User login
Structural changes may separate axial psoriatic arthritis from axial spondyloarthritis
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
Approximately 20% of adults with axial psoriatic arthritis (PsA) show active or structural spinal changes without changes in the sacroiliac joint, based on imaging data from 106 individuals.
Axial PsA has been historically grouped with axial spondyloarthritis (axSpA), but it has received more attention in recent years as a condition potentially distinct from axSpA, Henriette Käding, an MD and PhD student in the department of gastroenterology, infectiology, and rheumatology at Charité-Universitätsmedizin Berlin, said in her research presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). She added that the debate persists as to whether these conditions are on the same spectrum or should be separated.
Data from previous studies suggest differences in genetic, clinical, radiographic, and prognostic characteristics between axial PsA and axSpA that may affect patients’ response to available treatments. However, there are relatively little data available on distinguishing imaging and clinical features, and there’s a lack of classification criteria for axial PsA, Ms. Käding said.
Ms. Käding and colleagues prospectively collected data from 106 patients with axial PsA between August 2019 and June 2023 and presented the baseline data of this longitudinal project at the GRAPPA annual meeting in Dublin. At baseline, the researchers conducted clinical assessments of the participants, along with blood sampling, stool samples, and imaging protocols that included MRI of the whole spine and sacroiliac joint (SIJ).
The mean age of the included patients was 44.5 years; 55.7% were female. Inflammatory back pain was present in most of the patients at baseline (78.4%), and 48.1% were positive for HLA-B27, a genetic risk factor for both axSpA and axial PsA. Approximately one-third of the patients had elevated C-reactive protein (> 5 mg/L). In the baseline MRI scans, active inflammatory changes in the sacroiliac joints (SIJ) were seen in 51.9% of the patients and structural changes in 72.1%. MRI spine scans showed active changes in 58.7% of the patients. Notably, active and/or structural changes of the spine without changes in the SIJ appeared in 20% of the patients, Ms. Käding said.
With regard to existing classification criteria, the researchers observed that 92% of the patients met the CASPAR (Classification Criteria for Psoriatic Arthritis) criteria for PsA, 73% met the ASAS (Assessment of Spondyloarthritis International Society) criteria, while 66% of patients met both ASAS and CASPAR criteria.
The study will be the first to include longitudinal MRI scans of the whole spine and SIJ in addition to conventional radiographs, Ms. Käding said.
Better characterization should improve treatment
“Axial involvement in PsA might, on one hand, go unnoticed, but on the other hand, it could also be misdiagnosed in patients with degenerative spinal disease,” Denis Poddubnyy, MD, one of the study coauthors, also of Charité-Universitätsmedizin Berlin, said in an interview.
“By comprehending the unique characteristics, progression, and treatment responses within the axial domain, rheumatologists can customize interventions and therapies to effectively manage the psoriatic disease,” Dr. Poddubnyy said.
“One of the most significant findings [of the current study] is the relatively high frequency of spinal involvement without sacroiliac joint” involvement, Fabian Proft, MD, of Charité-Universitätsmedizin Berlin and senior author of the study, said in an interview. “This finding holds importance as, in primary axial SpA, the disease typically originates in the sacroiliac joints. In contrast, in PsA, the scenario differs, which has implications for the diagnostic approach in clinical practice.”
“In individuals with PsA, spinal involvement can occur independently of sacroiliac joint [involvement]. As a result, imaging studies conducted on patients suspected of having axial PsA should encompass not only the sacroiliac joints but also the spine,” Dr. Poddubnyy explained. “It is important to note, however, that imaging findings such as bony spurs and bone marrow edema might be caused by degeneration or mechanical issues and, therefore, need to be interpreted with caution within the clinical context.”
The study was supported in part by an unrestricted research grant from Novartis. Dr. Poddubnyy and Dr. Proft disclosed receiving research grants and consultancy payments from Novartis and serving on speaker bureaus for the company.
FROM GRAPPA 2023
Spondyloarthritis-related diseases share gut microbiota dysbiosis
TOPLINE:
Patients with spondyloarthritis (SpA) experience similar gut microbiota dysbiosis with related inflammatory conditions, such as acute anterior uveitis (AAU) and Crohn’s disease (CD), new data show.
METHODOLOGY:
- Researchers performed 16S rRNA sequencing on stool samples from 277 adult patients from the German Spondyloarthritis Inception Cohort (102 with SpA, 72 with CD, and 103 with AAU) and 62 control patients with chronic back pain for whom SpA had been ruled out.
- Patients were treatment naive to biologic disease-modifying antirheumatic drugs or had not received them for more than 3 months prior to study enrollment.
- The study is the first to identify the same microbiota in patients with SpA, AAU, and CD.
