User login
A history of head trauma may predict Parkinson’s disease progression
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a longitudinal online study, among patients with Parkinson’s disease who had a history of head injury, motor impairment developed 25% faster and cognitive impairment developed 45% faster than among those without such a history.
In addition, severe head injuries were associated with an even more rapid onset of impairment. The results give weight to the idea that “it’s head injuries themselves” prior to the development of Parkinson’s disease that might exacerbate motor and cognitive symptoms, said study investigator Ethan Brown, MD, assistant professor, Weill Institute of Neurosciences, department of neurology, University of California, San Francisco.
The findings emphasize the importance of “doing everything we can” to prevent falls and head injuries for patients with Parkinson’s disease, Dr. Brown said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Reverse causality concerns
Head injury is a risk factor for Parkinson’s disease, but its relationship to Parkinson’s disease progression is not well established. “There has always been this concern in Parkinson’s disease that maybe it’s problems with motor impairment that lead to head injuries, so reverse causality is an issue,” said Dr. Brown. “We wanted to look at whether risk factors we know relate to the development of Parkinson’s disease can also have a bearing on its progression,” he added.
The analysis was part of the online Fox Insight study that is evaluating motor and nonmotor symptoms in individuals with and those without Parkinson’s disease. The study included participants who had completed questionnaires on such things as head trauma.
The study included 1,065 patients (47% women; mean age, 63 years) with Parkinson’s disease who reported having had a head injury at least 5 years prior to their diagnosis. Among the participants, the mean duration of Parkinson’s disease was 7.5 years.
The investigators employed a 5-year lag time in their study to exclude head injuries caused by early motor dysfunction, they noted. “We wanted to look at people who had these head injuries we think might be part of the cause of Parkinson’s disease as opposed to a result of them,” Dr. Brown said.
In this head injury group, 51% had received one head injury, 28% had received two injuries, and 22% had received more than two injuries.
The study also included 1,457 participants (56% women; mean age, 65 years) with Parkinson’s disease who had not had a head injury prior to their diagnosis. Of these patients, the mean time with a Parkinson’s disease diagnosis was 8 years.
Dr. Brown noted that the age and sex distribution of the study group was “probably representative” of the general Parkinson’s disease population. However, because the participants had to be able to go online and complete questionnaires, it is unlikely that, among these patients, Parkinson’s disease was far advanced, he said.
The investigators adjusted for age, sex, years of education, and Parkinson’s disease duration.
Two-hit hypothesis?
The researchers compared time from diagnosis to the development of significant motor impairment, such as the need for assistance with walking, and cognitive impairment, such as having a score of less than 43 on the Penn Daily Activities Questionnaire.
They also examined the role of more severe head injuries. In the head injury group, over half (54%) had had a severe head injury, including 543 who had lost consciousness and others who had suffered a fracture or had had a seizure.
Results showed that the adjusted hazard ratio for developing motor impairment among those with a head injury, compared with those who had not had a head injury was 1.24 (95% confidence interval, 1.01-1.53; P = .037). For severe injuries, the aHR for motor impairment was 1.44 (95% CI, 1.13-1.83; P = .003).
For cognitive impairment, the aHR for those with versus without head injuries was 1.45 (95% CI, 1.14-1.86; P = .003); and for severe injuries, the aHR was 1.49 (95% CI, 1.11-2.0; P = .008).
Aside from severity, the researchers did not examine subgroups. However, Dr. Brown reported that his team would like to stratify results by sex and other variables in the future.
He noted that various mechanisms may explain why Parkinson’s disease progression is faster for patients who have a history of head injury, compared with others. Chronic inflammation due to the injury and “co-pathology” might play some role, he said. He noted that head injuries are associated with cognitive impairment in other conditions, including Alzheimer’s disease.
There is also the “two hit” hypothesis, Dr. Brown said. “A head injury could cause such broad damage that once people develop Parkinson’s disease, it’s harder for them to compensate.”
Dr. Brown also noted there might have been a “higher magnitude” of a difference between groups had the study captured participants with more severe symptoms.
‘Provocative’ findings
Michael S. Okun, MD, medical advisor at the Parkinson’s Foundation and professor and director at the Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the new data are “provocative.”
“The idea that a head injury may be important in predicting how quickly and how severely deficits will manifest could be important to the treating clinician,” said Dr. Okun, who was not involved with the research.
He noted that the results suggest clinicians should elicit more information from patients about head trauma. “They should be seeking more than a binary ‘yes or no’ answer to head injury when questioning patients,” he added.
Dr. Okun reiterated that head injury is a “known and important risk factor” not only for Parkinson’s disease but also for other neurodegenerative diseases. “It’s important to counsel patients about the association,” he said.
The study was supported by the Michael J. Fox Foundation. Dr. Brown reports having received grant support from the Michael J. Fox Foundation. Dr. Okun has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From MDS 2022
WHO releases six ‘action steps’ to combat global disparities in Parkinson’s disease
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Since 2000, Parkinson’s disease has increased 81% and related deaths have increased 100% globally. In addition, many patients affected by Parkinson’s disease live in low- and middle-income countries and experience large inequalities in access to neurologic care and essential medicines.
To address these issues, the Brain Health Unit at the WHO developed six “action steps” it says are urgently required to combat global disparities in Parkinson’s disease.
The need for action is great, said lead author Nicoline Schiess, MD, MPH, a neurologist and technical officer in the WHO’s Brain Health Unit in Geneva.
“In adults, disorders of the nervous system are the leading cause of disability adjusted life years, or DALYs, and the second leading cause of death globally, accounting for 9 million deaths per year,” Dr. Schiess said.
The WHO’s recommendations were published online recently as a “Special Communication” in JAMA Neurology.
Serious public health challenge
Parkinson’s disease is the fastest growing disorder in terms of death and disability, and it is estimated that it caused 329,000 deaths in 2019 – an increase of more than 100% since 2000.
“The rise in cases is thought to be multifactorial and is likely affected by factors such as aging populations and environmental exposures, such as certain pesticides. With these rapidly increasing numbers, compounded by a lack of specialists and medicines in low- and middle-income countries, Parkinson’s disease presents a serious public health challenge,” Dr. Schiess said.
The publication of the six action steps is targeted toward clinicians and researchers who work in Parkinson’s disease, she added. The steps address the following areas:
- 1. Disease burden
- 2. Advocacy and awareness
- 3. Prevention and risk reduction
- 4. Diagnosis, treatment, and care
- 5. Caregiver support
- 6. Research
Dr. Schiess noted that data on disease burden are lacking in certain areas of the world, such as low- and middle-income countries, and information “based on race and ethnicity are inconsistent. Studies are needed to establish more representative epidemiological data.”
She said that advocacy and awareness are particularly important since young people may not be aware they can also develop Parkinson’s disease, and sex and race differences can factor in to the potential for delays in diagnosis and care. “This is often due to the incorrect perception that Parkinson’s disease only affects older people,” she noted.
In addition, “a substantial need exists to identify risks for Parkinson’s disease – in particular the risks we can mitigate,” said Dr. Schiess, citing pesticide exposure as one example. “The evidence linking pesticide exposure, for example paraquat and chlorpyrifos, with the risk of developing Parkinson’s disease is substantial. And yet in many countries, these products are still being used.”
Under the heading of diagnosis, treatment, and care, Dr. Schiess noted that patients with Parkinson’s disease in “low resource settings” and low- to middle-income countries are unable to obtain “even the most basic medications” to treat Parkinson’s disease.
