User login
Endovascular treatment of acute IVC thrombosis found effective
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
FROM THE ANNALS OF VASCULAR SURGERY
Outpatient venography can be performed safely
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: Office-based iliac venography and stent placement may replace the need to perform these procedures in the hospital.
Major finding: Outpatient venography had the same therapeutic results and carried no greater risk as the same procedure done in an inpatient setting.
Data source: 245 patients who had undergone venography for the correction of suspected iliac vein stenosis in an office-based setting.
Disclosures: The researchers reported having no financial disclosures.
For high-risk SVT patients, anticoagulants may be effective option
Anticoagulation may be an effective option for patients with superficial venous thrombosis and are at high risk of venous thromboembolism (VTE), according to an evidence-based review by Dr. Joseph Raffetto and Dr. Robert Eberhardt.
In particular, they reviewed the results of three clinical trials with a total of nearly 1400 patients: STENOX, STEFLUX, and CALISTO.
Based on their assessment, Dr. Raffetto and Dr. Eberhardt, summarized that surgery and anticoagulants were both acceptable treatments for SVT patients at high risk of VTE, who had severe symptoms, who presented with close proximity to the saphenofemoral junction, or who had recurrence. Anticoagulants seemed to have fewer complications and a lower VTE rate than did surgery. However, treating all SVT patients with anticoagulants is not recommended because of cost concerns.
While anticoagulants do appear to be an effective treatment for SVT, “it is not known if SVT is causative of or an epiphenomenon for VTE. The optimal treatment of SVT is unknown with respect to selection of patient and vein, preferred therapy, and timing and duration of therapy,” the authors cautioned.
Find the full report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (doi: 10.1016/j.jvsv.2014.11.005).
Anticoagulation may be an effective option for patients with superficial venous thrombosis and are at high risk of venous thromboembolism (VTE), according to an evidence-based review by Dr. Joseph Raffetto and Dr. Robert Eberhardt.
In particular, they reviewed the results of three clinical trials with a total of nearly 1400 patients: STENOX, STEFLUX, and CALISTO.
Based on their assessment, Dr. Raffetto and Dr. Eberhardt, summarized that surgery and anticoagulants were both acceptable treatments for SVT patients at high risk of VTE, who had severe symptoms, who presented with close proximity to the saphenofemoral junction, or who had recurrence. Anticoagulants seemed to have fewer complications and a lower VTE rate than did surgery. However, treating all SVT patients with anticoagulants is not recommended because of cost concerns.
While anticoagulants do appear to be an effective treatment for SVT, “it is not known if SVT is causative of or an epiphenomenon for VTE. The optimal treatment of SVT is unknown with respect to selection of patient and vein, preferred therapy, and timing and duration of therapy,” the authors cautioned.
Find the full report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (doi: 10.1016/j.jvsv.2014.11.005).
Anticoagulation may be an effective option for patients with superficial venous thrombosis and are at high risk of venous thromboembolism (VTE), according to an evidence-based review by Dr. Joseph Raffetto and Dr. Robert Eberhardt.
In particular, they reviewed the results of three clinical trials with a total of nearly 1400 patients: STENOX, STEFLUX, and CALISTO.
Based on their assessment, Dr. Raffetto and Dr. Eberhardt, summarized that surgery and anticoagulants were both acceptable treatments for SVT patients at high risk of VTE, who had severe symptoms, who presented with close proximity to the saphenofemoral junction, or who had recurrence. Anticoagulants seemed to have fewer complications and a lower VTE rate than did surgery. However, treating all SVT patients with anticoagulants is not recommended because of cost concerns.
While anticoagulants do appear to be an effective treatment for SVT, “it is not known if SVT is causative of or an epiphenomenon for VTE. The optimal treatment of SVT is unknown with respect to selection of patient and vein, preferred therapy, and timing and duration of therapy,” the authors cautioned.
Find the full report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (doi: 10.1016/j.jvsv.2014.11.005).
FDA approves adhesive treatment for superficial varicose veins
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
The VenaSeal closure system, which uses an adhesive directly injected into the vein, has been approved as a permanent treatment for symptomatic, superficial varicose veins, the Food and Drug Administration announced on Feb. 20.
“This new system is the first to permanently treat varicose veins by sealing them with an adhesive,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the FDA’s statement. Because the system “does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising,” he added.
The VenaSeal system differs from other procedures used to treat varicose veins, which use drugs, lasers, radiofrequency, or incisions, the FDA statement points out. The complete sterile kit includes the adhesive (n-butyl-2-cyanoacrylate), which solidifies when injected directly into the target vein via a catheter, under ultrasound guidance. The additional system components include the catheter, the adhesive, a guidewire, dispenser gun, dispenser tips, and syringes.
Approval was based on data from three clinical trials sponsored by the manufacturer. In the U.S. study that compared results in 108 patients treated with the VenaSeal system and 114 patients treated with radiofrequency ablation therapy, the device was shown “to be safe and effective for vein closure for the treatment of symptomatic superficial varicose veins of the legs,” according to the FDA. In the study, adverse events associated with the VenaSeal treatment included phlebitis and paresthesias in the treated areas, which are “generally associated with treatments of this condition,” the FDA statement noted.
The agency reviewed the VenaSeal System as a class III medical device, considered the highest risk type of medical devices that are subjected to the highest level of regulatory control, and which must be approved before marketing.
VenaSeal is manufactured by Covidien, which acquired Sapheon, the company that developed VenaSeal, in 2014. The system has also been approved in Canada, Europe, and Hong Kong, according to a Covidien statement issued last year.
Even mild preop sepsis boosts postop thrombosis risk
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donze said at the annual meeting of the American College of Cardiology.
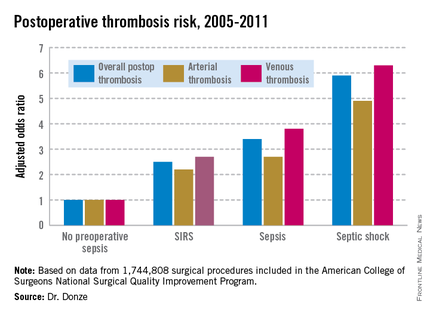
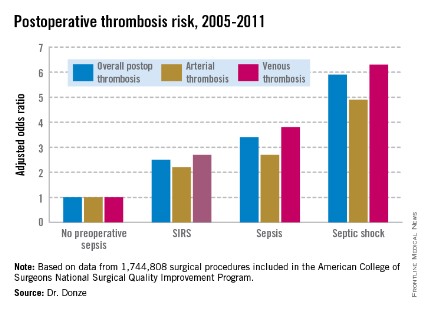
Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donze of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donze presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis.
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donze said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donze of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donze presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis.
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
WASHINGTON – Preoperative sepsis proved to be an important independent risk factor for both arterial and venous thrombosis during or after surgery in an analysis of nearly 1.75 million U.S. surgical procedures.
The take-home message here is that the risk-benefit assessment of surgical procedures should take into account the presence of sepsis. And if the surgery can’t be delayed, prophylaxis against arterial as well as venous thrombosis should be employed, Dr. Jacques Donze said at the annual meeting of the American College of Cardiology.

Another key finding in this study was that the risk of postoperative thrombosis varied according to the severity of preoperative sepsis. Even the early form of sepsis known as systemic inflammatory response syndrome, or SIRS, was associated with a 2.5-fold increased risk.
"Include even early signs of sepsis as a risk factor," urged Dr. Donze of Brigham and Women’s Hospital, Boston.
Also, preoperative sepsis was a risk factor for postoperative thrombosis in connection with outpatient elective surgery as well as inpatient operations, he added.
Dr. Donze presented an analysis of 1,744,808 surgical procedures performed during 2005-2011 at 314 U.S. hospitals participating in the American College of Surgeons National Surgical Quality Improvement Program. This large, prospective, observational registry is known for its high-quality data.
Within 48 hours prior to surgery, 7.8% of patients – totaling more than 136,000 – had SIRS, sepsis, or septic shock. Their postoperative thrombosis rate was 4.2%, compared with a 1.2% rate in patients without sepsis. In a multivariate regression analysis adjusted for potential confounding factors, the postoperative thrombosis risk climbed with increasing severity of preoperative sepsis.
SIRS was defined on the basis of temperature, heart rate, respiratory rate, WBC count, and/or the presence of anion gap acidosis. "Sepsis" was defined as SIRS plus infection. And septic shock required the presence of sepsis plus documented organ dysfunction, such as hypotension.
The importance of recognizing this newly spotlighted sepsis/postoperative thrombosis connection is that most of the other known risk factors for thrombosis in surgical patients, including age, cancer, renal failure, and immobilization, are nonmodifiable, Dr. Donze observed.
Among the factors known to contribute to thrombosis are a hypercoagulable state, a proinflammatory state, hypoxemia, hypotension, and endothelial dysfunction. "All of these factors can be triggered by sepsis," Dr. Donze noted.
He reported having no financial conflicts regarding this study.
AT ACC 14
Major finding: Preoperative sepsis is a strong independent risk factor for postoperative arterial and venous thrombosis; the more severe the sepsis, the greater the thrombosis risk.
Data source: This was an analysis of nearly 1.75 million surgical procedures at 314 U.S. hospitals detailed in the American College of Surgeons National Quality Improvement Program registry.
Disclosures: The presenter reported having no financial conflicts.
Endovascular coiling aids pelvic congestion syndrome
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
Pelvic venous congestion is misunderstood and frequently overlooked. Unfortunately pelvic pain is multifactorial. Even with significant reflux findings and encouraging results these patients, much like patients with other areas of venous insufficiency, frequently recur if followed longitudinally. Good markers to predict who will benefit from intervention and which interventions should be undertaken do not exist. This is an area that needs further study and development of standard outcome measures that can be followed sequentially.
Dr. Joann M. Lohr is associate program director, Good Samaritan Hospital Vascular Surgery Program She is also an associate medical editor for Vascular Specialist.
Pelvic venous congestion is misunderstood and frequently overlooked. Unfortunately pelvic pain is multifactorial. Even with significant reflux findings and encouraging results these patients, much like patients with other areas of venous insufficiency, frequently recur if followed longitudinally. Good markers to predict who will benefit from intervention and which interventions should be undertaken do not exist. This is an area that needs further study and development of standard outcome measures that can be followed sequentially.
Dr. Joann M. Lohr is associate program director, Good Samaritan Hospital Vascular Surgery Program She is also an associate medical editor for Vascular Specialist.
Pelvic venous congestion is misunderstood and frequently overlooked. Unfortunately pelvic pain is multifactorial. Even with significant reflux findings and encouraging results these patients, much like patients with other areas of venous insufficiency, frequently recur if followed longitudinally. Good markers to predict who will benefit from intervention and which interventions should be undertaken do not exist. This is an area that needs further study and development of standard outcome measures that can be followed sequentially.
Dr. Joann M. Lohr is associate program director, Good Samaritan Hospital Vascular Surgery Program She is also an associate medical editor for Vascular Specialist.
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
CHICAGO – Endovascular coiling should be offered to women with pelvic congestion syndrome as an effective treatment.
"The technical success rate is high, pain scores were significantly improved, and most importantly, the patient satisfaction with resolution of their symptoms is very high," Dr. Axel Thors said at the annual meeting of the Midwestern Vascular Surgical Society.
He reported on a 4-year review involving 15 women with pelvic congestion syndrome (PCS) who underwent endovenous coil embolization (n = 14) or stenting of the iliac vein (n = 1).
The diagnosis of PCS was made clinically by the presence of chronic pelvic pain for 6 months or more, sensations of pelvic fullness, dyspareunia, or perineal varicosities. There was no evidence of nutcracker syndrome or perirenal varicosities. Other pathologies had been previously ruled out.
"By the time these women got to us, we were probably the last provider they had seen and they had all undergone extensive evaluation for their pelvic pain, all the way from their primary providers to the ob.gyns.," said Dr. Thors of Ohio State University, Columbus.
Their average age was 36 years. Fourteen patients had a previous pregnancy, with an average parity of two.
Twelve patients presented with symptomatic vulvar varices and three with imaging or laproscopic findings of tubo-ovarian varices. All had complaints of chronic pelvic pain.
"Lower extremity venous insufficiency was closely associated with the incidence [of PCS], as was chronic dyspareunia," Dr. Thors said.
Gonadal vein venograms were performed during normal breath and the Valsalva maneuver. Embolization was performed if there was gonadal vein incompetence, congestion of the ovarian venous plexus, uterine venous congestion, cross-pelvic congestion, or marked enlargement of gonadal veins (minimum 6 mm). The average venality size was 7.3 mm.
In all, 13 gonadal veins were embolized with an average of three coils, ranging in size from 6 mm to 12 mm, Dr. Thors said.
Four gonadal veins were occluded using an Amplatzer plug (range 12-18 mm). One iliac vein was stented with a 16 mm by 60 mm stent.
Lower-extremity venous insufficiency was treated with ablation and subsequently followed clinically, he said.
Pain scores on a 10-point visual analog scale declined significantly from baseline for eight evaluable patients for pelvic pain (9.3 vs. 1.8), dyspareunia (8.875 vs. 1.5), painful vulvar varices (9.2 vs. 1.2), and lower extremity venous insufficiency (7 vs. 1), he said.
Two patients had recurrence, and their baseline pain score of 1.2 increased to 4.0 after a mean of 21 months.
All eight patients reported that they were "satisfied" or "very satisfied" with their procedure.
"Patients with chronic pelvic pain, vulvar varices, multiparity, and lower extremity venous insufficiency should be offered endovascular evaluation and treatment," Dr. Thors concluded.
Audience members said that the study represents an important concept in the management of these patients. It is a validation of a very old treatment that sometimes is not offered because of a lack of knowledge or perceived lack of data. A 2012 Agency for Healthcare Research and Quality review estimated that outpatient management of chronic pelvic pain cost $1.2 billion annually. The AHRQ review of 36 studies concluded that there is insufficient evidence to demonstrate the effectiveness of surgical approaches for chronic pelvic pain.
Dr. Thors and his coauthors reported having no financial disclosures.
AT MIDWESTERN VASCULAR 2013
Major finding: Key numerical finding (e.g., number needed to treat to prevent one death/event; number lived or died as result of intervention). Maximum 10 words/1 sentence.
Data source: Review of 15 women treated for pelvic congestion syndrome.
Disclosures: Dr. Thors and his coauthors reported having no financial disclosures.
Ten tips for chronic venous ulcers
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
The underlying pathophysiology of chronic venous insufficiency is complex and involves many factors. Studies have shown that average venous ulcers may need 6-12 months for complete healing with an anticipated recurrence rate exceeding 2/3 cases in 5 years. These numbers reflect the magnitude of the problem and mandate deploying all efforts to stop progression of the disease. Our group has found the following 10 tips have significantly improved our healing rates.
1. First, rule out any associated arterial, immunologic, endocrine, or other systemic causes for leg/foot ulceration.
2. Be aggressive to stop progression of the disease (fight CEAP 6): any local tenderness at the site of discolored skin at the gaiter area for venous ulcers should initiate a prompt reflux study to evaluate for incompetent perforators.
3. Venous ulcers are associated with an incompetent perforator within 2 cm of the ulcer area.
4. Recurrent venous ulcers at the same location may be associated with venous outflow obstruction, (May-Thurner syndrome is an underestimated pathology) which affects mainly the left leg.
5. When performing iliac vein venograms, make liberal use of intravascular ultrasound.
6. Exudative venous ulcers need multilayer compression dressings and appropriate antibiotics if infection exists.
7. Pentoxifylline (Trental) 800 mg, 3 times daily.
8. Frequent debridement and frequent objective evaluation for ulcer area with each office visit.
9. Bi-layered living cell treatment (Apligraf?) to promote healing.
10. Office/clinic visit every 3 months after complete healing (CEAP 5) and further testing as needed.
Dr. Mousa is an associate professor at the Department of Surgery, West Virginia University, Morgantown.
Hemodialysis AV graft patency similar for forearm, upper arm
SAN FRANCISCO – Outcomes of forearm and upper arm hemodialysis arteriovenous grafts are similar despite the fact that large caliber outflow veins are often encountered in the upper arm, results from a large trial showed.
"To preserve a maximal number of access sites, forearm location should always be considered before resorting to an upper arm graft," Dr. Alik Farber said at the Society for Vascular Surgery Annual Meeting.
The incidence and prevalence of end-stage renal disease in the United States has grown exponentially in the past 25 years, said Dr. Farber, chief of vascular and endovascular surgery at Boston University Medical Center. "In fact, in 2010 almost 400,000 patients were undergoing hemodialysis," he said. "At the same time, there has been a steady increase in the percent of AV fistulas placed and an associated decline in the percent of AV grafts placed in the United States. In 2010, 20% of patients were undergoing hemodialysis through AV grafts."
