User login
Hypermucoviscous K pneumoniae Shows Reduced Drug Resistance
TOPLINE:
Hypermucoviscous Klebsiella pneumoniae (hmKp) strains demonstrate a significantly lower prevalence of extended-spectrum beta-lactamase (ESBL) production and slightly lower carbapenem resistance than non-hmKp strains, according to a recent meta-analysis of 2049 clinical isolates.
METHODOLOGY:
- Researchers conducted a meta-analysis to assess the prevalence of ESBL-producing strains and carbapenem-resistant strains among the hmKp and non-hmKp clinical isolates.
- They included 15 studies published between 2014 and 2023, with 2049 clinical isolates of K pneumoniae identified using a string test to distinguish hypermucoviscous from non-hypermucoviscous strains.
- These studies spanned across four continents: Asia, Africa, Europe, and North America.
- The primary outcome was the prevalence of ESBL-producing and carbapenem-resistant strains, determined through antimicrobial susceptibility testing.
TAKEAWAY:
- The hmKp strains were associated with a significantly lower prevalence of ESBL-producing strains than non-hmKp strains (pooled odds ratio [OR], 0.26; P = .003).
- Similarly, hmKp strains were associated with a slightly lower prevalence of carbapenem-resistant strains than non-hmKp strains (pooled OR, 0.63; P = .038).
IN PRACTICE:
“Therapeutic options for CRKP [carbapenem-resistant K pneumoniae] infections are extremely limited due to the scarcity of effective antibacterial drugs. Therefore, it is crucial to consider the risks posed by CRKP strains when administering treatment to patients with hmKp infections and a history of the aforementioned risk factors,” the authors wrote.
SOURCE:
The study was led by Hiroki Namikawa, Department of Medical Education and General Practice, Graduate School of Medicine, Osaka Metropolitan University, Japan. It was published online on December 16, 2024, in Emerging Microbes & Infections.
LIMITATIONS:
Only three databases (PubMed, Scopus, and Cochrane Library) were searched for identifying studies, potentially missing relevant studies from other sources. Furthermore, only articles published in English were included, which may have restricted the scope of analysis. Additionally, geographical distribution was predominantly limited to Asia, limiting the global applicability of the results.
DISCLOSURES:
No funding sources were mentioned, and no conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Hypermucoviscous Klebsiella pneumoniae (hmKp) strains demonstrate a significantly lower prevalence of extended-spectrum beta-lactamase (ESBL) production and slightly lower carbapenem resistance than non-hmKp strains, according to a recent meta-analysis of 2049 clinical isolates.
METHODOLOGY:
- Researchers conducted a meta-analysis to assess the prevalence of ESBL-producing strains and carbapenem-resistant strains among the hmKp and non-hmKp clinical isolates.
- They included 15 studies published between 2014 and 2023, with 2049 clinical isolates of K pneumoniae identified using a string test to distinguish hypermucoviscous from non-hypermucoviscous strains.
- These studies spanned across four continents: Asia, Africa, Europe, and North America.
- The primary outcome was the prevalence of ESBL-producing and carbapenem-resistant strains, determined through antimicrobial susceptibility testing.
TAKEAWAY:
- The hmKp strains were associated with a significantly lower prevalence of ESBL-producing strains than non-hmKp strains (pooled odds ratio [OR], 0.26; P = .003).
- Similarly, hmKp strains were associated with a slightly lower prevalence of carbapenem-resistant strains than non-hmKp strains (pooled OR, 0.63; P = .038).
IN PRACTICE:
“Therapeutic options for CRKP [carbapenem-resistant K pneumoniae] infections are extremely limited due to the scarcity of effective antibacterial drugs. Therefore, it is crucial to consider the risks posed by CRKP strains when administering treatment to patients with hmKp infections and a history of the aforementioned risk factors,” the authors wrote.
SOURCE:
The study was led by Hiroki Namikawa, Department of Medical Education and General Practice, Graduate School of Medicine, Osaka Metropolitan University, Japan. It was published online on December 16, 2024, in Emerging Microbes & Infections.
LIMITATIONS:
Only three databases (PubMed, Scopus, and Cochrane Library) were searched for identifying studies, potentially missing relevant studies from other sources. Furthermore, only articles published in English were included, which may have restricted the scope of analysis. Additionally, geographical distribution was predominantly limited to Asia, limiting the global applicability of the results.
DISCLOSURES:
No funding sources were mentioned, and no conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Hypermucoviscous Klebsiella pneumoniae (hmKp) strains demonstrate a significantly lower prevalence of extended-spectrum beta-lactamase (ESBL) production and slightly lower carbapenem resistance than non-hmKp strains, according to a recent meta-analysis of 2049 clinical isolates.
METHODOLOGY:
- Researchers conducted a meta-analysis to assess the prevalence of ESBL-producing strains and carbapenem-resistant strains among the hmKp and non-hmKp clinical isolates.
- They included 15 studies published between 2014 and 2023, with 2049 clinical isolates of K pneumoniae identified using a string test to distinguish hypermucoviscous from non-hypermucoviscous strains.
- These studies spanned across four continents: Asia, Africa, Europe, and North America.
- The primary outcome was the prevalence of ESBL-producing and carbapenem-resistant strains, determined through antimicrobial susceptibility testing.
TAKEAWAY:
- The hmKp strains were associated with a significantly lower prevalence of ESBL-producing strains than non-hmKp strains (pooled odds ratio [OR], 0.26; P = .003).
- Similarly, hmKp strains were associated with a slightly lower prevalence of carbapenem-resistant strains than non-hmKp strains (pooled OR, 0.63; P = .038).
IN PRACTICE:
“Therapeutic options for CRKP [carbapenem-resistant K pneumoniae] infections are extremely limited due to the scarcity of effective antibacterial drugs. Therefore, it is crucial to consider the risks posed by CRKP strains when administering treatment to patients with hmKp infections and a history of the aforementioned risk factors,” the authors wrote.
SOURCE:
The study was led by Hiroki Namikawa, Department of Medical Education and General Practice, Graduate School of Medicine, Osaka Metropolitan University, Japan. It was published online on December 16, 2024, in Emerging Microbes & Infections.
LIMITATIONS:
Only three databases (PubMed, Scopus, and Cochrane Library) were searched for identifying studies, potentially missing relevant studies from other sources. Furthermore, only articles published in English were included, which may have restricted the scope of analysis. Additionally, geographical distribution was predominantly limited to Asia, limiting the global applicability of the results.
DISCLOSURES:
No funding sources were mentioned, and no conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Imipenem-Cilastatin-Relebactam, the New Go-To for Pneumonia?
TOPLINE:
In a multinational phase 3 trial, imipenem-cilastatin-relebactam demonstrated noninferiority to piperacillin-tazobactam in treating critically ill patients with hospital-acquired bacterial pneumonia (HABP) or ventilator-associated bacterial pneumonia (VABP), with a comparable safety profile.
METHODOLOGY:
- This multinational phase 3 trial, conducted between September 2018 and July 2022, compared imipenem-cilastatin-relebactam with piperacillin-tazobactam for HABP and VABP to support its use across multiple countries.
- Overall, 270 patients with HABP or VABP (mean age, 57.6 years; 73.3% men) were randomly assigned to receive either intravenous imipenem-cilastatin-relebactam (500 mg/250 mg) or piperacillin-tazobactam (4000 mg/500 mg) every 6 hours over 30 minutes for 7-14 days.
- Both treatment groups included critically ill patients, with 54.5% and 55.1% of patients in the imipenem-cilastatin-relebactam and piperacillin-tazobactam groups, respectively, having an Acute Physiology and Chronic Health Evaluation II score ≥ 15.
- The primary outcome was the 28-day all-cause mortality; secondary outcomes included the rates of clinical and microbiological responses, as well as the incidence of adverse events.
TAKEAWAY:
- Imipenem-cilastatin-relebactam was noninferior to piperacillin-tazobactam in terms of 28-day all-cause mortality (adjusted difference, 5.2%; 95% CI, −1.5-12.4; P = .024 for noninferiority).
- After treatment, microbiological response rates were 48.8% in the imipenem-cilastatin-relebactam group, whereas the rates were 47.9% in the piperacillin-tazobactam group.
- The incidence of drug-related adverse events was similar across the treatment groups, with diarrhea, increased levels of alanine aminotransferase and aspartate aminotransferase, and abnormal hepatic function being the most common events.
IN PRACTICE:
“These results support the use of IMI/REL [imipenem-cilastatin-relebactam] in MDR [multidrug-resistant] infections globally, including to expand the range of available treatments for critically ill patients with HABP/VABP in China, and provide additional data to inform the World Health Organization’s MDR pathogen strategy,” the authors wrote.
SOURCE:
This study was led by Junjie Li, Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. It was published online on December 12, 2024, in the International Journal of Infectious Diseases.
LIMITATIONS:
This study excluded patients with immunosuppression and those on intermittent hemodialysis, limiting the generalizability of the results to these populations.
DISCLOSURES:
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, New Jersey. Some authors served as employees of Merck Sharp & Dohme LLC, New Jersey, and MSD, China.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
In a multinational phase 3 trial, imipenem-cilastatin-relebactam demonstrated noninferiority to piperacillin-tazobactam in treating critically ill patients with hospital-acquired bacterial pneumonia (HABP) or ventilator-associated bacterial pneumonia (VABP), with a comparable safety profile.
METHODOLOGY:
- This multinational phase 3 trial, conducted between September 2018 and July 2022, compared imipenem-cilastatin-relebactam with piperacillin-tazobactam for HABP and VABP to support its use across multiple countries.
- Overall, 270 patients with HABP or VABP (mean age, 57.6 years; 73.3% men) were randomly assigned to receive either intravenous imipenem-cilastatin-relebactam (500 mg/250 mg) or piperacillin-tazobactam (4000 mg/500 mg) every 6 hours over 30 minutes for 7-14 days.
- Both treatment groups included critically ill patients, with 54.5% and 55.1% of patients in the imipenem-cilastatin-relebactam and piperacillin-tazobactam groups, respectively, having an Acute Physiology and Chronic Health Evaluation II score ≥ 15.
- The primary outcome was the 28-day all-cause mortality; secondary outcomes included the rates of clinical and microbiological responses, as well as the incidence of adverse events.
TAKEAWAY:
- Imipenem-cilastatin-relebactam was noninferior to piperacillin-tazobactam in terms of 28-day all-cause mortality (adjusted difference, 5.2%; 95% CI, −1.5-12.4; P = .024 for noninferiority).
- After treatment, microbiological response rates were 48.8% in the imipenem-cilastatin-relebactam group, whereas the rates were 47.9% in the piperacillin-tazobactam group.
- The incidence of drug-related adverse events was similar across the treatment groups, with diarrhea, increased levels of alanine aminotransferase and aspartate aminotransferase, and abnormal hepatic function being the most common events.
IN PRACTICE:
“These results support the use of IMI/REL [imipenem-cilastatin-relebactam] in MDR [multidrug-resistant] infections globally, including to expand the range of available treatments for critically ill patients with HABP/VABP in China, and provide additional data to inform the World Health Organization’s MDR pathogen strategy,” the authors wrote.
SOURCE:
This study was led by Junjie Li, Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. It was published online on December 12, 2024, in the International Journal of Infectious Diseases.
LIMITATIONS:
This study excluded patients with immunosuppression and those on intermittent hemodialysis, limiting the generalizability of the results to these populations.
DISCLOSURES:
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, New Jersey. Some authors served as employees of Merck Sharp & Dohme LLC, New Jersey, and MSD, China.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
In a multinational phase 3 trial, imipenem-cilastatin-relebactam demonstrated noninferiority to piperacillin-tazobactam in treating critically ill patients with hospital-acquired bacterial pneumonia (HABP) or ventilator-associated bacterial pneumonia (VABP), with a comparable safety profile.
METHODOLOGY:
- This multinational phase 3 trial, conducted between September 2018 and July 2022, compared imipenem-cilastatin-relebactam with piperacillin-tazobactam for HABP and VABP to support its use across multiple countries.
- Overall, 270 patients with HABP or VABP (mean age, 57.6 years; 73.3% men) were randomly assigned to receive either intravenous imipenem-cilastatin-relebactam (500 mg/250 mg) or piperacillin-tazobactam (4000 mg/500 mg) every 6 hours over 30 minutes for 7-14 days.
- Both treatment groups included critically ill patients, with 54.5% and 55.1% of patients in the imipenem-cilastatin-relebactam and piperacillin-tazobactam groups, respectively, having an Acute Physiology and Chronic Health Evaluation II score ≥ 15.
- The primary outcome was the 28-day all-cause mortality; secondary outcomes included the rates of clinical and microbiological responses, as well as the incidence of adverse events.
TAKEAWAY:
- Imipenem-cilastatin-relebactam was noninferior to piperacillin-tazobactam in terms of 28-day all-cause mortality (adjusted difference, 5.2%; 95% CI, −1.5-12.4; P = .024 for noninferiority).
- After treatment, microbiological response rates were 48.8% in the imipenem-cilastatin-relebactam group, whereas the rates were 47.9% in the piperacillin-tazobactam group.
- The incidence of drug-related adverse events was similar across the treatment groups, with diarrhea, increased levels of alanine aminotransferase and aspartate aminotransferase, and abnormal hepatic function being the most common events.
IN PRACTICE:
“These results support the use of IMI/REL [imipenem-cilastatin-relebactam] in MDR [multidrug-resistant] infections globally, including to expand the range of available treatments for critically ill patients with HABP/VABP in China, and provide additional data to inform the World Health Organization’s MDR pathogen strategy,” the authors wrote.
SOURCE:
This study was led by Junjie Li, Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. It was published online on December 12, 2024, in the International Journal of Infectious Diseases.
LIMITATIONS:
This study excluded patients with immunosuppression and those on intermittent hemodialysis, limiting the generalizability of the results to these populations.
DISCLOSURES:
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, New Jersey. Some authors served as employees of Merck Sharp & Dohme LLC, New Jersey, and MSD, China.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
ACIP Recommends Pneumococcal Vaccine for Adults 50 Years or Older
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) now recommends a pneumococcal conjugate vaccine (PCV) for all PCV-naive adults aged 50 years or older. The new recommendation, which passed with an ACIP member vote of 14 for and one against, expands the current age-based recommendations, which include children younger than 5 years and adults older than 65 years, as well as adults aged 19-64 years with underlying conditions or risk factors who have not received a PCV and certain adults who have received PCV13 but not PCV20.
The decision was based in part on economic analyses of the use of PCV in adults aged 50-64 years in the United States. Miwako Kobayashi, MD, presented the summary of the Pneumococcal Vaccines Work Group’s interpretation of the evidence and the proposed recommendation in a meeting of the ACIP on October 23, 2024, when the ACIP voting occurred.
Data from the CDC show an increase in the relative burden of pneumococcal disease in adults aged 50-64 years based in part on the success of the pediatric PCV program, she said.
Health equity was another main factor in the Work Group’s decision to recommend vaccination for adults aged 50 years or older. “Disparities in pneumococcal vaccine coverage by race and ethnicity exist for both age-based and risk-based indications,” Kobayashi noted in her presentation. The Work Group acknowledged that the overall effect of a vaccine recommendation on health equity is complex, but the majority agreed that the update would improve health equity by increasing vaccine coverage for those with known or unknown risk factors and providing protection at an earlier age when some populations already experience elevated disease rates, she said.
As for safety, the Work Group concluded that the undesirable anticipated effects of PCVs are minimal, despite the potential signal for Guillain-Barré Syndrome, and the CDC and US Food and Drug Administration will continue to monitor post-licensure safety of PCVs.
Support Not Universal
A majority of the ACIP Pneumococcal Vaccines Work Group supported the approved option, but agreed that a future booster dose may be needed, Work Group Chair James Loehr, MD, said in his introductory presentation.
Overall, key uncertainties remain, including indirect effects of new pediatric pneumococcal vaccines on adults, data on the duration of protection with adult vaccinations, and the impact new higher-valency vaccines have on adults, several of which are in development, Loehr said.
A new 21-valent PCV, known as PCV 21, was approved by the FDA for adults aged 18 years or older in June 2024, said Loehr. “PCV21 is not PCV20 with one additional serotype” and provides additional protection, he emphasized. The Work Group examined models involving PCV21 and the existing PCV20. However, a majority of the Work Group agreed that having age-based recommendations based on vaccine product would be more challenging to implement and that insurance coverage may be a factor given the recent approval of PCV21. Therefore, the proposal submitted to the full ACIP was not for a specific PCV.
Notably, Loehr said that, although as Work Group Chair he was tasked with making the motion in favor of the recommendation, he voted against it as a voting member because of his strong opinion that only the PCV21 vaccine is needed for vaccine-naive adults aged 50 or older. “I think that PCV21 is a better vaccine that targets more serotypes,” he said during the discussion. Data presented at the February 2024 ACIP meeting showed more than 80% coverage vs less than 60% coverage for invasive pneumococcal disease with PCV21 vs PCV20 among adults aged 65 years or older and those aged 19-64 years with a risk-based indication, Loehr said.
A version of this article appeared on Medscape.com.
Clozapine and Respiratory Infection Risk: What to Know
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Anticipated Effects of Pneumococcal Vaccines on Otitis
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).
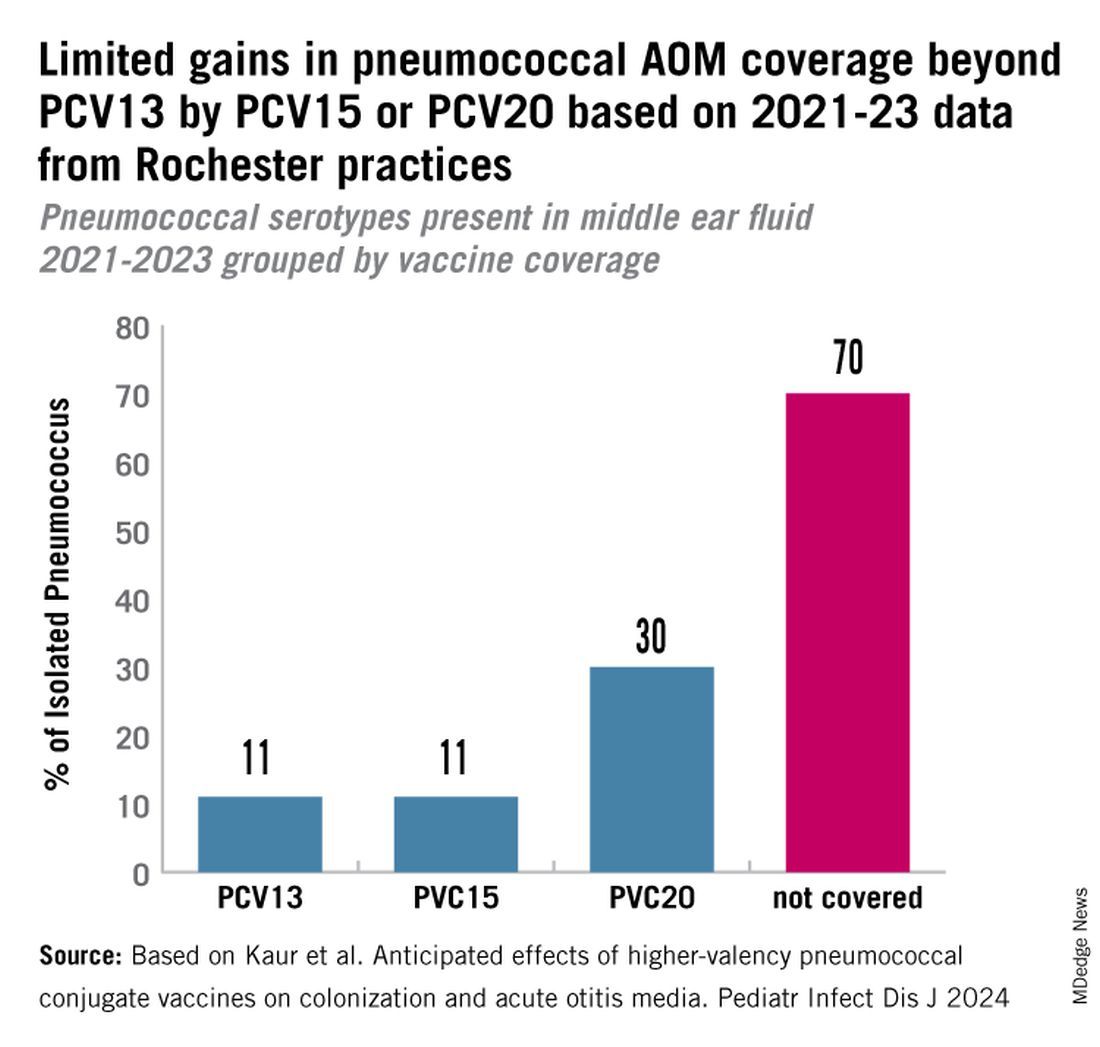
Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
Acute otitis media (AOM) is caused by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Since the introduction of pneumococcal conjugate vaccines (PCVs) shifts in the proportion of these three bacteria as causes of AOM and their antibiotic susceptibility profiles and strain diversity have occurred due to multiple factors including the PCVs and antibiotic selection pressure.
The 7-valent PCV (PCV7) was introduced in 2000 and was proven to be efficacious in preventing AOM, but no subsequent PCV has received an indication for prevention of AOM because the FDA required a tympanocentesis study to prove efficacy and that approval was not achieved for PCV13, PCV15, or PCV20. This is a little known fact. After introduction of PCV7, replacement pneumococcal strains expressing serotypes not in PCV7 emerged and antibiotic non-susceptible strains became predominant causes of AOM, especially antibiotic-resistant serotype 19A. To address the phenomena of pneumococcal serotype replacement, PCV13 was introduced in 2010. But serotype replacement continued to occur under PCV13 pressure, replacement serotypes increasingly caused AOM, and antibiotic-resistant serotype 35B emerged. Now we have two new higher valency PCVs: PCV15 (Merck) where serotypes 22F and 33F were added to the PCV13 serotypes and PCV20 (Pfizer) where 22F, 33F, 8, 10A, 11A, 12F, 15B were added to PCV13. Note that neither PCV15 nor PCV20 includes the most common serotype causing AOM – serotype 35B.1
While PCV15 and PCV20 should provide protection against more pneumococcal serotypes, increasing serotypes in both vaccines decreased immunogenicity of certain shared serotypes, more so with the addition of seven more in PCV20 than two more in PCV15, compared with PCV13. Whether lower antibody concentrations will make a difference clinically in terms of vaccine failure to prevent nasopharyngeal colonization, AOM, and/or invasive pneumococcal infections is currently unknown.
Our group from greater Rochester, New York, is the only one in the United States performing tympanocentesis to determine the etiology of AOM infections. Children between ages 6 and 36 months are studied. We recently reported our results for the time span September 2021 to September 2023, the immediate 2 years prior to recommendations for use of PCV15 and PCV20 in young children.2 Tympanocentesis was performed in 139 (78%) of 179 episodes of AOM, yielding 216 middle ear fluid samples (the higher number of middle ear fluids was due to bilateral tympanocentesis in some children). H. influenzae (40%) was the most common bacterial isolate, followed by S. pneumonia (19%) and M. catarrhalis (17%), with the remainder no growth. Polymerase chain reactions (PCR) was positive in many of those culture negative samples, suggesting prior use of antibiotics before tympanocentesis was performed. Among the pneumococcal isolates, 46% were oxacillin non-susceptible. Among the H. influenzae isolates, 27% were beta-lactamase producing and all M. catarrhalis were beta-lactamase-producing.
As we previously reported,1 we once again found that serotype 35B was the most frequent non-PCV15, non-PCV20, serotype. Other frequently detected non-PCV20 pneumococcal serotypes were 23A, 23B, 35D, 35F and 15C.2
Projected Pneumococcal Serotype Coverage by PCV15 and PCV20
PCV13 serotypes were identified in 9% of middle ear fluids, consistent with vaccine failure. Assuming 100% vaccine-type effectiveness, PCV15 will provide about 11% coverage of pneumococci causing AOM, the same PCV13 and PCV20 will provide 30% coverage, leaving 70% of pneumococci causing AOM in young children uncovered (Figure).

Thus, the high proportion of pneumococcal serotype 35B and other non-PCV15 or non-PCV20 serotypes will result in a relatively small incremental benefit over PCV13 in young children for AOM.
AOM is the most common cause of pediatric outpatient visits and antibiotic prescriptions in the United States that contributes to selection of antibiotic-resistant microbes.3 The economic burden of AOM is high, estimated at about $3 billion annually in the United States, when direct and indirect costs are calculated,4 thereby making AOM a major factor in calculations of cost effectiveness analyses of PCV immunizations in children.
While PCV15 and PCV20 include common serotypes associated with invasive pneumococcal diseases, their effectiveness in preventing AOM, acute sinusitis, and non-bacteremic community-acquired pneumonia is currently unknown because these vaccines were licensed based on safety and immunogenicity data, not proven efficacy.
The data on antibiotic susceptibility of pneumococci and H. influenza and M. catarrhalis isolated in the late post PCV13 era from young children in a pediatric primary-care setting raise a question about empiric antibiotic choice for AOM today. For penicillin non-susceptible pneumococcal strains, higher dosages of amoxicillin can improve eradication. However, higher dosages of amoxicillin cannot overcome beta-lactamase production by H. influenza and M. catarrhalis. Based on the mix of pathogens causing AOM and the antibiotic susceptibility of those bacteria, high-dose amoxicillin/clavulanate or alternative cephalosporin drugs active against pneumococci and beta-lactamase producing H. influenza and M. catarrhalis would be a better empiric choice over high-dose amoxicillin.
Limitations of our study include that it occurred in one center in New York, although we have previously shown results of tympanocentesis at our center are similar to those in Virginia and Pennsylvania5 and our study population was composed of children living in urban, suburban, and rural households of all economic levels. Because this study was conducted during a relatively short time frame (2021-2023), the numbers of subjects and samples were sometimes insufficient to identify statistically significant differences in some comparisons. Some children were lost to follow-up, and not every participant was consented for tympanocentesis. Some participants received antibiotics prior to middle ear fluid specimen collection.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Dynamic Changes in Otopathogens Colonizing the Nasopharynx and Causing Acute Otitis Media in Children After 13-Valent (PCV13) Pneumococcal Conjugate Vaccination During 2015-2019. Eur J Clin Microbiol Infect Dis. 2022 Jan;41(1):37-44. doi: 10.1007/s10096-021-04324-0.
2. Kaur R et al. Anticipated Effects of Higher-valency Pneumococcal Conjugate Vaccines on Colonization and Acute Otitis Media. Pediatr Infect Dis J. 2024 Oct 1;43(10):1004-1010. doi: 10.1097/INF.0000000000004413.
3. King LM et al. Pediatric Outpatient Visits and Antibiotic Use Attributable to Higher Valency Pneumococcal Conjugate Vaccine Serotypes. medRxiv [Preprint]. 2023 Aug 25:2023.08.24.23294570. doi: 10.1101/2023.08.24.23294570.
4. Ahmed S et al. Incremental Health Care Utilization and Costs for Acute Otitis Media in Children. Laryngoscope. 2014 Jan;124(1):301-5. doi: 10.1002/lary.24190.
5. Pichichero ME et al. Pathogens Causing Recurrent and Difficult-to-Treat Acute Otitis Media, 2003-2006. Clin Pediatr (Phila). 2008 Nov;47(9):901-6. doi: 10.1177/0009922808319966.
FDA Approves New Pneumococcal Vaccine
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
Seniors in Households with Children Have Sixfold Higher Risk for Pneumococcal Disease
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
BARCELONA, SPAIN — Streptococcus pneumoniae, the bacteria that causes pneumococcal disease, is sixfold more likely to colonize adults older than 60 years who have regular contact with children than those who do not, data from a community-based study showed.
However, there is “no clear evidence of adult-to-adult transmission,” and the researchers, led by Anne L. Wyllie, PhD, from the Yale School of Public Health, New Haven, Connecticut, noted that the study results suggest “the main benefit of adult pneumococcal conjugate vaccine (PCV) immunization is to directly protect adults who are exposed to children, who still carry and transmit some vaccine-type pneumococci despite successful pediatric national immunization programs.”
The data show that relatively high pneumococcus carriage rates are seen in people who have regular contact with children, who have had contact in the previous 2 weeks, and who have had contact for extended periods, Dr. Wyllie explained.
Preschoolers in particular were found to be most likely to transmit pneumococcus to older adults. “It is the 24- to 59-month-olds who are most associated with pneumococcal carriage, more than 1- to 2-year-olds,” she reported. However, transmission rates from children younger than 1 year are higher than those from children aged 1-2 years, she added.
The findings were presented at the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) 2024 global conference, formerly known as the ECCMID conference.
Originally Designed to Investigate Adult-to-Adult Transmission
The researchers wanted to understand the sources and dynamics of transmission, as well as the risk factors for pneumococcal disease in older adults, to help predict the effect of PCVs in people older than 60 years.
Although “we designed the study to specifically look at transmission between adults, in the end, we were presented with a very unique scenario” — restricted social mixing as a result of the COVID pandemic — during which “no community activities were happening,” Dr. Wyllie said. Because of this, the team was able to determine “the source of acquisition or transmission to the older adults was, very likely, coming from contact with children.”
Pneumococci are commonly found in respiratory tracts of healthy people. The US Centers for Disease Control and Prevention estimated that 20%-60% of school-aged children may be colonized compared with only 5%-10% of adults without children.
The longitudinal study was conducted among household pairs, such as married couples who were both aged at least 60 years and who did not have people younger than 60 years living in the household, in New Haven over two winter seasons: 2020-2021 and 2021-2022.
Self-collected saliva samples were assessed, and surveys on social behaviors and health were completed every 2 weeks for a 10-week period (with six study visits). The saliva sampling method was used because the researchers considered it to be more effective than samples from nasopharyngeal swabs. Quantitative polymerase chain reaction assays were used to test the saliva samples for the presence of pneumococcal DNA (pneumococcus genes piaB and lytA) and the diversity of pneumococcal strains (36 serotypes were targeted).
Strongly Suggestive of Transmission From Children to Older Adults
Of the 121 adults living in 61 households who were enrolled in the study, 62 adults participated in both seasons. Mean age was 70.9 years (range, 60-86 years), 51% of participants were women, and 85% were White.
Overall, 52 of 1088 (4.8%) samples tested positive for pneumococcus, and 27 of 121 (22.3%) adults were colonized on at least one sampling visit. Some were colonized at multiple timepoints, and two were colonized throughout the 10-week sampling period. Of the two participants who were colonized at five of six timepoints, one reported daily contact with children younger than 5 years and children aged 5-9 years in the two study seasons. This person was also positive at three of six sampling points during the first study season.
There were five instances in which both members of the household were carriers in the same season, although not necessarily at the same timepoint. Numbers were too small to determine whether transmission had occurred between the household pairs.
Contact with a 24- to 59-month-old child (older than 2 years but younger than 5 years) had the strongest association with elevated odds of carrying pneumococcus, the authors reported in their preprint, although the frequency and intensity of contact also mattered.
At any sampled time (point prevalence), pneumococcal carriage was substantially — just over sixfold — higher among older adults who had contact with children daily or every few days (10%) than among those who had no contact with children (1.6%).
In particular, contact between adults and children younger than 5 years and children aged 5-9 years was found to lead to elevated point prevalences of 13.8% and 14.1%, respectively. Pneumococcal carriage in children older than 10 years was lower, with a point prevalence of 8.3%.
The younger the child, the greater the point prevalence; point prevalences were 13.8% for samples from children aged 1 year and younger, 10.5% for samples from children aged 1-2 years, and 17.8% for children aged 2-5 years.
Carriage prevalence was higher in older adults who reported daily contact with children (15.7%) or contact every few days (14.0%) than in those who reported contact with children only once or twice a month (4.5%) or never (1.8%), they wrote.
“Older people who have a lot of contact with kids and are more susceptible to respiratory viruses can get a secondary infection from pneumococcus, especially during the cold and flu seasons. Vaccination can help to protect them or lessen severity of the illness,” Wyllie pointed out.
However, adult PCV immunization may not have a major impact on onward transmission to other adults, the authors wrote in their preprint.
This study supports prior work demonstrating that pneumococcal colonization is greater in households with children than in those without, said Stephen Pelton, MD, a pediatric infectious disease specialist from Boston University schools of medicine and public health. “The unique aspect is that Dr. Wyllie’s group has looked at individuals over age 60 and used the most sensitive methods currently available to detect pneumococcal carriage.”
“At the most recent ISPPD [International Society of Pneumonia and Pneumococcal Diseases conference], the role of adult-to-adult transmission in the community was discussed. This study confirms the critical role children play in community transmission of the pneumococcus,” Dr. Pelton noted.
Dr. Wyllie received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Primary Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project and is the principal investigator on research grants from Pfizer, Merck, NIH RADx-UP, and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io, and Shield T3 to SalivaDirect, Inc. Dr. Pelton received honoraria from Merck, Pfizer, Sanofi, and GSK for participation in Pneumococcal Advisory Boards and DSMB (Sanofi). Boston Medical Center received grant funding for investigator-initiated research from Merck and Pfizer.
A version of this article appeared on Medscape.com.
FROM ESCMID GLOBAL 2024
Automated Risk Assessment Tool Reduces Antibiotic Prescribing Rates
An algorithm-driven risk assessment embedded in an electronic health record (EHR) helped clinicians reduce inappropriate broad-spectrum antibiotic prescribing by 17.4% and 28.4% in patients with UTIs and pneumonia, respectively, according to two related studies published in JAMA.
The randomized control trials included more than 200,000 adult patients with non–life threatening pneumonia or urinary tract infections (UTIs) in 59 hospitals owned by HCA Healthcare across the country.
Researchers analyzed baseline prescribing behaviors over an 18-month period starting in April 2017, and data from a 15-month period of implementation of the new antibiotic system starting in April 2019.
, according to lead author Shruti K. Gohil, MD, MPH, associate medical director of epidemiology and infection prevention, infectious diseases at the University of California Irvine School of Medicine.
“When a patient comes in with pneumonia or a UTI, it’s precisely because we are concerned that our patients have a multidrug-resistant organism that we end up using broad-spectrum antibiotics,” she said.
Despite growing awareness of the need to reduce unnecessary antibiotic use, clinicians have still been slow to adopt a more conservative approach to prescribing, Dr. Gohil said.
“What physicians have been needing is something to hang their hat on, to be able to say, ‘Okay, well, this one’s a low-risk person,’ ” Dr. Gohil said.
The trials compared the impact of routine antibiotic activities with a stewardship bundle, called INSPIRE (Intelligent Stewardship Prompts to Improve Real-time Empiric Antibiotic Selection).
Both groups received educational materials, quarterly coaching calls, prospective evaluations for antibiotic use, and were required to select a reason for prescribing an antibiotic.
But prescribers in the intervention group took part in monthly coaching calls and feedback reports. In addition, if a clinician ordered a broad-spectrum antibiotic to treat pneumonia or a UTI outside of the intensive care unit within 72 hours of admission, an EHR prompt would pop up. The pop-up suggested a standard-spectrum antibiotic instead if patient risk for developing a multidrug-resistant (MDRO) version of either condition was less than 10%.
An algorithm used data from the EHR calculated risk, using factors like patient demographics and history and MDRO infection at the community and hospital level.
Prescribing rates were based on the number of days a patient received a broad-spectrum antibiotic during the first 72 hours of hospitalization.
For the UTI intervention group, rates dropped by 17.4% (rate ratio [RR], 0.83; 95% CI, 0.77-0.89; P < .001), and 28.4% reduction in the pneumonia group (RR, 0.72; 95% CI, 0.66-0.78; P < .001).
“We cannot know which element — prompt, education, or feedback — worked, but the data suggests that the prompt was the main driver,” Dr. Gohil said.
“In antibiotic stewardship, we have learned not only that doctors want to do the right thing, but that we as stewards need to make it easy for them do the right thing,” said Paul Pottinger, MD, professor of medicine at the Division of Allergy and Infectious Diseases at the University of Washington Medical Center in Seattle.
The prompt “is your easy button,” said Dr. Pottinger, who was not involved with either study. “The researchers made it simple, fast, and straightforward, so people don’t have to think about it too much.”
The studies showed similar safety outcomes for the control and intervention groups. Among patients with a UTI, those in the control group were transferred to the ICU after an average of 6.6 days compared to 7 days in the intervention group. Among patients with pneumonia, the average days to ICU transfer were 6.5 for the control group and 7.1 for the intervention group.
“This study is a proof of concept that physicians want to do the right thing and are willing to trust this information,” Dr. Pottinger said. “And this also shows us that this tool can be refined and made even more precise over time.”
The study was funded by the US Centers for Disease Control and Prevention and was led by the University of California Irvine, Harvard Pilgrim Healthcare Institute, and HCA Healthcare System. Various authors report funding and support from entities outside the submitted work. The full list can be found with the original articles.
A version of this article appeared on Medscape.com.
An algorithm-driven risk assessment embedded in an electronic health record (EHR) helped clinicians reduce inappropriate broad-spectrum antibiotic prescribing by 17.4% and 28.4% in patients with UTIs and pneumonia, respectively, according to two related studies published in JAMA.
The randomized control trials included more than 200,000 adult patients with non–life threatening pneumonia or urinary tract infections (UTIs) in 59 hospitals owned by HCA Healthcare across the country.
Researchers analyzed baseline prescribing behaviors over an 18-month period starting in April 2017, and data from a 15-month period of implementation of the new antibiotic system starting in April 2019.
, according to lead author Shruti K. Gohil, MD, MPH, associate medical director of epidemiology and infection prevention, infectious diseases at the University of California Irvine School of Medicine.
“When a patient comes in with pneumonia or a UTI, it’s precisely because we are concerned that our patients have a multidrug-resistant organism that we end up using broad-spectrum antibiotics,” she said.
Despite growing awareness of the need to reduce unnecessary antibiotic use, clinicians have still been slow to adopt a more conservative approach to prescribing, Dr. Gohil said.
“What physicians have been needing is something to hang their hat on, to be able to say, ‘Okay, well, this one’s a low-risk person,’ ” Dr. Gohil said.
The trials compared the impact of routine antibiotic activities with a stewardship bundle, called INSPIRE (Intelligent Stewardship Prompts to Improve Real-time Empiric Antibiotic Selection).
Both groups received educational materials, quarterly coaching calls, prospective evaluations for antibiotic use, and were required to select a reason for prescribing an antibiotic.
But prescribers in the intervention group took part in monthly coaching calls and feedback reports. In addition, if a clinician ordered a broad-spectrum antibiotic to treat pneumonia or a UTI outside of the intensive care unit within 72 hours of admission, an EHR prompt would pop up. The pop-up suggested a standard-spectrum antibiotic instead if patient risk for developing a multidrug-resistant (MDRO) version of either condition was less than 10%.
An algorithm used data from the EHR calculated risk, using factors like patient demographics and history and MDRO infection at the community and hospital level.
Prescribing rates were based on the number of days a patient received a broad-spectrum antibiotic during the first 72 hours of hospitalization.
For the UTI intervention group, rates dropped by 17.4% (rate ratio [RR], 0.83; 95% CI, 0.77-0.89; P < .001), and 28.4% reduction in the pneumonia group (RR, 0.72; 95% CI, 0.66-0.78; P < .001).
“We cannot know which element — prompt, education, or feedback — worked, but the data suggests that the prompt was the main driver,” Dr. Gohil said.
“In antibiotic stewardship, we have learned not only that doctors want to do the right thing, but that we as stewards need to make it easy for them do the right thing,” said Paul Pottinger, MD, professor of medicine at the Division of Allergy and Infectious Diseases at the University of Washington Medical Center in Seattle.
The prompt “is your easy button,” said Dr. Pottinger, who was not involved with either study. “The researchers made it simple, fast, and straightforward, so people don’t have to think about it too much.”
The studies showed similar safety outcomes for the control and intervention groups. Among patients with a UTI, those in the control group were transferred to the ICU after an average of 6.6 days compared to 7 days in the intervention group. Among patients with pneumonia, the average days to ICU transfer were 6.5 for the control group and 7.1 for the intervention group.
“This study is a proof of concept that physicians want to do the right thing and are willing to trust this information,” Dr. Pottinger said. “And this also shows us that this tool can be refined and made even more precise over time.”
The study was funded by the US Centers for Disease Control and Prevention and was led by the University of California Irvine, Harvard Pilgrim Healthcare Institute, and HCA Healthcare System. Various authors report funding and support from entities outside the submitted work. The full list can be found with the original articles.
A version of this article appeared on Medscape.com.
An algorithm-driven risk assessment embedded in an electronic health record (EHR) helped clinicians reduce inappropriate broad-spectrum antibiotic prescribing by 17.4% and 28.4% in patients with UTIs and pneumonia, respectively, according to two related studies published in JAMA.
The randomized control trials included more than 200,000 adult patients with non–life threatening pneumonia or urinary tract infections (UTIs) in 59 hospitals owned by HCA Healthcare across the country.
Researchers analyzed baseline prescribing behaviors over an 18-month period starting in April 2017, and data from a 15-month period of implementation of the new antibiotic system starting in April 2019.
, according to lead author Shruti K. Gohil, MD, MPH, associate medical director of epidemiology and infection prevention, infectious diseases at the University of California Irvine School of Medicine.
“When a patient comes in with pneumonia or a UTI, it’s precisely because we are concerned that our patients have a multidrug-resistant organism that we end up using broad-spectrum antibiotics,” she said.
Despite growing awareness of the need to reduce unnecessary antibiotic use, clinicians have still been slow to adopt a more conservative approach to prescribing, Dr. Gohil said.
“What physicians have been needing is something to hang their hat on, to be able to say, ‘Okay, well, this one’s a low-risk person,’ ” Dr. Gohil said.
The trials compared the impact of routine antibiotic activities with a stewardship bundle, called INSPIRE (Intelligent Stewardship Prompts to Improve Real-time Empiric Antibiotic Selection).
Both groups received educational materials, quarterly coaching calls, prospective evaluations for antibiotic use, and were required to select a reason for prescribing an antibiotic.
But prescribers in the intervention group took part in monthly coaching calls and feedback reports. In addition, if a clinician ordered a broad-spectrum antibiotic to treat pneumonia or a UTI outside of the intensive care unit within 72 hours of admission, an EHR prompt would pop up. The pop-up suggested a standard-spectrum antibiotic instead if patient risk for developing a multidrug-resistant (MDRO) version of either condition was less than 10%.
An algorithm used data from the EHR calculated risk, using factors like patient demographics and history and MDRO infection at the community and hospital level.
Prescribing rates were based on the number of days a patient received a broad-spectrum antibiotic during the first 72 hours of hospitalization.
For the UTI intervention group, rates dropped by 17.4% (rate ratio [RR], 0.83; 95% CI, 0.77-0.89; P < .001), and 28.4% reduction in the pneumonia group (RR, 0.72; 95% CI, 0.66-0.78; P < .001).
“We cannot know which element — prompt, education, or feedback — worked, but the data suggests that the prompt was the main driver,” Dr. Gohil said.
“In antibiotic stewardship, we have learned not only that doctors want to do the right thing, but that we as stewards need to make it easy for them do the right thing,” said Paul Pottinger, MD, professor of medicine at the Division of Allergy and Infectious Diseases at the University of Washington Medical Center in Seattle.
The prompt “is your easy button,” said Dr. Pottinger, who was not involved with either study. “The researchers made it simple, fast, and straightforward, so people don’t have to think about it too much.”
The studies showed similar safety outcomes for the control and intervention groups. Among patients with a UTI, those in the control group were transferred to the ICU after an average of 6.6 days compared to 7 days in the intervention group. Among patients with pneumonia, the average days to ICU transfer were 6.5 for the control group and 7.1 for the intervention group.
“This study is a proof of concept that physicians want to do the right thing and are willing to trust this information,” Dr. Pottinger said. “And this also shows us that this tool can be refined and made even more precise over time.”
The study was funded by the US Centers for Disease Control and Prevention and was led by the University of California Irvine, Harvard Pilgrim Healthcare Institute, and HCA Healthcare System. Various authors report funding and support from entities outside the submitted work. The full list can be found with the original articles.
A version of this article appeared on Medscape.com.
Antibiotics of Little Benefit in Lower Respiratory Tract Infection
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
Antibiotics had no measurable effect on the severity or duration of coughs due to acute lower respiratory tract infection (LRTI, or acute bronchitis), a large prospective study found.
In fact, those receiving an antibiotic in the primary- and urgent-care setting had a small but significant increase in overall length of illness (17.5 vs 15.9 days; P = .05) — largely because patients with longer illness before the index visit were more likely to receive these drugs. The study adds further support for reducing the prescription of antibiotics for LRTIs.
“Importantly, the pathogen data demonstrated that the length of time until illness resolution for those with bacterial infection was the same as for those not receiving an antibiotic versus those receiving one (17.3 vs 17.4 days),” researchers led by Daniel J. Merenstein, MD, a professor and director of research programs, family medicine, at Georgetown University Medical Center in Washington, wrote in the Journal of General Internal Medicine (doi: 10.1007/s11606-024-08758-y).
Patients believed an antibiotic would shorten their illness by an average of about 4 days, from 13.4 days to 9.7 days, whereas the average duration of all coughs was more than 2 weeks regardless of pathogen type or receipt of an antibiotic.
“Patients had unrealistic expectations regarding the duration of LRTI and the effect of antibiotics, which should be the target of antibiotic stewardship efforts,” the group wrote.
LRTIs can, however, be dangerous, with 3%-5% progressing to pneumonia, “but not everyone has easy access at an initial visit to an x-ray, which may be the reason clinicians still give antibiotics without any other evidence of a bacterial infection,” Dr. Merenstein said in a news release. “Patients have come to expect antibiotics for a cough, even if it doesn’t help. Basic symptom-relieving medications plus time bring a resolution to most people’s infections.”
The authors noted that cough is the most common reason for an ambulatory care visit, accounting for 2.7 million outpatient visits and more than 4 million emergency department visits annually.
Risks
Overuse of antibiotics can result in dizziness, nausea, diarrhea, and rash, along with a roughly 4% chance of serious adverse effects including anaphylaxis; Stevens-Johnson syndrome, a serious skin and mucous membrane disorder; and Clostridioides difficile-associated diarrhea.
An estimated half of all antibiotic prescriptions for acute respiratory conditions are unnecessary. Before the COVID-19 pandemic, antibiotics were prescribed about 70% of the time for a diagnosis of uncomplicated cough and LRTI. The viral pandemic did not change this practice according to a meta-analysis of 130 studies showing that 78% of COVID-19 patients were prescribed an antibiotic.
The study
The study looked at a cohort of 718 patients, with a mean age of 38.9 years, 65.3% female, of whom 207 received an antibiotic and 511 did not. Of those with baseline data, 29% had an antibiotic prescribed at baseline, the most common (in 85%) being amoxicillin-clavulanate, azithromycin, doxycycline, and amoxicillin. Antibiotics had no effect on the duration or overall severity of cough in viral, bacterial, or mixed infections. Receipt of an antibiotic did, however, reduce the likelihood of a follow-up visit: 14.1% vs 8.2% (adjusted odds ratio, 0.47; 95% confidence interval, 0.26-0.84) — perhaps because it removed the motivation for seeking another consultation. Antibiotic recipients were more likely to receive a systemic corticosteroid (31.9% vs 4.5%, P <.001) and were also more likely to receive an albuterol inhaler (22.7% vs 7.6%, P <.001).
Jeffrey A. Linder, MD, MPH, a primary care physician and chief of internal medicine and geriatrics at Northwestern University Feinberg School of Medicine in Chicago, agrees that in the vast majority of LRTIs — usually acute bronchitis — antibiotics do not speed the healing process. “Forty years of research show that antibiotics do not make acute bronchitis go away any faster,” Dr. Linder, who was not involved in the current study, said in an interview. “There’s even growing evidence that a lot of pneumonia is viral as well, and 10 or 20 years from now we may often not be giving antibiotics for pneumonia because we’ll be able to see better if it’s caused by a virus.”
A large 2018 review by Dr. Linder and associates reported that 46% of antibiotics were prescribed without any infection-related diagnosis code and 20% without an office visit.
Dr. Linder routinely informs patients requesting an antibiotic about the risks of putting an ineffective chemical into their body. “I stress that it can cause rash and other allergic reactions, and even promote C diff infection,” he said. “And I also say it messes with the good bacteria in the microbiome, and they usually come around.”
Patients need to know, Dr. Linder added, that the normal course of healing the respiratory tract after acute bronchitis takes weeks. While a wet cough with sputum or phlegm will last a few days, it’s replaced with a dry annoying cough that persists for up to 3 weeks. “As long as they’re feeling generally better, that cough is normal,” he said. “A virus has run roughshod over their airways and they need a long time to heal and the cough is part of the healing process. Think how long it takes to heal a cut on a finger.”
In an era of escalating antimicrobial resistance fueled by antibiotic overuse, it’s become increasingly important to reserve antibiotics for necessary cases. According to a recent World Health Organization call to action, “Uncontrolled antimicrobial resistance is expected to lower life expectancy and lead to unprecedented health expenditure and economic losses.”
That said, there is important clinical work to be done to determine if there is a limited role for antibiotics in patients with cough, perhaps based on age and baseline severity. “Serious cough symptoms and how to treat them properly needs to be studied more, perhaps in a randomized clinical trial as this study was observational and there haven’t been any randomized trials looking at this issue since about 2012,” Dr. Merenstein said.
This research was funded by the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to declare. Dr. Linder reported stock ownership in pharmaceutical companies but none that make antibiotics or other infectious disease drugs.
FROM JOURNAL OF GENERAL INTERNAL MEDICINE
What Markers Are Helpful to Diagnose Infection in Tocilizumab Users?
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Eosinopenia and low ratio between eosinophil count (EC) and neutrophil count (NC) are potential indicators of infection for patients with inflammatory disease who are treated with tocilizumab.
METHODOLOGY:
- The researchers reviewed data from 163 patients treated for an inflammatory disease (mostly rheumatoid arthritis) with tocilizumab at a single center between 2009 and 2020.
- The study population included 41 patients with unscheduled hospitalizations for suspected infections. Patients’ median age was 59 years, and 83% were female.
- The researchers assessed the association in tocilizumab-treated patients between infections and eosinopenia (defined as EC < 0.05 g/L) and a low ratio between EC and NC, defined as EC/NC × 1000 < 11.8.
TAKEAWAY:
- Infectious diseases were diagnosed in 20 of the hospitalized patients (49%); the most common diseases were pneumonia (30%), joint or bone infections (25%), and gastrointestinal tract infections (15%).
- The median absolute EC at hospital admission was significantly lower for patients with infections than for those without infections (0.06 g/L vs 0.20 g/L).
- The median EC/NC × 1000 ratios were significantly lower in infected patients vs noninfected patients (6.54 vs 48.50).
- No differences appeared between patients with and without infections in age, sex, type of inflammatory disease, and steroid treatment.
IN PRACTICE:
“This original study suggests that all those easily available parameters should be used to maximize [sensitivity] in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists,” the researchers wrote.
SOURCE:
The lead author on the study was Audrey Glatre, MD, of University Hospital Centre Reims, France. The study was published online in RMD Open on February 9.
LIMITATIONS:
The retrospective, observational design; relatively small study population; and use of data from a single center were potential limitations of the findings.
DISCLOSURES:
The study received no outside funding. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.