“Strengthening health and social systems, and building capacity to improve medical care, including rehabilitation and palliative care and medication access, are vital. Also, education and training of primary health care professionals, growing the neurological workforce, and increasing the use of digital technology such as telemedicine, are key mechanisms to improving diagnosis and sustainability of care,” she said.
For caregiver support, Dr. Schiess pointed out that the progressive nature of the disease and timing of onset are contributors to increased caregiver burden. Other contributors, as the disease advances in a patient, include the development of cognitive impairment, psychiatric manifestations, and sleep disruption.
“Solutions that could decrease the burden on caregivers include providing an accurate and timely diagnosis and training and education to caregivers, such as the WHO iSUPPORT program, as well as psychosocial, financial, and community-based support,” said Dr. Schiess.
For research, she noted that the amount of studies in the field of Parkinson’s disease has grown because of increased funding and a greater number of initiatives over the past 2 decades.
“Continuing to build on this momentum is important in order to generate new treatment options, better care, and research capacity, especially in low- and middle-income countries,” she said.
Dr. Schiess emphasized the urgency for adopting these measures as cases of Parkinson’s disease continue to rise.
“The take-away message for clinicians is that Parkinson disease is a growing global public health issue. There is a pressing need for a global public health response to address health and social requirements for people with Parkinson’s disease,” she said.
Dr. Schiess reports having received grants from the Edmond J. Safra Foundation paid to her institution during the conduct of the study.
A version of this article first appeared on Medscape.com.
Novel combination treatment improves function in early Parkinson’s disease
Results from a phase 3 trial found that P2B001 was superior to its components in improving motor symptoms and daily function and was comparable with marketed doses of pramipexole.
P2B001 also produced less daytime sleepiness and fewer dopaminergic effects, said the investigators, who presented findings at the International Congress of Parkinson’s Disease and Movement Disorders.
The treatment studied, P2B001, is a proprietary, fixed-dose combination of extended-release (ER) formulations of pramipexole and rasagiline. Neither dose is currently available on the market.
Investigators wanted to test the hypothesis that two anti-Parkinsonian drugs that act through different mechanisms could work synergistically, providing benefits comparable with pramipexole but with fewer side effects, said lead study author Warren Olanow, MD, professor emeritus in the neurology and neuroscience departments at the Icahn School of Medicine at Mount Sinai, New York.
Pramipexole is problematic in that it causes dopaminergic and sleep-related side effects.
Laboratory studies have shown that low doses of pramipexole and rasagiline act synergistically, said Dr. Olanow. “A previous double-blind controlled study demonstrated that P2B001 was significantly superior to placebo with respect to efficacy (P < .001) and had a good safety and tolerability profile.”
P2B001 outperforms other formulations
The multicenter phase 3 study (NCT03329508) enrolled 544 patients aged 35-80 with early Parkinson’s disease to assess efficacy and safety of a daily dose of P2B001, compared with its components. Patients were randomized 2:2:2:1 to 12 weeks of treatment with P2B001; pramipexole ER 0.6 mg; rasagiline ER 0.75 mg, or to a calibration arm of marketed pramipexole-ER titrated to optimal dose (mean dose, 3.2 mg).
The primary endpoint compared baseline with week 12 changes in Unified Parkinson’s Disease Rating Scale total scores for P2B001 versus its individual components. The secondary endpoint compared baseline changes in Epworth Sleepiness Scale (ESS) for P2B001 versus pramipexole-ER.
P2B001 showed superior efficacy to each of its individual components and comparable efficacy with marketed doses of pramipexole-ER. It also yielded fewer adverse events related to dopaminergic side effects and less daytime sleepiness as measured by ESS. “Further, the drug is administered once a day and does not require titration,” said Dr. Olanow.
Levodopa-related benefits
Another advantage of starting early-stage patients on P2B001 is that it would give patients more time to be on an effective therapy with fewer side effects before going on levodopa, the current gold standard for Parkinson’s disease treatment.
Although the American Academy of Neurology recommends levodopa as initial therapy for Parkinson’s disease, the drug has been associated with a risk of developing motor complications.
“This opinion, however, was formulated prior to the availability of the results of the P2B001 study and should be reassessed in the light of the present study,” said Dr. Olanow. Longer-term studies should assess when and if patients will require levodopa therapy, as well as the long-term effects of P2B001 on the development of motor complications in patients with early Parkinson’s disease patients.
Investigators are preparing a regulatory market approval filing for P2B001 with the Food and Drug Administration.
Dr. Olanow is CEO of Clintrex Research Corporation, which has provided services to Pharma2B, sponsor of the phase 3 study. Pharma Two B is a private, late clinical-stage pharmaceutical company in Rehovot, Israel, that owns worldwide granted patents for P2B001 pharmaceutical composition and method of treatment.
Results from a phase 3 trial found that P2B001 was superior to its components in improving motor symptoms and daily function and was comparable with marketed doses of pramipexole.
P2B001 also produced less daytime sleepiness and fewer dopaminergic effects, said the investigators, who presented findings at the International Congress of Parkinson’s Disease and Movement Disorders.
The treatment studied, P2B001, is a proprietary, fixed-dose combination of extended-release (ER) formulations of pramipexole and rasagiline. Neither dose is currently available on the market.
Investigators wanted to test the hypothesis that two anti-Parkinsonian drugs that act through different mechanisms could work synergistically, providing benefits comparable with pramipexole but with fewer side effects, said lead study author Warren Olanow, MD, professor emeritus in the neurology and neuroscience departments at the Icahn School of Medicine at Mount Sinai, New York.
Pramipexole is problematic in that it causes dopaminergic and sleep-related side effects.
Laboratory studies have shown that low doses of pramipexole and rasagiline act synergistically, said Dr. Olanow. “A previous double-blind controlled study demonstrated that P2B001 was significantly superior to placebo with respect to efficacy (P < .001) and had a good safety and tolerability profile.”
P2B001 outperforms other formulations
The multicenter phase 3 study (NCT03329508) enrolled 544 patients aged 35-80 with early Parkinson’s disease to assess efficacy and safety of a daily dose of P2B001, compared with its components. Patients were randomized 2:2:2:1 to 12 weeks of treatment with P2B001; pramipexole ER 0.6 mg; rasagiline ER 0.75 mg, or to a calibration arm of marketed pramipexole-ER titrated to optimal dose (mean dose, 3.2 mg).
The primary endpoint compared baseline with week 12 changes in Unified Parkinson’s Disease Rating Scale total scores for P2B001 versus its individual components. The secondary endpoint compared baseline changes in Epworth Sleepiness Scale (ESS) for P2B001 versus pramipexole-ER.
P2B001 showed superior efficacy to each of its individual components and comparable efficacy with marketed doses of pramipexole-ER. It also yielded fewer adverse events related to dopaminergic side effects and less daytime sleepiness as measured by ESS. “Further, the drug is administered once a day and does not require titration,” said Dr. Olanow.
Levodopa-related benefits
Another advantage of starting early-stage patients on P2B001 is that it would give patients more time to be on an effective therapy with fewer side effects before going on levodopa, the current gold standard for Parkinson’s disease treatment.
Although the American Academy of Neurology recommends levodopa as initial therapy for Parkinson’s disease, the drug has been associated with a risk of developing motor complications.
“This opinion, however, was formulated prior to the availability of the results of the P2B001 study and should be reassessed in the light of the present study,” said Dr. Olanow. Longer-term studies should assess when and if patients will require levodopa therapy, as well as the long-term effects of P2B001 on the development of motor complications in patients with early Parkinson’s disease patients.
Investigators are preparing a regulatory market approval filing for P2B001 with the Food and Drug Administration.
Dr. Olanow is CEO of Clintrex Research Corporation, which has provided services to Pharma2B, sponsor of the phase 3 study. Pharma Two B is a private, late clinical-stage pharmaceutical company in Rehovot, Israel, that owns worldwide granted patents for P2B001 pharmaceutical composition and method of treatment.
Results from a phase 3 trial found that P2B001 was superior to its components in improving motor symptoms and daily function and was comparable with marketed doses of pramipexole.
P2B001 also produced less daytime sleepiness and fewer dopaminergic effects, said the investigators, who presented findings at the International Congress of Parkinson’s Disease and Movement Disorders.
The treatment studied, P2B001, is a proprietary, fixed-dose combination of extended-release (ER) formulations of pramipexole and rasagiline. Neither dose is currently available on the market.
Investigators wanted to test the hypothesis that two anti-Parkinsonian drugs that act through different mechanisms could work synergistically, providing benefits comparable with pramipexole but with fewer side effects, said lead study author Warren Olanow, MD, professor emeritus in the neurology and neuroscience departments at the Icahn School of Medicine at Mount Sinai, New York.
Pramipexole is problematic in that it causes dopaminergic and sleep-related side effects.
Laboratory studies have shown that low doses of pramipexole and rasagiline act synergistically, said Dr. Olanow. “A previous double-blind controlled study demonstrated that P2B001 was significantly superior to placebo with respect to efficacy (P < .001) and had a good safety and tolerability profile.”
P2B001 outperforms other formulations
The multicenter phase 3 study (NCT03329508) enrolled 544 patients aged 35-80 with early Parkinson’s disease to assess efficacy and safety of a daily dose of P2B001, compared with its components. Patients were randomized 2:2:2:1 to 12 weeks of treatment with P2B001; pramipexole ER 0.6 mg; rasagiline ER 0.75 mg, or to a calibration arm of marketed pramipexole-ER titrated to optimal dose (mean dose, 3.2 mg).
The primary endpoint compared baseline with week 12 changes in Unified Parkinson’s Disease Rating Scale total scores for P2B001 versus its individual components. The secondary endpoint compared baseline changes in Epworth Sleepiness Scale (ESS) for P2B001 versus pramipexole-ER.
P2B001 showed superior efficacy to each of its individual components and comparable efficacy with marketed doses of pramipexole-ER. It also yielded fewer adverse events related to dopaminergic side effects and less daytime sleepiness as measured by ESS. “Further, the drug is administered once a day and does not require titration,” said Dr. Olanow.
Levodopa-related benefits
Another advantage of starting early-stage patients on P2B001 is that it would give patients more time to be on an effective therapy with fewer side effects before going on levodopa, the current gold standard for Parkinson’s disease treatment.
Although the American Academy of Neurology recommends levodopa as initial therapy for Parkinson’s disease, the drug has been associated with a risk of developing motor complications.
“This opinion, however, was formulated prior to the availability of the results of the P2B001 study and should be reassessed in the light of the present study,” said Dr. Olanow. Longer-term studies should assess when and if patients will require levodopa therapy, as well as the long-term effects of P2B001 on the development of motor complications in patients with early Parkinson’s disease patients.
Investigators are preparing a regulatory market approval filing for P2B001 with the Food and Drug Administration.
Dr. Olanow is CEO of Clintrex Research Corporation, which has provided services to Pharma2B, sponsor of the phase 3 study. Pharma Two B is a private, late clinical-stage pharmaceutical company in Rehovot, Israel, that owns worldwide granted patents for P2B001 pharmaceutical composition and method of treatment.
From MDS 2022
Baseline neuromotor abnormalities persist in schizophrenia
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
New Parkinson’s test developed thanks to woman who could smell the disease
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
The test has been years in the making after academics realized that Joy Milne could smell the condition.
The 72-year-old from Perth, Scotland, has a rare condition that gives her a heightened sense of smell.
She noticed that her late husband Les developed a different odor when he was 33 – some 12 years before he was diagnosed with the disease, which leads to parts of the brain become progressively damaged over many years.
Mrs. Milne, dubbed ‘the woman who can smell Parkinson’s, described a “musky” aroma, different from his normal scent.
Her observation piqued the interest of scientists who decided to research what she could smell, and whether this could be harnessed to help identify people with the neurological condition.
‘Early phases of research’
Years later, academics at the University of Manchester (England) have made a breakthrough by developing a test that can identify people with Parkinson’s disease using a simple cotton bud run along the back of the neck.
Researchers can examine the sample to identify molecules linked to the disease to help diagnose whether someone has the disease.
While still in the early phases of research, scientists are excited about the prospect of the NHS being able to deploy a simple test for the disease.
There is currently no definitive test for Parkinson’s disease, with diagnosis based on a patient’s symptoms and medical history.
If the new skin swab is successful outside laboratory conditions it could be rolled out to achieve faster diagnosis.
Mrs. Milne told the PA news agency that it was “not acceptable” that people with Parkinson’s had such high degrees of neurologic damage at the time of diagnosis, adding: “I think it has to be detected far earlier – the same as cancer and diabetes, earlier diagnosis means far more efficient treatment and a better lifestyle for people.
“It has been found that exercise and change of diet can make a phenomenal difference.”
She said her husband, a former doctor, was “determined” to find the right researcher to examine the link between odor and Parkinson’s and they sought out Tilo Kunath, PhD, at the University of Edinburgh in 2012.
Chemical change in sebum
Dr. Kunath paired up with Perdita Barran, PhD, to examine Mrs. Milne’s sense of smell.
The scientists believed that the scent may be caused by a chemical change in skin oil, known as sebum, that is triggered by the disease.
In their preliminary work they asked Mrs. Milne to smell t-shirts worn by people who have Parkinson’s and those who did not.
Mrs. Milne correctly identified the t-shirts worn by Parkinson’s patients but she also said that one from the group of people without Parkinson’s smelled like the disease – 8 months later the individual who wore the t-shirt was diagnosed with Parkinson’s.
Researchers hoped the finding could lead to a test being developed to detect Parkinson’s, working under the assumption that if they were able to identify a unique chemical signature in the skin linked to Parkinson’s, they may eventually be able to diagnose the condition from simple skin swabs.
In 2019 researchers at the University of Manchester, led by Dr. Barran, announced that they had identified molecules linked to the disease found in skin swabs.
And now the scientists have developed a test using this information.
The tests have been successfully conducted in research labs and now scientists are assessing whether they can be used in hospital settings.
If successful, the test could potentially be used in the NHS so GPs can refer patients for Parkinson’s tests.
The findings, which have been published in the Journal of the American Chemical Society, detail how sebum can be analyzed with mass spectrometry – a method which weighs molecules – to identify the disease.
Some molecules are present only in people who have Parkinson’s disease.
Researchers compared swabs from 79 people with Parkinson’s with a healthy control group of 71 people.
Dr. Barran told the PA news agency: “At the moment, there are no cures for Parkinson’s, but a confirmatory diagnostic would allow them to get the right treatment and get the drugs that will help to alleviate their symptoms.