Most grafts in the upper extremity are based on the brachial artery. Some are in the forearm while others are in the upper arm. "In the forearm most grafts are looped," Dr. Farber said. "In the upper arm some are looped and some are straight. As it turns out, the optimal graft configuration is unknown. The optimal venous outflow in the upper extremity is unknown. And the optimal location of the first-time AV graft is controversial."
He went on to note that the forearm AV graft "saves the upper arm for a future graft site and has a potential advantage of increasing the suitability of upper arm veins for future native fistula. On the other hand, there is some evidence in the literature that forearm grafts have lower patency rates. The upper arm graft may have higher patency rates because they are ‘sawn into’ large caliber veins. However, surgeons who preferentially place upper arm grafts tend to skip potential distal access sites."
Given the dearth of information on this topic, Dr. Farber and his associates set out to compare outcomes of forearm and upper arm grafts and to evaluate the association between upper extremity AV graft configuration, location, venous outflow, and patency in 649 patients from a multicenter trial conducted by the Dialysis Access Consortium (DAC). This was a randomized, controlled trial of dipyridamole versus placebo in patients with new AV grafts. It found that dipyridamole increased primary unassisted graft patency (N. Engl. J. Med. 2009;360:2191-201). "The important thing for us was that this is the largest randomized, controlled trial of AV grafts conducted to date," Dr. Farber said.
He presented results from 522 patients with AV grafts that were based on the brachial artery. Of the 522 patients, 269 had a forearm graft (fAVG) and 253 had an upper arm graft (uAVG). The primary outcome was loss of primary unassisted patency. "This was defined as a first occurrence of graft thrombosis, an access procedure to correct a greater than 50% stenosis, or other surgical graft modification," Dr. Farber explained. The secondary outcome was cumulative graft failure, "which was defined as the time from randomization to complete loss of access site for dialysis." Kaplan-Meier curves and Cox models were used to examine the effects of access location and configuration on study outcomes.
Compared with patients in the fAVG group, those in the uAVG group were more likely to be male (43% vs. 34%), to be African-American (78% vs. 62%), to have a lower body mass index (mean of 29 kg/m2 vs. a mean of 32 kg/m2), to have a lower baseline systolic blood pressure (139 mm Hg vs. 146 mm Hg), to have hemodialysis initiated before graft placement (80% vs. 64%), and to be on dialysis for a longer period of time (a mean of 2.59 years vs. a mean of 2.51 years).
Unadjusted analyses showed that there was no significant difference in the loss of primary unassisted graft patency or cumulative graft failure between the fAVG and uAVG groups.
Multivariate analyses of outcomes controlled for covariates revealed that the risk of loss of primary unassisted graft patency was not significantly higher in the uAVG group, compared with the fAVG group (hazard ratio of 1.24; P = .15). However, there was a suggestion of an association of increased risk of cumulative graft failure among upper arm grafts (HR 1.37; P = .09).
In a comparison of straight vs. looped grafts, straight configuration grafts "appeared to have a lower risk of primary and secondary failure, compared with looped figuration grafts, [but] this difference was not statistically significant," he said.
When compared to forearm looped grafts, which were used as a reference, there was no significant difference in the risk of primary and secondary failure among straight fAVGs, straight uAVGs, and looped uAVGs. There was a suggestion of increased risk of failure among upper arm looped grafts (HR 1.47; P = .06). There were also no significant differences between the two groups in adverse events and complications at 30 days.
Dr. Farber acknowledged certain limitations of the study. "Like any observational comparison of treatment groups, analysis was susceptible to uncontrolled confounding [variables]," he said. "We did a post hoc analysis of a randomized trial which did not answer the questions that we posed. Preoperative artery and vein diameters were not recorded and the reasons for graft selection are not known. Lastly, access interventions were followed for only 30 days beyond the occurrence of the primary endpoint, so we couldn’t really use access intervention to thoroughly evaluate the determinants of cumulative graft failure."
Dr. Farber said that he had no disclosures.
An aphorism in dialysis procedures is that one should start distal and move proximally only after all distal procedures have been exhausted. Occlusion of a proximal site may preclude a more distal site that might have originally been useable. However some surgeons have favored an upper arm graft because of perceived improved long term patency. This review shows that is not necessarily the case. However, most of us who do a significant amount of dialysis realize there are many variables that enter into the decision process as to where to place the graft. In the end it is probably more “art” than “science” that colors our decisions!
Dr. Russell Samson is the Medical Editor, Vascular Specialist.
An aphorism in dialysis procedures is that one should start distal and move proximally only after all distal procedures have been exhausted. Occlusion of a proximal site may preclude a more distal site that might have originally been useable. However some surgeons have favored an upper arm graft because of perceived improved long term patency. This review shows that is not necessarily the case. However, most of us who do a significant amount of dialysis realize there are many variables that enter into the decision process as to where to place the graft. In the end it is probably more “art” than “science” that colors our decisions!
Dr. Russell Samson is the Medical Editor, Vascular Specialist.
An aphorism in dialysis procedures is that one should start distal and move proximally only after all distal procedures have been exhausted. Occlusion of a proximal site may preclude a more distal site that might have originally been useable. However some surgeons have favored an upper arm graft because of perceived improved long term patency. This review shows that is not necessarily the case. However, most of us who do a significant amount of dialysis realize there are many variables that enter into the decision process as to where to place the graft. In the end it is probably more “art” than “science” that colors our decisions!
Dr. Russell Samson is the Medical Editor, Vascular Specialist.
SAN FRANCISCO – Outcomes of forearm and upper arm hemodialysis arteriovenous grafts are similar despite the fact that large caliber outflow veins are often encountered in the upper arm, results from a large trial showed.
"To preserve a maximal number of access sites, forearm location should always be considered before resorting to an upper arm graft," Dr. Alik Farber said at the Society for Vascular Surgery Annual Meeting.
The incidence and prevalence of end-stage renal disease in the United States has grown exponentially in the past 25 years, said Dr. Farber, chief of vascular and endovascular surgery at Boston University Medical Center. "In fact, in 2010 almost 400,000 patients were undergoing hemodialysis," he said. "At the same time, there has been a steady increase in the percent of AV fistulas placed and an associated decline in the percent of AV grafts placed in the United States. In 2010, 20% of patients were undergoing hemodialysis through AV grafts."
Most grafts in the upper extremity are based on the brachial artery. Some are in the forearm while others are in the upper arm. "In the forearm most grafts are looped," Dr. Farber said. "In the upper arm some are looped and some are straight. As it turns out, the optimal graft configuration is unknown. The optimal venous outflow in the upper extremity is unknown. And the optimal location of the first-time AV graft is controversial."
He went on to note that the forearm AV graft "saves the upper arm for a future graft site and has a potential advantage of increasing the suitability of upper arm veins for future native fistula. On the other hand, there is some evidence in the literature that forearm grafts have lower patency rates. The upper arm graft may have higher patency rates because they are ‘sawn into’ large caliber veins. However, surgeons who preferentially place upper arm grafts tend to skip potential distal access sites."
Given the dearth of information on this topic, Dr. Farber and his associates set out to compare outcomes of forearm and upper arm grafts and to evaluate the association between upper extremity AV graft configuration, location, venous outflow, and patency in 649 patients from a multicenter trial conducted by the Dialysis Access Consortium (DAC). This was a randomized, controlled trial of dipyridamole versus placebo in patients with new AV grafts. It found that dipyridamole increased primary unassisted graft patency (N. Engl. J. Med. 2009;360:2191-201). "The important thing for us was that this is the largest randomized, controlled trial of AV grafts conducted to date," Dr. Farber said.
He presented results from 522 patients with AV grafts that were based on the brachial artery. Of the 522 patients, 269 had a forearm graft (fAVG) and 253 had an upper arm graft (uAVG). The primary outcome was loss of primary unassisted patency. "This was defined as a first occurrence of graft thrombosis, an access procedure to correct a greater than 50% stenosis, or other surgical graft modification," Dr. Farber explained. The secondary outcome was cumulative graft failure, "which was defined as the time from randomization to complete loss of access site for dialysis." Kaplan-Meier curves and Cox models were used to examine the effects of access location and configuration on study outcomes.
Compared with patients in the fAVG group, those in the uAVG group were more likely to be male (43% vs. 34%), to be African-American (78% vs. 62%), to have a lower body mass index (mean of 29 kg/m2 vs. a mean of 32 kg/m2), to have a lower baseline systolic blood pressure (139 mm Hg vs. 146 mm Hg), to have hemodialysis initiated before graft placement (80% vs. 64%), and to be on dialysis for a longer period of time (a mean of 2.59 years vs. a mean of 2.51 years).
Unadjusted analyses showed that there was no significant difference in the loss of primary unassisted graft patency or cumulative graft failure between the fAVG and uAVG groups.
Multivariate analyses of outcomes controlled for covariates revealed that the risk of loss of primary unassisted graft patency was not significantly higher in the uAVG group, compared with the fAVG group (hazard ratio of 1.24; P = .15). However, there was a suggestion of an association of increased risk of cumulative graft failure among upper arm grafts (HR 1.37; P = .09).
In a comparison of straight vs. looped grafts, straight configuration grafts "appeared to have a lower risk of primary and secondary failure, compared with looped figuration grafts, [but] this difference was not statistically significant," he said.
When compared to forearm looped grafts, which were used as a reference, there was no significant difference in the risk of primary and secondary failure among straight fAVGs, straight uAVGs, and looped uAVGs. There was a suggestion of increased risk of failure among upper arm looped grafts (HR 1.47; P = .06). There were also no significant differences between the two groups in adverse events and complications at 30 days.
Dr. Farber acknowledged certain limitations of the study. "Like any observational comparison of treatment groups, analysis was susceptible to uncontrolled confounding [variables]," he said. "We did a post hoc analysis of a randomized trial which did not answer the questions that we posed. Preoperative artery and vein diameters were not recorded and the reasons for graft selection are not known. Lastly, access interventions were followed for only 30 days beyond the occurrence of the primary endpoint, so we couldn’t really use access intervention to thoroughly evaluate the determinants of cumulative graft failure."
Dr. Farber said that he had no disclosures.
SAN FRANCISCO – Outcomes of forearm and upper arm hemodialysis arteriovenous grafts are similar despite the fact that large caliber outflow veins are often encountered in the upper arm, results from a large trial showed.
"To preserve a maximal number of access sites, forearm location should always be considered before resorting to an upper arm graft," Dr. Alik Farber said at the Society for Vascular Surgery Annual Meeting.
The incidence and prevalence of end-stage renal disease in the United States has grown exponentially in the past 25 years, said Dr. Farber, chief of vascular and endovascular surgery at Boston University Medical Center. "In fact, in 2010 almost 400,000 patients were undergoing hemodialysis," he said. "At the same time, there has been a steady increase in the percent of AV fistulas placed and an associated decline in the percent of AV grafts placed in the United States. In 2010, 20% of patients were undergoing hemodialysis through AV grafts."
Most grafts in the upper extremity are based on the brachial artery. Some are in the forearm while others are in the upper arm. "In the forearm most grafts are looped," Dr. Farber said. "In the upper arm some are looped and some are straight. As it turns out, the optimal graft configuration is unknown. The optimal venous outflow in the upper extremity is unknown. And the optimal location of the first-time AV graft is controversial."
He went on to note that the forearm AV graft "saves the upper arm for a future graft site and has a potential advantage of increasing the suitability of upper arm veins for future native fistula. On the other hand, there is some evidence in the literature that forearm grafts have lower patency rates. The upper arm graft may have higher patency rates because they are ‘sawn into’ large caliber veins. However, surgeons who preferentially place upper arm grafts tend to skip potential distal access sites."
Given the dearth of information on this topic, Dr. Farber and his associates set out to compare outcomes of forearm and upper arm grafts and to evaluate the association between upper extremity AV graft configuration, location, venous outflow, and patency in 649 patients from a multicenter trial conducted by the Dialysis Access Consortium (DAC). This was a randomized, controlled trial of dipyridamole versus placebo in patients with new AV grafts. It found that dipyridamole increased primary unassisted graft patency (N. Engl. J. Med. 2009;360:2191-201). "The important thing for us was that this is the largest randomized, controlled trial of AV grafts conducted to date," Dr. Farber said.
He presented results from 522 patients with AV grafts that were based on the brachial artery. Of the 522 patients, 269 had a forearm graft (fAVG) and 253 had an upper arm graft (uAVG). The primary outcome was loss of primary unassisted patency. "This was defined as a first occurrence of graft thrombosis, an access procedure to correct a greater than 50% stenosis, or other surgical graft modification," Dr. Farber explained. The secondary outcome was cumulative graft failure, "which was defined as the time from randomization to complete loss of access site for dialysis." Kaplan-Meier curves and Cox models were used to examine the effects of access location and configuration on study outcomes.
Compared with patients in the fAVG group, those in the uAVG group were more likely to be male (43% vs. 34%), to be African-American (78% vs. 62%), to have a lower body mass index (mean of 29 kg/m2 vs. a mean of 32 kg/m2), to have a lower baseline systolic blood pressure (139 mm Hg vs. 146 mm Hg), to have hemodialysis initiated before graft placement (80% vs. 64%), and to be on dialysis for a longer period of time (a mean of 2.59 years vs. a mean of 2.51 years).
Unadjusted analyses showed that there was no significant difference in the loss of primary unassisted graft patency or cumulative graft failure between the fAVG and uAVG groups.
Multivariate analyses of outcomes controlled for covariates revealed that the risk of loss of primary unassisted graft patency was not significantly higher in the uAVG group, compared with the fAVG group (hazard ratio of 1.24; P = .15). However, there was a suggestion of an association of increased risk of cumulative graft failure among upper arm grafts (HR 1.37; P = .09).
In a comparison of straight vs. looped grafts, straight configuration grafts "appeared to have a lower risk of primary and secondary failure, compared with looped figuration grafts, [but] this difference was not statistically significant," he said.
When compared to forearm looped grafts, which were used as a reference, there was no significant difference in the risk of primary and secondary failure among straight fAVGs, straight uAVGs, and looped uAVGs. There was a suggestion of increased risk of failure among upper arm looped grafts (HR 1.47; P = .06). There were also no significant differences between the two groups in adverse events and complications at 30 days.
Dr. Farber acknowledged certain limitations of the study. "Like any observational comparison of treatment groups, analysis was susceptible to uncontrolled confounding [variables]," he said. "We did a post hoc analysis of a randomized trial which did not answer the questions that we posed. Preoperative artery and vein diameters were not recorded and the reasons for graft selection are not known. Lastly, access interventions were followed for only 30 days beyond the occurrence of the primary endpoint, so we couldn’t really use access intervention to thoroughly evaluate the determinants of cumulative graft failure."
Dr. Farber said that he had no disclosures.
AT THE SVS ANNUAL MEETING
Major finding: The risk of loss of primary unassisted graft patency was not significantly higher in patients who had an upper arm arteriovenous graft compared with those who had a forearm AV graft (hazard ratio of 1.24; P = .15). However, there was a suggestion of an association of increased risk of cumulative graft failure among upper arm grafts (HR 1.37; P = .09).
Data source: A study of 522 hemodialysis patients with AV grafts based on the brachial artery. Of these, 269 had a forearm graft and 253 had an upper arm graft.
Disclosures: Dr. Farber said that he had no disclosures.
Use of 'liberation therapy' may make MS worse
SAN DIEGO -- Percutaneous transluminal venous angioplasty – also known as "liberation therapy" -- doesn't help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
The technique "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chest and neck that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
The possibility of a causal relationship between MS and CCSVI gave patients with that chronic, debilitating, relapsing disease a glimmer of hope. Anecdotal reports of dramatic improvement with "liberation therapy" for treatment of MS raised expectations even further and created a demand for CCSVI interventions. Still, many vascular and neurological specialists remained skeptical of this approach, citing the lack of high-level evidence to support it. Some were even accused (mostly by patients) of withholding an effective treatment, while advocates of intervention tended to minimize the risks and cost involved. In spite of the controversy surrounding MS and CCSVI, there should be agreement that the "gold standard" for determining treatment efficacy is the randomized controlled clinical trial, and liberation therapy must be held to that standard. While not definitive, the small trial summarized here is a step in the right direction.
Dr. Robert Eugene Zierler, MD, is at the University of Washington, Seattle.
The possibility of a causal relationship between MS and CCSVI gave patients with that chronic, debilitating, relapsing disease a glimmer of hope. Anecdotal reports of dramatic improvement with "liberation therapy" for treatment of MS raised expectations even further and created a demand for CCSVI interventions. Still, many vascular and neurological specialists remained skeptical of this approach, citing the lack of high-level evidence to support it. Some were even accused (mostly by patients) of withholding an effective treatment, while advocates of intervention tended to minimize the risks and cost involved. In spite of the controversy surrounding MS and CCSVI, there should be agreement that the "gold standard" for determining treatment efficacy is the randomized controlled clinical trial, and liberation therapy must be held to that standard. While not definitive, the small trial summarized here is a step in the right direction.
Dr. Robert Eugene Zierler, MD, is at the University of Washington, Seattle.
The possibility of a causal relationship between MS and CCSVI gave patients with that chronic, debilitating, relapsing disease a glimmer of hope. Anecdotal reports of dramatic improvement with "liberation therapy" for treatment of MS raised expectations even further and created a demand for CCSVI interventions. Still, many vascular and neurological specialists remained skeptical of this approach, citing the lack of high-level evidence to support it. Some were even accused (mostly by patients) of withholding an effective treatment, while advocates of intervention tended to minimize the risks and cost involved. In spite of the controversy surrounding MS and CCSVI, there should be agreement that the "gold standard" for determining treatment efficacy is the randomized controlled clinical trial, and liberation therapy must be held to that standard. While not definitive, the small trial summarized here is a step in the right direction.
Dr. Robert Eugene Zierler, MD, is at the University of Washington, Seattle.
SAN DIEGO -- Percutaneous transluminal venous angioplasty – also known as "liberation therapy" -- doesn't help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
The technique "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chest and neck that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
SAN DIEGO -- Percutaneous transluminal venous angioplasty – also known as "liberation therapy" -- doesn't help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
The technique "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chest and neck that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
CCSVI Controversy: A Call for New Research
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
To say that the relationship between CCSVI and multiple sclerosis is controversial is an understatement. This article introduces both sides of the story and summarizes the current evidence. While many physicians remain understandably skeptical, it is worth remembering that "conventional wisdom" on the pathophysiology of some diseases has turned out to be way off the mark - consider peptic ulcers. Clearly, more objective data is needed before routine interventions for CCSVI can be recommended, and hopefully, ongoing registries and trials will provide that data. In the meantime, those of us involved with the vascular laboratory should be prepared to evaluate patients for the abnormalities described in CCSVI. Detection of reflux and obstruction in the internal jugular and vertebral veins by duplex ultrasound (in both supine and seated positions) is based on the same general principles as the more familiar upper extremity venous and carotid duplex examinations. Ideally, patients should be screened and entered into a clinical study to establish an evidence base for future decision-making. Until then, we will be stuck between the "liberators" and the "nihilists", and our patients will be caught in the middle.
Robert Eugene Zierler, M.D., is professor of surgery, division of vascular surgery, department of surgery, and medical director, Vascular Diagnostic Service, University of Washington School of Medicine, Seattle, Wash. He is also an associate medical editor for Vascular Specialist.
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.
Multiple sclerosis patients and endovascular interventionalists were elated when Italian researchers reported in 2009 that they had found evidence of chronic cerebrospinal venous insufficiency in nearly every MS patient they had studied and that in many cases, balloon angioplasty and sometimes stent placement of central thoracic veins reduced or eliminated signs of the disease. Neurologists, on the other hand, suggested that hope might be eclipsing reason in the rush to advocate the vascular procedure, given the single-center study's small sample size and nonrandomized, uncontrolled design.
The opposing perspectives incited an apparent turf war within the MS community fueled by accusations on both sides, according to Dr. Jack Burks, chief medical officer of the Multiple Sclerosis Association of America. At issue, he said, is the validity not only of the study results but also of the underlying hypothesis that toxic iron overload in the brain due to chronic cerebrospinal venous insufficiency (CCSVI) might have a primary role in the pathogenesis of MS - a hypothesis that contradicts the compelling body of evidence suggesting that MS is primarily an autoimmune condition.
On one side of the debate are the MS patients and endovascular interventionalists, dubbed the "liberators" by Dr. Burks because of their unflappable advocacy for what has become known as the liberation procedure - the endovascular surgery designed to open the lesions causing the venous insufficiency, he said.
"Neurologists believe the interventionalists are overstating the possible value of CCSVI and that commercial interests are overriding scientific inquiry," according to Dr. Burks, a neurologist and clinical professor of medicine at the University of Nevada, Reno. Patients, armed with anecdotal evidence downloaded from the Internet, are certain that CCSVI surgery is the miracle they've been waiting for and perceive the hesitancy of U.S. and Canadian neurologists to embrace the treatment as evidence of a possible conspiracy with pharmaceutical companies who stand to lose billions of dollars if the surgery becomes a first-line treatment, he said. Further, he noted, advocates of CCSVI claim that neurologists who refuse patients' demands for diagnostic testing and surgical referral for CCSVI are jeopardizing the safety of those patients, who are traveling to foreign countries to get the care that they cannot receive in North America.
To date, the majority of the evidence regarding CCSVI diagnosis and treatment in MS is inconsistent, and can be confusing, Dr. Burks noted. In the initial study, Dr. Paolo Zamboni of the University of Ferrara in Italy, and colleagues, used Doppler ultrasound to examine venous drainage of the brain and spinal cord in 65 patients with different types of MS and 235 controls without MS and observed abnormal venous flow in all of the MS patients and none of the controls. The patterns of venous obstruction differed depending on MS stage and course, although there was no apparent relationship between disease severity and extent of venous obstruction, and MS treatment status did not influence the signs of CCSVI in any of the patients, the authors wrote (J. Neurol. Neurosurg. Psychiatry 2009;80:392-9).
The researchers went on to conduct an open pilot study to determine whether percutaneous transluminal angioplasty could safely and effectively treat the narrowing of the extracranial cerebrospinal veins in the 65 MS patients in which the condition was observed - 35 with relapsing-remitting MS, 20 with secondary progressive MS, and 10 with primary progressive MS. They reported significant improvements in MS clinical outcome measures, significant reductions in new brain lesions on MRI, and significant reductions in the number of relapses experienced by some of the patients.
The findings were limited, however, not only by the study design, but also by the fact that patients remained on their disease-modifying antirheumatic drug therapy during the study period and the timing and type of MRI scans varied among the patients, according to the authors. They also noted that restenosis of the internal jugular veins occurred in nearly half of the patients (J. Vasc. Surg. 2009;50:1348-58).
Since the initial paper, a number of CCSVI studies of various designs have been undertaken, with contradictory results. Following are some of the investigations reported within the past year:
P Researchers at the University of Buffalo found that up to 62% of the 280 patients with MS enrolled in the Combined Transcranial and Extracranial Venous Doppler Evaluation study - the first randomized clinical trial to evaluate MS patients for CCSVI - had the characteristic narrowing of the extracranial veins compared with approximately 22% of 220 healthy controls. While the results, which were reported at the annual meeting of the American Academy of Neurology, did not establish causation, they showed "that narrowing of the extracranial veins, at the very least, is an important association in multiple sclerosis," principal investigator Dr. Robert Zivadinov said in a statement. He acknowledged that the finding of vascular narrowing in nearly a quarter of the healthy controls warranted additional investigation (www.buffalo.edu/news/fast-execute.cgi/article-page.html?article=109370009).
P In an open-label study of extracranial Doppler criteria of CCSVI in 70 MS patients in Poland - 49 with relapsing-remitting MS, 5 with primary progressive MS, and 16 with secondary progressive MS - investigators detected at least two of four extracranial criteria in 90% of the patients. They concluded that, while the extracranial abnormalities could exist in various combinations, "the most common pathology in our patients was the presence of an inverted valve or another pathologic structure [like membranaceous or netlike septum] in the area of junction of the [internal jugular vein] with the brachiocephalic vein (Int. Angiol. 2010;29:109-14).
P A comparison of the internal jugular vein hemodynamics and morphology in 25 patients with MS and 25 controls identified abnormal findings in 92% of the MS patients and 24% of the controls, and evidence of CCSVI in 84% of the MS patients and none of the controls, leading the investigators to conclude that both hemodynamic abnormalities and morphologic changes in the internal jugular vein "are strongly associated with MS" (Int. Angiol. 2010;29:115-20).
P An extended extra- and transcranial color-coded sonography study in 56 MS patients and 20 controls detected no internal jugular vein stenosis and normal blood flow direction in all but 1 patient. There were no between-group differences in intracranial veins and during Valsalva maneuver, and none of the patients fulfilled more than one CCSVI criterion, according to the authors. They concluded that their findings "challenge the hypothesis that cerebral venous congestion plays a significant role in the pathogenesis of MS" (Ann. Neurol. 2010;68:173-83).
P Swedish investigators used phase-contrast MRI to study 21 relapsing-remitting MS patients and 20 healthy controls and found no differences in internal jugular venous outflow, aqueductal cerebrospinal fluid flow, or the presence of internal jugular blood reflux between the two groups. Although contrast-enhanced MR angiography showed internal jugular vein stenosis in 3 of the 21 MS patients, the authors stated they found no evidence "confirming the suggested vascular multiple sclerosis hypothesis" (Ann. Neurol. 2010;68:255-9).
P The authors of an MR venography and flow quantification study in The Netherlands compared the intracranial and extracranial venous anatomy and the intracerebral venous flow profiles of 20 MS patients and 20 age- and gender-matched controls, with image analysis performed by blinded interventional neuroradiologists. They identified venous system anomalies in 50% of the MS patients and 40% of the healthy controls and no venous backflow in either group. "Given the normal intracranial venous flow quantification results, it is likely that these findings reflect anatomical variants of venous drainage rather than clinically relevant venous outflow obstructions," the authors wrote (J. Neurol. Neurosurg. Psychiatry 2010 Oct. 27 [doi: 10.1136/jnnp.2010.223479]).
P Italian researchers investigating the occurrence of CCSVI in 50 consecutive patients with clinically isolated syndromes suggestive of MS reviewed the patients' extracranial and transcranial venous echo-color Doppler sonographs and compared the findings to those of 50 age- and gender-matched healthy controls as well as those of 60 patients with transient global amnesia (TGA) and 60 healthy controls matched to the TGA patients. They found extracranial Doppler sonographic abnormalities in 52% of patients with possible MS, 68.3% of patients with TGA, and 31.8% of the healthy controls. While eight of the patients with possible MS fulfilled the CCSVI criteria, selective phlebography showed no venous anomalies in seven of them. The authors concluded that there was no evidence of CCSVI at MS onset but recommended further studies to "clarify whether CCSVI is associated with later disease stages and characterizes the progressive forms of MS" (Ann. Neurol. 2011;69:90-9).
In a position statement, the Society of Interventional Radiology stated that at present, the published literature is "inconclusive on whether CCSVI is a clinically important factor and on whether balloon angioplasty and/or stent placement are effective in patients with MS" (J. Vasc. Interv. Radiol. 2010;21:1335-7).
Additionally, in a commentary on the treatment of CCSVI, representatives of the Cardiovascular and Interventional Radiological Society of Europe acknowledged that although several centers worldwide are promoting and performing balloon dilatation, with or without stenting for CCSVI, "We believe that until real scientific data are available for CCSVI and balloon dilatation, this treatment should not be offered to MS patients outside of a well designed clinical trial" (Cardiovasc. Intervent. Radiol. 2011;34:1-2).
Toward that end, the National Multiple Sclerosis Society of the United States and the MS Society of Canada have pledged $2.4 million in support of seven CCSVI research studies, including projects designed to evaluate venous abnormalities in children and teens with MS, patients with early and late stage MS, and those at risk for MS.
An international review panel comprising radiologists, vascular surgeons, and neurologists evaluated research applications via an expedited review process, according to the societies.
Dr. Burks disclosed relationships with various pharmaceutical companies. Dr. Baracchini had no relevant conflicts.