User login
Proinflammatory microbiome tied to colorectal adenoma
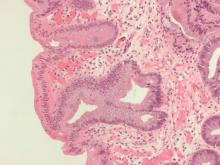
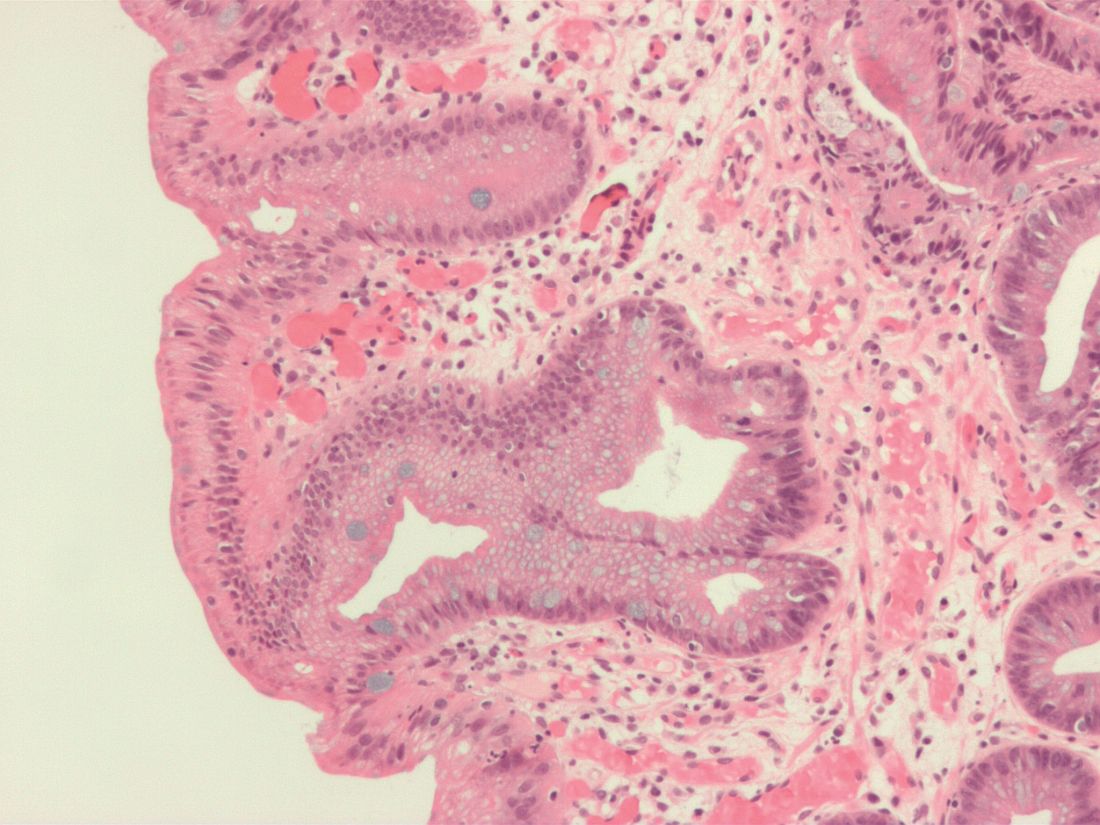
MIAMI – The fecal presence of least two genes harbored by toxin-producing or proinflammatory bacteria conferred a nearly 200% rise in the odds of colorectal adenoma, investigators reported.
Moreover, the fecal presence of usp (uropathogenic-specific protein), a bacterial gene encoding a genotoxin that damages DNA, correlated with nearly 1,200% greater odds of colorectal adenoma (P =.08), said senior investigator María González-Pons, PhD, of the University of Puerto Rico Comprehensive Cancer Center in San Juan.
“We are continuing to enlarge this study. We need more power to assess statistical significance and look at associations for individual combinations of bacterial genes,” Dr. Pons said in an interview. “Our ultimate goal is to risk-stratify patients so that we know whom to target for [colorectal cancer] prevention.”
Dr. Pons and the study’s lead author, Noe Crespo-Hernandez, of the University of Puerto Rico, presented the findings with their associates in a poster at the the annual Gut Microbiota for Health World Summit.
Colorectal cancer is the most lethal cancer and the second-most common malignancy in Puerto Rico. Despite recommendations for screening colonoscopy, many patients are diagnosed in late-stage disease, when treatment options are limited. Intestinal inflammation is itself key to colorectal carcinogenesis and also promotes the enteric proliferation of gram-negative bacteria that produce potentially carcinogenic toxins. Thus, gut inflammation and the microbiome are of great interest to researchers who are working to develop reliable, minimally invasive tests that assess future colorectal cancer risk.
For their study presented at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Pons and her coinvestigators compared stool samples from 67 adults with colonoscopically confirmed colorectal adenomas and 39 controls with negative screening colonoscopies. Both groups were captured in the Puerto Rico Familial Colorectal Cancer Registry. The researchers used TaqMan SNP Genotyping to look for single-nucleotide polymorphisms (SNPs) from promoter regions of genes encoding interleukin-1 beta, IL-6, and IL-10, cytokines that regulate gut inflammation. They found nonsignificant associations between colorectal adenoma and two of the three SNPs: rs143627 (encoding IL-1B) and rs1800795 (IL-6).
The real-time polymerase chain reaction results were even more striking. Using SYBR Green, the researchers tested stool for six genotoxic or proinflammatory bacterial genes and identified five, each of which correlated with colorectal adenoma. Colorectal adenoma also was linked with the fecal presence of a nonpathogenic housekeeping gene that is a surrogate for a mucolytic bacterium abundant in the stool of colorectal cancer patients.
Odds ratios for these associations ranged from 1.17 (for cnf, or cytotoxic necrotizing factor) to 12.83 (for usp), the researchers reported. Dr. Pons commented that Hurricane Maria greatly delayed this study and thus the cohort was underpowered to test for statistical significance. Nonetheless, P values approached significance for the usp gene (OR, 12.83; 95% confidence interval, 0.73-226.8; P = .08) and the fecal presence of at least two genes in combination (OR, 2.84; 95% CI, 1.01-8.90; P = .05).
Next, Dr. Pons and her team will expand the study to assess links between colorectal adenoma and these pathogenic bacterial genes, individually and in various combinations. They also plan to compare genes in normal and adenomatous colon tissue and to use enteroid (small intestinal organoid) models to tease out the carcinogenic mechanisms of these bacterial toxins.
The National Institutes of Health provided funding. The researchers disclosed no competing interests.
MIAMI – The fecal presence of least two genes harbored by toxin-producing or proinflammatory bacteria conferred a nearly 200% rise in the odds of colorectal adenoma, investigators reported.
Moreover, the fecal presence of usp (uropathogenic-specific protein), a bacterial gene encoding a genotoxin that damages DNA, correlated with nearly 1,200% greater odds of colorectal adenoma (P =.08), said senior investigator María González-Pons, PhD, of the University of Puerto Rico Comprehensive Cancer Center in San Juan.
“We are continuing to enlarge this study. We need more power to assess statistical significance and look at associations for individual combinations of bacterial genes,” Dr. Pons said in an interview. “Our ultimate goal is to risk-stratify patients so that we know whom to target for [colorectal cancer] prevention.”
Dr. Pons and the study’s lead author, Noe Crespo-Hernandez, of the University of Puerto Rico, presented the findings with their associates in a poster at the the annual Gut Microbiota for Health World Summit.
Colorectal cancer is the most lethal cancer and the second-most common malignancy in Puerto Rico. Despite recommendations for screening colonoscopy, many patients are diagnosed in late-stage disease, when treatment options are limited. Intestinal inflammation is itself key to colorectal carcinogenesis and also promotes the enteric proliferation of gram-negative bacteria that produce potentially carcinogenic toxins. Thus, gut inflammation and the microbiome are of great interest to researchers who are working to develop reliable, minimally invasive tests that assess future colorectal cancer risk.
For their study presented at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Pons and her coinvestigators compared stool samples from 67 adults with colonoscopically confirmed colorectal adenomas and 39 controls with negative screening colonoscopies. Both groups were captured in the Puerto Rico Familial Colorectal Cancer Registry. The researchers used TaqMan SNP Genotyping to look for single-nucleotide polymorphisms (SNPs) from promoter regions of genes encoding interleukin-1 beta, IL-6, and IL-10, cytokines that regulate gut inflammation. They found nonsignificant associations between colorectal adenoma and two of the three SNPs: rs143627 (encoding IL-1B) and rs1800795 (IL-6).
The real-time polymerase chain reaction results were even more striking. Using SYBR Green, the researchers tested stool for six genotoxic or proinflammatory bacterial genes and identified five, each of which correlated with colorectal adenoma. Colorectal adenoma also was linked with the fecal presence of a nonpathogenic housekeeping gene that is a surrogate for a mucolytic bacterium abundant in the stool of colorectal cancer patients.
Odds ratios for these associations ranged from 1.17 (for cnf, or cytotoxic necrotizing factor) to 12.83 (for usp), the researchers reported. Dr. Pons commented that Hurricane Maria greatly delayed this study and thus the cohort was underpowered to test for statistical significance. Nonetheless, P values approached significance for the usp gene (OR, 12.83; 95% confidence interval, 0.73-226.8; P = .08) and the fecal presence of at least two genes in combination (OR, 2.84; 95% CI, 1.01-8.90; P = .05).
Next, Dr. Pons and her team will expand the study to assess links between colorectal adenoma and these pathogenic bacterial genes, individually and in various combinations. They also plan to compare genes in normal and adenomatous colon tissue and to use enteroid (small intestinal organoid) models to tease out the carcinogenic mechanisms of these bacterial toxins.
The National Institutes of Health provided funding. The researchers disclosed no competing interests.
MIAMI – The fecal presence of least two genes harbored by toxin-producing or proinflammatory bacteria conferred a nearly 200% rise in the odds of colorectal adenoma, investigators reported.
Moreover, the fecal presence of usp (uropathogenic-specific protein), a bacterial gene encoding a genotoxin that damages DNA, correlated with nearly 1,200% greater odds of colorectal adenoma (P =.08), said senior investigator María González-Pons, PhD, of the University of Puerto Rico Comprehensive Cancer Center in San Juan.
“We are continuing to enlarge this study. We need more power to assess statistical significance and look at associations for individual combinations of bacterial genes,” Dr. Pons said in an interview. “Our ultimate goal is to risk-stratify patients so that we know whom to target for [colorectal cancer] prevention.”
Dr. Pons and the study’s lead author, Noe Crespo-Hernandez, of the University of Puerto Rico, presented the findings with their associates in a poster at the the annual Gut Microbiota for Health World Summit.
Colorectal cancer is the most lethal cancer and the second-most common malignancy in Puerto Rico. Despite recommendations for screening colonoscopy, many patients are diagnosed in late-stage disease, when treatment options are limited. Intestinal inflammation is itself key to colorectal carcinogenesis and also promotes the enteric proliferation of gram-negative bacteria that produce potentially carcinogenic toxins. Thus, gut inflammation and the microbiome are of great interest to researchers who are working to develop reliable, minimally invasive tests that assess future colorectal cancer risk.
For their study presented at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Pons and her coinvestigators compared stool samples from 67 adults with colonoscopically confirmed colorectal adenomas and 39 controls with negative screening colonoscopies. Both groups were captured in the Puerto Rico Familial Colorectal Cancer Registry. The researchers used TaqMan SNP Genotyping to look for single-nucleotide polymorphisms (SNPs) from promoter regions of genes encoding interleukin-1 beta, IL-6, and IL-10, cytokines that regulate gut inflammation. They found nonsignificant associations between colorectal adenoma and two of the three SNPs: rs143627 (encoding IL-1B) and rs1800795 (IL-6).
The real-time polymerase chain reaction results were even more striking. Using SYBR Green, the researchers tested stool for six genotoxic or proinflammatory bacterial genes and identified five, each of which correlated with colorectal adenoma. Colorectal adenoma also was linked with the fecal presence of a nonpathogenic housekeeping gene that is a surrogate for a mucolytic bacterium abundant in the stool of colorectal cancer patients.
Odds ratios for these associations ranged from 1.17 (for cnf, or cytotoxic necrotizing factor) to 12.83 (for usp), the researchers reported. Dr. Pons commented that Hurricane Maria greatly delayed this study and thus the cohort was underpowered to test for statistical significance. Nonetheless, P values approached significance for the usp gene (OR, 12.83; 95% confidence interval, 0.73-226.8; P = .08) and the fecal presence of at least two genes in combination (OR, 2.84; 95% CI, 1.01-8.90; P = .05).