“There would also be nonpharmaceutical interventions, including movement and also nutritional classes, which can really help.
“And I think most critically, it will allow them to have a confirmed diagnosis to actually know what’s wrong with them.”
She added: “What we are now doing is seeing if [hospital laboratories] can do what we’ve done in a research lab in a hospital lab. Once that’s happened then we want to see if we can make this a confirmatory diagnostic that could be used along with the referral process from a GP to a consultant. At the moment in Greater Manchester there are about 18,000 people waiting for a neurological consult and just to clear that list, without any new people joining it, will take up to 2 years. Of those 10%-15% are suspect Parkinson’s. Our test would be able to tell them whether they did or whether they didn’t [have Parkinson’s] and allow them to be referred to the right specialist. So at the moment, we’re talking about being able to refer people in a timely manner to the right specialism and that will be transformative.”
Mrs. Milne may be able to smell other diseases
Mrs. Milne is now working with scientists around the world to see if she can smell other diseases like cancer and tuberculosis.
“I have to go shopping very early or very late because of people’s perfumes, I can’t go into the chemical aisle in the supermarket,” she told the PA news agency. “So yes, a curse sometimes but I have also been out to Tanzania and have done research on TB, and research on cancer in the U.S. – just preliminary work. So it is a curse and a benefit.”
She said that she can sometimes smell people who have Parkinson’s while in the supermarket or walking down the street but has been told by medical ethicists she cannot tell them. “Which GP would accept a man or a woman walking in saying ‘the woman who smells Parkinson’s has told me I have it?’ Maybe in the future but not now.”
Mrs. Milne said that her husband, who died 7 years ago, was like a “changed man” after researchers found the link between Parkinson’s and odor.
A version of this article first appeared on Medscape UK.
Parkinson’s disease: Is copper culpable?
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.
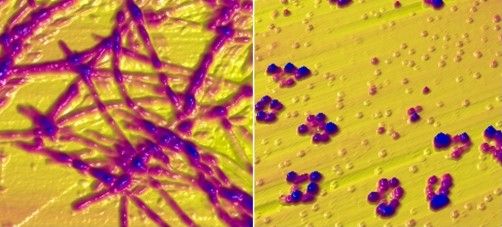
These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
FROM ACS CHEMICAL NEUROSCIENCE
COVID-19 tied to increased risk for Alzheimer’s disease and Parkinson’s disease
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests. However, the research also showed there was no excess risk of these neurologic disorders following COVID than other respiratory infections such as influenza or community-acquired bacterial pneumonia.
Considering these results, study investigator Pardis Zarifkar, MD, department of neurology, Rigshospitalet, Copenhagen University Hospital, urged doctors to “keep an eye on” COVID patients and use “a critical mindset” if these patients present with neurologic issues.
“They should consider whether the patient’s condition is something new or if there were already signs and symptoms before they had COVID-19,” she said.
The findings were presented at the 2022 congress of the European Academy of Neurology and published online in Frontiers in Neurology.
‘Surprising’ increased risk
Previous research shows more than 80% of patients hospitalized with COVID-19 have neurologic symptoms including anosmia, dysgeusia, headache, dizziness, memory and concentration difficulties, fatigue, and irritability.
However, it’s unclear whether COVID-19 affects the risk for specific neurologic diseases and if so, whether this association differs from other respiratory infections.
From electronic health records covering about half the Danish population, researchers identified adults who were tested for COVID-19 or diagnosed with community-acquired bacterial pneumonia from February 2020 to November 2021. They also flagged individuals with influenza in the corresponding prepandemic period (February 2018–November 2019).
Dr. Zarifkar noted influenza A or B and community-acquired bacterial pneumonia are two of the most common respiratory tract infections.
The investigators tracked neurologic diseases up to 12 months after a positive test. They looked at two neurodegenerative diseases, Alzheimer’s disease and Parkinson’s disease, as well as cerebrovascular disorders including ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
The study included 43,262 individuals with a positive COVID test without a history of influenza A/B in the past year and 876,356 without a positive COVID test. It also included 1,474 individuals with community-acquired pneumonia without a history of COVID and 8,102 with influenza A or B.
“We wanted to investigate whether COVID-19 is really that much worse than all these other common respiratory infections that we have had for ages and see every single year,” said Dr. Zarifkar.
After 12 months, the relative risk for Alzheimer’s disease was 3.4 (95% confidence interval, 2.3-5.1) in the COVID-positive group versus the COVID-negative group. The risks were greater among inpatients versus outpatients.
These results were rather unexpected, said Dr. Zarifkar. “I would have expected a small increase, but the extent of the increase was quite surprising.”
However, there was no difference when comparing the COVID-19 group with the influenza or bacterial pneumonia groups, which Dr. Zarifkar said was “very reassuring.”
The findings were similar for Parkinson’s disease, where there was a 2.2-fold increased risk of a Parkinson’s disease diagnosis within the first 12 months in COVID-positive individuals, compared with COVID-negative people (RR, 2.2; 95% CI, 1.5-3.4). Again, there was no excess risk, compared with influenza or bacterial pneumonia.
Potential mechanisms
Dr. Zarifkar believes a “constellation” of factors may explain higher risks of these diagnoses in COVID patients. Part of it could be a result of neuroinflammation, which can lead to a toxic accumulation of beta amyloid in Alzheimer’s disease and alpha-synuclein in Parkinson’s disease.
“It can accelerate a neurodegenerative disease already in the making,” she said. But perhaps the biggest driver of differences between the groups is the “scientific focus” on COVID patients. “In Denmark, almost everyone who has had COVID-19, especially severe COVID-19, is offered some sort of cognitive testing, and if you hand out MoCAs [Montreal Cognitive Assessments] which is the cognitive test we use, to almost everyone you’re meeting, you’re going to catch these disorders earlier than you might have otherwise.”
As for cerebrovascular disorders, the study showed an increased risk of ischemic stroke in COVID-positive versus COVID-negative subjects at 12 months (RR, 2.87; 95% confidence interval, 2.2-3.2).
The relatively strong inflammatory response associated with COVID-19, which may create a hypercoagulable state, may help explain the increased ischemic stroke risk in COVID patients, said Dr. Zarifkar.
The study did not show an increased risk for subarachnoid hemorrhage in COVID-positive, compared with COVID-negative, subjects but did reveal an increased risk of intracerebral hemorrhage after 12 months (RR, 4.8; 95% CI, 1.8-12.9).
This could be explained by COVID-positive subjects having a higher risk for ischemic stroke and receiving thrombolysis that may increase risk for bleeding in the brain. However, an analysis accounting for medication use found differences in thrombolysis rates didn’t change the result, said Dr. Zarifkar.
It’s also possible that extracorporeal membrane oxygenation and mechanical ventilation – interventions more frequently used in COVID-19 patients – may increase the risk for bleeding in brain, she added.
The researchers did not find an increased risk for multiple sclerosis, myasthenia gravis, Guillain-Barré syndrome, or narcolepsy in COVID patients. However, Dr. Zarifkar noted that it can take years to detect an association with autoimmune disorders.
The investigators did not stratify risk by disease severity, although this would be an important step, she said. “The threshold of being admitted to the hospital with COVID-19 has been much lower than for influenza or bacterial pneumonia where you’re typically quite ill before you’re admitted, so this might actually dilute the findings and underestimate our findings.”
A national, registry-based study that includes the entire Danish population and additional information on vaccination status, virus variants, socioeconomic status, and comorbidities is needed, said Dr. Zarifkar.
The study was supported by Lundbeck Foundation and Novo Nordisk. Dr. Zarifkar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NEUROLOGY
Motor abnormalities drive decreased function in schizophrenia
Approximately half of adults with schizophrenia suffer from motor abnormalities that may impair their ability to work and decrease their quality of life, wrote Niluja Nadesalingam, MD, of the University of Bern, Switzerland, and colleagues. “Although previous reports show strong associations between single movement abnormalities and global as well as social functioning, we still struggle to understand the contribution of various motor domains,” they said.
The impact of these abnormalities on social and global functioning and on functional capacity remains unclear, but the researchers proposed that motor abnormalities would be associated with worse functional outcomes in schizophrenia patients.
In a study published in Comprehensive Psychiatry, the researchers identified patients with diagnosed schizophrenia spectrum disorders who were treated on an inpatient or outpatient basis at a single center. They collected data on five motor abnormalities: parkinsonism, catatonia, dyskinesia, neurological soft signs (NSS), and psychomotor slowing (PS). They assessed functional outcomes using the Global Assessment of Functioning (GAF), the Social and Occupational Functioning Assessment Scale (SOFAS), and the UCSD Performance-Based Skills Assessment (UPSA-B). The average age of the participants was 37.9 years and 88 of the 156 were male. The average duration of illness was 12.5 years.
Overall, patients with catatonia and parkinsonism scored significantly lower on GAF and SOFAS scale compared to those without catatonia and parkinsonism (P < .035 and P < .027, respectively).
No significant differences in functional outcomes appeared between patients with and without dyskinesia.
However, significant negative correlations were identified for parkinsonism and PS with GAF, SOFAS, and UPSA-B (P < .036 for all). “Our study further found that parkinsonism and psychomotor slowing also impair the functional capacity of patients,” which may be influenced by factors including deficits in social interaction and cognitive impairment, the researchers said.
Overall, the study findings demonstrate that motor abnormalities in patients with schizophrenia are strongly associated with poor functional outcomes, and the stronger the motor impairment, the worse the global and social functioning, the researchers said.
As for potential pathways, “motor abnormalities are readily observable signs, allowing laypersons to perceive subjects with schizophrenia as somebody with severe mental illness. Thus, motor abnormalities might lead to stigmatization of patients suffering from schizophrenia,” they wrote in their discussion.
The researchers emphasized the need to explore alternative treatment options that might improve motor abnormalities, such as transcranial magnetic stimulation, given the potential of antipsychotic medications to introduce additional motor abnormalities.
The study findings were limited by several factors including the potential for missed confounding variables, the small number of patients with dyskinesia, and the inability to deduce the course of illness because most of the patients were in psychotic episodes, the researchers noted.
However, the results suggest that specific motor abnormalities are associated with poor global and social functioning, and with reduced functional capacity, in adults with schizophrenia, the researchers said. “Future studies need to test whether amelioration of motor abnormalities may improve community functioning,” they concluded.
The study was supported by the Swiss National Science Foundation, the Bangerter Rhyner Foundation, and the Adrian and Simone Frutiger Foundation. Lead author Dr. Nadesalingam had no financial conflicts to disclose.
Approximately half of adults with schizophrenia suffer from motor abnormalities that may impair their ability to work and decrease their quality of life, wrote Niluja Nadesalingam, MD, of the University of Bern, Switzerland, and colleagues. “Although previous reports show strong associations between single movement abnormalities and global as well as social functioning, we still struggle to understand the contribution of various motor domains,” they said.
The impact of these abnormalities on social and global functioning and on functional capacity remains unclear, but the researchers proposed that motor abnormalities would be associated with worse functional outcomes in schizophrenia patients.
In a study published in Comprehensive Psychiatry, the researchers identified patients with diagnosed schizophrenia spectrum disorders who were treated on an inpatient or outpatient basis at a single center. They collected data on five motor abnormalities: parkinsonism, catatonia, dyskinesia, neurological soft signs (NSS), and psychomotor slowing (PS). They assessed functional outcomes using the Global Assessment of Functioning (GAF), the Social and Occupational Functioning Assessment Scale (SOFAS), and the UCSD Performance-Based Skills Assessment (UPSA-B). The average age of the participants was 37.9 years and 88 of the 156 were male. The average duration of illness was 12.5 years.
Overall, patients with catatonia and parkinsonism scored significantly lower on GAF and SOFAS scale compared to those without catatonia and parkinsonism (P < .035 and P < .027, respectively).
No significant differences in functional outcomes appeared between patients with and without dyskinesia.
However, significant negative correlations were identified for parkinsonism and PS with GAF, SOFAS, and UPSA-B (P < .036 for all). “Our study further found that parkinsonism and psychomotor slowing also impair the functional capacity of patients,” which may be influenced by factors including deficits in social interaction and cognitive impairment, the researchers said.
Overall, the study findings demonstrate that motor abnormalities in patients with schizophrenia are strongly associated with poor functional outcomes, and the stronger the motor impairment, the worse the global and social functioning, the researchers said.
As for potential pathways, “motor abnormalities are readily observable signs, allowing laypersons to perceive subjects with schizophrenia as somebody with severe mental illness. Thus, motor abnormalities might lead to stigmatization of patients suffering from schizophrenia,” they wrote in their discussion.
The researchers emphasized the need to explore alternative treatment options that might improve motor abnormalities, such as transcranial magnetic stimulation, given the potential of antipsychotic medications to introduce additional motor abnormalities.
The study findings were limited by several factors including the potential for missed confounding variables, the small number of patients with dyskinesia, and the inability to deduce the course of illness because most of the patients were in psychotic episodes, the researchers noted.
However, the results suggest that specific motor abnormalities are associated with poor global and social functioning, and with reduced functional capacity, in adults with schizophrenia, the researchers said. “Future studies need to test whether amelioration of motor abnormalities may improve community functioning,” they concluded.
The study was supported by the Swiss National Science Foundation, the Bangerter Rhyner Foundation, and the Adrian and Simone Frutiger Foundation. Lead author Dr. Nadesalingam had no financial conflicts to disclose.
Approximately half of adults with schizophrenia suffer from motor abnormalities that may impair their ability to work and decrease their quality of life, wrote Niluja Nadesalingam, MD, of the University of Bern, Switzerland, and colleagues. “Although previous reports show strong associations between single movement abnormalities and global as well as social functioning, we still struggle to understand the contribution of various motor domains,” they said.
The impact of these abnormalities on social and global functioning and on functional capacity remains unclear, but the researchers proposed that motor abnormalities would be associated with worse functional outcomes in schizophrenia patients.
In a study published in Comprehensive Psychiatry, the researchers identified patients with diagnosed schizophrenia spectrum disorders who were treated on an inpatient or outpatient basis at a single center. They collected data on five motor abnormalities: parkinsonism, catatonia, dyskinesia, neurological soft signs (NSS), and psychomotor slowing (PS). They assessed functional outcomes using the Global Assessment of Functioning (GAF), the Social and Occupational Functioning Assessment Scale (SOFAS), and the UCSD Performance-Based Skills Assessment (UPSA-B). The average age of the participants was 37.9 years and 88 of the 156 were male. The average duration of illness was 12.5 years.
Overall, patients with catatonia and parkinsonism scored significantly lower on GAF and SOFAS scale compared to those without catatonia and parkinsonism (P < .035 and P < .027, respectively).
No significant differences in functional outcomes appeared between patients with and without dyskinesia.
However, significant negative correlations were identified for parkinsonism and PS with GAF, SOFAS, and UPSA-B (P < .036 for all). “Our study further found that parkinsonism and psychomotor slowing also impair the functional capacity of patients,” which may be influenced by factors including deficits in social interaction and cognitive impairment, the researchers said.
Overall, the study findings demonstrate that motor abnormalities in patients with schizophrenia are strongly associated with poor functional outcomes, and the stronger the motor impairment, the worse the global and social functioning, the researchers said.
As for potential pathways, “motor abnormalities are readily observable signs, allowing laypersons to perceive subjects with schizophrenia as somebody with severe mental illness. Thus, motor abnormalities might lead to stigmatization of patients suffering from schizophrenia,” they wrote in their discussion.
The researchers emphasized the need to explore alternative treatment options that might improve motor abnormalities, such as transcranial magnetic stimulation, given the potential of antipsychotic medications to introduce additional motor abnormalities.
The study findings were limited by several factors including the potential for missed confounding variables, the small number of patients with dyskinesia, and the inability to deduce the course of illness because most of the patients were in psychotic episodes, the researchers noted.
However, the results suggest that specific motor abnormalities are associated with poor global and social functioning, and with reduced functional capacity, in adults with schizophrenia, the researchers said. “Future studies need to test whether amelioration of motor abnormalities may improve community functioning,” they concluded.
The study was supported by the Swiss National Science Foundation, the Bangerter Rhyner Foundation, and the Adrian and Simone Frutiger Foundation. Lead author Dr. Nadesalingam had no financial conflicts to disclose.
FROM COMPREHENSIVE PSYCHIATRY
Blood-based assay may offer new way of diagnosing Parkinson’s disease
A novel blood-based assay could one day be used to diagnose Parkinson’s disease and possibly other chronic inflammatory conditions, according to investigators. In addition to being highly accurate, the assay, which detects changes in expression of cytochrome P450s, is faster and easier to perform than other Parkinson’s disease assays under investigation, reported lead author Kohei Ihara, PhD, of Kobe University, Japan, and colleagues.
“Effective diagnostic systems and biomarkers for patients without subjective motor symptoms have not yet been established,” the investigators wrote in Nature Scientific Reports. “Consequently, the poor diagnostic options for Parkinson’s disease delay the development of therapeutic approaches and medication. Therefore, the development of efficient diagnostic systems and biomarkers is crucial for overcoming Parkinson’s disease.”
According to Dr. Ihara and colleagues, various cytochrome P450 expression patterns and associated serum metabolites correlate with chronic conditions, making them possible markers of disease. To detect these changes, they developed the present assay. It relies upon recombinant P450s expressed on the surface of Escherichia coli. By mixing the E. coli with serum and Vivid, a fluorescent substrate, the investigators can measure “the inhibition rate of the Vivid decomposition reaction” that was driven by “serum metabolites associated with P450s,” revealing underlying expression and, if present, disease.
After some promising initial experiments with mouse models of ulcerative colitis and diabetes, Dr. Ihara and colleagues focused on a rat model of Parkinson’s disease. Evaluating inhibition rates associated with four P450s revealed area-under-the-curve (AUC) values of 0.814-0.914. Two of those P450s were also associated with progression of disease symptoms.
“Therefore, we concluded that the P450 inhibition assay could discriminate between Parkinson’s disease model rats and control rats,” the investigators wrote.
Next, the investigators tested the approach with a case-control study involving 20 patients with Parkinson’s disease and 20 healthy volunteers. Twelve P450s were analyzed, three of which revealed significant differences between patients with Parkinson’s disease and controls, with AUCs ranging from 0.740-0.775. Each of the three P450 enzymes also correlated significantly with stage of disease on the Hoehn & Yahr scale, although severity and frequency of symptoms were not reported.
To increase accuracy of the technique, the investigators developed a logistic regression model using two of the three P450s, generating an AUC of 0.910. Further testing showed that the P450 inhibition assay could distinguish between patients with Parkinson’s disease and Alzheimer’s disease, as well as other chronic inflammatory diseases.
“The P450 inhibition assay is easier to perform and is faster than other assays because this assay does not require pretreatment, such as purification of exosomes, and it involves a single enzymatic reaction,” the investigators wrote, suggesting that the assay may be suitable for real-world diagnosis.
‘Promising’ findings need replication
According to Douglas Galasko, MD, a neurologist and professor of neurosciences at UC San Diego Health, the reported accuracy of the assay “seems spectacular,” and the findings are “promising,” but they need to be replicated, “particularly in early-stage patients where the diagnosis [of Parkinson’s disease] is more difficult and important to make.” In practice, the assay would likely see greatest usage for “early diagnosis or diagnosis of unusual or challenging cases,” so accuracy testing needs to be conducted in this setting, he said.
Dr. Galasko, who was not involved in the study, predicted that liquid biopsy for detecting Parkinson’s disease is unlikely to hit the clinic floor anytime soon. “We’re not really close with blood-based biomarkers for Parkinson’s disease,” he said, “unlike the situation for Alzheimer’s disease, where there are several promising blood-based biomarkers.”
For diagnosing Parkinson’s disease, Dr. Galasko suggested that assays using skin biopsies to measure alpha-synuclein accumulation may be closer to approval.
The study was supported by JSPS KAKENHI Grant Number 20K20223 and the Sumitomo Electric Industries Group Corporate Social Responsibility Foundation. The investigators disclosed no conflicts of interest.
A novel blood-based assay could one day be used to diagnose Parkinson’s disease and possibly other chronic inflammatory conditions, according to investigators. In addition to being highly accurate, the assay, which detects changes in expression of cytochrome P450s, is faster and easier to perform than other Parkinson’s disease assays under investigation, reported lead author Kohei Ihara, PhD, of Kobe University, Japan, and colleagues.
“Effective diagnostic systems and biomarkers for patients without subjective motor symptoms have not yet been established,” the investigators wrote in Nature Scientific Reports. “Consequently, the poor diagnostic options for Parkinson’s disease delay the development of therapeutic approaches and medication. Therefore, the development of efficient diagnostic systems and biomarkers is crucial for overcoming Parkinson’s disease.”
According to Dr. Ihara and colleagues, various cytochrome P450 expression patterns and associated serum metabolites correlate with chronic conditions, making them possible markers of disease. To detect these changes, they developed the present assay. It relies upon recombinant P450s expressed on the surface of Escherichia coli. By mixing the E. coli with serum and Vivid, a fluorescent substrate, the investigators can measure “the inhibition rate of the Vivid decomposition reaction” that was driven by “serum metabolites associated with P450s,” revealing underlying expression and, if present, disease.
After some promising initial experiments with mouse models of ulcerative colitis and diabetes, Dr. Ihara and colleagues focused on a rat model of Parkinson’s disease. Evaluating inhibition rates associated with four P450s revealed area-under-the-curve (AUC) values of 0.814-0.914. Two of those P450s were also associated with progression of disease symptoms.
“Therefore, we concluded that the P450 inhibition assay could discriminate between Parkinson’s disease model rats and control rats,” the investigators wrote.
Next, the investigators tested the approach with a case-control study involving 20 patients with Parkinson’s disease and 20 healthy volunteers. Twelve P450s were analyzed, three of which revealed significant differences between patients with Parkinson’s disease and controls, with AUCs ranging from 0.740-0.775. Each of the three P450 enzymes also correlated significantly with stage of disease on the Hoehn & Yahr scale, although severity and frequency of symptoms were not reported.
To increase accuracy of the technique, the investigators developed a logistic regression model using two of the three P450s, generating an AUC of 0.910. Further testing showed that the P450 inhibition assay could distinguish between patients with Parkinson’s disease and Alzheimer’s disease, as well as other chronic inflammatory diseases.
“The P450 inhibition assay is easier to perform and is faster than other assays because this assay does not require pretreatment, such as purification of exosomes, and it involves a single enzymatic reaction,” the investigators wrote, suggesting that the assay may be suitable for real-world diagnosis.
‘Promising’ findings need replication
According to Douglas Galasko, MD, a neurologist and professor of neurosciences at UC San Diego Health, the reported accuracy of the assay “seems spectacular,” and the findings are “promising,” but they need to be replicated, “particularly in early-stage patients where the diagnosis [of Parkinson’s disease] is more difficult and important to make.” In practice, the assay would likely see greatest usage for “early diagnosis or diagnosis of unusual or challenging cases,” so accuracy testing needs to be conducted in this setting, he said.
Dr. Galasko, who was not involved in the study, predicted that liquid biopsy for detecting Parkinson’s disease is unlikely to hit the clinic floor anytime soon. “We’re not really close with blood-based biomarkers for Parkinson’s disease,” he said, “unlike the situation for Alzheimer’s disease, where there are several promising blood-based biomarkers.”
For diagnosing Parkinson’s disease, Dr. Galasko suggested that assays using skin biopsies to measure alpha-synuclein accumulation may be closer to approval.
The study was supported by JSPS KAKENHI Grant Number 20K20223 and the Sumitomo Electric Industries Group Corporate Social Responsibility Foundation. The investigators disclosed no conflicts of interest.
A novel blood-based assay could one day be used to diagnose Parkinson’s disease and possibly other chronic inflammatory conditions, according to investigators. In addition to being highly accurate, the assay, which detects changes in expression of cytochrome P450s, is faster and easier to perform than other Parkinson’s disease assays under investigation, reported lead author Kohei Ihara, PhD, of Kobe University, Japan, and colleagues.
“Effective diagnostic systems and biomarkers for patients without subjective motor symptoms have not yet been established,” the investigators wrote in Nature Scientific Reports. “Consequently, the poor diagnostic options for Parkinson’s disease delay the development of therapeutic approaches and medication. Therefore, the development of efficient diagnostic systems and biomarkers is crucial for overcoming Parkinson’s disease.”
According to Dr. Ihara and colleagues, various cytochrome P450 expression patterns and associated serum metabolites correlate with chronic conditions, making them possible markers of disease. To detect these changes, they developed the present assay. It relies upon recombinant P450s expressed on the surface of Escherichia coli. By mixing the E. coli with serum and Vivid, a fluorescent substrate, the investigators can measure “the inhibition rate of the Vivid decomposition reaction” that was driven by “serum metabolites associated with P450s,” revealing underlying expression and, if present, disease.
After some promising initial experiments with mouse models of ulcerative colitis and diabetes, Dr. Ihara and colleagues focused on a rat model of Parkinson’s disease. Evaluating inhibition rates associated with four P450s revealed area-under-the-curve (AUC) values of 0.814-0.914. Two of those P450s were also associated with progression of disease symptoms.
“Therefore, we concluded that the P450 inhibition assay could discriminate between Parkinson’s disease model rats and control rats,” the investigators wrote.
Next, the investigators tested the approach with a case-control study involving 20 patients with Parkinson’s disease and 20 healthy volunteers. Twelve P450s were analyzed, three of which revealed significant differences between patients with Parkinson’s disease and controls, with AUCs ranging from 0.740-0.775. Each of the three P450 enzymes also correlated significantly with stage of disease on the Hoehn & Yahr scale, although severity and frequency of symptoms were not reported.
To increase accuracy of the technique, the investigators developed a logistic regression model using two of the three P450s, generating an AUC of 0.910. Further testing showed that the P450 inhibition assay could distinguish between patients with Parkinson’s disease and Alzheimer’s disease, as well as other chronic inflammatory diseases.
“The P450 inhibition assay is easier to perform and is faster than other assays because this assay does not require pretreatment, such as purification of exosomes, and it involves a single enzymatic reaction,” the investigators wrote, suggesting that the assay may be suitable for real-world diagnosis.
‘Promising’ findings need replication
According to Douglas Galasko, MD, a neurologist and professor of neurosciences at UC San Diego Health, the reported accuracy of the assay “seems spectacular,” and the findings are “promising,” but they need to be replicated, “particularly in early-stage patients where the diagnosis [of Parkinson’s disease] is more difficult and important to make.” In practice, the assay would likely see greatest usage for “early diagnosis or diagnosis of unusual or challenging cases,” so accuracy testing needs to be conducted in this setting, he said.
Dr. Galasko, who was not involved in the study, predicted that liquid biopsy for detecting Parkinson’s disease is unlikely to hit the clinic floor anytime soon. “We’re not really close with blood-based biomarkers for Parkinson’s disease,” he said, “unlike the situation for Alzheimer’s disease, where there are several promising blood-based biomarkers.”
For diagnosing Parkinson’s disease, Dr. Galasko suggested that assays using skin biopsies to measure alpha-synuclein accumulation may be closer to approval.
The study was supported by JSPS KAKENHI Grant Number 20K20223 and the Sumitomo Electric Industries Group Corporate Social Responsibility Foundation. The investigators disclosed no conflicts of interest.
FROM NATURE SCIENTIFIC REPORTS
Deep brain stimulation fails to halt depression in Parkinson’s disease
Treatment with deep brain stimulation improved motor function and quality of life, but depression scores increased after 1 year, based on data from 20 adults.
Subthalamic nucleus deep brain stimulation (STN-DBS) has emerged as an effective treatment for Parkinson’s disease symptoms, with evidence supporting improved motor symptoms and quality of life, wrote Francesca Mameli, PsyD, of Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and colleagues.
However, the effect of STN-DBS on personality in Parkinson’s disease (PD) has not been well investigated they said.
In a study published in Neuromodulation, the researchers reviewed data from 12 women and 8 men with PD who underwent bilateral STN-DBS.
Depression was assessed via the Montgomery-Asberg Depressive Rating Scale (MADRS), personality characteristics were assessed via the Minnesota Multiphasic Personality Inventory–2 (MMPI-2), and motor disabilities were assessed via UPDRS-III-Motor. The motor disabilities score was obtained in medication on and medication off conditions; the off condition followed a 12-hour overnight withdrawal of dopaminergic medication. Quality of life was assessed via the Parkinson’s Disease Questionnaire–8 (PDQ-8).
After 12 months, scores on the MMPI-2 were significantly higher on the D subscale, increased from a baseline mean of 56.05 to a 12-month mean of 61.90 (P = .015).
Other MMPI-2 scales showing significant increases included the DEP scale, LSE scale, WRK scale, and TRT scale. No differences appeared between male and female patients.
No significant changes occurred from pre-DBS baseline to the 12-month follow-up in MADRS scale assessment, with mean scores of 8.18 and 9.22, respectively.
A 40% improvement in UPDRS measures of motor function occurred among patients in the “medication-off” condition, although there was no significant change following DBS in the medication-on condition, the researchers said. Among 18 patients with PDQ-8 assessments, quality of life scores were significantly higher at 12 months’ post DBS compared to baseline pre DBS (40.15 vs. 30.73, P = .011).
The researchers also examined the relationship between the total electrical energy delivered (TEED) and the occurrence of personality trait shift. In the TEED analysis, “only the energy on the right side was inversely correlated with the changes in depression,” they wrote.
“Because of the complexity of psychiatric phenomena, it would be advisable to take a cautious approach by including psychiatric evaluation by interview for a better selection of patients who score close to the pathological cutoffs in MADRS and MMPI-2,” the researchers wrote in their discussion.
The study findings were limited by several factors including the small sample size, lack of data on the prevalence and severity of apathy, the use of scales based on self-reports, and inability to control for all factors that might affect depressive traits, the researchers noted. In addition, more research is needed to explore the correlation between TEED and personality trait changes, they said.
However, the results support the value of DBS in PD, but emphasize the need to manage expectations, they emphasized. “Expectations should never be unrealistic, and the caring team should ensure not only that patients fully understand the risks and potential benefits of the DBS but also that it will not stop the neurodegenerative progression of the disease,” they said.
The study was supported in part by the Italian Ministry of Health. The researchers had no financial conflicts to disclose.
Treatment with deep brain stimulation improved motor function and quality of life, but depression scores increased after 1 year, based on data from 20 adults.
Subthalamic nucleus deep brain stimulation (STN-DBS) has emerged as an effective treatment for Parkinson’s disease symptoms, with evidence supporting improved motor symptoms and quality of life, wrote Francesca Mameli, PsyD, of Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and colleagues.
However, the effect of STN-DBS on personality in Parkinson’s disease (PD) has not been well investigated they said.
In a study published in Neuromodulation, the researchers reviewed data from 12 women and 8 men with PD who underwent bilateral STN-DBS.
Depression was assessed via the Montgomery-Asberg Depressive Rating Scale (MADRS), personality characteristics were assessed via the Minnesota Multiphasic Personality Inventory–2 (MMPI-2), and motor disabilities were assessed via UPDRS-III-Motor. The motor disabilities score was obtained in medication on and medication off conditions; the off condition followed a 12-hour overnight withdrawal of dopaminergic medication. Quality of life was assessed via the Parkinson’s Disease Questionnaire–8 (PDQ-8).
After 12 months, scores on the MMPI-2 were significantly higher on the D subscale, increased from a baseline mean of 56.05 to a 12-month mean of 61.90 (P = .015).
Other MMPI-2 scales showing significant increases included the DEP scale, LSE scale, WRK scale, and TRT scale. No differences appeared between male and female patients.
No significant changes occurred from pre-DBS baseline to the 12-month follow-up in MADRS scale assessment, with mean scores of 8.18 and 9.22, respectively.
A 40% improvement in UPDRS measures of motor function occurred among patients in the “medication-off” condition, although there was no significant change following DBS in the medication-on condition, the researchers said. Among 18 patients with PDQ-8 assessments, quality of life scores were significantly higher at 12 months’ post DBS compared to baseline pre DBS (40.15 vs. 30.73, P = .011).
The researchers also examined the relationship between the total electrical energy delivered (TEED) and the occurrence of personality trait shift. In the TEED analysis, “only the energy on the right side was inversely correlated with the changes in depression,” they wrote.
“Because of the complexity of psychiatric phenomena, it would be advisable to take a cautious approach by including psychiatric evaluation by interview for a better selection of patients who score close to the pathological cutoffs in MADRS and MMPI-2,” the researchers wrote in their discussion.
The study findings were limited by several factors including the small sample size, lack of data on the prevalence and severity of apathy, the use of scales based on self-reports, and inability to control for all factors that might affect depressive traits, the researchers noted. In addition, more research is needed to explore the correlation between TEED and personality trait changes, they said.
However, the results support the value of DBS in PD, but emphasize the need to manage expectations, they emphasized. “Expectations should never be unrealistic, and the caring team should ensure not only that patients fully understand the risks and potential benefits of the DBS but also that it will not stop the neurodegenerative progression of the disease,” they said.
The study was supported in part by the Italian Ministry of Health. The researchers had no financial conflicts to disclose.
Treatment with deep brain stimulation improved motor function and quality of life, but depression scores increased after 1 year, based on data from 20 adults.
Subthalamic nucleus deep brain stimulation (STN-DBS) has emerged as an effective treatment for Parkinson’s disease symptoms, with evidence supporting improved motor symptoms and quality of life, wrote Francesca Mameli, PsyD, of Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, and colleagues.
However, the effect of STN-DBS on personality in Parkinson’s disease (PD) has not been well investigated they said.
In a study published in Neuromodulation, the researchers reviewed data from 12 women and 8 men with PD who underwent bilateral STN-DBS.
Depression was assessed via the Montgomery-Asberg Depressive Rating Scale (MADRS), personality characteristics were assessed via the Minnesota Multiphasic Personality Inventory–2 (MMPI-2), and motor disabilities were assessed via UPDRS-III-Motor. The motor disabilities score was obtained in medication on and medication off conditions; the off condition followed a 12-hour overnight withdrawal of dopaminergic medication. Quality of life was assessed via the Parkinson’s Disease Questionnaire–8 (PDQ-8).
After 12 months, scores on the MMPI-2 were significantly higher on the D subscale, increased from a baseline mean of 56.05 to a 12-month mean of 61.90 (P = .015).
Other MMPI-2 scales showing significant increases included the DEP scale, LSE scale, WRK scale, and TRT scale. No differences appeared between male and female patients.
No significant changes occurred from pre-DBS baseline to the 12-month follow-up in MADRS scale assessment, with mean scores of 8.18 and 9.22, respectively.
A 40% improvement in UPDRS measures of motor function occurred among patients in the “medication-off” condition, although there was no significant change following DBS in the medication-on condition, the researchers said. Among 18 patients with PDQ-8 assessments, quality of life scores were significantly higher at 12 months’ post DBS compared to baseline pre DBS (40.15 vs. 30.73, P = .011).
The researchers also examined the relationship between the total electrical energy delivered (TEED) and the occurrence of personality trait shift. In the TEED analysis, “only the energy on the right side was inversely correlated with the changes in depression,” they wrote.
“Because of the complexity of psychiatric phenomena, it would be advisable to take a cautious approach by including psychiatric evaluation by interview for a better selection of patients who score close to the pathological cutoffs in MADRS and MMPI-2,” the researchers wrote in their discussion.
The study findings were limited by several factors including the small sample size, lack of data on the prevalence and severity of apathy, the use of scales based on self-reports, and inability to control for all factors that might affect depressive traits, the researchers noted. In addition, more research is needed to explore the correlation between TEED and personality trait changes, they said.
However, the results support the value of DBS in PD, but emphasize the need to manage expectations, they emphasized. “Expectations should never be unrealistic, and the caring team should ensure not only that patients fully understand the risks and potential benefits of the DBS but also that it will not stop the neurodegenerative progression of the disease,” they said.
The study was supported in part by the Italian Ministry of Health. The researchers had no financial conflicts to disclose.
FROM NEUROMODULATION