TAKEAWAY:
- “Our results showed a shared depletion of predominately Lachnospiraceae taxa, most notably Fusicatenibacter, which partially mediated increased CRP [C-reactive protein], and was most abundant in controls receiving NSAID monotherapy,” the researchers wrote.
- Among patients who tested positive for HLA-B27, an allele associated with SpA and other spondyloarthropathies, levels of Faecalibacterium were increased; among patients with SpA, levels of Collinsella were enriched; and among patients with CD, there was an abundance of beneficial Ruminococcus bacteria.
- The results suggest the diagnostic and therapeutic potential of the gut microbiome for mediating disease activity for patients with autoimmune diseases.
- Additional research is needed to clarify the roles of different bacteria in gut-joint inflammation and to understand the relationship between genetics and gut microbes.
IN PRACTICE:
The study is too preliminary to have applications for practice.
SOURCE:
Co–first authors Morgan Essex, MSc, and Valeria Rios Rodriguez, MD, of Charité–Universitätsmedizin Berlin and colleagues conducted the study, which was published online July 20, 2023, in Arthritis and Rheumatology.
LIMITATIONS:
- The results were limited by several factors, including the restriction to amplicon sequencing, which prevented in-depth characterization of the gut microbiome.
- More studies are needed to validate the findings, especially regarding gut bacteria as potential mediators of inflammation or disease activity. The researchers recommended studies with whole-genome sequencing and fecal metabolite quantification.
DISCLOSURES:
The study was supported in part by the Deutsche Forschungsgemeinschaft. Additional funding came from the German Federal Ministry for Health and Research and the Berlin Institute of Health. Two patient cohorts were partially and separately supported by grants from Novartis and AbbVie.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with spondyloarthritis (SpA) experience similar gut microbiota dysbiosis with related inflammatory conditions, such as acute anterior uveitis (AAU) and Crohn’s disease (CD), new data show.
METHODOLOGY:
- Researchers performed 16S rRNA sequencing on stool samples from 277 adult patients from the German Spondyloarthritis Inception Cohort (102 with SpA, 72 with CD, and 103 with AAU) and 62 control patients with chronic back pain for whom SpA had been ruled out.
- Patients were treatment naive to biologic disease-modifying antirheumatic drugs or had not received them for more than 3 months prior to study enrollment.
- The study is the first to identify the same microbiota in patients with SpA, AAU, and CD.
TAKEAWAY:
- “Our results showed a shared depletion of predominately Lachnospiraceae taxa, most notably Fusicatenibacter, which partially mediated increased CRP [C-reactive protein], and was most abundant in controls receiving NSAID monotherapy,” the researchers wrote.
- Among patients who tested positive for HLA-B27, an allele associated with SpA and other spondyloarthropathies, levels of Faecalibacterium were increased; among patients with SpA, levels of Collinsella were enriched; and among patients with CD, there was an abundance of beneficial Ruminococcus bacteria.
- The results suggest the diagnostic and therapeutic potential of the gut microbiome for mediating disease activity for patients with autoimmune diseases.
- Additional research is needed to clarify the roles of different bacteria in gut-joint inflammation and to understand the relationship between genetics and gut microbes.
IN PRACTICE:
The study is too preliminary to have applications for practice.
SOURCE:
Co–first authors Morgan Essex, MSc, and Valeria Rios Rodriguez, MD, of Charité–Universitätsmedizin Berlin and colleagues conducted the study, which was published online July 20, 2023, in Arthritis and Rheumatology.
LIMITATIONS:
- The results were limited by several factors, including the restriction to amplicon sequencing, which prevented in-depth characterization of the gut microbiome.
- More studies are needed to validate the findings, especially regarding gut bacteria as potential mediators of inflammation or disease activity. The researchers recommended studies with whole-genome sequencing and fecal metabolite quantification.
DISCLOSURES:
The study was supported in part by the Deutsche Forschungsgemeinschaft. Additional funding came from the German Federal Ministry for Health and Research and the Berlin Institute of Health. Two patient cohorts were partially and separately supported by grants from Novartis and AbbVie.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with spondyloarthritis (SpA) experience similar gut microbiota dysbiosis with related inflammatory conditions, such as acute anterior uveitis (AAU) and Crohn’s disease (CD), new data show.
METHODOLOGY:
- Researchers performed 16S rRNA sequencing on stool samples from 277 adult patients from the German Spondyloarthritis Inception Cohort (102 with SpA, 72 with CD, and 103 with AAU) and 62 control patients with chronic back pain for whom SpA had been ruled out.
- Patients were treatment naive to biologic disease-modifying antirheumatic drugs or had not received them for more than 3 months prior to study enrollment.
- The study is the first to identify the same microbiota in patients with SpA, AAU, and CD.