Next, Dr. Pons and her team will expand the study to assess links between colorectal adenoma and these pathogenic bacterial genes, individually and in various combinations. They also plan to compare genes in normal and adenomatous colon tissue and to use enteroid (small intestinal organoid) models to tease out the carcinogenic mechanisms of these bacterial toxins.
The National Institutes of Health provided funding. The researchers disclosed no competing interests.
REPORTING FROM GUT 2019
For patients with end-stage liver disease, acute care incurs steep costs
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
End-of-life care for patients with end-stage liver disease cost more than four of the five most expensive chronic medical conditions, according to the findings of a population-based study in Canada.
During their final year of life, patients with end-stage liver disease incurred a median of $51,191 Canadian dollars in health care costs (interquartile range, $28,510-$86,659) – approximately $2,360 more than ischemic heart disease, $1,830 more than diabetes, $1,600 more than mental health disorders, and $600 more than congestive heart failure, Erin M. Kelly, MD, of the University of Ottawa, and her associates wrote in Clinical Gastroenterology and Hepatology. Only chronic renal disease cost more (median, $55,453). Most health care costs of end-stage liver disease covered the final 90 days of life and were tied to high use of hospital resources, the researchers said.
In the United States, more than 150,000 patients are hospitalized for end-stage liver disease every year at a price tag of $4 billion, Dr. Kelly and her associates noted. This price tag is expected to rise further because of epidemic levels of obesity and related nonalcoholic fatty liver disease. The shortage of livers for transplantation and the fact that many patients with cirrhosis are not transplantation candidates leave many in end-of-life care. Given the lack of population-level data on costs of this care, the researchers studied data for all individuals who died in Ontario – Canada’s largest province – between April 2010 and March 2013. The data source was the Institute for Clinical Evaluative Sciences, a nonprofit group that tracks diagnoses, health care, outcomes, and costs.
Among 264,723 decedents, 5,087 (1.9%) had a diagnosis of end-stage liver disease. These patients died a median of 15 years earlier than other patients (median age of death, 65 vs. 80 years old). During the last year of life, 99% visited the emergency department or were hospitalized, compared with 86% of other patients. Importantly, health care costs for the two groups were similar up until the final 90 days of life, when there was “a clear divergence,” the researchers said. A total of 51% of the costs of the final 12 months of care related to acute care during the final 90 days of life. Consequently, during their last 3 months, patients with end-stage liver disease cost the health care system 46% more than other individuals, the difference remained statistically significant after accounting for demographics and comorbidities, and the picture changed little after excluding transplantation patients and those with hepatocellular carcinoma.
Medical care for patients with end-stage liver disease is complex – often involving serious infections, gastrointestinal bleeding, renal dysfunction, electrolyte disturbances, and worsening encephalopathy – and often involves frequent hospital readmissions, the researchers noted. Nonetheless, the findings highlight the need to consider steps such as advanced care planning and palliative care to help keep patients with end-stage liver disease from dying in acute care settings, they concluded. Such steps “may direct services toward more appropriate sectors, while reducing costs.”
The Ontario Ministry of Health and Long-Term Care supported the work. The researchers reported having no competing interests.
SOURCE: Kelly EM et al. Clin Gastroenterol Hepatol. 2019 Jan 28. doi: 10.1016/j.cgh.2019.01.046.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Semaglutide plus SGLT2 inhibitors improves glycemic control in type 2 diabetes
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes already being treated with sodium-glucose cotransporter-2 (SGLT2) inhibitors meet their glycemic targets and lose weight, researchers reported.
“Combining the distinct modes of action of these two drug classes has beneficial effects on glucose and weight outcomes,” wrote Bernard Zinman, MD, of Mount Sinai Hospital, Toronto, and his coauthors. Their report is in Lancet Diabetes and Endocrinology.
In the international, double-blind, phase 3 SUSTAIN 9 trial, 302 patients with type 2 diabetes whose hemoglobin A1c (HbA1c) levels were 7.0% to 10.0% (53 to 86 mmol/mol) despite at least 90 days of SGLT2-inhibitor therapy (alone or with metformin or sulfonylurea) were randomly assigned to add either once-weekly semaglutide (1-mg injection) or placebo to their regimen.
A total of 294 patients completed the trial. After 30 weeks, those who had received adjunctive semaglutide had significantly greater reductions in their HbA1c levels (estimated treatment difference, –1.42%; P less than .0001). They also lost about 3.81 kg more bodyweight than did patients in the placebo group and they had significantly greater reductions in mean body mass index, waist circumference, fasting and self-measured blood glucose, systolic blood pressure, pulse rate, total cholesterol, and low-density lipoprotein and triglyceride levels.
The most commonly reported adverse effects of semaglutide were nausea, diarrhea, vomiting, and constipation, which were usually mild in severity. Severe or blood-glucose–confirmed hypoglycemia occurred in 2.7% patients on semaglutide and none on placebo. However, most patients who received semaglutide achieved an HbA1c of 7.0% (53 mmol/mol) or less without weight gain or severe or blood-glucose–confirmed hypoglycemia (P less than .0001 vs. placebo).
Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
SOURCE: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
FROM LANCET DIABETES AND ENDOCRINOLOGY
Key clinical point: Adding once-weekly treatment with the glucagon-like peptide-1 receptor agonist (GLP-1 RA) semaglutide helped most patients with type 2 diabetes on sodium-glucose contransporter-2 (SGLT2) inhibitors meet glycemic targets and lose weight.
Major finding: After 30 weeks, the estimated treatment difference for hemoglobin A1c (HbA1c) favored semaglutide over placebo (–1.42; P less than .0001). Patients also lost about 3.81 kg more bodyweight with adjunctive semaglutide, compared with placebo (P less than .0001)
Study details: International, double-blind, phase 3 trial of 302 patients with type 2 diabetes with HbA1c levels of 7.0% to 10.0% despite having received at least 90 days of SGLT2-inhibitor therapy (SUSTAIN 9).
Disclosures: Novo Nordisk funded the study. Three of the authors were employees of Novo Nordisk at the time of the study, and the remaining authors reported that they and/or their institutions had received funding from the company.
Source: Zinman B et al. Lancet Diabetes Endocrinol. 2019 Mar 1. doi: 10.1016/S2213-8587(19)30066-X.
Golimumab plus methotrexate looks good in early psoriatic arthritis
For patients with early psoriatic arthritis, starting the tumor necrosis factor inhibitor golimumab (Simponi) at the same time as methotrexate nearly doubled the chances of remission, compared with methotrexate monotherapy, researchers reported in Annals of the Rheumatic Diseases.
In this multicenter, double-blind trial, 51 adults with CASPAR-defined psoriatic arthritis who were naive to methotrexate and biologic disease-modifying antirheumatic drugs were randomly assigned to receive monthly golimumab (50 mg subcutaneously) or placebo, in addition to methotrexate (15 mg/week, increased to 25 mg/week over 8 weeks). All patients had current active disease: At baseline, most had at least five swollen joints and at least nine tender joints.
Among 45 patients who completed the study, rates of Disease Activity Score (DAS) remission (DAS C-reactive protein score less than 1.6) at week 22 were 81% for golimumab-methotrexate and 42% for methotrexate-placebo (P = .004). “This difference in DAS remission was already observed at week 8,” wrote Leonieke J.J. van Mens, MD, of AMC/University of Amsterdam and her colleagues.
Golimumab-methotrexate also topped methotrexate monotherapy on secondary outcome measures. By week 22, median swollen joint counts were 0 with combined therapy versus 3 with methotrexate monotherapy (P = .04). Median tender joint counts were 0 and 2, respectively (P = .02). Combined golimumab-methotrexate therapy also produced significantly higher rates of low disease activity based on Disease Activity in Psoriatic Arthritis score (92% vs. 54%, respectively), Minimal Disease Activity (81% vs. 29%), and ACR20, 50, or 70 response (85% vs. 58%, 81% vs. 33%, and 58% vs. 13%, respectively).
Most differences were already statistically significant by week 8, and many were more pronounced by week 22, the researchers said. “It remains unknown if the responses – in particular the stringent responses such as remission – have already plateaued at week 22 or could even further increase over time,” they added. “Similarly, it remains to be determined if the combination of tumor necrosis factor inhibitor and methotrexate is only needed for the induction of remission or is also needed to maintain this state of remission over time.”
They explained that golimumab (or placebo) was stopped at week 22 in patients who achieved DAS CRP remission. An extension of the current study will assess whether methotrexate monotherapy can maintain responses for up to 50 weeks.
The only serious adverse event in the study occurred in the methotrexate arm and consisted of spinal stenosis that was not seen as treatment related. Rates of other adverse events were similar between arms, and those that required a dose halt or dose reduction were related to methotrexate, not golimumab. There were no deaths on trial.
Merck Sharp & Dohme provided medication and unrestricted funding for the study. Dr. van Mens and two coinvestigators reported having no disclosures. Several other coinvestigators disclosed ties to UCB, AbbVie, Novartis, Janssen, Eli Lilly, and other pharmaceutical companies.
SOURCE: van Mens LJJ et al. Ann Rheum Dis. 2019 Feb 26. doi: 10.1136/annrheumdis-2018-214746.
For patients with early psoriatic arthritis, starting the tumor necrosis factor inhibitor golimumab (Simponi) at the same time as methotrexate nearly doubled the chances of remission, compared with methotrexate monotherapy, researchers reported in Annals of the Rheumatic Diseases.
In this multicenter, double-blind trial, 51 adults with CASPAR-defined psoriatic arthritis who were naive to methotrexate and biologic disease-modifying antirheumatic drugs were randomly assigned to receive monthly golimumab (50 mg subcutaneously) or placebo, in addition to methotrexate (15 mg/week, increased to 25 mg/week over 8 weeks). All patients had current active disease: At baseline, most had at least five swollen joints and at least nine tender joints.
Among 45 patients who completed the study, rates of Disease Activity Score (DAS) remission (DAS C-reactive protein score less than 1.6) at week 22 were 81% for golimumab-methotrexate and 42% for methotrexate-placebo (P = .004). “This difference in DAS remission was already observed at week 8,” wrote Leonieke J.J. van Mens, MD, of AMC/University of Amsterdam and her colleagues.
Golimumab-methotrexate also topped methotrexate monotherapy on secondary outcome measures. By week 22, median swollen joint counts were 0 with combined therapy versus 3 with methotrexate monotherapy (P = .04). Median tender joint counts were 0 and 2, respectively (P = .02). Combined golimumab-methotrexate therapy also produced significantly higher rates of low disease activity based on Disease Activity in Psoriatic Arthritis score (92% vs. 54%, respectively), Minimal Disease Activity (81% vs. 29%), and ACR20, 50, or 70 response (85% vs. 58%, 81% vs. 33%, and 58% vs. 13%, respectively).
Most differences were already statistically significant by week 8, and many were more pronounced by week 22, the researchers said. “It remains unknown if the responses – in particular the stringent responses such as remission – have already plateaued at week 22 or could even further increase over time,” they added. “Similarly, it remains to be determined if the combination of tumor necrosis factor inhibitor and methotrexate is only needed for the induction of remission or is also needed to maintain this state of remission over time.”
They explained that golimumab (or placebo) was stopped at week 22 in patients who achieved DAS CRP remission. An extension of the current study will assess whether methotrexate monotherapy can maintain responses for up to 50 weeks.
The only serious adverse event in the study occurred in the methotrexate arm and consisted of spinal stenosis that was not seen as treatment related. Rates of other adverse events were similar between arms, and those that required a dose halt or dose reduction were related to methotrexate, not golimumab. There were no deaths on trial.
Merck Sharp & Dohme provided medication and unrestricted funding for the study. Dr. van Mens and two coinvestigators reported having no disclosures. Several other coinvestigators disclosed ties to UCB, AbbVie, Novartis, Janssen, Eli Lilly, and other pharmaceutical companies.
SOURCE: van Mens LJJ et al. Ann Rheum Dis. 2019 Feb 26. doi: 10.1136/annrheumdis-2018-214746.
For patients with early psoriatic arthritis, starting the tumor necrosis factor inhibitor golimumab (Simponi) at the same time as methotrexate nearly doubled the chances of remission, compared with methotrexate monotherapy, researchers reported in Annals of the Rheumatic Diseases.
In this multicenter, double-blind trial, 51 adults with CASPAR-defined psoriatic arthritis who were naive to methotrexate and biologic disease-modifying antirheumatic drugs were randomly assigned to receive monthly golimumab (50 mg subcutaneously) or placebo, in addition to methotrexate (15 mg/week, increased to 25 mg/week over 8 weeks). All patients had current active disease: At baseline, most had at least five swollen joints and at least nine tender joints.
Among 45 patients who completed the study, rates of Disease Activity Score (DAS) remission (DAS C-reactive protein score less than 1.6) at week 22 were 81% for golimumab-methotrexate and 42% for methotrexate-placebo (P = .004). “This difference in DAS remission was already observed at week 8,” wrote Leonieke J.J. van Mens, MD, of AMC/University of Amsterdam and her colleagues.
Golimumab-methotrexate also topped methotrexate monotherapy on secondary outcome measures. By week 22, median swollen joint counts were 0 with combined therapy versus 3 with methotrexate monotherapy (P = .04). Median tender joint counts were 0 and 2, respectively (P = .02). Combined golimumab-methotrexate therapy also produced significantly higher rates of low disease activity based on Disease Activity in Psoriatic Arthritis score (92% vs. 54%, respectively), Minimal Disease Activity (81% vs. 29%), and ACR20, 50, or 70 response (85% vs. 58%, 81% vs. 33%, and 58% vs. 13%, respectively).
Most differences were already statistically significant by week 8, and many were more pronounced by week 22, the researchers said. “It remains unknown if the responses – in particular the stringent responses such as remission – have already plateaued at week 22 or could even further increase over time,” they added. “Similarly, it remains to be determined if the combination of tumor necrosis factor inhibitor and methotrexate is only needed for the induction of remission or is also needed to maintain this state of remission over time.”
They explained that golimumab (or placebo) was stopped at week 22 in patients who achieved DAS CRP remission. An extension of the current study will assess whether methotrexate monotherapy can maintain responses for up to 50 weeks.
The only serious adverse event in the study occurred in the methotrexate arm and consisted of spinal stenosis that was not seen as treatment related. Rates of other adverse events were similar between arms, and those that required a dose halt or dose reduction were related to methotrexate, not golimumab. There were no deaths on trial.
Merck Sharp & Dohme provided medication and unrestricted funding for the study. Dr. van Mens and two coinvestigators reported having no disclosures. Several other coinvestigators disclosed ties to UCB, AbbVie, Novartis, Janssen, Eli Lilly, and other pharmaceutical companies.
SOURCE: van Mens LJJ et al. Ann Rheum Dis. 2019 Feb 26. doi: 10.1136/annrheumdis-2018-214746.
FROM ANNALS OF THE RHEUMATIC DISEASES
One-time, universal hepatitis C testing cost effective, researchers say
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
Universal one-time screening for hepatitis C virus infection is cost effective, compared with birth cohort screening alone, according to the results of a study published in Clinical Gastroenterology and Hepatology.
The Centers for Disease Control and Prevention and the U.S. Preventive Services Task Force recommend testing all individuals born between 1945 and 1965 in addition to injection drug users and other high-risk individuals. But so-called birth cohort screening does not reflect the recent spike in hepatitis C virus (HCV) cases among younger persons in the United States, nor the current recommendation to treat nearly all chronic HCV cases, wrote Mark H. Eckman, MD, of the University of Cincinnati, and his associates.
Using a computer program called Decision Maker, they modeled the cost-effectiveness of universal one-time testing, birth cohort screening, and no screening based on quality-adjusted life-years (QALYS) and 2017 U.S. dollars. They assumed that all HCV-infected patients were treatment naive, treatment eligible, and asymptomatic (for example, had no decompensated cirrhosis). They used efficacy data from the ASTRAL trials of sofosbuvir-velpatasvir as well as the ENDURANCE, SURVEYOR, and EXPEDITION trials of glecaprevir-pibrentasvir. In the model, patients who did not achieve a sustained viral response to treatment went on to complete a 12-week triple direct-acting antiviral (DAA) regimen (sofosbuvir, velpatasvir, and voxilaprevir).
Based on these assumptions, universal one-time screening and treatment of infected individuals cost less than $50,000 per QALY gained, making it highly cost effective, compared with no screening, the investigators wrote. Universal screening also was highly cost effective when compared with birth cohort screening, costing $11,378 for each QALY gained.
“Analyses performed during the era of first-generation DAAs and interferon-based treatment regimens found birth-cohort screening to be ‘cost effective,’ ” the researchers wrote. “However, the availability of a new generation of highly effective, non–interferon-based oral regimens, with fewer side effects and shorter treatment courses, has altered the dynamic around the question of screening.” They pointed to another recent study in which universal one-time HCV testing was more cost effective than birth cohort screening.
Such findings have spurred experts to revisit guidelines on HCV screening, but universal testing is controversial when some states, counties, and communities have a low HCV prevalence. In the model, universal one-time HCV screening was cost effective (less than $50,000 per QALY gained), compared with birth cohort screening as long as prevalence exceeded 0.07% among adults not born between 1945 and 1965. The current prevalence estimate in this group is 0.29%, which is probably low because it does not account for the rising incidence among younger adults, the researchers wrote. In an ideal world, all clinics and hospitals would implement an HCV testing program, but in the real world of scarce resources, “data regarding the cost-effectiveness threshold can guide local policy decisions by directing testing services to settings in which they generate sufficient benefit for the cost.”
Partial funding came from the National Foundation for the Centers for Disease Control and Prevention (CDC Foundation), with funding provided through multiple donors to the CDC Foundation’s Viral Hepatitis Action Coalition. Dr. Eckman reported grant support from Merck and one coinvestigator reported ties to AbbVie, Gilead, Merck, and several other pharmaceutical companies.
SOURCE: Eckman MH et al. Clin Gastroenterol Hepatol. 2018 Sep 7. doi: 10.1016/j.cgh.2018.08.080.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Novel capsid assembly modulator shows promise in HBV
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
For adults with chronic hepatitis B virus infection, treatment with a novel investigational capsid assembly modulator was well tolerated and showed antiviral activity against HBV, according to the results of a phase 1 study of 73 patients.
“Substantial and correlated reductions in serum HBV DNA and HBV RNA levels were observed consistently with the higher-dose cohorts and were notably greatest for combination treatment with NVR 3-778 and pegIFN [pegylated interferon],” Man Fung Yuen, MD, of the University of Hong Kong, and his associates wrote in a report published in Gastroenterology. Hence, this first-in-class capsid assembly modulator might help prolong treatment responses, “most likely as a component of new combination treatment regimens for HBV-infected patients.” However, one patient developed severe rash immediately after completing treatment that took 6 months of intensive outpatient treatment to resolve, they noted.
Chronic viral hepatitis due to HBV is a major cause of early death worldwide, and new therapies are needed to help prevent severe liver disease and liver death from this infection. Current treatments for HBV infection consist of nucleoside or nucleotide analogs or pegylated interferon. These suppress HBV replication in many patients, but most patients do not achieve durable responses. Consequently, most patients require long-term treatment with HBV nucleosides and nucleotide analogs, which they may find difficult to tolerate or adhere to and to which their infections can become resistant, the researchers said.
The HBV virion contains a viral core protein (HBc) that is required to encapsidate viral polymerase and pregenomic HBV RNA into a nucleocapsid. To target this process, researchers developed NVR 3-778, a first-in-class, orally bioavailable small molecule that binds HBc so that HBc forms a defective capsid that lacks nuclear material. Hence, NVR 3-778 is intended to stop the production of HBV nucleocapsids and keep infected cells from releasing the enveloped infectious viral particles that perpetuate HBV infection.
To assess the safety, pharmacokinetics, and antiviral activity of NVR 3-778, the researchers conducted a phase 1 study of 73 patients with chronic HBV infection who tested positive for hepatitis B e-antigen (HBeAg) and had no detectable cirrhosis. Patients were randomly assigned to receive oral NVR 3-778 (100 mg, 200 mg, or 400 mg daily or 600 mg or 1,000 mg twice daily ) or placebo for 28 days. Some patients received combination therapy with pegylated interferon plus either NVR 3-778 (600 mg twice daily) or placebo. Treatment was generally well tolerated, and adverse events were usually mild and deemed unrelated to therapy. No patient stopped treatment for adverse effects.
The only serious adverse event in the study consisted of grade 3 rash that developed in a 42-year-old male after 22 days of treatment at the lowest dose of NVR 3-778 (100 mg per day). This patient completed treatment and ultimately developed a severe papulovesicular rash with a predominantly acral distribution over the hands, arm, side of neck, and one leg (palmar plantar erythrodysesthesia), the researchers said. “There were no perioral or mucosal lesions, no ecchymotic skin involvement, no bullae, and no systemic manifestations or hematological abnormalities,” they wrote. “The rash was subsequently managed with a psoriasis-like treatment regimen of psoralen, ultraviolet light, and topical steroid ointment during outpatient follow-up and resolved after approximately 6 months.”
Another three cases of “minor” skin rash were considered probably related to treatment in the cohort that received 600 mg NVR 3-778 b.i.d. plus pegylated interferon, the investigators said. Two additional cases of mild rash were deemed unrelated to treatment.
“The observed reductions in HBV RNA confirmed the novel mechanism of NVR 3-778,” the researchers concluded. “This class of compounds can also inhibit replenishment of intranuclear covalently closed circular DNA over time and may have immunomodulatory properties.” Longer treatment periods would be needed to study these mechanisms and to quantify reductions in serum HBsAg and HBeAG, they noted.
Novira Therapeutics developed NVR 3-778 and is a Janssen Pharmaceutical Company. Janssen provided funding for editorial support. Dr. Yuen disclosed relationships with AbbVie, Biocartis, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Ionis, Roche, Vir Biotechnology, and several other pharmaceutical companies. Other coinvestigators disclosed ties to pharmaceutical companies; eight reported employment by Novira or a Janssen company.
SOURCE: Yuen MF et al. Gastroenterology. 2019 Jan 5. doi: 10.1053/j.gastro.2018.12.023.
FROM GASTROENTEROLOGY
AGA Clinical Practice Update: Surgical risk assessment and perioperative management in cirrhosis
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
Patients with cirrhosis should be risk stratified and counseled accordingly before all but the most urgent surgeries, cautions a clinical practice update from the American Gastroenterological Association.
These risks, which include mortality and reflect “the profound effects of hepatic synthetic dysfunction and portal hypertension,” require presurgical evaluation based on CTP score (Child-Pugh class), Model for End-Stage Liver Disease (MELD) score, Mayo Postoperative Mortality Risk Score, or another proven risk-stratification system, writes Patrick G. Northup, MD, of the University of Virginia, Charlottesville, together with his associates. “There is no single definitive risk-stratification system to determine operative risk in all patients with cirrhosis, and we recommend using multiple methods,” they elaborated in Clinical Gastroenterology and Hepatology.
The prevalence of cirrhosis is rising, affected patients are living longer, and liver disease is more advanced and may involve comorbidities that merit consideration of surgery, noted Dr. Northup and his associates. However, cirrhosis increases the risk for serious postoperative complications, including hepatic decompensation, worsening of liver synthetic function, exacerbated portal hypertension, wound dehiscence, pleural effusions, pneumonia, bacterial peritonitis, bleeding, and multiple organ failure. Because clinical trials of surgery in cirrhotic patients are lacking, the experts stress the need for case-by-case management.
There is no definite threshold that precludes all surgeries in cases of cirrhosis, but a Child-Pugh class C (CTP score over 10) or MELD score over 20 greatly increases the risk of postoperative decompensation and death. For these patients, “all but the most urgent and life-saving procedures” should be canceled or postponed until after liver transplantation, the experts wrote. For less severe cirrhosis, it is key to consider the type and anatomic site of the proposed surgery. Hepatobiliary surgeries, other intra-abdominal surgeries, cardiovascular surgeries, and thoracic procedures are most likely to lead to serious complications.
Preoperative care should emphasize control of ascites, variceal bleeding risk, and hepatic encephalopathy. Bleeding and clotting safety thresholds in cirrhosis are unknown, and individualized management, ideally with viscoelastic testing–directed therapy, is warranted instead of protocol transfusions to a target international normalized ratio (INR). Bleeding events are more common in critically ill patients with plasma fibrinogen ratios under 100 mg/dL.
Segmental hepatic resection (usually for malignancy), the most studied procedure in cirrhosis, is generally safe in the absence of clinically significant portal hypertension. For patients who do have portal hypertension, transjugular intrahepatic portosystemic shunt (TIPS) has not clearly been shown to outperform conservative management, although small case series have found that TIPS during deep pelvic or colonic resection decompresses abdominal collaterals.
Because of the risk of poor outcomes, patients with cirrhosis and incompletely controlled ascites should not undergo abdominal hernia repair unless they have an incarceration that is not manually reducible or suspected strangulation. Bariatric surgery is contraindicated in cases of clinically significant portal hypertension but otherwise can be performed at a center with cirrhosis expertise. Sleeve gastrectomy at the same time as liver transplantation is also an option for select patients with obesity.
Elective cholecystectomy should be avoided, and required cases should be performed in experienced centers. “The gallbladder wall may appear thickened on imaging, which may lead to the erroneous diagnosis of acute cholecystitis,” the experts noted. Hence, the diagnosis “should be made only in the appropriate clinical setting, usually in the presence of biliary pain.”
Hepatic decompensation after surgery can be severe enough to merit liver transplantation. There is no agreed-on MELD score that mandates liver transplant evaluation before elective surgery, but the experts recommend doing so if the MELD score is 15 or greater or if risk of mortality within 3 months after surgery exceeds 15%.
Postoperative management of patients with cirrhosis should include aggressive measures to prevent portal hypertension. Monitor renal function closely and avoid volume depletion or overload, the experts advised. Patients should receive only short-acting benzodiazepines and lower opiate doses, administered less often, than in the general population. Avoiding constipation is vital to minimize hepatic encephalopathy, which makes oral rifaximin a better choice than lactulose. Patients should not receive NSAIDs, which can impair renal blood flow. To prevent liver toxicity, they should not be discharged on opiate/acetaminophen combinations, which they might unknowingly take along with another drug that contains acetaminophen.
The experts disclosed no external funding sources and reported having no conflicts of interest.
SOURCE: Northup PG et al. Clin Gastroenterol Hepatol. 2018 Sep 28. doi: 10.1016/j.cgh.2018.09.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA Clinical Practice Update: Changing utility of serology and histologic measures in celiac disease
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
FROM GASTROENTEROLOGY
Interactive online module improved detection of Barrett’s esophagus neoplasia
An online educational tool for endoscopists helped improve their detection of Barrett’s esophagus–related neoplasia (BORN), researchers reported in the April issue of Gastroenterology.
In tests administered before and after training, endoscopists increased their rates of BORN detection by a median of 30% (P less than .001), reported J.J. Bergman, MD, PhD, of the University of Amsterdam, together with his associates. “To our knowledge, this is the first validated online, interactive endoscopic training program in our field,” they wrote. “Widespread use of this tool might improve management of Barrett’s esophagus by general endoscopists.”
To develop the program, the investigators recorded high-definition videos of upper endoscopies of patients with either BORN or nondysplastic Barrett’s esophagus. They sent these videos to three experts, who used special tools to superimpose their delineations of lesions.
Next, 68 general endoscopists (fellows, early-career general gastroenterologists, and senior general gastroenterologists) watched four batches of 20 videos each. The researchers compared the assessors’ interpretations with the experts’ to identify the 25 videos with the most educational impact. These were then shown in four batches of five to 121 new assessors (five videos were reserved for pre- and post testing).
From the first to the fourth batch of training videos, assessors sequentially improved their scores for detection, delineation, agreement delineation, and relative delineation of BORN, the researchers said. Among the 121 assessors in the second phase of development, median rates of detection of BORN rose by 30% after training. Furthermore, from baseline to the end of the study, scores rose by 46% for detection, 129% for delineation, 105% for agreement delineation, and 106% for relative delineation (all P less than .001). These improvements did not depend on the country of origin of the assessors or their level of endoscopic experience.
This module requires the use of high-definition videos whose resolution is not lost during replay or when viewed on the web, the researchers emphasized. They noted that the module is active, not passive – learners select the video frame to position a biopsy mark and delineate the lesion, and the software then gives them tailored feedback on their choice. Learners also can add and remove the experts’ delineations as well as their own during feedback sessions at the end of each batch of videos. This enables them to “fully appreciate the subtle appearance of the lesion on the selected time frame,” the investigators wrote.
By completing the training module, “general endoscopists with a wide range of experience and from different countries of origin can substantially and conveniently increase their skills for detection and delineation of early BORN lesions,” they concluded. “Therefore, the module could provide training in an essential upper gastrointestinal endoscopic skill that is not otherwise readily available.”
The investigators disclosed no external funding sources. They reported having no conflicts of interest.
SOURCE: Bergman JJ et al. Gastroenterology. 2019 Jan 2. doi: 10.1053/j.gastro.2018.12.021.
Endoscopic mucosal resection and ablation strategies offer the potential for minimally invasive, curative treatment for patients with Barrett’s esophagus–associated intramucosal neoplasia. For the gastroenterologist interested in endoscopic prevention and management of esophageal cancer, however, achieving proficiency in performance of these endoscopic techniques represents only part of the requisite preparatory experience. Acquisition of cognitive skills in lesion recognition is a fundamental and underappreciated component to a successful endoscopic treatment paradigm.
General endoscopist assessors were grouped into three groups based on level of experience. Following completion of the training module, scores in lesion detection and delineation increased irrespective of level of endoscopist experience.
The module is free, CME-accredited, and available for online use. Any endoscopist who performs Barrett’s screening, surveillance, and therapy should be motivated and incentivized to engage with this important educational tool.
Patrick Yachimski, MD, MPH, AGAF, is associate professor of medicine, director of pancreatobiliary endoscopy, division of gastroenterology, hepatology & nutrition, Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Endoscopic mucosal resection and ablation strategies offer the potential for minimally invasive, curative treatment for patients with Barrett’s esophagus–associated intramucosal neoplasia. For the gastroenterologist interested in endoscopic prevention and management of esophageal cancer, however, achieving proficiency in performance of these endoscopic techniques represents only part of the requisite preparatory experience. Acquisition of cognitive skills in lesion recognition is a fundamental and underappreciated component to a successful endoscopic treatment paradigm.
General endoscopist assessors were grouped into three groups based on level of experience. Following completion of the training module, scores in lesion detection and delineation increased irrespective of level of endoscopist experience.
The module is free, CME-accredited, and available for online use. Any endoscopist who performs Barrett’s screening, surveillance, and therapy should be motivated and incentivized to engage with this important educational tool.
Patrick Yachimski, MD, MPH, AGAF, is associate professor of medicine, director of pancreatobiliary endoscopy, division of gastroenterology, hepatology & nutrition, Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
Endoscopic mucosal resection and ablation strategies offer the potential for minimally invasive, curative treatment for patients with Barrett’s esophagus–associated intramucosal neoplasia. For the gastroenterologist interested in endoscopic prevention and management of esophageal cancer, however, achieving proficiency in performance of these endoscopic techniques represents only part of the requisite preparatory experience. Acquisition of cognitive skills in lesion recognition is a fundamental and underappreciated component to a successful endoscopic treatment paradigm.
General endoscopist assessors were grouped into three groups based on level of experience. Following completion of the training module, scores in lesion detection and delineation increased irrespective of level of endoscopist experience.
The module is free, CME-accredited, and available for online use. Any endoscopist who performs Barrett’s screening, surveillance, and therapy should be motivated and incentivized to engage with this important educational tool.
Patrick Yachimski, MD, MPH, AGAF, is associate professor of medicine, director of pancreatobiliary endoscopy, division of gastroenterology, hepatology & nutrition, Vanderbilt University Medical Center, Nashville, Tenn. He has no conflicts.
An online educational tool for endoscopists helped improve their detection of Barrett’s esophagus–related neoplasia (BORN), researchers reported in the April issue of Gastroenterology.
In tests administered before and after training, endoscopists increased their rates of BORN detection by a median of 30% (P less than .001), reported J.J. Bergman, MD, PhD, of the University of Amsterdam, together with his associates. “To our knowledge, this is the first validated online, interactive endoscopic training program in our field,” they wrote. “Widespread use of this tool might improve management of Barrett’s esophagus by general endoscopists.”
To develop the program, the investigators recorded high-definition videos of upper endoscopies of patients with either BORN or nondysplastic Barrett’s esophagus. They sent these videos to three experts, who used special tools to superimpose their delineations of lesions.
Next, 68 general endoscopists (fellows, early-career general gastroenterologists, and senior general gastroenterologists) watched four batches of 20 videos each. The researchers compared the assessors’ interpretations with the experts’ to identify the 25 videos with the most educational impact. These were then shown in four batches of five to 121 new assessors (five videos were reserved for pre- and post testing).
From the first to the fourth batch of training videos, assessors sequentially improved their scores for detection, delineation, agreement delineation, and relative delineation of BORN, the researchers said. Among the 121 assessors in the second phase of development, median rates of detection of BORN rose by 30% after training. Furthermore, from baseline to the end of the study, scores rose by 46% for detection, 129% for delineation, 105% for agreement delineation, and 106% for relative delineation (all P less than .001). These improvements did not depend on the country of origin of the assessors or their level of endoscopic experience.
This module requires the use of high-definition videos whose resolution is not lost during replay or when viewed on the web, the researchers emphasized. They noted that the module is active, not passive – learners select the video frame to position a biopsy mark and delineate the lesion, and the software then gives them tailored feedback on their choice. Learners also can add and remove the experts’ delineations as well as their own during feedback sessions at the end of each batch of videos. This enables them to “fully appreciate the subtle appearance of the lesion on the selected time frame,” the investigators wrote.
By completing the training module, “general endoscopists with a wide range of experience and from different countries of origin can substantially and conveniently increase their skills for detection and delineation of early BORN lesions,” they concluded. “Therefore, the module could provide training in an essential upper gastrointestinal endoscopic skill that is not otherwise readily available.”
The investigators disclosed no external funding sources. They reported having no conflicts of interest.
SOURCE: Bergman JJ et al. Gastroenterology. 2019 Jan 2. doi: 10.1053/j.gastro.2018.12.021.
An online educational tool for endoscopists helped improve their detection of Barrett’s esophagus–related neoplasia (BORN), researchers reported in the April issue of Gastroenterology.
In tests administered before and after training, endoscopists increased their rates of BORN detection by a median of 30% (P less than .001), reported J.J. Bergman, MD, PhD, of the University of Amsterdam, together with his associates. “To our knowledge, this is the first validated online, interactive endoscopic training program in our field,” they wrote. “Widespread use of this tool might improve management of Barrett’s esophagus by general endoscopists.”
To develop the program, the investigators recorded high-definition videos of upper endoscopies of patients with either BORN or nondysplastic Barrett’s esophagus. They sent these videos to three experts, who used special tools to superimpose their delineations of lesions.
Next, 68 general endoscopists (fellows, early-career general gastroenterologists, and senior general gastroenterologists) watched four batches of 20 videos each. The researchers compared the assessors’ interpretations with the experts’ to identify the 25 videos with the most educational impact. These were then shown in four batches of five to 121 new assessors (five videos were reserved for pre- and post testing).
From the first to the fourth batch of training videos, assessors sequentially improved their scores for detection, delineation, agreement delineation, and relative delineation of BORN, the researchers said. Among the 121 assessors in the second phase of development, median rates of detection of BORN rose by 30% after training. Furthermore, from baseline to the end of the study, scores rose by 46% for detection, 129% for delineation, 105% for agreement delineation, and 106% for relative delineation (all P less than .001). These improvements did not depend on the country of origin of the assessors or their level of endoscopic experience.
This module requires the use of high-definition videos whose resolution is not lost during replay or when viewed on the web, the researchers emphasized. They noted that the module is active, not passive – learners select the video frame to position a biopsy mark and delineate the lesion, and the software then gives them tailored feedback on their choice. Learners also can add and remove the experts’ delineations as well as their own during feedback sessions at the end of each batch of videos. This enables them to “fully appreciate the subtle appearance of the lesion on the selected time frame,” the investigators wrote.
By completing the training module, “general endoscopists with a wide range of experience and from different countries of origin can substantially and conveniently increase their skills for detection and delineation of early BORN lesions,” they concluded. “Therefore, the module could provide training in an essential upper gastrointestinal endoscopic skill that is not otherwise readily available.”
The investigators disclosed no external funding sources. They reported having no conflicts of interest.
SOURCE: Bergman JJ et al. Gastroenterology. 2019 Jan 2. doi: 10.1053/j.gastro.2018.12.021.
FROM GASTROENTEROLOGY
Barrett’s esophagus uncommon in patients with uncomplicated GERD
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY