User login
Adolescents’ moderate use of digital technology tied to positive mental well-being
The moderate use of digital technology is not intrinsically harmful for adolescents and may be good for their mental well-being.
Those are the results of a study of 120,115 adolescents from the United Kingdom’s Department for Education National Pupil Database who were asked to complete questionnaires about their mental well-being and digital screen time, reported Andrew K. Przybylski, PhD, of the University of Oxford (England), and Netta Weinstein, PhD, of Cardiff (Wales) University.
The researchers began the study using what they called the “digital Goldilocks hypothesis.”
At the beginning of the study, Dr. Przybylski, Dr. Weinstein, and their team asked 15-year-olds from across England to complete the Warick-Edinburgh Mental Well-Being Scale, a 14-item self-report that seeks to measure factors such as happiness, life satisfaction, and psychological and social functioning. Meanwhile, the adolescents’ screen time was assessed through four questions asking about “watching films and other media, playing games, and using computers, and smartphones.”
About 20% of the participants reported a sum of more than 12 hours of digital engagement on weekdays, and 35% of the sample reported a total of more than 12 hours on weekend days. Girls reported spending more time using smartphones, using computers, and watching videos, and the boys devoted more time to playing computer and console games. Smartphones, however, were used more often daily among both girls and boys, Dr. Przybylski and Dr. Weinstein said.
After comparing the well-being and screen time data, the reseachers found that “the relations between screen time and mental well-being were either positive (P less than or equal to .001) or flat (P greater than .183), except for a negative link in the case of weekend smartphone use,” they said.
The investigators said their findings inform current guidelines that seek to limit adolescents’ technology use. “Future research and recommendations building on the Goldilocks hypothesis would be sensitive to the various types and contexts of media use and would be based on peaks and drops in well-being as well as other meaningful outcomes identified systematically,” they wrote.
Dr. Przybylski and Dr. Weinstein reported that they had no conflicts of interest to disclose.
The moderate use of digital technology is not intrinsically harmful for adolescents and may be good for their mental well-being.
Those are the results of a study of 120,115 adolescents from the United Kingdom’s Department for Education National Pupil Database who were asked to complete questionnaires about their mental well-being and digital screen time, reported Andrew K. Przybylski, PhD, of the University of Oxford (England), and Netta Weinstein, PhD, of Cardiff (Wales) University.
The researchers began the study using what they called the “digital Goldilocks hypothesis.”
At the beginning of the study, Dr. Przybylski, Dr. Weinstein, and their team asked 15-year-olds from across England to complete the Warick-Edinburgh Mental Well-Being Scale, a 14-item self-report that seeks to measure factors such as happiness, life satisfaction, and psychological and social functioning. Meanwhile, the adolescents’ screen time was assessed through four questions asking about “watching films and other media, playing games, and using computers, and smartphones.”
About 20% of the participants reported a sum of more than 12 hours of digital engagement on weekdays, and 35% of the sample reported a total of more than 12 hours on weekend days. Girls reported spending more time using smartphones, using computers, and watching videos, and the boys devoted more time to playing computer and console games. Smartphones, however, were used more often daily among both girls and boys, Dr. Przybylski and Dr. Weinstein said.
After comparing the well-being and screen time data, the reseachers found that “the relations between screen time and mental well-being were either positive (P less than or equal to .001) or flat (P greater than .183), except for a negative link in the case of weekend smartphone use,” they said.
The investigators said their findings inform current guidelines that seek to limit adolescents’ technology use. “Future research and recommendations building on the Goldilocks hypothesis would be sensitive to the various types and contexts of media use and would be based on peaks and drops in well-being as well as other meaningful outcomes identified systematically,” they wrote.
Dr. Przybylski and Dr. Weinstein reported that they had no conflicts of interest to disclose.
The moderate use of digital technology is not intrinsically harmful for adolescents and may be good for their mental well-being.
Those are the results of a study of 120,115 adolescents from the United Kingdom’s Department for Education National Pupil Database who were asked to complete questionnaires about their mental well-being and digital screen time, reported Andrew K. Przybylski, PhD, of the University of Oxford (England), and Netta Weinstein, PhD, of Cardiff (Wales) University.
The researchers began the study using what they called the “digital Goldilocks hypothesis.”
At the beginning of the study, Dr. Przybylski, Dr. Weinstein, and their team asked 15-year-olds from across England to complete the Warick-Edinburgh Mental Well-Being Scale, a 14-item self-report that seeks to measure factors such as happiness, life satisfaction, and psychological and social functioning. Meanwhile, the adolescents’ screen time was assessed through four questions asking about “watching films and other media, playing games, and using computers, and smartphones.”
About 20% of the participants reported a sum of more than 12 hours of digital engagement on weekdays, and 35% of the sample reported a total of more than 12 hours on weekend days. Girls reported spending more time using smartphones, using computers, and watching videos, and the boys devoted more time to playing computer and console games. Smartphones, however, were used more often daily among both girls and boys, Dr. Przybylski and Dr. Weinstein said.
After comparing the well-being and screen time data, the reseachers found that “the relations between screen time and mental well-being were either positive (P less than or equal to .001) or flat (P greater than .183), except for a negative link in the case of weekend smartphone use,” they said.
The investigators said their findings inform current guidelines that seek to limit adolescents’ technology use. “Future research and recommendations building on the Goldilocks hypothesis would be sensitive to the various types and contexts of media use and would be based on peaks and drops in well-being as well as other meaningful outcomes identified systematically,” they wrote.
Dr. Przybylski and Dr. Weinstein reported that they had no conflicts of interest to disclose.
FROM PSYCHOLOGICAL SCIENCE
57% drop in central venous catheter–related infections after QI interventions
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
Quality improvement (QI) interventions related to the use of central venous catheters (CVCs) were, on average, associated with 57% fewer infections and $1.85 million in net savings to hospitals within 1-3 years of implementation, based on the results of a meta-analysis of data from 113 hospitals.
“Hospitals that have already attained very low infection rates (through the use of quality improvement checklists) would likely see smaller clinical benefits and savings than in the studies we have reviewed,” said Dr. Teryl Nuckols of Cedars-Sinai Medical Center, Los Angeles. “Nonetheless, we found that QI interventions can be associated with declines in CLABSI (central line-associated bloodstream infection) and/or CRBSI (catheter-related bloodstream infection) and net savings when checklists are already in use, and when hospitals have CLABSI rates as low as 1.7-3.7 per 1,000 CVC-days.”
Studies were eligible for the analysis if they reported or estimated the quality improvement intervention’s clinical effectiveness, measured or modeled its costs, compared alternatives to the intervention, and reported both program and infection-related costs.
Insertion checklists were examined in 12 studies, physician education in 11 studies, ultrasound-guided placement of catheters in 3 studies, all-inclusive catheter kits in 5 studies, sterile dressings in 5 studies, chlorhexidine gluconate sponge or antimicrobial dressing in 2 studies, and antimicrobial catheters in 2 studies.
Overall, the weighted mean incidence rate ratio was 0.43 (95% confidence interval, 0.35-0.51) and incremental net savings were $1.85 million (95% CI, $1.30 million to $2.40 million) per hospital over 3 years (2015 U.S. dollars). Each $100,000 increase in program cost was associated with $315,000 greater savings (95% CI, $166 000-$464 000; P less than .001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7-3.7 per 1,000 catheter-days. (doi: 10.1001/jamainternmed.2016.6610)
Dr. Nuckols acknowledged that the price tag for achieving these savings “may be burdensome for hospitals with limited financial resources … wages and benefits account for two-thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years. We found that, for CLABSI- and CRBSI-prevention interventions, median program costs were about $270,000 per hospital over 3 years – but reached $500,000 to $750,000 in some studies.”
The researchers recommended that “future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.”
First drug for spinal muscular atrophy approved
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
The antisense oligonucleotide drug nusinersen is the first therapy approved by the U.S. Food and Drug Administration to treat children and adults with spinal muscular atrophy.
The drug was developed by Ionis Pharmaceuticals and will be marketed by Biogen under the brand name Spinraza. The antisense oligonucleotide drug is administered via an intrathecal injection and promotes transcription of the full-length survival motor neuron (SMN) protein from the SMN2 gene.
The efficacy of nusinersen was tested during a randomized clinical trial in 121 patients with infantile-onset spinal muscular atrophy who were diagnosed before the age of 6 months or were younger than 7 months at the time of their first dose. Patients were randomized 2:1 to receive an injection of nusinersen into the fluid surrounding the spinal cord or undergo a mock procedure without drug injection (a skin prick).
A total of 82 of 121 patients who were randomized were eligible for an interim analysis of the results that was requested by the FDA. The results showed 40% of patients treated with nusinersen achieved improvement in motor milestones as defined in the study, whereas none of the control patients did. The side effects during the clinical trials among patients on nusinersen included upper respiratory infection, lower respiratory infection, and constipation. Warnings and precautions include low blood platelet count and renal toxicity. Neurotoxicity was observed in animal studies.
Read the full announcement from the agency here.
Children in Taiwan being undervaccinated for pertussis
Up to one-fifth of pertussis cases in Taiwanese children aged 3-35 months from 1996 to 2012 could have been prevented with on-time vaccination, reported Wan-Ting Huang and coauthors at the Taiwan Centers for Disease Control, Taipei.
Although vaccination coverage was 97.4% for three or more pertussis vaccine doses among Taiwanese children aged 12-23 months, many had not received proper vaccination at the right time.
“The study indicated that in Taiwan, undervaccination was decreasing but had placed young children at risk for pertussis,” the authors wrote.
Read the full study from Human Vaccines & Immunotherapeutics (2016. doi: 10.1080/21645515.2016.1249552).
Up to one-fifth of pertussis cases in Taiwanese children aged 3-35 months from 1996 to 2012 could have been prevented with on-time vaccination, reported Wan-Ting Huang and coauthors at the Taiwan Centers for Disease Control, Taipei.
Although vaccination coverage was 97.4% for three or more pertussis vaccine doses among Taiwanese children aged 12-23 months, many had not received proper vaccination at the right time.
“The study indicated that in Taiwan, undervaccination was decreasing but had placed young children at risk for pertussis,” the authors wrote.
Read the full study from Human Vaccines & Immunotherapeutics (2016. doi: 10.1080/21645515.2016.1249552).
Up to one-fifth of pertussis cases in Taiwanese children aged 3-35 months from 1996 to 2012 could have been prevented with on-time vaccination, reported Wan-Ting Huang and coauthors at the Taiwan Centers for Disease Control, Taipei.
Although vaccination coverage was 97.4% for three or more pertussis vaccine doses among Taiwanese children aged 12-23 months, many had not received proper vaccination at the right time.
“The study indicated that in Taiwan, undervaccination was decreasing but had placed young children at risk for pertussis,” the authors wrote.
Read the full study from Human Vaccines & Immunotherapeutics (2016. doi: 10.1080/21645515.2016.1249552).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Do fellowship programs help prepare general surgery residents for board exams?
WASHINGTON – Pass rates on the general surgery board exams were significantly higher for programs in which residents trained alongside surgical fellows, according to a retrospective study presented at the annual clinical congress of the American College of Surgeons.
The study adds another perspective to the ongoing debate about the impact of fellowships on general surgery residency and residency programs. Mohammed J. Al Fayyadh, MD, of the University of Texas Health Science Center at San Antonio and his associates investigated the impact of fellowships on program pass rates for general surgery boards. They reviewed American Board of Surgery exam data for the classes of 2010-2014, which included 242 programs (a total of 5,191 resident examinees), of which 148 had fellows participating (3,767 resident examinees).
The findings suggest that having fellows in a program has a positive impact on the pass rates of those taking the ABS general surgery exam. Pass rates were significantly higher for general surgery programs with fellows. This trend held for all measures studied: Qualifying (written) exam (88% vs. 86%), certifying (oral) exam (83% vs. 80%), and combined exams (74% vs. 69%). Differences between the groups were statistically significant.
The pass rates tended to be higher in programs with higher Fel:Res ratios. For example, programs with a 1.5:1 Fel:Res ratio had the highest pass rates for the qualifying, certifying, and combined exams.
The impact of subspecialty fellowships on general surgery residencies has been the subject of research and debate in recent years. Some studies have suggested that subspecialty training for fellows has meant that general surgery residents have less opportunity to operate in areas such as trauma (“Trauma operative training declining for general surgery residents,” ACS Surgery News, Oct. 4, 2016) and vascular surgery (Ann Vasc Surg. 2016;33;98-102).
Taken in this context, it is possible that the programs with more fellows simply recruit more competitive residents who tend to do better on exams. However, this could also reflect more resources in fellowship programs that are available to all trainees or better mentorship opportunities, said Dr. Al Fayyadh. He concluded that more research is needed to tease out the impact of fellowship programs on general surgery resident education.
Dr. Al Fayyadh had no disclosures.
WASHINGTON – Pass rates on the general surgery board exams were significantly higher for programs in which residents trained alongside surgical fellows, according to a retrospective study presented at the annual clinical congress of the American College of Surgeons.
The study adds another perspective to the ongoing debate about the impact of fellowships on general surgery residency and residency programs. Mohammed J. Al Fayyadh, MD, of the University of Texas Health Science Center at San Antonio and his associates investigated the impact of fellowships on program pass rates for general surgery boards. They reviewed American Board of Surgery exam data for the classes of 2010-2014, which included 242 programs (a total of 5,191 resident examinees), of which 148 had fellows participating (3,767 resident examinees).
The findings suggest that having fellows in a program has a positive impact on the pass rates of those taking the ABS general surgery exam. Pass rates were significantly higher for general surgery programs with fellows. This trend held for all measures studied: Qualifying (written) exam (88% vs. 86%), certifying (oral) exam (83% vs. 80%), and combined exams (74% vs. 69%). Differences between the groups were statistically significant.
The pass rates tended to be higher in programs with higher Fel:Res ratios. For example, programs with a 1.5:1 Fel:Res ratio had the highest pass rates for the qualifying, certifying, and combined exams.
The impact of subspecialty fellowships on general surgery residencies has been the subject of research and debate in recent years. Some studies have suggested that subspecialty training for fellows has meant that general surgery residents have less opportunity to operate in areas such as trauma (“Trauma operative training declining for general surgery residents,” ACS Surgery News, Oct. 4, 2016) and vascular surgery (Ann Vasc Surg. 2016;33;98-102).
Taken in this context, it is possible that the programs with more fellows simply recruit more competitive residents who tend to do better on exams. However, this could also reflect more resources in fellowship programs that are available to all trainees or better mentorship opportunities, said Dr. Al Fayyadh. He concluded that more research is needed to tease out the impact of fellowship programs on general surgery resident education.
Dr. Al Fayyadh had no disclosures.
WASHINGTON – Pass rates on the general surgery board exams were significantly higher for programs in which residents trained alongside surgical fellows, according to a retrospective study presented at the annual clinical congress of the American College of Surgeons.
The study adds another perspective to the ongoing debate about the impact of fellowships on general surgery residency and residency programs. Mohammed J. Al Fayyadh, MD, of the University of Texas Health Science Center at San Antonio and his associates investigated the impact of fellowships on program pass rates for general surgery boards. They reviewed American Board of Surgery exam data for the classes of 2010-2014, which included 242 programs (a total of 5,191 resident examinees), of which 148 had fellows participating (3,767 resident examinees).
The findings suggest that having fellows in a program has a positive impact on the pass rates of those taking the ABS general surgery exam. Pass rates were significantly higher for general surgery programs with fellows. This trend held for all measures studied: Qualifying (written) exam (88% vs. 86%), certifying (oral) exam (83% vs. 80%), and combined exams (74% vs. 69%). Differences between the groups were statistically significant.
The pass rates tended to be higher in programs with higher Fel:Res ratios. For example, programs with a 1.5:1 Fel:Res ratio had the highest pass rates for the qualifying, certifying, and combined exams.
The impact of subspecialty fellowships on general surgery residencies has been the subject of research and debate in recent years. Some studies have suggested that subspecialty training for fellows has meant that general surgery residents have less opportunity to operate in areas such as trauma (“Trauma operative training declining for general surgery residents,” ACS Surgery News, Oct. 4, 2016) and vascular surgery (Ann Vasc Surg. 2016;33;98-102).
Taken in this context, it is possible that the programs with more fellows simply recruit more competitive residents who tend to do better on exams. However, this could also reflect more resources in fellowship programs that are available to all trainees or better mentorship opportunities, said Dr. Al Fayyadh. He concluded that more research is needed to tease out the impact of fellowship programs on general surgery resident education.
Dr. Al Fayyadh had no disclosures.
AT THE ACS CLINICAL CONGRESS
School-located influenza vaccination programs can be effective
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
School-located influenza vaccination (SLIV) increased seasonal influenza vaccination rates countywide and in both suburban and urban settings, a study found.
“Schools have a stake in influenza vaccination because immunization of schoolchildren can reduce absenteeism throughout the community. Nevertheless, only 6% of childhood influenza vaccinations occur at school. SLIV poses logistical challenges: obtaining parental consent, ordering and administering vaccine, and billing,” said Peter G. Szilagyi, MD, of Mattel Children’s Hospital, Los Angeles, and his associates.
From 2014 to 2015, 44 elementary schools were randomized in upstate New York in an organized cluster-randomized trial in which 19,776 children were eligible candidates. Seven percent of SLIV school students, 5% of suburban SLIV school students, and 9% of urban SLIV students were vaccinated at SLIV clinics. Children in SLIV schools had higher flu vaccination rates than did children in control schools countywide (54% vs. 47%, P less than .001) and in suburban (62% vs. 54%, P less than .001) and urban schools (44% vs. 39%; P less than .001).
SLIV did substitute for vaccination for urban settings serving more Vaccines for Children–covered students, but did not substitute for practice-based vaccination in the suburbs, where pediatricians often preorder influenza vaccine.
“SLIV, using Web-based consent, is a potential strategy to improve influenza vaccination coverage among large populations of children,” the researchers concluded.
Read the full story here: Pediatrics. 2016. doi: 10.1542/peds.2016-1746.
FROM PEDIATRICS
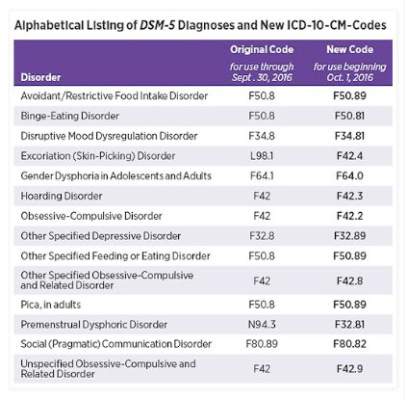
Changes in DSM-5 codes in ICD-10 go into effect Oct. 1
Earlier this month, the American Psychiatric Association released a supplement to the DSM-5 that updated codes for 14 diagnoses, including binge-eating disorder, disruptive mood dysregulation disorder, hoarding disorder, and premenstrual dysphoric disorder. On Oct. 1, those DSM-5 coding changes will be reflected within the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM).
Some of the changes are minor. For example, the code for binge-eating disorder is now F50.8 and will become F50.81 on Oct. 1. Disruptive mood dysregulation disorder will change from F34.8 to F34.81, and hoarding disorder will change from F42 to F42.3.
However, some of the changes are significant. The code for premenstrual dysphoric disorder, for example, will change from N94.3 to F32.81. The changes are aimed at improving diagnostic recording, communication among clinicians, and the collection of prevalence data.
To access the DSM-5 supplement, click here. A concise listing of the updated ICD-10-CM changes can be found here.
Earlier this month, the American Psychiatric Association released a supplement to the DSM-5 that updated codes for 14 diagnoses, including binge-eating disorder, disruptive mood dysregulation disorder, hoarding disorder, and premenstrual dysphoric disorder. On Oct. 1, those DSM-5 coding changes will be reflected within the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM).
Some of the changes are minor. For example, the code for binge-eating disorder is now F50.8 and will become F50.81 on Oct. 1. Disruptive mood dysregulation disorder will change from F34.8 to F34.81, and hoarding disorder will change from F42 to F42.3.
However, some of the changes are significant. The code for premenstrual dysphoric disorder, for example, will change from N94.3 to F32.81. The changes are aimed at improving diagnostic recording, communication among clinicians, and the collection of prevalence data.
To access the DSM-5 supplement, click here. A concise listing of the updated ICD-10-CM changes can be found here.
Earlier this month, the American Psychiatric Association released a supplement to the DSM-5 that updated codes for 14 diagnoses, including binge-eating disorder, disruptive mood dysregulation disorder, hoarding disorder, and premenstrual dysphoric disorder. On Oct. 1, those DSM-5 coding changes will be reflected within the International Classification of Diseases, Tenth Edition, Clinical Modification (ICD-10-CM).
Some of the changes are minor. For example, the code for binge-eating disorder is now F50.8 and will become F50.81 on Oct. 1. Disruptive mood dysregulation disorder will change from F34.8 to F34.81, and hoarding disorder will change from F42 to F42.3.
However, some of the changes are significant. The code for premenstrual dysphoric disorder, for example, will change from N94.3 to F32.81. The changes are aimed at improving diagnostic recording, communication among clinicians, and the collection of prevalence data.
To access the DSM-5 supplement, click here. A concise listing of the updated ICD-10-CM changes can be found here.
Merck Manual available as free app
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
The Merck Manual professional app is now available for free for use on either Android or Apple devices.
“In today’s mobile world, health care professionals expect critical health information at their fingertips, with or without an Internet connection,” said Robert S. Porter, MD, Merck Manuals’ editor-in-chief.
The app contains more than 1,000 photos and illustrations, and offline content can be stored on the device. A Wi-Fi connection is not needed to access the manual. When a cell phone is connected to the Internet, the app allows access to features such as videos of procedures, quizzes, medical news, editorials, and drug reference information.
Ninlaro receives approval for use in Canada
Health Canada has approved ixazomib (Ninlaro) for use in combination with lenalidomide and dexamethasone for the treatment of adults with relapsed/refractory multiple myeloma.
The U.S. Food and Drug Administration approved ixazomib in November 2015 for patients with relapsed/refractory multiple myeloma, based on data from TOURMALINE-MM1 that showed extended progression-free survival with a manageable safety profile.
“The approval of Ninlaro offers a much-needed new option for Canadian patients with multiple myeloma who have received at least one prior therapy. Its oral delivery may help multiple myeloma patients overcome some of the logistical burdens they may face with current therapies, which are typically administered in-clinic or in-hospital requiring significant travel and time constraints,” said Donna Reece, M.D., professor and director of the program for multiple myeloma and related diseases in the department of medical oncology and haematology at Princess Margaret Hospital/University of Toronto.
Ninlaro is marketed by Takeda. Click here to read the press release.
Health Canada has approved ixazomib (Ninlaro) for use in combination with lenalidomide and dexamethasone for the treatment of adults with relapsed/refractory multiple myeloma.
The U.S. Food and Drug Administration approved ixazomib in November 2015 for patients with relapsed/refractory multiple myeloma, based on data from TOURMALINE-MM1 that showed extended progression-free survival with a manageable safety profile.
“The approval of Ninlaro offers a much-needed new option for Canadian patients with multiple myeloma who have received at least one prior therapy. Its oral delivery may help multiple myeloma patients overcome some of the logistical burdens they may face with current therapies, which are typically administered in-clinic or in-hospital requiring significant travel and time constraints,” said Donna Reece, M.D., professor and director of the program for multiple myeloma and related diseases in the department of medical oncology and haematology at Princess Margaret Hospital/University of Toronto.
Ninlaro is marketed by Takeda. Click here to read the press release.
Health Canada has approved ixazomib (Ninlaro) for use in combination with lenalidomide and dexamethasone for the treatment of adults with relapsed/refractory multiple myeloma.
The U.S. Food and Drug Administration approved ixazomib in November 2015 for patients with relapsed/refractory multiple myeloma, based on data from TOURMALINE-MM1 that showed extended progression-free survival with a manageable safety profile.
“The approval of Ninlaro offers a much-needed new option for Canadian patients with multiple myeloma who have received at least one prior therapy. Its oral delivery may help multiple myeloma patients overcome some of the logistical burdens they may face with current therapies, which are typically administered in-clinic or in-hospital requiring significant travel and time constraints,” said Donna Reece, M.D., professor and director of the program for multiple myeloma and related diseases in the department of medical oncology and haematology at Princess Margaret Hospital/University of Toronto.
Ninlaro is marketed by Takeda. Click here to read the press release.
Pertussis often goes undiagnosed, especially in adults
A majority of pertussis cases in the United States may go undetected in people under the age of 50, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pa., and associates. Symptoms may be misdiagnosed as other respiratory illnesses, infected individuals may not seek treatment, and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, the investigator used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for ICD-9 diagnosed pertussis, found an annual incidence rate of 9/100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21/100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649/100,000 population, which is 58-93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures – such as increased vaccination – against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
The study was funded by GlaxoSmithKline Vaccines.
Read the full study in Human Vaccines & Immunotherapeutics (2016 May. doi: 10.1080/21645515.2016.1186313).
A majority of pertussis cases in the United States may go undetected in people under the age of 50, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pa., and associates. Symptoms may be misdiagnosed as other respiratory illnesses, infected individuals may not seek treatment, and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, the investigator used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for ICD-9 diagnosed pertussis, found an annual incidence rate of 9/100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21/100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649/100,000 population, which is 58-93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures – such as increased vaccination – against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
The study was funded by GlaxoSmithKline Vaccines.
Read the full study in Human Vaccines & Immunotherapeutics (2016 May. doi: 10.1080/21645515.2016.1186313).
A majority of pertussis cases in the United States may go undetected in people under the age of 50, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pa., and associates. Symptoms may be misdiagnosed as other respiratory illnesses, infected individuals may not seek treatment, and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, the investigator used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for ICD-9 diagnosed pertussis, found an annual incidence rate of 9/100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21/100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649/100,000 population, which is 58-93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures – such as increased vaccination – against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
The study was funded by GlaxoSmithKline Vaccines.
Read the full study in Human Vaccines & Immunotherapeutics (2016 May. doi: 10.1080/21645515.2016.1186313).
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS