm because of false negatives and concerns for diagnostic inaccuracy with FNA.8 Although data were mixed, Shin and colleagues reported higher incidence of false negative FNA results in larger nodules.8 Given the authors’ findings and earlier conflicting results, the decision for surgical intervention cannot be made solely on nodule size and requires consideration of additional factors including FNA results, nodule characteristics, patient risk factors, and patient preference.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.
1. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564-570.
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-33.
3. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96(2):329-349.
4. Amrikachi M, Ramzy I, Rubenfeld S, Wheeler TM. Accuracy of fine needle aspiration of thyroid. Arch Pathol Lab Med. 2001;125(4):484-488.
5. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009;31(7):856-866.
6. Kim JH, Kim NK, Oh YL, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasound characteristics. Endocrinol Metab (Seoul). 2014;29(4):545-552.
7. Wharry LI, McCoy KL, Stang MT, et al. Thyroid nodules (≥4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614-621.
8. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009;144(7):649-655.
9. Megwalu UC. Risk of malignancy in thyroid nodules 4 cm or larger. Endocrinol Metab (Seoul). 2017;32(1):77-82.
10. Magister MJ, Chaikhoutdinov I, Schaefer E, et al. Association of thyroid nodule size and Bethesda class with rate of malignant disease. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1089-1095.
11. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22(12):1251-1256.