TAKEAWAY:
- “Our results showed a shared depletion of predominately Lachnospiraceae taxa, most notably Fusicatenibacter, which partially mediated increased CRP [C-reactive protein], and was most abundant in controls receiving NSAID monotherapy,” the researchers wrote.
- Among patients who tested positive for HLA-B27, an allele associated with SpA and other spondyloarthropathies, levels of Faecalibacterium were increased; among patients with SpA, levels of Collinsella were enriched; and among patients with CD, there was an abundance of beneficial Ruminococcus bacteria.
- The results suggest the diagnostic and therapeutic potential of the gut microbiome for mediating disease activity for patients with autoimmune diseases.
- Additional research is needed to clarify the roles of different bacteria in gut-joint inflammation and to understand the relationship between genetics and gut microbes.
IN PRACTICE:
The study is too preliminary to have applications for practice.
SOURCE:
Co–first authors Morgan Essex, MSc, and Valeria Rios Rodriguez, MD, of Charité–Universitätsmedizin Berlin and colleagues conducted the study, which was published online July 20, 2023, in Arthritis and Rheumatology.
LIMITATIONS:
- The results were limited by several factors, including the restriction to amplicon sequencing, which prevented in-depth characterization of the gut microbiome.
- More studies are needed to validate the findings, especially regarding gut bacteria as potential mediators of inflammation or disease activity. The researchers recommended studies with whole-genome sequencing and fecal metabolite quantification.
DISCLOSURES:
The study was supported in part by the Deutsche Forschungsgemeinschaft. Additional funding came from the German Federal Ministry for Health and Research and the Berlin Institute of Health. Two patient cohorts were partially and separately supported by grants from Novartis and AbbVie.
A version of this article appeared on Medscape.com.
Could risk stratifying methotrexate users lead to less frequent testing?
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
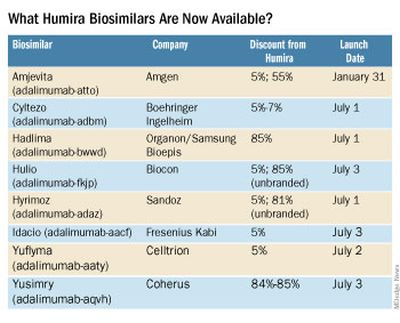
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Does colchicine have a role in treating excess ASCVD risk in patients with chronic inflammatory conditions?
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
Methotrexate does not impair sperm quality, small study finds
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
After Yusimry’s steep discount, little clarity on future adalimumab biosimilar pricing
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
High-intensity interval training has sustainable effects in patients with inflammatory arthritis
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MILAN – High-intensity interval training (HIIT) has been shown to enhance cardiorespiratory fitness (CRF) and mitigate cardiovascular disease (CVD) risk factors in patients with inflammatory joint diseases (IJD) in a randomized trial. Notably, the positive response in CRF did not coincide with changes in pain or fatigue.
Kristine Norden, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Norwegian National Advisory Unit on Rehabilitation in Rheumatology, Diakonhjemmet Hospital, Oslo, presented the late-breaking results of the ExeHeart trial at the annual European Congress of Rheumatology. The trial aimed to evaluate the short- and long-term effects of 12 weeks of supervised HIIT in patients with IJD.
Ms. Norden said in an interview that “HIIT is a feasible physiotherapeutic intervention with sustainable effects in patients with IJD. It does not exacerbate symptoms of IJD and can be implemented in primary care settings.”
The trial
The ExeHeart trial is a randomized controlled trial designed to assess the effects of HIIT on CRF, CVD risk, and disease activity in patients with IJD. The trial is a collaborative effort with patient research partners and aligns with patients’ requests for effective nonpharmacologic treatments. The outcomes being evaluated include CRF (primary outcome), CVD risk factors, anthropometric measures, disease activity, and patient-reported outcomes related to pain, fatigue, disease, physical activity, and exercise.
A total of 60 patients with IJD were recruited from the Preventive Cardio-Rheuma clinic at Diakonhjemmet. They were randomly assigned to receive either standard care (including relevant lifestyle advice and cardiopreventive medication) or standard care along with a 12-week HIIT intervention supervised by physiotherapists. Assessments were conducted at baseline, at 3 months (primary endpoint), and at 6 months post baseline. There was no supervised intervention between the 3- and 6-month time points.
The median age of the participants was 59 years, with 34 participants (57%) being women. The types of IJD among the participants included rheumatoid arthritis in 45%, spondyloarthritis in 32%, and psoriatic arthritis in 23%. Furthermore, 49 patients (82%) had a high risk for CVD.
The participants were divided into two groups: a control group (n = 30) and a HIIT group (n = 30). The HIIT group underwent a 12-week intervention consisting of twice-a-week supervised 4x4-minute HIIT sessions at 90%-95% of peak heart rate, alternated with moderate activity at 70%. The control group engaged in unsupervised moderate-intensity exercise sessions. The primary outcome measured was the change in CRF, assessed through peak oxygen uptake (VO2 max) using a cardiopulmonary exercise test. Secondary outcomes – pain and fatigue – were evaluated using a questionnaire (Numeric Rating Scale 0-10, where 0 represents no pain or fatigue).
Following HIIT, a statistically significant difference was observed in VO2 max (2.5 mL/kg per min; P < .01) in favor of the exercise group at 3 months, while no significant differences were found in pain and fatigue. This discrepancy in VO2 max between the groups was maintained at 6 months (2.6 mL/kg per min; P < .01), with no notable disparities in pain and fatigue. A per-protocol analysis at 3 months demonstrated a difference in VO2 max between the groups (3.2 mL/kg per min; P < .01).
Ms. Norden concluded that the clinical implications of these findings are significant, as increased CRF achieved through HIIT reflects an improvement in the body’s ability to deliver oxygen to working muscles. Consequently, this enhancement in CRF can lead to overall health improvements and a reduced risk for CVD.
Long-lasting effects
Christopher Edwards, MBBS, MD, honorary consultant rheumatologist at University Hospital Southampton (England) NHS Foundation Trust Medicine, University of Southampton, was concerned about future maintenance of increased CRF. “I really wish we had data on these patients at 12 months as well, so we could see if the effects last even longer. Regarding intensity, there are clear indications that engaging in moderate and high-intensity workouts is more beneficial,” Dr. Norden said. “So, I would certainly recommend at least one high-intensity exercise session per week for those patients, while also incorporating lower and moderate-intensity exercises if desired. However, for individuals aiming to maximize their oxygen uptake, high-intensity exercise is considered the most effective approach.”
There is compelling evidence supporting the benefits of physical activity in improving disease activity among patients with IJD, making it a critical component of nonpharmacologic treatment. However, individuals with rheumatic and musculoskeletal conditions generally exhibit lower levels of physical activity, compared with their healthy counterparts. Recognizing the importance of CVD prevention in patients with IJD, EULAR recommends routine CVD screening for individuals diagnosed with IJD.
Ms. Norden and coauthors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EULAR 2023
Early axial spondyloarthritis diagnosis in referred patients remains stable in most
MILAN – Most people with recent-onset chronic back pain who are referred to a rheumatologist and then diagnosed with definite axial spondyloarthritis (axSpA) maintain that diagnosis over the next 2 years, but for those with residual diagnostic uncertainty for axSpA, particular characteristics may help to identify those who will or will not go on to receive a definite diagnosis, according to presentations given at the annual European Congress of Rheumatology.
Although a rheumatologist’s early axSpA diagnosis is reliable, new research also presented at the meeting reveals that the axSpA clinical phenotype presentation has great heterogeneity around the world, adding to the challenge.
These findings also dovetail with the consensus of an expert panel from the Assessment of SpondyloArthritis international Society (ASAS) that determined early axSpA should be defined by a duration of axial symptoms of less than 2 years, a move that should make research studies of early disease more consistent.
Diagnosis at first sight
To help in overcoming the long diagnostic delay typically encountered by patients with axSpA, researchers involved in the longitudinal Spondyloarthritis Caught Early (SPACE) cohort have sought to measure the prevalence of axSpA and the reliability of an early diagnosis in patients with chronic back pain (CBP). SPACE researcher Mary Lucy Marques, MD, a rheumatologist at Coimbra (Portugal) Hospital and University Center, and PhD student at Leiden (the Netherlands) University Medical Center, presented the main results of the study, which included patients younger than 45 years with CBP of unknown origin lasting 3 months to 2 years.
Patients referred to rheumatologists were judged at each visit for the presence or absence of axSpA, and the baseline judgment was reviewed after 2 years to assess its reliability. Baseline diagnostic judgments remained rather stable, and definite axSpA was present in one-third of the patients referred to the rheumatologist (175 out of 555 patients; 32%). After 2 years, the number of patients with definite axSpA diagnosis changed to 165, due to 5% of the definite diagnoses being refuted and 8% gaining a definite axSpA diagnosis. Among the features related to axSpA, the presence (or absence) of imaging-detected sacroiliitis at baseline was the best discriminator for a definite diagnosis at 2 years.
In commenting on these findings, Alexandre Sepriano, MD, PhD, assistant professor of rheumatology, NOVA Medical School, Lisbon, Portugal, who was not involved in the study, said: “These data show that the key is likely the referral of the ‘right patients’ to tertiary care centers. The [ASAS] has developed referral criteria that can be used for this purpose. According to these, patients with chronic low back pain starting before 45 years of age should be referred to a rheumatologist if at least one additional SpA feature is present.
“It should be acknowledged that axSpA is not a disease of males only. In fact, there is a 1:1 ratio between males and females in the full spectrum of the disease. Also, although imaging findings are important, not all patients will have these. Similarly, not all patients with imaging abnormalities will have the disease, and their sole presence without other SpA features does not suffice for diagnosis.”
Repeated assessment: Is it worth it?
Despite the positive findings described above, residual diagnostic uncertainty remained for 15% of patients, representing an obstacle to initiating an appropriate treatment. Therefore, it is important to understand whether and how the repeated assessment of axSpA features is of value for a definite diagnosis.
This last question was addressed in a second abstract also presented by Dr. Marques that focused on the yield of repeated assessment in CBP patients with suspected axSpA from the SPACE cohort. The main outcome of the study was the clinical diagnosis of definite axSpA at 2 years. Compared with baseline, at the 2-year evaluation 32 patients changed their diagnosis and were classified as definite axSpA: Sixteen were previously described as uncertain axSpA at baseline, 11 as uncertain no axSpA, and 5 as definite no axSpA.
On average, three axSpA features were present at baseline with one or two adjunctive features found throughout the study that led to the final diagnosis of definite axSpA. These adjunctive features were most commonly response to NSAIDs and sacroiliitis on MRI. Dr. Marques and colleagues concluded that the yield of repeated assessment in this setting was modest for a new diagnosis of definite axSpA. “Usefulness of repeating MRI in terms of diagnostic yield is low but can be considered in HLA-B27+ patients, especially if male,” Dr. Marques said, commenting on the analysis of SpA features in patients who changed their diagnosis to definite axSpA at 2 years.
“The early diagnosis of axial spondyloarthritis remains a challenge,” Dr. Sepriano said in commenting on the second SPACE study. “Probably one of the main reasons is the yet suboptimal awareness of the [full spectrum] of the disease in a primary care setting, in which most patients will first show up to get medical care. It is now well-known that patients do not always have changes in pelvic radiographs and that waiting for these to make a diagnosis of [radiographic] axSpA results in further delay and in missing many patients who will never develop these changes.
“Still, recognizing the clinical picture of early axSpA and differentiating it from other more common causes of chronic back pain (e.g., degenerative spinal disease) can sometimes be difficult. Continuous efforts in raising awareness and in education will likely result in further reducing the diagnostic delay gap and, as such, improve the prognosis of this often-debilitating rheumatic inflammatory disease.”
One epidemiologic size does not fit all
According to data from the International Map of Axial Spondyloarthritis (IMAS), axSpA clinical phenotype presentation shows great heterogeneity around the world. Marco Garrido-Cumbrera, PhD, of the University of Seville in Spain, presented the results of an analysis of an IMAS online survey (2017-2022).
The study, supported by Novartis, aimed at exploring differences in axSpA clinical phenotype presentation in a large sample of unselected patients: a total of 5,557 individuals from 27 countries across five regions. The results showed statistically significant differences among countries in almost all the analyzed characteristics, from age at onset of symptoms (the highest in Latin America) to HLA-B27 positivity frequency (lowest in Latin America and highest in Asia).
Differences also emerged in the presence of a positive family history of the disease (most common in Europe) and of physical and mental comorbidities (common in Africa). The authors also reported treatment data showing that most of the patients had used NSAIDs, and almost half of the patients had ever taken biologic disease-modifying antirheumatic drugs. Data also showed a mean delay in diagnosis of 7 years, with the longest values observed in South Africa and the lowest in Asia.
A consensus definition of early AxSpA
Early axSpA for the first time has been defined based on ASAS expert consensus, and the definition was presented at the meeting by Victoria Navarro-Compán, MD, PhD, of La Paz University Hospital, Madrid. An international working group came to a definition based on the symptom duration and taking solely axial symptoms into account. At the end of a five-step process, the group successfully developed the first consensus definition of early axSpA: “patients with diagnosis of axSpA with axial symptoms duration of ≤ 2 years.” Also to be noted are axial symptoms as assessed by a rheumatologist, which should include spinal/buttock pain or morning stiffness.
As reported by the authors, this ASAS definition is based on expert consensus, with the limitation of a lack of scientific evidence to support it, especially with regard to the specific duration of symptoms from the time of disease onset. Nonetheless, ASAS recommends the use of this definition in studies referring to early axSpA.
Dr. Marques reports no relevant financial relationships. Dr. Navarro-Compán reports serving on the speakers bureau for AbbVie, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; consulting for AbbVie, Eli Lilly, Galapagos, MoonLake, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; and receiving grant/research support from AbbVie and Novartis. Dr. Garrido-Cumbrera reports receiving grant or research support from Novartis.
A version of this article originally appeared on Medscape.com.
MILAN – Most people with recent-onset chronic back pain who are referred to a rheumatologist and then diagnosed with definite axial spondyloarthritis (axSpA) maintain that diagnosis over the next 2 years, but for those with residual diagnostic uncertainty for axSpA, particular characteristics may help to identify those who will or will not go on to receive a definite diagnosis, according to presentations given at the annual European Congress of Rheumatology.
Although a rheumatologist’s early axSpA diagnosis is reliable, new research also presented at the meeting reveals that the axSpA clinical phenotype presentation has great heterogeneity around the world, adding to the challenge.
These findings also dovetail with the consensus of an expert panel from the Assessment of SpondyloArthritis international Society (ASAS) that determined early axSpA should be defined by a duration of axial symptoms of less than 2 years, a move that should make research studies of early disease more consistent.
Diagnosis at first sight
To help in overcoming the long diagnostic delay typically encountered by patients with axSpA, researchers involved in the longitudinal Spondyloarthritis Caught Early (SPACE) cohort have sought to measure the prevalence of axSpA and the reliability of an early diagnosis in patients with chronic back pain (CBP). SPACE researcher Mary Lucy Marques, MD, a rheumatologist at Coimbra (Portugal) Hospital and University Center, and PhD student at Leiden (the Netherlands) University Medical Center, presented the main results of the study, which included patients younger than 45 years with CBP of unknown origin lasting 3 months to 2 years.
Patients referred to rheumatologists were judged at each visit for the presence or absence of axSpA, and the baseline judgment was reviewed after 2 years to assess its reliability. Baseline diagnostic judgments remained rather stable, and definite axSpA was present in one-third of the patients referred to the rheumatologist (175 out of 555 patients; 32%). After 2 years, the number of patients with definite axSpA diagnosis changed to 165, due to 5% of the definite diagnoses being refuted and 8% gaining a definite axSpA diagnosis. Among the features related to axSpA, the presence (or absence) of imaging-detected sacroiliitis at baseline was the best discriminator for a definite diagnosis at 2 years.
In commenting on these findings, Alexandre Sepriano, MD, PhD, assistant professor of rheumatology, NOVA Medical School, Lisbon, Portugal, who was not involved in the study, said: “These data show that the key is likely the referral of the ‘right patients’ to tertiary care centers. The [ASAS] has developed referral criteria that can be used for this purpose. According to these, patients with chronic low back pain starting before 45 years of age should be referred to a rheumatologist if at least one additional SpA feature is present.
“It should be acknowledged that axSpA is not a disease of males only. In fact, there is a 1:1 ratio between males and females in the full spectrum of the disease. Also, although imaging findings are important, not all patients will have these. Similarly, not all patients with imaging abnormalities will have the disease, and their sole presence without other SpA features does not suffice for diagnosis.”
Repeated assessment: Is it worth it?
Despite the positive findings described above, residual diagnostic uncertainty remained for 15% of patients, representing an obstacle to initiating an appropriate treatment. Therefore, it is important to understand whether and how the repeated assessment of axSpA features is of value for a definite diagnosis.
This last question was addressed in a second abstract also presented by Dr. Marques that focused on the yield of repeated assessment in CBP patients with suspected axSpA from the SPACE cohort. The main outcome of the study was the clinical diagnosis of definite axSpA at 2 years. Compared with baseline, at the 2-year evaluation 32 patients changed their diagnosis and were classified as definite axSpA: Sixteen were previously described as uncertain axSpA at baseline, 11 as uncertain no axSpA, and 5 as definite no axSpA.
On average, three axSpA features were present at baseline with one or two adjunctive features found throughout the study that led to the final diagnosis of definite axSpA. These adjunctive features were most commonly response to NSAIDs and sacroiliitis on MRI. Dr. Marques and colleagues concluded that the yield of repeated assessment in this setting was modest for a new diagnosis of definite axSpA. “Usefulness of repeating MRI in terms of diagnostic yield is low but can be considered in HLA-B27+ patients, especially if male,” Dr. Marques said, commenting on the analysis of SpA features in patients who changed their diagnosis to definite axSpA at 2 years.
“The early diagnosis of axial spondyloarthritis remains a challenge,” Dr. Sepriano said in commenting on the second SPACE study. “Probably one of the main reasons is the yet suboptimal awareness of the [full spectrum] of the disease in a primary care setting, in which most patients will first show up to get medical care. It is now well-known that patients do not always have changes in pelvic radiographs and that waiting for these to make a diagnosis of [radiographic] axSpA results in further delay and in missing many patients who will never develop these changes.
“Still, recognizing the clinical picture of early axSpA and differentiating it from other more common causes of chronic back pain (e.g., degenerative spinal disease) can sometimes be difficult. Continuous efforts in raising awareness and in education will likely result in further reducing the diagnostic delay gap and, as such, improve the prognosis of this often-debilitating rheumatic inflammatory disease.”
One epidemiologic size does not fit all
According to data from the International Map of Axial Spondyloarthritis (IMAS), axSpA clinical phenotype presentation shows great heterogeneity around the world. Marco Garrido-Cumbrera, PhD, of the University of Seville in Spain, presented the results of an analysis of an IMAS online survey (2017-2022).
The study, supported by Novartis, aimed at exploring differences in axSpA clinical phenotype presentation in a large sample of unselected patients: a total of 5,557 individuals from 27 countries across five regions. The results showed statistically significant differences among countries in almost all the analyzed characteristics, from age at onset of symptoms (the highest in Latin America) to HLA-B27 positivity frequency (lowest in Latin America and highest in Asia).
Differences also emerged in the presence of a positive family history of the disease (most common in Europe) and of physical and mental comorbidities (common in Africa). The authors also reported treatment data showing that most of the patients had used NSAIDs, and almost half of the patients had ever taken biologic disease-modifying antirheumatic drugs. Data also showed a mean delay in diagnosis of 7 years, with the longest values observed in South Africa and the lowest in Asia.
A consensus definition of early AxSpA
Early axSpA for the first time has been defined based on ASAS expert consensus, and the definition was presented at the meeting by Victoria Navarro-Compán, MD, PhD, of La Paz University Hospital, Madrid. An international working group came to a definition based on the symptom duration and taking solely axial symptoms into account. At the end of a five-step process, the group successfully developed the first consensus definition of early axSpA: “patients with diagnosis of axSpA with axial symptoms duration of ≤ 2 years.” Also to be noted are axial symptoms as assessed by a rheumatologist, which should include spinal/buttock pain or morning stiffness.
As reported by the authors, this ASAS definition is based on expert consensus, with the limitation of a lack of scientific evidence to support it, especially with regard to the specific duration of symptoms from the time of disease onset. Nonetheless, ASAS recommends the use of this definition in studies referring to early axSpA.
Dr. Marques reports no relevant financial relationships. Dr. Navarro-Compán reports serving on the speakers bureau for AbbVie, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; consulting for AbbVie, Eli Lilly, Galapagos, MoonLake, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; and receiving grant/research support from AbbVie and Novartis. Dr. Garrido-Cumbrera reports receiving grant or research support from Novartis.
A version of this article originally appeared on Medscape.com.
MILAN – Most people with recent-onset chronic back pain who are referred to a rheumatologist and then diagnosed with definite axial spondyloarthritis (axSpA) maintain that diagnosis over the next 2 years, but for those with residual diagnostic uncertainty for axSpA, particular characteristics may help to identify those who will or will not go on to receive a definite diagnosis, according to presentations given at the annual European Congress of Rheumatology.
Although a rheumatologist’s early axSpA diagnosis is reliable, new research also presented at the meeting reveals that the axSpA clinical phenotype presentation has great heterogeneity around the world, adding to the challenge.
These findings also dovetail with the consensus of an expert panel from the Assessment of SpondyloArthritis international Society (ASAS) that determined early axSpA should be defined by a duration of axial symptoms of less than 2 years, a move that should make research studies of early disease more consistent.
Diagnosis at first sight
To help in overcoming the long diagnostic delay typically encountered by patients with axSpA, researchers involved in the longitudinal Spondyloarthritis Caught Early (SPACE) cohort have sought to measure the prevalence of axSpA and the reliability of an early diagnosis in patients with chronic back pain (CBP). SPACE researcher Mary Lucy Marques, MD, a rheumatologist at Coimbra (Portugal) Hospital and University Center, and PhD student at Leiden (the Netherlands) University Medical Center, presented the main results of the study, which included patients younger than 45 years with CBP of unknown origin lasting 3 months to 2 years.
Patients referred to rheumatologists were judged at each visit for the presence or absence of axSpA, and the baseline judgment was reviewed after 2 years to assess its reliability. Baseline diagnostic judgments remained rather stable, and definite axSpA was present in one-third of the patients referred to the rheumatologist (175 out of 555 patients; 32%). After 2 years, the number of patients with definite axSpA diagnosis changed to 165, due to 5% of the definite diagnoses being refuted and 8% gaining a definite axSpA diagnosis. Among the features related to axSpA, the presence (or absence) of imaging-detected sacroiliitis at baseline was the best discriminator for a definite diagnosis at 2 years.
In commenting on these findings, Alexandre Sepriano, MD, PhD, assistant professor of rheumatology, NOVA Medical School, Lisbon, Portugal, who was not involved in the study, said: “These data show that the key is likely the referral of the ‘right patients’ to tertiary care centers. The [ASAS] has developed referral criteria that can be used for this purpose. According to these, patients with chronic low back pain starting before 45 years of age should be referred to a rheumatologist if at least one additional SpA feature is present.
“It should be acknowledged that axSpA is not a disease of males only. In fact, there is a 1:1 ratio between males and females in the full spectrum of the disease. Also, although imaging findings are important, not all patients will have these. Similarly, not all patients with imaging abnormalities will have the disease, and their sole presence without other SpA features does not suffice for diagnosis.”
Repeated assessment: Is it worth it?
Despite the positive findings described above, residual diagnostic uncertainty remained for 15% of patients, representing an obstacle to initiating an appropriate treatment. Therefore, it is important to understand whether and how the repeated assessment of axSpA features is of value for a definite diagnosis.
This last question was addressed in a second abstract also presented by Dr. Marques that focused on the yield of repeated assessment in CBP patients with suspected axSpA from the SPACE cohort. The main outcome of the study was the clinical diagnosis of definite axSpA at 2 years. Compared with baseline, at the 2-year evaluation 32 patients changed their diagnosis and were classified as definite axSpA: Sixteen were previously described as uncertain axSpA at baseline, 11 as uncertain no axSpA, and 5 as definite no axSpA.
On average, three axSpA features were present at baseline with one or two adjunctive features found throughout the study that led to the final diagnosis of definite axSpA. These adjunctive features were most commonly response to NSAIDs and sacroiliitis on MRI. Dr. Marques and colleagues concluded that the yield of repeated assessment in this setting was modest for a new diagnosis of definite axSpA. “Usefulness of repeating MRI in terms of diagnostic yield is low but can be considered in HLA-B27+ patients, especially if male,” Dr. Marques said, commenting on the analysis of SpA features in patients who changed their diagnosis to definite axSpA at 2 years.
“The early diagnosis of axial spondyloarthritis remains a challenge,” Dr. Sepriano said in commenting on the second SPACE study. “Probably one of the main reasons is the yet suboptimal awareness of the [full spectrum] of the disease in a primary care setting, in which most patients will first show up to get medical care. It is now well-known that patients do not always have changes in pelvic radiographs and that waiting for these to make a diagnosis of [radiographic] axSpA results in further delay and in missing many patients who will never develop these changes.
“Still, recognizing the clinical picture of early axSpA and differentiating it from other more common causes of chronic back pain (e.g., degenerative spinal disease) can sometimes be difficult. Continuous efforts in raising awareness and in education will likely result in further reducing the diagnostic delay gap and, as such, improve the prognosis of this often-debilitating rheumatic inflammatory disease.”
One epidemiologic size does not fit all
According to data from the International Map of Axial Spondyloarthritis (IMAS), axSpA clinical phenotype presentation shows great heterogeneity around the world. Marco Garrido-Cumbrera, PhD, of the University of Seville in Spain, presented the results of an analysis of an IMAS online survey (2017-2022).
The study, supported by Novartis, aimed at exploring differences in axSpA clinical phenotype presentation in a large sample of unselected patients: a total of 5,557 individuals from 27 countries across five regions. The results showed statistically significant differences among countries in almost all the analyzed characteristics, from age at onset of symptoms (the highest in Latin America) to HLA-B27 positivity frequency (lowest in Latin America and highest in Asia).
Differences also emerged in the presence of a positive family history of the disease (most common in Europe) and of physical and mental comorbidities (common in Africa). The authors also reported treatment data showing that most of the patients had used NSAIDs, and almost half of the patients had ever taken biologic disease-modifying antirheumatic drugs. Data also showed a mean delay in diagnosis of 7 years, with the longest values observed in South Africa and the lowest in Asia.
A consensus definition of early AxSpA
Early axSpA for the first time has been defined based on ASAS expert consensus, and the definition was presented at the meeting by Victoria Navarro-Compán, MD, PhD, of La Paz University Hospital, Madrid. An international working group came to a definition based on the symptom duration and taking solely axial symptoms into account. At the end of a five-step process, the group successfully developed the first consensus definition of early axSpA: “patients with diagnosis of axSpA with axial symptoms duration of ≤ 2 years.” Also to be noted are axial symptoms as assessed by a rheumatologist, which should include spinal/buttock pain or morning stiffness.
As reported by the authors, this ASAS definition is based on expert consensus, with the limitation of a lack of scientific evidence to support it, especially with regard to the specific duration of symptoms from the time of disease onset. Nonetheless, ASAS recommends the use of this definition in studies referring to early axSpA.
Dr. Marques reports no relevant financial relationships. Dr. Navarro-Compán reports serving on the speakers bureau for AbbVie, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; consulting for AbbVie, Eli Lilly, Galapagos, MoonLake, Merck Sharp & Dohme, Novartis, Pfizer, and UCB; and receiving grant/research support from AbbVie and Novartis. Dr. Garrido-Cumbrera reports receiving grant or research support from Novartis.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
Enthesitis, arthritis, tenosynovitis linked to dupilumab use for atopic dermatitis
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY