User login
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13
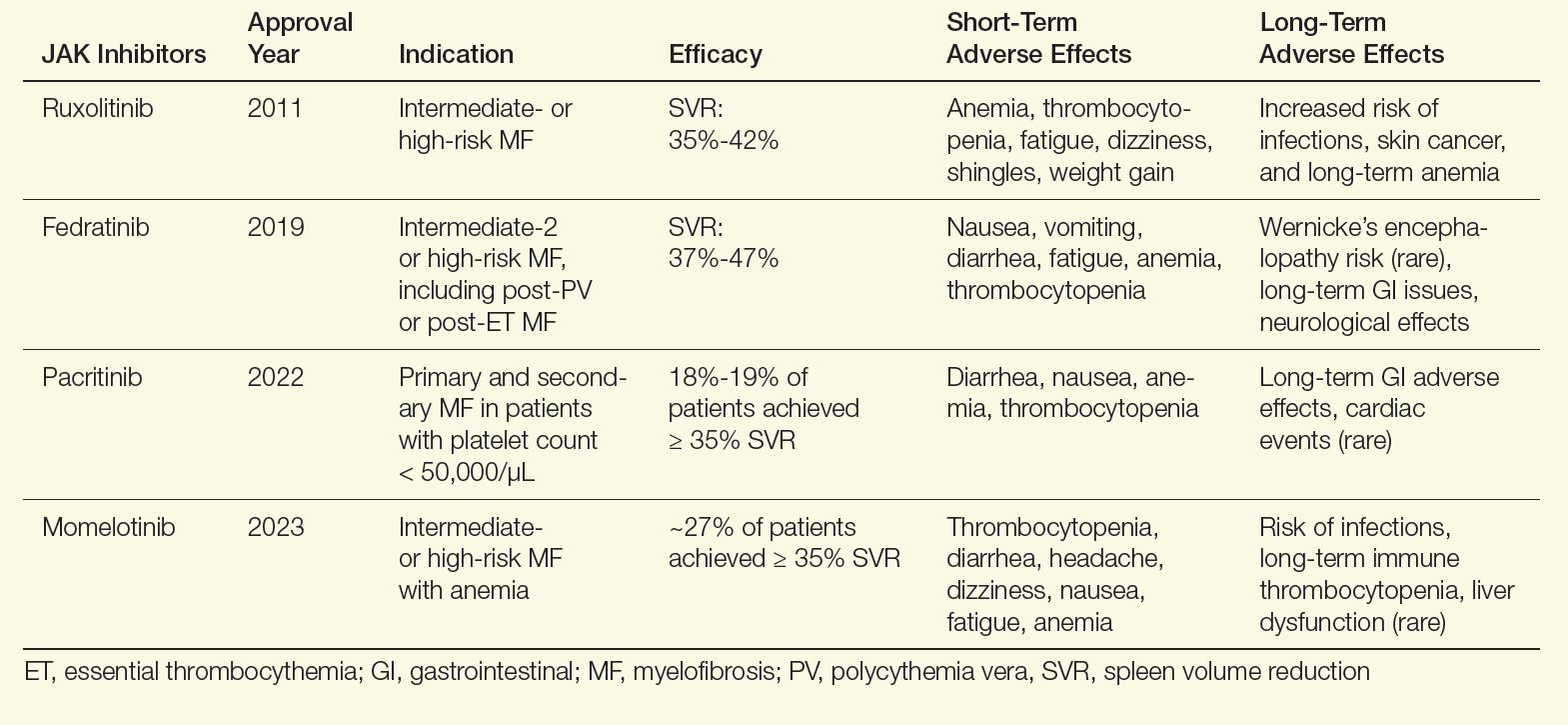
Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13

Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13

Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
Project’s Improvement in JIA Outcome Disparities Sets Stage for Further Interventions
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
WASHINGTON — A quality improvement project aimed at reducing racial disparities in juvenile idiopathic arthritis (JIA) led to a modest reduction in the overall clinical Juvenile Arthritis Disease Activity Score (cJADAS) and a 17% reduction in the disparity gap between Black and White patients, according to a study presented at the annual meeting of the American College of Rheumatology.
“Our work has led to initial progress in all groups, but we did not fully close the gap in outcomes,” Dori Abel, MD, MSHP, an attending rheumatologist at Children’s Hospital of Philadelphia in Pennsylvania, told attendees. But the project still revealed that it’s feasible to improve outcomes and reduce disparities with a “multipronged, equity-driven approach,” she said. “Stratifying data by demographic variables can reveal important differences in health care delivery and outcomes, catalyzing improvement efforts.”
Giya Harry, MD, MPH, MSc, an associate professor of pediatric rheumatology at Wake Forest University School of Medicine in Winston-Salem, North Carolina, was not involved in the study but praised both the effort and the progress made.
“The results are promising and suggest that with additional interventions targeting other key drivers, the team may be successful in completely eliminating the disparity in outcomes,” Harry said in an interview. “I applaud the hard work of Dr Abel and the other members of the team for doing the important work of characterizing the very complex issue of disparities in JIA outcomes across different race groups.”
It will now be important to build upon what the physicians learned during this process, said Harry, also the chair of the Diversity, Equity, Inclusion, and Accessibility committee of the Childhood Arthritis and Rheumatology Research Alliance.
“Patience is needed as they cycle through interventions with an emphasis on other key drivers” of disparities, Harry said.
Targeting Factors That Clinicians Can Potentially Influence
In her presentation, Abel discussed the various barriers that interfere with patients’ ability to move up the “JIA escalator” of getting referred and diagnosed, starting treatment and getting control of the disease, and monitoring and managing the disease and flares. These barriers include difficulties with access, trust, finances, insurance, caregivers’ missed work, medication burden, side effects, system barriers, and exhaustion and depression among caregivers and patients.
These barriers then contribute to disparities in JIA outcomes. In the STOP-JIA study, for example, Black children had greater polyarthritis disease activity in the first year and greater odds of radiographic damage, Abel noted. At her own institution, despite a mean cJADAS of 2.9 for the whole population of patients with JIA, the average was 5.0 for non-Hispanic Black patients, compared with 2.6 for non-Hispanic White patients.
The team therefore developed and implemented a quality improvement initiative aimed at improving the overall mean cJADAS and narrowing the gap between Black and White patients. The goal was to reduce the mean cJADAS to 2.7 by July 2024 and simultaneously reduce the cJADAS in Black patients by 1.2 units, or 50% of the baseline disparity gap, without increasing the existing gap.
The team first explored the many overlapping and interacting drivers of disparities within the realms of community characteristics, JIA treatment course, patient/family characteristics, organizational infrastructure, divisional infrastructure, and provider characteristics. While many of the individual factors driving disparities are outside clinicians’ control, “there are some domains clinicians may be able to directly influence, such as provider characteristics, JIA treatment course, and possibly divisional infrastructure,” Harry noted, and the team appeared to choose goals that fell under domains within clinicians’ potential influence.
The research team focused their efforts on four areas: Consistent outcome documentation, application of JIA best practices, providing access to at-risk patients, and team awareness and agency.
As part of improving consistent outcome documentation, they integrated outcome metrics into data visualization tools so that gaps were more evident. Applying JIA best practices included standardizing their approach to assessing medication adherence and barriers, with changes to the JIA note templates in the electronic health record and updates to medication adherence handouts.
Providing access to at-risk patients included several components:
- Creating a population management team
- Defining a target population to engage with for earlier follow-up
- Using a monthly batch outreach to defined patients
- Having a coordinator or social worker reach out to those who don’t make appointments
- Using a new JIA/high disease activity video follow-up program.
Finally, team awareness and agency involved giving physicians monthly access to mean cJADAS values for their own patients and at the division level. They also held quarterly disparity mitigation workshops.
Although the institution’s JIA population grew 13%, from 776 to 878 patients, over the course of the study, from January 2023 to May 2024, there was minimal change in the characteristics of the patient population. By May 2024, two thirds of patients (68%) were women, and 23% had public insurance. The population included 67% non-Hispanic White, 9% Hispanic/Latino, 7% non-Hispanic Black, and 4% Asian patients.
One third of the patients (32%) had the oligoarticular subtype, and other subtypes included enthesitis-related at 16%, polyarticular rheumatoid factor (RF)–negative at 15%, systemic at 7%, psoriatic at 6%, undifferentiated at 5%, and polyarticular RF-positive at 4%; data on subtype were unavailable for 14%. Most of their patients (71%) were in a high or very high quintile of the Childhood Opportunity Index, and 12% were in a low or very low quintile.
Results of the Quality Improvement Project
As of May 2024, the team had reached most of the goals they had set in terms of individual metrics. They met their goal of having a complete cJADAS calculated in more than 80% of JIA visits each month. With a goal of having over 90% of JIA monthly visits include disease activity target attestations, they reached 95% by May.
They aimed to have over half of JIA monthly visits include documentation of medication adherence/barrier assessment, and 75% of monthly visits had one. For their monthly outreach goal for overdue visits, they aimed to contact more than 75% of patients within 30 days if they were newly overdue for a follow-up visit but had only reached 47% by May 2024. The team had also completed 154 Maintenance of Certification assessments by May.
From initiation of project planning in January 2023 through May 2024, the overall JIA patient population experienced an improvement in cJADAS from 2.9 to 2.54. In individual cJADAS components, the mean patient global score improved from 1.71 to 1.47, the physician global score improved from 0.81 to 0.75, and the joint count score improved from 0.71 to 0.68.
In the non-Hispanic Black population, the mean cJADAS improved from 5.06 in January 2023 to 4.31 in May 2024. Mean cJADAS in the non-Hispanic White population fell from 2.63 to 2.29. With a difference of 0.4 points fewer between the Black and White populations, the disparity gap closed by 17%.
One of the team’s next steps will be to focus on the Hispanic population in 2024-2025 by optimizing language services, working toward greater family involvement to better understand barriers to care, and ongoing population management.
Abel and Harry had no disclosures. No external funding was noted.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Could Biomarkers Help to Detect Lung Disease Earlier in Systemic JIA?
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
WASHINGTON — Children who have systemic juvenile idiopathic arthritis with lung disease (sJIA-LD) have distinct biomarker profiles that may hold potential in eventually detecting LD earlier and identifying personalized treatment, according to research presented at the American College of Rheumatology (ACR) 2024 Annual Meeting.
Established risk factors for LD, which affects up to 1 in every 20 patients with sJIA, include being of a younger age, having more macrophage activation syndrome (MAS) episodes, and having more adverse reactions to biologics, Esraa Eloseily, MD, MS, an assistant professor of pediatrics at UT Southwestern Children’s Medical Center, Dallas, told attendees.
“The pathophysiology remains unclear and debate centers around elevated IL-18 [interleukin 18] and T-cell activation in association with HLA-DRB1*15/DRESS [drug reaction with eosinophilia and systemic symptoms] reactions to biologics, and thus, we have urgent unmet needs to understand the prevalence, the pathogenesis, disease biomarkers, and influence of biologics,” Eloseily said.
Their study confirmed that patients with LD tended to be younger and have more MAS. The researchers also found lower hemoglobin and higher white blood cell counts and platelets in patients with sJIA-LD, as well as a higher medication burden, particularly with steroids, biologics, and Janus kinase (JAK) inhibitors.
Randy Cron, MD, PhD, director of the Division of Pediatric Rheumatology at the University of Alabama at Birmingham, attended the presentation and noted that every additional piece of information is helpful in filling out the picture of what we know and can predict about sJIA-LD development.
“We’re all learning as we go, so the more people that study this, the better,” Cron told Medscape Medical News. “Even if it’s just seeing things that other groups have seen or really solidifying the risk factors for the development of lung disease, I think, at this point, that’s one of the most clinically relevant things: Do we screen? Who do we screen? Certainly, kids who have very young age of onset, children who develop macrophage activation syndrome, children who have high IL-18 levels.”
Study Results
The study compared 37 patients with sJIA-LD from 16 Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry sites with 141 patients with sJIA but without LD who had similar follow-up durations in the CARRA Registry.
Disease duration for patients with sJIA-LD was defined as the time from their initial sJIA diagnosis to their baseline sJIA-LD cohort visit, which was considered their index visit. In patients without LD, duration was from their enrollment in the CARRA Registry to their last CARRA Registry visit, which was considered their index visit.
The patients with sJIA-LD were significantly younger — a median age of 1 year — at onset of sJIA than those without LD, who had a median age of 5 years (P < .0019). The patients with sJIA-LD were also younger (median age, 7 years) at their index visit than those without LD (median age, 10 years) (P < .0001).
There were also significant differences in medication usage between those with and without LD. While 40.5% of patients with sJIA-LD were using steroids at their index visit, only 11.4% of those without LD were using steroids (P < .0001). Yet the mean dose of steroids was significantly lower in those with LD (5.45 mg/d) than in those without (20.7 mg/d). In addition, nearly half the patients with sJIA-LD had ever used cyclosporin A (45.7%) compared with 2.8% of those without LD (P < .0001), and 17.1% of patients with sJIA-LD had used mycophenolate mofetil compared with 0.7% of those without LD (P = .0002).
Similar disparities were seen for usage of biologics and JAK inhibitors: Anakinra (82.9% vs 56.7%; P = .0036), abatacept (8.6% vs 1.4%; P = .053), tofacitinib (57.1% vs 5.7%; P < .0001), ruxolitinib (25.7% vs 0%; P < .0001), baricitinib (8.6% vs 0%; P = .007), and emapalumab (23% vs 0.7%; P < .0001). Further, 5.7% of those with sJIA-LD had taken etoposide and 22.9% had received intravenous immunoglobulin compared with 0% and 4.3%, respectively, in those without LD (P = .04 and P = .001).
Laboratory parameters of patients with sJIA-LD were also significantly different from those of patients without LD, including a higher white blood cell count (8.8 × 109/L vs 8.1 × 109/L; P = .01), higher platelets (316.5 × 109/L vs 311.2 × 109/L; P = .03), and lower hemoglobin (11.5 g/dL vs 12.6 g/dL; P = .007). Ferritin levels trended nonsignificantly higher in patients with sJIA-LD (506 ng/mL vs 173.2 ng/mL; P = .09), and aspartate aminotransferase levels were significantly higher (37 U/L vs 28.72 U/L; P < .0001).
Patients’ overall well-being was “unexpectedly” higher in patients with sJIA-LD (P = .007), Eloseily noted, including the parent/patient rating (P = .027). However, more of the patients without LD had an excellent (61%) or very good (20.4%) health-related quality of life compared with those with LD (13% and 39%), and nearly one third of patients with sJIA-LD (30.4%) had only fair health-related quality of life compared with 5.5% without LD (P = .0002).
In line with known risk factors, most of the patients with sJIA-LD had a prior MAS episode (67.6%) compared with 10.6% of those without LD (P < .0001). Mortality was also higher in those with LD, two of whom died, whereas none without LD died (P = .04).
While existing biomarkers have been reported, they lack independent validation, Eloseily told attendees. Among the known key biomarkers are IL-18/interferon (IFN)-gamma axis, which are known to drive MAS and pulmonary inflammation, especially in those with MAS and LD; ICAM-5 and MMP-7, which is linked to fibrosis and tissue remodeling; and CCL11, CCL17, and GDF-15, which is linked to fibrosis and inflammation.
Because the CARRA Registry has limited availability of biosamples for most patients, the researchers used plasma samples from the FROST study for 27 patients with sJIA-LD and 46 patients without LD. When they compared 23 biomarkers between the groups, most of the known key biomarkers, as well as several other biomarkers, were significantly elevated in those with LD compared with in those without:
- MMP-7 (P = .001)
- IFN gamma (P = .008)
- CCL11 (P < .0001)
- CCL17 (P = .002)
- CCL15 (P < .0001)
- MCP-1 (P = .0003)
- MCP-3 (P = .02)
- CCL25 (P < .0001)
- CD25 (P < .0001)
- GDF-15 (P < .0001)
- TRAIL (P < .0001)
- IL-23 (P = .002)
They found that IL-18 only trended higher (P = .07), and there were not adequate data for ICAM-5.
The study was limited by the differences in disease duration between the compared groups and the limited availability of biosamples, which they only had from patients enrolled in the FROST study.
The research was funded by CARRA and the Arthritis Foundation. Eloseily reported no disclosures. Cron reported serving as an adviser for AbbVie/Abbott and Sobi, receiving grant funding and speaking and consulting fees from Pfizer, and receiving royalties from Springer.
A version of this article appeared on Medscape.com.
FROM ACR 2024
Real-World Data Question Low-Dose Steroid Use in ANCA Vasculitis
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Compared with a standard dosing regimen, a reduced-dose glucocorticoid regimen is associated with an increased risk for disease progression, relapse, death, or kidney failure in antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis, particularly affecting patients receiving rituximab or those with elevated creatinine levels.
METHODOLOGY:
- The PEXIVAS trial demonstrated that a reduced-dose glucocorticoid regimen was noninferior to standard dosing in terms of death or end-stage kidney disease in ANCA-associated vasculitis. However, the trial did not include disease progression or relapse as a primary endpoint, and cyclophosphamide was the primary induction therapy.
- Researchers conducted this retrospective study across 19 hospitals (18 in France and one in Luxembourg) between January 2018 and November 2022 to compare the effectiveness of a reduced-dose glucocorticoid regimen, as used in the PEXIVAS trial, with a standard-dose regimen in patients with ANCA-associated vasculitis in the real-world setting.
- They included 234 patients aged > 15 years (51% men) with severe granulomatosis with polyangiitis (n = 141) or microscopic polyangiitis (n = 93) who received induction therapy with rituximab or cyclophosphamide; 126 and 108 patients received reduced-dose and standard-dose glucocorticoid regimens, respectively.
- Most patients (70%) had severe renal involvement.
- The primary composite outcome encompassed minor relapse, major relapse, disease progression before remission, end-stage kidney disease requiring dialysis for > 12 weeks or transplantation, and death within 12 months post-induction.
TAKEAWAY:
- The primary composite outcome occurred in a higher proportion of patients receiving reduced-dose glucocorticoid therapy than in those receiving standard-dose therapy (33.3% vs 18.5%; hazard ratio [HR], 2.20; 95% CI, 1.23-3.94).
- However, no significant association was found between reduced-dose glucocorticoids and the risk for death or end-stage kidney disease or the occurrence of serious infections.
- Among patients receiving reduced-dose glucocorticoids, serum creatinine levels > 300 μmol/L were associated with an increased risk for the primary composite outcome (adjusted HR, 3.02; 95% CI, 1.28-7.11).
- In the rituximab induction subgroup, reduced-dose glucocorticoid was associated with an increased risk for the primary composite outcome (adjusted HR, 2.36; 95% CI, 1.18-4.71), compared with standard-dose glucocorticoids.
IN PRACTICE:
“Our data suggest increased vigilance when using the [reduced-dose glucocorticoid] regimen, especially in the two subgroups of patients at higher risk of failure, that is, those receiving [rituximab] as induction therapy and those with a baseline serum creatinine greater than 300 μmol/L,” the authors wrote.
SOURCE:
The study was led by Sophie Nagle, MD, National Referral Centre for Rare Autoimmune and Systemic Diseases, Department of Internal Medicine, Hôpital Cochin, Paris, France. It was published online on November 20, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The retrospective nature of this study may have introduced inherent limitations and potential selection bias. The study lacked data on patient comorbidities, which could have influenced treatment choice and outcomes. Additionally, about a quarter of patients did not receive methylprednisolone pulses prior to oral glucocorticoids, unlike the PEXIVAS trial protocol. The group receiving standard-dose glucocorticoids showed heterogeneity in glucocorticoid regimens, and the minimum follow-up was only 6 months.
DISCLOSURES:
This study did not report any source of funding. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
First Brain-Injected Gene Therapy Approved by FDA
The US Food and Drug Administration (FDA) has granted fast-track approval for a groundbreaking gene therapy indicated for a rare genetic disorder called aromatic L-amino acid decarboxylase (AADC) deficiency. The gene therapy, marketed under the brand name Kebilidi, is the first in the United States to be injected directly into the brain. It is approved for children with fully developed skulls and for adults.
AADC is an enzyme that helps the body make dopamine. AADC deficiency affects patients’ physical, mental, and behavioral health from infancy, leading to severe disabilities and shorter lifespan. Children with AADC may also experience painful seizure-like episodes called oculogyric crises.
Kebilidi (generic name: eladocagene exuparvovec) is injected into a specific area of the brain where it boosts AADC, restoring dopamine production and gradually improving movement-related symptoms. This surgery is to be performed only by brain surgeons in specialized centers.
The FDA approval was based on the therapy’s safety and effectiveness as shown in an ongoing clinical trial involving 13 children diagnosed with AADC deficiency. According to PTC Therapeutics, the maker of Kebilidi, long-term follow-up studies of the participants are still needed, and additional proof of the therapy’s benefits are required for full FDA approval.
Common side effects of Kebilidi therapy may include involuntary movements (dyskinesia), anemia, fever, low blood pressure, excessive salivation, problems sleeping, low blood levels of certain minerals, and complications after the injection, including breathing or heart problems. The surgical procedure for injecting Kebilidi also carries certain risks, such as cerebrospinal fluid leaks, bleeding in the brain, inflammation, strokes, and infections.
A version of this article appeared on WebMD.com.
The US Food and Drug Administration (FDA) has granted fast-track approval for a groundbreaking gene therapy indicated for a rare genetic disorder called aromatic L-amino acid decarboxylase (AADC) deficiency. The gene therapy, marketed under the brand name Kebilidi, is the first in the United States to be injected directly into the brain. It is approved for children with fully developed skulls and for adults.
AADC is an enzyme that helps the body make dopamine. AADC deficiency affects patients’ physical, mental, and behavioral health from infancy, leading to severe disabilities and shorter lifespan. Children with AADC may also experience painful seizure-like episodes called oculogyric crises.
Kebilidi (generic name: eladocagene exuparvovec) is injected into a specific area of the brain where it boosts AADC, restoring dopamine production and gradually improving movement-related symptoms. This surgery is to be performed only by brain surgeons in specialized centers.
The FDA approval was based on the therapy’s safety and effectiveness as shown in an ongoing clinical trial involving 13 children diagnosed with AADC deficiency. According to PTC Therapeutics, the maker of Kebilidi, long-term follow-up studies of the participants are still needed, and additional proof of the therapy’s benefits are required for full FDA approval.
Common side effects of Kebilidi therapy may include involuntary movements (dyskinesia), anemia, fever, low blood pressure, excessive salivation, problems sleeping, low blood levels of certain minerals, and complications after the injection, including breathing or heart problems. The surgical procedure for injecting Kebilidi also carries certain risks, such as cerebrospinal fluid leaks, bleeding in the brain, inflammation, strokes, and infections.
A version of this article appeared on WebMD.com.
The US Food and Drug Administration (FDA) has granted fast-track approval for a groundbreaking gene therapy indicated for a rare genetic disorder called aromatic L-amino acid decarboxylase (AADC) deficiency. The gene therapy, marketed under the brand name Kebilidi, is the first in the United States to be injected directly into the brain. It is approved for children with fully developed skulls and for adults.
AADC is an enzyme that helps the body make dopamine. AADC deficiency affects patients’ physical, mental, and behavioral health from infancy, leading to severe disabilities and shorter lifespan. Children with AADC may also experience painful seizure-like episodes called oculogyric crises.
Kebilidi (generic name: eladocagene exuparvovec) is injected into a specific area of the brain where it boosts AADC, restoring dopamine production and gradually improving movement-related symptoms. This surgery is to be performed only by brain surgeons in specialized centers.
The FDA approval was based on the therapy’s safety and effectiveness as shown in an ongoing clinical trial involving 13 children diagnosed with AADC deficiency. According to PTC Therapeutics, the maker of Kebilidi, long-term follow-up studies of the participants are still needed, and additional proof of the therapy’s benefits are required for full FDA approval.
Common side effects of Kebilidi therapy may include involuntary movements (dyskinesia), anemia, fever, low blood pressure, excessive salivation, problems sleeping, low blood levels of certain minerals, and complications after the injection, including breathing or heart problems. The surgical procedure for injecting Kebilidi also carries certain risks, such as cerebrospinal fluid leaks, bleeding in the brain, inflammation, strokes, and infections.
A version of this article appeared on WebMD.com.
Need for Low-Dose Steroids to Prevent Relapse in GPA Vasculitis Depends on Treatment Regimen
WASHINGTON — Patients with granulomatosis with polyangiitis (GPA) completely tapered off prednisone have a more than fourfold risk of relapse by 6 months, compared with those tapered to 5 mg/day of prednisone; however, this benefit was only seen in patients not on rituximab, according to new research presented at the annual meeting of the American College of Rheumatology (ACR).
“For patients treated with rituximab, fully tapering off glucocorticoids is reasonable to consider as the first approach,” said Peter Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, during his presentation of the findings.
Although a low dose of glucocorticoids can prevent some minor relapses in patients on other treatment regimens such as methotrexate or azathioprine, “fully tapering off prednisone presents relatively little risk of major relapse, and that major relapse can be treated rather quickly,” Merkel added.
The Assessment of Prednisone in Remission (TAPIR) trial enrolled 143 patients with GPA who were in remission (defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis [BVAS/WG] of 0) within 1 year of treatment to induce remission for active disease and who were taking 5-10 mg of prednisone per day. After all patients tapered to 5 mg/day of prednisone, 71 patients completely tapered off prednisone over 4 weeks and remained off glucocorticoids until month 6. The remaining patients maintained a 5-mg/day dose over the study period. Placement in either treatment group was randomized, and patients continued other immunosuppressive therapy during the study.
Researchers evaluated the rate of relapse by 6 months, defined as a physician’s decision to increase the dose of glucocorticoids to treat GPA, in both groups.
Across all participants, the median age was 58 years, and 52% of patients were male. Most patients were White, and 47% of all patients were prescribed rituximab.
At 6 months, 15.5% of participants who completely tapered off prednisone experienced a relapse of GPA, compared with 4.2% of those taking low-dose prednisone. Time to relapse was also shorter in the 0-mg prednisone group (P = .026), and relapses occurred continually over 6 months, Merkel said.
When stratified by rituximab use, relapse rates at 6 months between the 5-mg and 0-mg prednisone groups in patients taking rituximab showed no difference. Among patients not taking rituximab, those who completely stopped prednisone were nine and a half times as likely to experience relapse as those in the low-dose group.
Despite these differences in relapse rates, “surprisingly, there were no differences in patient-reported outcomes [such as pain interference, physical function, and fatigue],” Merkel said.
Across all patients, all but one relapse was characterized as minor. There were five serious adverse events and 10 infections in the 0-mg group versus one adverse event and 4 infections in the 5-mg group, but these differences were not statistically significant.
In patients who relapsed, musculoskeletal and ear, nose, and throat manifestations of GPA were most common, and these are “the kind of stuff we see that is helped by low-dose glucocorticoids,” Merkel said.
It’s a good sign that for patients who were completely weaned off glucocorticoids, nearly all relapses were minor, Galina Marder, MD, a rheumatologist and associate professor of medicine at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said in an interview. She was not involved with the research.
The study “can reinforce the message [of] trying to get them off steroids completely [when possible],” she said.
The findings also provide insight for future clinical trials, Merkel noted. For patients taking non–rituximab-based regimens, completely tapering off glucocorticoids or maintaining a low dose can affect study outcomes.
“[These data are] even more important for clinical trials because they are [reinforcing] the fact that you can have a diminishing signal if you allow some patients to stay on 5 mg prednisone” when GPA flares are the primary outcome, Marder added.
The Vasculitis Clinical Research Consortium received funding for this research through grants from the National Institutes of Health. Merkel has disclosed financial relationships with AbbVie/Abbott, Amgen, argenx, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cabaletta, ChemoCentryx, CSL Behring, Dynacure, Eicos, Electra, EMD Serono, Forbius, Genentech/Roche, Genzyme/Sanofi, GSK, HI-Bio, Inmagene, InflaRx, Janssen, Kiniksa, Kyverna, Magenta, MiroBio, Neutrolis, Novartis, NS Pharma, Pfizer, Regeneron, Sanofi, Sparrow, Takeda, Talaris, UpToDate, and Visterra. Marder consults for Amgen and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
WASHINGTON — Patients with granulomatosis with polyangiitis (GPA) completely tapered off prednisone have a more than fourfold risk of relapse by 6 months, compared with those tapered to 5 mg/day of prednisone; however, this benefit was only seen in patients not on rituximab, according to new research presented at the annual meeting of the American College of Rheumatology (ACR).
“For patients treated with rituximab, fully tapering off glucocorticoids is reasonable to consider as the first approach,” said Peter Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, during his presentation of the findings.
Although a low dose of glucocorticoids can prevent some minor relapses in patients on other treatment regimens such as methotrexate or azathioprine, “fully tapering off prednisone presents relatively little risk of major relapse, and that major relapse can be treated rather quickly,” Merkel added.
The Assessment of Prednisone in Remission (TAPIR) trial enrolled 143 patients with GPA who were in remission (defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis [BVAS/WG] of 0) within 1 year of treatment to induce remission for active disease and who were taking 5-10 mg of prednisone per day. After all patients tapered to 5 mg/day of prednisone, 71 patients completely tapered off prednisone over 4 weeks and remained off glucocorticoids until month 6. The remaining patients maintained a 5-mg/day dose over the study period. Placement in either treatment group was randomized, and patients continued other immunosuppressive therapy during the study.
Researchers evaluated the rate of relapse by 6 months, defined as a physician’s decision to increase the dose of glucocorticoids to treat GPA, in both groups.
Across all participants, the median age was 58 years, and 52% of patients were male. Most patients were White, and 47% of all patients were prescribed rituximab.
At 6 months, 15.5% of participants who completely tapered off prednisone experienced a relapse of GPA, compared with 4.2% of those taking low-dose prednisone. Time to relapse was also shorter in the 0-mg prednisone group (P = .026), and relapses occurred continually over 6 months, Merkel said.
When stratified by rituximab use, relapse rates at 6 months between the 5-mg and 0-mg prednisone groups in patients taking rituximab showed no difference. Among patients not taking rituximab, those who completely stopped prednisone were nine and a half times as likely to experience relapse as those in the low-dose group.
Despite these differences in relapse rates, “surprisingly, there were no differences in patient-reported outcomes [such as pain interference, physical function, and fatigue],” Merkel said.
Across all patients, all but one relapse was characterized as minor. There were five serious adverse events and 10 infections in the 0-mg group versus one adverse event and 4 infections in the 5-mg group, but these differences were not statistically significant.
In patients who relapsed, musculoskeletal and ear, nose, and throat manifestations of GPA were most common, and these are “the kind of stuff we see that is helped by low-dose glucocorticoids,” Merkel said.
It’s a good sign that for patients who were completely weaned off glucocorticoids, nearly all relapses were minor, Galina Marder, MD, a rheumatologist and associate professor of medicine at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said in an interview. She was not involved with the research.
The study “can reinforce the message [of] trying to get them off steroids completely [when possible],” she said.
The findings also provide insight for future clinical trials, Merkel noted. For patients taking non–rituximab-based regimens, completely tapering off glucocorticoids or maintaining a low dose can affect study outcomes.
“[These data are] even more important for clinical trials because they are [reinforcing] the fact that you can have a diminishing signal if you allow some patients to stay on 5 mg prednisone” when GPA flares are the primary outcome, Marder added.
The Vasculitis Clinical Research Consortium received funding for this research through grants from the National Institutes of Health. Merkel has disclosed financial relationships with AbbVie/Abbott, Amgen, argenx, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cabaletta, ChemoCentryx, CSL Behring, Dynacure, Eicos, Electra, EMD Serono, Forbius, Genentech/Roche, Genzyme/Sanofi, GSK, HI-Bio, Inmagene, InflaRx, Janssen, Kiniksa, Kyverna, Magenta, MiroBio, Neutrolis, Novartis, NS Pharma, Pfizer, Regeneron, Sanofi, Sparrow, Takeda, Talaris, UpToDate, and Visterra. Marder consults for Amgen and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
WASHINGTON — Patients with granulomatosis with polyangiitis (GPA) completely tapered off prednisone have a more than fourfold risk of relapse by 6 months, compared with those tapered to 5 mg/day of prednisone; however, this benefit was only seen in patients not on rituximab, according to new research presented at the annual meeting of the American College of Rheumatology (ACR).
“For patients treated with rituximab, fully tapering off glucocorticoids is reasonable to consider as the first approach,” said Peter Merkel, MD, MPH, chief of the division of rheumatology at the University of Pennsylvania, Philadelphia, during his presentation of the findings.
Although a low dose of glucocorticoids can prevent some minor relapses in patients on other treatment regimens such as methotrexate or azathioprine, “fully tapering off prednisone presents relatively little risk of major relapse, and that major relapse can be treated rather quickly,” Merkel added.
The Assessment of Prednisone in Remission (TAPIR) trial enrolled 143 patients with GPA who were in remission (defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis [BVAS/WG] of 0) within 1 year of treatment to induce remission for active disease and who were taking 5-10 mg of prednisone per day. After all patients tapered to 5 mg/day of prednisone, 71 patients completely tapered off prednisone over 4 weeks and remained off glucocorticoids until month 6. The remaining patients maintained a 5-mg/day dose over the study period. Placement in either treatment group was randomized, and patients continued other immunosuppressive therapy during the study.
Researchers evaluated the rate of relapse by 6 months, defined as a physician’s decision to increase the dose of glucocorticoids to treat GPA, in both groups.
Across all participants, the median age was 58 years, and 52% of patients were male. Most patients were White, and 47% of all patients were prescribed rituximab.
At 6 months, 15.5% of participants who completely tapered off prednisone experienced a relapse of GPA, compared with 4.2% of those taking low-dose prednisone. Time to relapse was also shorter in the 0-mg prednisone group (P = .026), and relapses occurred continually over 6 months, Merkel said.
When stratified by rituximab use, relapse rates at 6 months between the 5-mg and 0-mg prednisone groups in patients taking rituximab showed no difference. Among patients not taking rituximab, those who completely stopped prednisone were nine and a half times as likely to experience relapse as those in the low-dose group.
Despite these differences in relapse rates, “surprisingly, there were no differences in patient-reported outcomes [such as pain interference, physical function, and fatigue],” Merkel said.
Across all patients, all but one relapse was characterized as minor. There were five serious adverse events and 10 infections in the 0-mg group versus one adverse event and 4 infections in the 5-mg group, but these differences were not statistically significant.
In patients who relapsed, musculoskeletal and ear, nose, and throat manifestations of GPA were most common, and these are “the kind of stuff we see that is helped by low-dose glucocorticoids,” Merkel said.
It’s a good sign that for patients who were completely weaned off glucocorticoids, nearly all relapses were minor, Galina Marder, MD, a rheumatologist and associate professor of medicine at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said in an interview. She was not involved with the research.
The study “can reinforce the message [of] trying to get them off steroids completely [when possible],” she said.
The findings also provide insight for future clinical trials, Merkel noted. For patients taking non–rituximab-based regimens, completely tapering off glucocorticoids or maintaining a low dose can affect study outcomes.
“[These data are] even more important for clinical trials because they are [reinforcing] the fact that you can have a diminishing signal if you allow some patients to stay on 5 mg prednisone” when GPA flares are the primary outcome, Marder added.
The Vasculitis Clinical Research Consortium received funding for this research through grants from the National Institutes of Health. Merkel has disclosed financial relationships with AbbVie/Abbott, Amgen, argenx, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cabaletta, ChemoCentryx, CSL Behring, Dynacure, Eicos, Electra, EMD Serono, Forbius, Genentech/Roche, Genzyme/Sanofi, GSK, HI-Bio, Inmagene, InflaRx, Janssen, Kiniksa, Kyverna, Magenta, MiroBio, Neutrolis, Novartis, NS Pharma, Pfizer, Regeneron, Sanofi, Sparrow, Takeda, Talaris, UpToDate, and Visterra. Marder consults for Amgen and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
FROM ACR 2024
First Phase 3 Drug Trial in IgG4-Related Disease Has Success
WASHINGTON — The B cell–depleting agent inebilizumab (Uplizna) dramatically reduced the risk of flares and increased year-long remission of IgG4-related disease (RD), new research has found.
In a phase 3, multicenter, double-blind, randomized, placebo-controlled trial of 135 adults with active IgG4-RD, treatment with inebilizumab resulted in a significant 87% reduction in flare risk and nearly fivefold greater likelihood of flare-free remission at 1 year. The results were published online November 14 in The New England Journal of Medicine and were presented at the annual meeting of the American College of Rheumatology (ACR).
The drug’s manufacturer, Amgen, released top-line results of the trial, called MITIGATE, in June 2024.
Until now, the mainstay of management for the chronic multiorgan disease IgG4-RD has been glucocorticoids, which can cause numerous adverse effects. “It is hoped that inebilizumab can be used as an important steroid-sparing medication in this disease to reduce steroid toxicity,” lead author John H. Stone, MD, professor of medicine at Harvard Medical School, Boston, Massachusetts, said in an interview, noting that it may not entirely eliminate the need for steroid treatment, but for many, it appears to work after the remission induction period as a monotherapy without steroids.
Asked to comment, Leonard H. Calabrese, DO, head of the Section of Clinical Immunology and manager of the Clinical Immunology Clinic at the Cleveland Clinic, Ohio, said: “There has been anecdotal or observational evidence for some effect with other immunosuppressive agents, including rituximab, but no robust clinical trial until this study. This clearly has demonstrated efficacy by reducing the risk of flares. And most importantly, putting people into remission means no active disease in any given organ. ... This gives us another tool in the toolbox to attack B cell–directed diseases, and I think it really makes a lot of sense.”
Calabrese cautioned, though, that “this is a disease that extends over many years. This is just a 1-year study. Label extensions will be important.”
And several questions remain, Calabrese noted: “How long do patients need to remain on drug? What will happen when the drug is stopped? Can they be retreated? These are the natural questions that arise in any sentinel study like this. But this is extremely encouraging. And I think it’s great for patients. I also think it’s a clarion call to increase awareness about this disease since there’s now strong evidence of effective treatment.”
Underrecognized, Often Misdiagnosed as Cancer
Indeed, IgG4-RD, a chronic, relapsing, autoimmune, fibro-inflammatory multiorgan disease, was only first described in Japan in 2003. Since then, it has been reported all over the world yet remains vastly underrecognized. It is often misdiagnosed as cancer because it produces lesions in multiple organs. It received an ICD-10 code only about a year ago. A previous study estimated a prevalence of about 5.3 persons per 100,000 but that is likely to be a three- to fourfold underestimate, said Stone, who is also executive chairman of the IgG4ward! Foundation.
“Nobody had heard of the disease until about 20 years ago. ... And there are many people in the world who have still not heard of it despite the fact that it is a multiorgan autoimmune disease and is probably as common, or more common, than many other diseases that rheumatologists spend a lot of time thinking about, such as scleroderma.”
While knowledge about the disease is increasing in rheumatology circles, it’s less well-recognized among many of the specialties where patients present, depending on the location of their lesions. These include gastroenterology, ophthalmology, pulmonary medicine, neurology, and nephrology. “All would be likely to see this disease,” Stone said.
The disease can be mistaken for tumors in many of those locations and even as metastatic cancer, he noted, adding that “any time a patient has a mass lesion in a typical organ, the pancreas, the major salivary glands, the lungs, or the kidneys, this should be on the differential diagnosis.”
The diagnosis of IgG4-RD is a clinical one, involving “quadrangulation between clinical features, serological findings, IgG4 levels in the blood, radiology studies, and then pathology biopsies when those are available,” Stone said.
Calabrese characterized the current situation as “we’re all blind men on the elephant. To the neurologist or the neurosurgeon, it’s a mass in the brain. It could present to the ophthalmologist as an [eye] tumor. It can be thyroid gland failure, pulmonary disease, retroperitoneal fibrosis, hepatobiliary disease, and beyond. So, whoever sees that patient, there’s often a long lag time in recognizing it.”
And interestingly, Stone noted that unlike other autoimmune diseases, IgG4-RD primarily affects middle-aged men rather than younger-to-middle-aged women. And when IgG4-RD is diagnosed, glucocorticoid treatment can be particularly toxic when the pancreas is involved, heightening the risk for hyperglycemia and potentially causing diabetes.
Dramatic Improvement in Flares, Remission Achievement
MITIGATE is a phase 3, multicenter, double-blind, randomized, placebo-controlled trial in which 135 adults (mean age 58.2 years, 88 men) with active IgG4-RD were randomized 1:1 to receive 300-mg intravenous infusions of inebilizumab or placebo on days 1 and 15, and again at week 26. At baseline, 62 (45.9%) participants had newly diagnosed IgG4-RD and 73 (54.1%) had recurrent disease.
Both groups received identical glucocorticoid tapers. Overall, 127 (94.1%) completed the 52 weeks of treatment.
By 52 weeks, only seven patients in the inebilizumab group (10%) had experienced disease flares vs 40 (60%) in the placebo group, a significant difference with a hazard ratio of 0.13 (P < .001).
The percentage of participants achieving flare-free, treatment-free complete remission was 59 with inebilizumab (57%), compared with just 15 (22%) in the placebo group (odds ratio [OR], 4.68; P < .001). And for flare-free, glucocorticoid-free complete remission, those proportions were 40 (59%) vs 15 (22%), respectively (OR, 4.96; P < .001).
Excluding the 8-week glucocorticoid taper period, mean total glucocorticoid use was 1264.2 mg less in the inebilizumab than the placebo group, a significant reduction. Overall, 61 participants (90%) were able to entirely discontinue glucocorticoids during the trial, compared with just 25 (37%) in the placebo group.
Adverse events of grade 3 or higher occurred in 12 participants (18%) in the inebilizumab group and 8 (12%) in the placebo group; serious adverse events occurred in 12 (18%) and 6 (9%), respectively. However, no serious adverse event occurred in more than one participant, and there were no deaths. Adverse events led to withdrawal from the trial in six patients (9%) in the inebilizumab group and three patients (4%) in the placebo group.
Adverse events that occurred in more than 10% of participants in the inebilizumab group were COVID-19 in 16 participants (24%), lymphopenia in 11 (16%), and urinary tract infection in 8 (12%).
Importantly, Stone noted, B-cell depletion can reduce responses to vaccines, so patients should receive all recommended vaccinations, including COVID-19, influenza, respiratory syncytial virus, and others, prior to initiating therapy.
Uplizna (inebilizumab-cdon) was approved by the Food and Drug Administration (FDA) for the treatment of neuromyelitis optica spectrum disorder in 2020. In October 2024, the FDA granted Amgen breakthrough therapy designation for use in IgG4-RD. The company is also developing the drug for use in myasthenia gravis.
The study was funded by Amgen. Stone has reported being a consultant for Amgen, Zenas, Argenx, Bristol Myers Squibb, Novartis, Sanofi, and Horizon Pharma. Calabrese has reported being a consultant and/or speaker for Amgen, AstraZeneca, Jansen, Sanofi, and UCB.
A version of this article first appeared on Medscape.com.
WASHINGTON — The B cell–depleting agent inebilizumab (Uplizna) dramatically reduced the risk of flares and increased year-long remission of IgG4-related disease (RD), new research has found.
In a phase 3, multicenter, double-blind, randomized, placebo-controlled trial of 135 adults with active IgG4-RD, treatment with inebilizumab resulted in a significant 87% reduction in flare risk and nearly fivefold greater likelihood of flare-free remission at 1 year. The results were published online November 14 in The New England Journal of Medicine and were presented at the annual meeting of the American College of Rheumatology (ACR).
The drug’s manufacturer, Amgen, released top-line results of the trial, called MITIGATE, in June 2024.
Until now, the mainstay of management for the chronic multiorgan disease IgG4-RD has been glucocorticoids, which can cause numerous adverse effects. “It is hoped that inebilizumab can be used as an important steroid-sparing medication in this disease to reduce steroid toxicity,” lead author John H. Stone, MD, professor of medicine at Harvard Medical School, Boston, Massachusetts, said in an interview, noting that it may not entirely eliminate the need for steroid treatment, but for many, it appears to work after the remission induction period as a monotherapy without steroids.
Asked to comment, Leonard H. Calabrese, DO, head of the Section of Clinical Immunology and manager of the Clinical Immunology Clinic at the Cleveland Clinic, Ohio, said: “There has been anecdotal or observational evidence for some effect with other immunosuppressive agents, including rituximab, but no robust clinical trial until this study. This clearly has demonstrated efficacy by reducing the risk of flares. And most importantly, putting people into remission means no active disease in any given organ. ... This gives us another tool in the toolbox to attack B cell–directed diseases, and I think it really makes a lot of sense.”
Calabrese cautioned, though, that “this is a disease that extends over many years. This is just a 1-year study. Label extensions will be important.”
And several questions remain, Calabrese noted: “How long do patients need to remain on drug? What will happen when the drug is stopped? Can they be retreated? These are the natural questions that arise in any sentinel study like this. But this is extremely encouraging. And I think it’s great for patients. I also think it’s a clarion call to increase awareness about this disease since there’s now strong evidence of effective treatment.”
Underrecognized, Often Misdiagnosed as Cancer
Indeed, IgG4-RD, a chronic, relapsing, autoimmune, fibro-inflammatory multiorgan disease, was only first described in Japan in 2003. Since then, it has been reported all over the world yet remains vastly underrecognized. It is often misdiagnosed as cancer because it produces lesions in multiple organs. It received an ICD-10 code only about a year ago. A previous study estimated a prevalence of about 5.3 persons per 100,000 but that is likely to be a three- to fourfold underestimate, said Stone, who is also executive chairman of the IgG4ward! Foundation.
“Nobody had heard of the disease until about 20 years ago. ... And there are many people in the world who have still not heard of it despite the fact that it is a multiorgan autoimmune disease and is probably as common, or more common, than many other diseases that rheumatologists spend a lot of time thinking about, such as scleroderma.”
While knowledge about the disease is increasing in rheumatology circles, it’s less well-recognized among many of the specialties where patients present, depending on the location of their lesions. These include gastroenterology, ophthalmology, pulmonary medicine, neurology, and nephrology. “All would be likely to see this disease,” Stone said.
The disease can be mistaken for tumors in many of those locations and even as metastatic cancer, he noted, adding that “any time a patient has a mass lesion in a typical organ, the pancreas, the major salivary glands, the lungs, or the kidneys, this should be on the differential diagnosis.”
The diagnosis of IgG4-RD is a clinical one, involving “quadrangulation between clinical features, serological findings, IgG4 levels in the blood, radiology studies, and then pathology biopsies when those are available,” Stone said.
Calabrese characterized the current situation as “we’re all blind men on the elephant. To the neurologist or the neurosurgeon, it’s a mass in the brain. It could present to the ophthalmologist as an [eye] tumor. It can be thyroid gland failure, pulmonary disease, retroperitoneal fibrosis, hepatobiliary disease, and beyond. So, whoever sees that patient, there’s often a long lag time in recognizing it.”
And interestingly, Stone noted that unlike other autoimmune diseases, IgG4-RD primarily affects middle-aged men rather than younger-to-middle-aged women. And when IgG4-RD is diagnosed, glucocorticoid treatment can be particularly toxic when the pancreas is involved, heightening the risk for hyperglycemia and potentially causing diabetes.
Dramatic Improvement in Flares, Remission Achievement
MITIGATE is a phase 3, multicenter, double-blind, randomized, placebo-controlled trial in which 135 adults (mean age 58.2 years, 88 men) with active IgG4-RD were randomized 1:1 to receive 300-mg intravenous infusions of inebilizumab or placebo on days 1 and 15, and again at week 26. At baseline, 62 (45.9%) participants had newly diagnosed IgG4-RD and 73 (54.1%) had recurrent disease.
Both groups received identical glucocorticoid tapers. Overall, 127 (94.1%) completed the 52 weeks of treatment.
By 52 weeks, only seven patients in the inebilizumab group (10%) had experienced disease flares vs 40 (60%) in the placebo group, a significant difference with a hazard ratio of 0.13 (P < .001).
The percentage of participants achieving flare-free, treatment-free complete remission was 59 with inebilizumab (57%), compared with just 15 (22%) in the placebo group (odds ratio [OR], 4.68; P < .001). And for flare-free, glucocorticoid-free complete remission, those proportions were 40 (59%) vs 15 (22%), respectively (OR, 4.96; P < .001).
Excluding the 8-week glucocorticoid taper period, mean total glucocorticoid use was 1264.2 mg less in the inebilizumab than the placebo group, a significant reduction. Overall, 61 participants (90%) were able to entirely discontinue glucocorticoids during the trial, compared with just 25 (37%) in the placebo group.
Adverse events of grade 3 or higher occurred in 12 participants (18%) in the inebilizumab group and 8 (12%) in the placebo group; serious adverse events occurred in 12 (18%) and 6 (9%), respectively. However, no serious adverse event occurred in more than one participant, and there were no deaths. Adverse events led to withdrawal from the trial in six patients (9%) in the inebilizumab group and three patients (4%) in the placebo group.
Adverse events that occurred in more than 10% of participants in the inebilizumab group were COVID-19 in 16 participants (24%), lymphopenia in 11 (16%), and urinary tract infection in 8 (12%).
Importantly, Stone noted, B-cell depletion can reduce responses to vaccines, so patients should receive all recommended vaccinations, including COVID-19, influenza, respiratory syncytial virus, and others, prior to initiating therapy.
Uplizna (inebilizumab-cdon) was approved by the Food and Drug Administration (FDA) for the treatment of neuromyelitis optica spectrum disorder in 2020. In October 2024, the FDA granted Amgen breakthrough therapy designation for use in IgG4-RD. The company is also developing the drug for use in myasthenia gravis.
The study was funded by Amgen. Stone has reported being a consultant for Amgen, Zenas, Argenx, Bristol Myers Squibb, Novartis, Sanofi, and Horizon Pharma. Calabrese has reported being a consultant and/or speaker for Amgen, AstraZeneca, Jansen, Sanofi, and UCB.
A version of this article first appeared on Medscape.com.
WASHINGTON — The B cell–depleting agent inebilizumab (Uplizna) dramatically reduced the risk of flares and increased year-long remission of IgG4-related disease (RD), new research has found.
In a phase 3, multicenter, double-blind, randomized, placebo-controlled trial of 135 adults with active IgG4-RD, treatment with inebilizumab resulted in a significant 87% reduction in flare risk and nearly fivefold greater likelihood of flare-free remission at 1 year. The results were published online November 14 in The New England Journal of Medicine and were presented at the annual meeting of the American College of Rheumatology (ACR).
The drug’s manufacturer, Amgen, released top-line results of the trial, called MITIGATE, in June 2024.
Until now, the mainstay of management for the chronic multiorgan disease IgG4-RD has been glucocorticoids, which can cause numerous adverse effects. “It is hoped that inebilizumab can be used as an important steroid-sparing medication in this disease to reduce steroid toxicity,” lead author John H. Stone, MD, professor of medicine at Harvard Medical School, Boston, Massachusetts, said in an interview, noting that it may not entirely eliminate the need for steroid treatment, but for many, it appears to work after the remission induction period as a monotherapy without steroids.
Asked to comment, Leonard H. Calabrese, DO, head of the Section of Clinical Immunology and manager of the Clinical Immunology Clinic at the Cleveland Clinic, Ohio, said: “There has been anecdotal or observational evidence for some effect with other immunosuppressive agents, including rituximab, but no robust clinical trial until this study. This clearly has demonstrated efficacy by reducing the risk of flares. And most importantly, putting people into remission means no active disease in any given organ. ... This gives us another tool in the toolbox to attack B cell–directed diseases, and I think it really makes a lot of sense.”
Calabrese cautioned, though, that “this is a disease that extends over many years. This is just a 1-year study. Label extensions will be important.”
And several questions remain, Calabrese noted: “How long do patients need to remain on drug? What will happen when the drug is stopped? Can they be retreated? These are the natural questions that arise in any sentinel study like this. But this is extremely encouraging. And I think it’s great for patients. I also think it’s a clarion call to increase awareness about this disease since there’s now strong evidence of effective treatment.”
Underrecognized, Often Misdiagnosed as Cancer
Indeed, IgG4-RD, a chronic, relapsing, autoimmune, fibro-inflammatory multiorgan disease, was only first described in Japan in 2003. Since then, it has been reported all over the world yet remains vastly underrecognized. It is often misdiagnosed as cancer because it produces lesions in multiple organs. It received an ICD-10 code only about a year ago. A previous study estimated a prevalence of about 5.3 persons per 100,000 but that is likely to be a three- to fourfold underestimate, said Stone, who is also executive chairman of the IgG4ward! Foundation.
“Nobody had heard of the disease until about 20 years ago. ... And there are many people in the world who have still not heard of it despite the fact that it is a multiorgan autoimmune disease and is probably as common, or more common, than many other diseases that rheumatologists spend a lot of time thinking about, such as scleroderma.”
While knowledge about the disease is increasing in rheumatology circles, it’s less well-recognized among many of the specialties where patients present, depending on the location of their lesions. These include gastroenterology, ophthalmology, pulmonary medicine, neurology, and nephrology. “All would be likely to see this disease,” Stone said.
The disease can be mistaken for tumors in many of those locations and even as metastatic cancer, he noted, adding that “any time a patient has a mass lesion in a typical organ, the pancreas, the major salivary glands, the lungs, or the kidneys, this should be on the differential diagnosis.”
The diagnosis of IgG4-RD is a clinical one, involving “quadrangulation between clinical features, serological findings, IgG4 levels in the blood, radiology studies, and then pathology biopsies when those are available,” Stone said.
Calabrese characterized the current situation as “we’re all blind men on the elephant. To the neurologist or the neurosurgeon, it’s a mass in the brain. It could present to the ophthalmologist as an [eye] tumor. It can be thyroid gland failure, pulmonary disease, retroperitoneal fibrosis, hepatobiliary disease, and beyond. So, whoever sees that patient, there’s often a long lag time in recognizing it.”
And interestingly, Stone noted that unlike other autoimmune diseases, IgG4-RD primarily affects middle-aged men rather than younger-to-middle-aged women. And when IgG4-RD is diagnosed, glucocorticoid treatment can be particularly toxic when the pancreas is involved, heightening the risk for hyperglycemia and potentially causing diabetes.
Dramatic Improvement in Flares, Remission Achievement
MITIGATE is a phase 3, multicenter, double-blind, randomized, placebo-controlled trial in which 135 adults (mean age 58.2 years, 88 men) with active IgG4-RD were randomized 1:1 to receive 300-mg intravenous infusions of inebilizumab or placebo on days 1 and 15, and again at week 26. At baseline, 62 (45.9%) participants had newly diagnosed IgG4-RD and 73 (54.1%) had recurrent disease.
Both groups received identical glucocorticoid tapers. Overall, 127 (94.1%) completed the 52 weeks of treatment.
By 52 weeks, only seven patients in the inebilizumab group (10%) had experienced disease flares vs 40 (60%) in the placebo group, a significant difference with a hazard ratio of 0.13 (P < .001).
The percentage of participants achieving flare-free, treatment-free complete remission was 59 with inebilizumab (57%), compared with just 15 (22%) in the placebo group (odds ratio [OR], 4.68; P < .001). And for flare-free, glucocorticoid-free complete remission, those proportions were 40 (59%) vs 15 (22%), respectively (OR, 4.96; P < .001).
Excluding the 8-week glucocorticoid taper period, mean total glucocorticoid use was 1264.2 mg less in the inebilizumab than the placebo group, a significant reduction. Overall, 61 participants (90%) were able to entirely discontinue glucocorticoids during the trial, compared with just 25 (37%) in the placebo group.
Adverse events of grade 3 or higher occurred in 12 participants (18%) in the inebilizumab group and 8 (12%) in the placebo group; serious adverse events occurred in 12 (18%) and 6 (9%), respectively. However, no serious adverse event occurred in more than one participant, and there were no deaths. Adverse events led to withdrawal from the trial in six patients (9%) in the inebilizumab group and three patients (4%) in the placebo group.
Adverse events that occurred in more than 10% of participants in the inebilizumab group were COVID-19 in 16 participants (24%), lymphopenia in 11 (16%), and urinary tract infection in 8 (12%).
Importantly, Stone noted, B-cell depletion can reduce responses to vaccines, so patients should receive all recommended vaccinations, including COVID-19, influenza, respiratory syncytial virus, and others, prior to initiating therapy.
Uplizna (inebilizumab-cdon) was approved by the Food and Drug Administration (FDA) for the treatment of neuromyelitis optica spectrum disorder in 2020. In October 2024, the FDA granted Amgen breakthrough therapy designation for use in IgG4-RD. The company is also developing the drug for use in myasthenia gravis.
The study was funded by Amgen. Stone has reported being a consultant for Amgen, Zenas, Argenx, Bristol Myers Squibb, Novartis, Sanofi, and Horizon Pharma. Calabrese has reported being a consultant and/or speaker for Amgen, AstraZeneca, Jansen, Sanofi, and UCB.
A version of this article first appeared on Medscape.com.
FROM ACR 2024
Scurvy: A Diagnosis Still Relevant Today
“Petechial rash often prompts further investigation into hematological, dermatological, or vasculitis causes. However, if the above investigations are negative and skin biopsy has not revealed a cause, there is a Renaissance-era diagnosis that is often overlooked but is easily investigated and treated,” wrote Andrew Dermawan, MD, and colleagues from Sir Charles Gairdner Hospital in Nedlands, Australia, in BMJ Case Reports. The diagnosis they highlight is scurvy, a disease that has faded from common medical concern but is reemerging, partly because of the rise in bariatric surgery.
Diagnosing Scurvy in the 2020s
In their article, Dermawan and colleagues present the case of a 50-year-old man with a bilateral petechial rash on his lower limbs, without any history of trauma. The patient, who exhibited no infectious symptoms, also had gross hematuria, microcytic anemia, mild neutropenia, and lymphopenia. Tests for autoimmune and hematological diseases were negative, as were abdominal and leg CT scans, ruling out abdominal hemorrhage and vasculitis. Additionally, a skin biopsy showed no causative findings.
The doctors noted that the patient had undergone sleeve gastrectomy, prompting them to inquire about his diet. They discovered that, because of financial difficulties, his diet primarily consisted of processed foods with little to no fruits or vegetables, and he had stopped taking supplements recommended by his gastroenterologist. Further tests revealed a vitamin D deficiency and a severe deficiency in vitamin C. With the diagnosis of scurvy confirmed, the doctors treated the patient with 1000 mg of ascorbic acid daily, along with cholecalciferol, folic acid, and a multivitamin complex, leading to a complete resolution of his symptoms.
Risk Factors Then and Now
It can cause mucosal and gastric hemorrhages, and if left untreated, it can lead to fatal bleeding.
Historically known as “sailors’ disease,” scurvy plagued men on long voyages who lacked access to fresh fruits or vegetables and thus did not get enough vitamin C. In 1747, James Lind, a British physician in the Royal Navy, demonstrated that the consumption of oranges and lemons could combat scurvy.
Today’s risk factors for scurvy include malnutrition, gastrointestinal disorders (eg, chronic inflammatory bowel diseases), alcohol and tobacco use, eating disorders, psychiatric illnesses, dialysis, and the use of medications that reduce the absorption of ascorbic acid (such as corticosteroids and proton pump inhibitors).
Scurvy remains more common among individuals with unfavorable socioeconomic conditions. The authors of the study emphasize how the rising cost of living — specifically in Australia but applicable elsewhere — is changing eating habits, leading to a high consumption of low-cost, nutritionally poor foods.
Poverty has always been a risk factor for scurvy, but today there may be an additional cause: bariatric surgery. Patients undergoing these procedures are at a risk for deficiencies in fat-soluble vitamins A, D, E, and K, and if their diet is inadequate, they may also experience a vitamin C deficiency. Awareness of this can facilitate the timely diagnosis of scurvy in these patients.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“Petechial rash often prompts further investigation into hematological, dermatological, or vasculitis causes. However, if the above investigations are negative and skin biopsy has not revealed a cause, there is a Renaissance-era diagnosis that is often overlooked but is easily investigated and treated,” wrote Andrew Dermawan, MD, and colleagues from Sir Charles Gairdner Hospital in Nedlands, Australia, in BMJ Case Reports. The diagnosis they highlight is scurvy, a disease that has faded from common medical concern but is reemerging, partly because of the rise in bariatric surgery.
Diagnosing Scurvy in the 2020s
In their article, Dermawan and colleagues present the case of a 50-year-old man with a bilateral petechial rash on his lower limbs, without any history of trauma. The patient, who exhibited no infectious symptoms, also had gross hematuria, microcytic anemia, mild neutropenia, and lymphopenia. Tests for autoimmune and hematological diseases were negative, as were abdominal and leg CT scans, ruling out abdominal hemorrhage and vasculitis. Additionally, a skin biopsy showed no causative findings.
The doctors noted that the patient had undergone sleeve gastrectomy, prompting them to inquire about his diet. They discovered that, because of financial difficulties, his diet primarily consisted of processed foods with little to no fruits or vegetables, and he had stopped taking supplements recommended by his gastroenterologist. Further tests revealed a vitamin D deficiency and a severe deficiency in vitamin C. With the diagnosis of scurvy confirmed, the doctors treated the patient with 1000 mg of ascorbic acid daily, along with cholecalciferol, folic acid, and a multivitamin complex, leading to a complete resolution of his symptoms.
Risk Factors Then and Now
It can cause mucosal and gastric hemorrhages, and if left untreated, it can lead to fatal bleeding.
Historically known as “sailors’ disease,” scurvy plagued men on long voyages who lacked access to fresh fruits or vegetables and thus did not get enough vitamin C. In 1747, James Lind, a British physician in the Royal Navy, demonstrated that the consumption of oranges and lemons could combat scurvy.
Today’s risk factors for scurvy include malnutrition, gastrointestinal disorders (eg, chronic inflammatory bowel diseases), alcohol and tobacco use, eating disorders, psychiatric illnesses, dialysis, and the use of medications that reduce the absorption of ascorbic acid (such as corticosteroids and proton pump inhibitors).
Scurvy remains more common among individuals with unfavorable socioeconomic conditions. The authors of the study emphasize how the rising cost of living — specifically in Australia but applicable elsewhere — is changing eating habits, leading to a high consumption of low-cost, nutritionally poor foods.
Poverty has always been a risk factor for scurvy, but today there may be an additional cause: bariatric surgery. Patients undergoing these procedures are at a risk for deficiencies in fat-soluble vitamins A, D, E, and K, and if their diet is inadequate, they may also experience a vitamin C deficiency. Awareness of this can facilitate the timely diagnosis of scurvy in these patients.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“Petechial rash often prompts further investigation into hematological, dermatological, or vasculitis causes. However, if the above investigations are negative and skin biopsy has not revealed a cause, there is a Renaissance-era diagnosis that is often overlooked but is easily investigated and treated,” wrote Andrew Dermawan, MD, and colleagues from Sir Charles Gairdner Hospital in Nedlands, Australia, in BMJ Case Reports. The diagnosis they highlight is scurvy, a disease that has faded from common medical concern but is reemerging, partly because of the rise in bariatric surgery.
Diagnosing Scurvy in the 2020s
In their article, Dermawan and colleagues present the case of a 50-year-old man with a bilateral petechial rash on his lower limbs, without any history of trauma. The patient, who exhibited no infectious symptoms, also had gross hematuria, microcytic anemia, mild neutropenia, and lymphopenia. Tests for autoimmune and hematological diseases were negative, as were abdominal and leg CT scans, ruling out abdominal hemorrhage and vasculitis. Additionally, a skin biopsy showed no causative findings.
The doctors noted that the patient had undergone sleeve gastrectomy, prompting them to inquire about his diet. They discovered that, because of financial difficulties, his diet primarily consisted of processed foods with little to no fruits or vegetables, and he had stopped taking supplements recommended by his gastroenterologist. Further tests revealed a vitamin D deficiency and a severe deficiency in vitamin C. With the diagnosis of scurvy confirmed, the doctors treated the patient with 1000 mg of ascorbic acid daily, along with cholecalciferol, folic acid, and a multivitamin complex, leading to a complete resolution of his symptoms.
Risk Factors Then and Now
It can cause mucosal and gastric hemorrhages, and if left untreated, it can lead to fatal bleeding.
Historically known as “sailors’ disease,” scurvy plagued men on long voyages who lacked access to fresh fruits or vegetables and thus did not get enough vitamin C. In 1747, James Lind, a British physician in the Royal Navy, demonstrated that the consumption of oranges and lemons could combat scurvy.
Today’s risk factors for scurvy include malnutrition, gastrointestinal disorders (eg, chronic inflammatory bowel diseases), alcohol and tobacco use, eating disorders, psychiatric illnesses, dialysis, and the use of medications that reduce the absorption of ascorbic acid (such as corticosteroids and proton pump inhibitors).
Scurvy remains more common among individuals with unfavorable socioeconomic conditions. The authors of the study emphasize how the rising cost of living — specifically in Australia but applicable elsewhere — is changing eating habits, leading to a high consumption of low-cost, nutritionally poor foods.
Poverty has always been a risk factor for scurvy, but today there may be an additional cause: bariatric surgery. Patients undergoing these procedures are at a risk for deficiencies in fat-soluble vitamins A, D, E, and K, and if their diet is inadequate, they may also experience a vitamin C deficiency. Awareness of this can facilitate the timely diagnosis of scurvy in these patients.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Myasthenia Gravis: Where Does Traditional Therapy Fit In?
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
SAVANNAH, GEORGIA —
In a debate at American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM) 2024, a pair of neurologists who specialize in neuromuscular disorders laid out opposing evidence for each approach.
On one hand, Benjamin Claytor, MD, of Cleveland Clinic, Cleveland, argued that “traditional therapy is very effective for the majority of myasthenia gravis patients,” and he said it should be considered first-line.
But Amanda C. Guidon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, responded that “the immunosuppression of traditional therapies is too broad: The time to benefit is too long, the burden of side effects is too high, and the cancer risk is too elevated.”
Traditional Therapy: Affordable, Tolerable, and Safe?
Claytor said ideal myasthenia gravis therapies are effective, tolerable, and safe. They’re also affordable, convenient (such as a pill), lead to sustained remission, and can have dosages reduced.
Only traditional therapies — corticosteroids, azathioprine, mycophenolate, and rituximab — meet those last three criteria, he said. Newer therapies, he said, do not.
Claytor highlighted a 2023 Duke University study that tracked 367 patients with MG who were treated with traditional therapies after the year 2000. Of those, 72% reached the treatment goal of minimal manifestations in a median of less than 2 years.
In addition, Claytor noted that the percentage of patients with myasthenia gravis who reach minimal symptom expression ranges from 45% (6 months) to 60% or more (2 years), while studies suggest that newer treatments such as eculizumab (Soliris), efgartigimod (Vyvgart), rozanolixizumab (Rystiggo), and zilucoplan (Zilbrysq) haven’t reached those levels.
As for specific traditional therapies, Claytor said the corticosteroid prednisone is “extremely affordable,” effective, and takes fewer than 2 weeks to work. All patients with myasthenia gravis can take it, he said, and at least 75% of those with mild/moderate disease respond to low doses.
Nonsteroidal Agents, Immune Globulin, Rituximab
He acknowledged side effects from corticosteroids but said doses can be tapered once severity improves. Calcium and vitamin D can be helpful to support bone health, he added.
As for nonsteroidal immunosuppressive treatments, he said they’re easy to administer, increase the likelihood of reaching minimal manifestation status, can be effective at lower doses, and may allow patients to discontinue steroids.
Two other traditional therapies, immune globulin and plasmapheresis, can be appropriate in crisis or impending crisis situations, he said, or as an add-on therapy if steroids and nonsteroidal immunosuppressive therapies don’t work.
What about rituximab? “We’re learning that patients with new-onset disease and younger patients seem to respond better,” Claytor said. While rituximab is expensive, it’s “not even in the same realm” as newer agents if only a dose or two are given, he said.
Steroids Are Ideal in MG? Not So Fast
In her response, Guidon noted that she was assigned to offer a counter-perspective in her presentation, and “personal opinions are not being represented here fully.” She then listed the weaknesses of traditional therapy in myasthenia gravis.
For one thing, she said the drugs don’t work well. She highlighted a 2019 registry study that found “many myasthenia gravis patients remain negatively impacted despite treatment.”
In addition, “we can’t predict who will respond to which therapy. ... We start drugs and don’t know if we’ll have benefit from 6 months up to 18 months. We also can’t determine minimally effective dose a priori. Some patients require higher doses, and some subtherapeutic doses are actually therapeutic for our patients.”
Broad immunosuppression, she added, boosts the risk for serious infections. “We’ve all heard from our patients that the side effects can be worse than the myasthenia, and next we’re going to talk about the role of corticosteroids in myasthenia.”
As for corticosteroids in particular, “they’re really the best treatment and also the worst treatment.” Efficacy and side effects battle for supremacy in patients, she said, “and you don’t know which is going to win out.”
Kicking Traditional Therapy to the Curb
There are many possible side effects from steroids, she said, including steroid-induced diabetes, which is “profound.” Some patients never recover from it.
On top of all these risks, she said, 20%-30% of patients are resistant to steroids.
As for other treatments, immune globulin and plasmapheresis “aren’t really benign,” Guidon said. They come with potentially serious side effects of their own, as do nonsteroidal immunosuppressive treatments.
Guidon said better treatments are needed to minimize the risks from traditional therapies. “We need targeted therapies that drive disease into remission, can be tapered, are delivered orally or with infrequent self-injections, and don’t require frequent lab monitoring.”
In addition, ideal treatments should “have a good safety data in pregnancy and for breastfeeding and have a favorable side effect profile with no significant long-term cancer risks.”
Claytor had no disclosures. Guidon disclosed consulting/medical advisory board (Alexion Pharmaceuticals, argenx, Regeneron, and UCB), publishing royalties (Oakstone), and other research support (Myasthenia Gravis Foundation of America, Myasthenia Gravis Rare Disease Network, National Institutes of Health, and National Institute of Neurological Disorders and Stroke/BioSensics).
A version of this article appeared on Medscape.com.
FROM AANEM 2024



